Construction of Zeolite-Loaded Fluorescent Supramolecular on-off Probes for Corrosion Detection Based on a Cation Exchange Mechanism
Abstract
1. Introduction
2. Materials and Experiments
2.1. Preparation of RBA and RBA/ZEO
2.2. Characterization of RBA and RBA/ZEO
2.3. UV-Vis Absorption and Fluorescence Behavior
2.4. Release Behavior of RBA from RBA/ZEO
2.5. Fluorescence Detection of RBA/ZEO/Epoxy Coatings
2.6. Anticorrosion Performance of RBA/ZEO/Epoxy Coatings
3. Results and Discussion
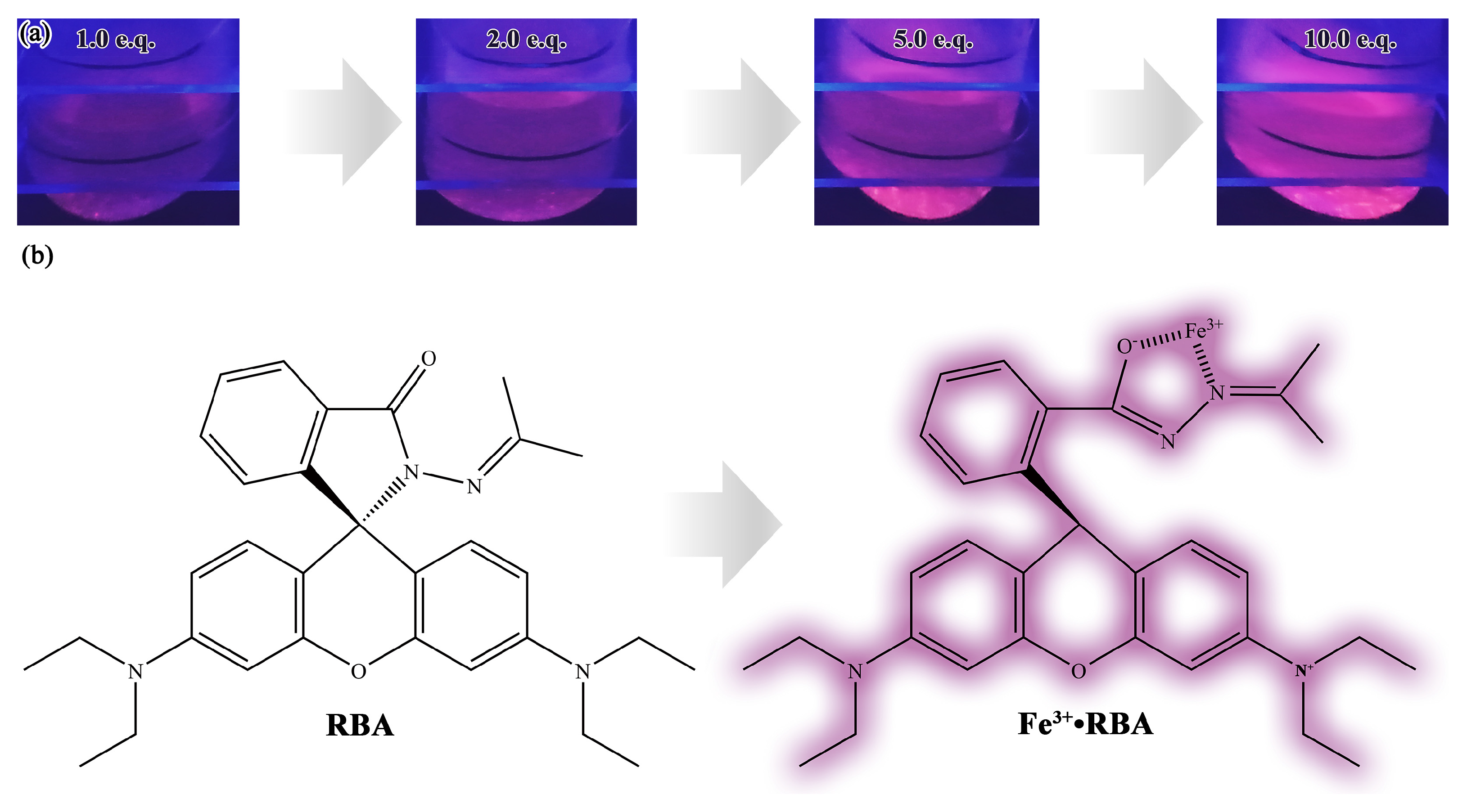
3.1. Structural Analysis of RBA
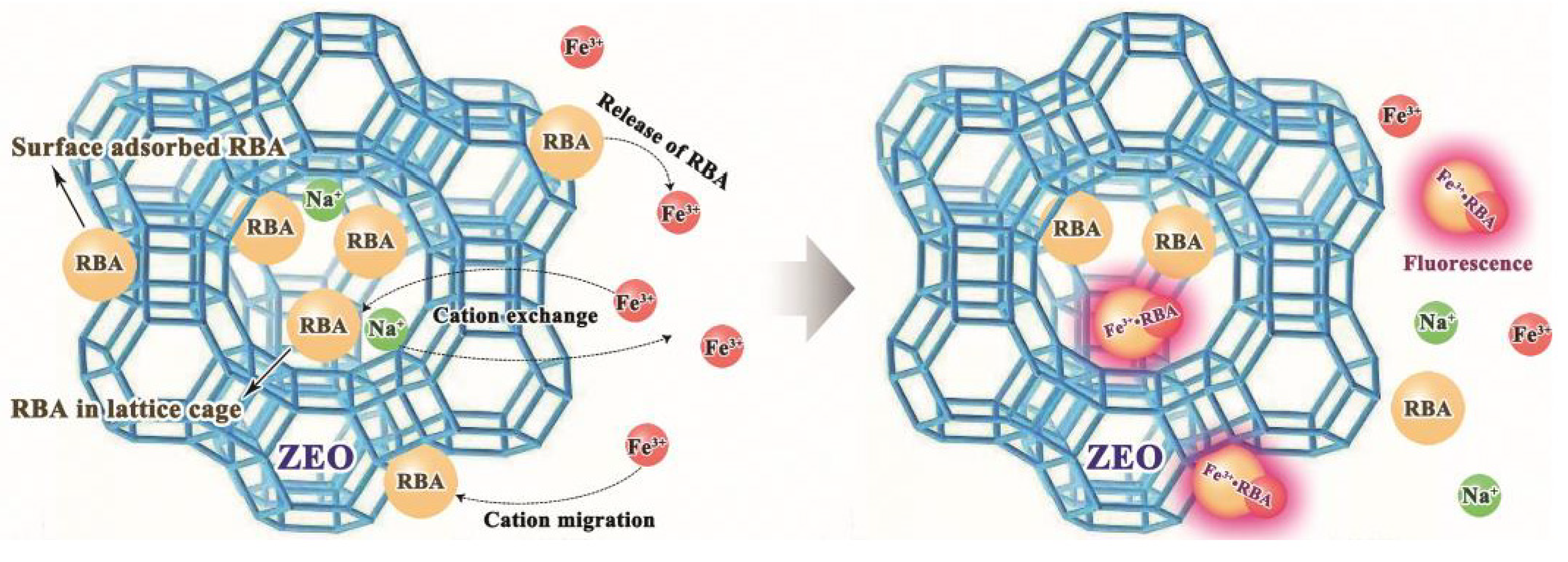
3.2. Loading Behavior and Characterization of ZEO/RBA
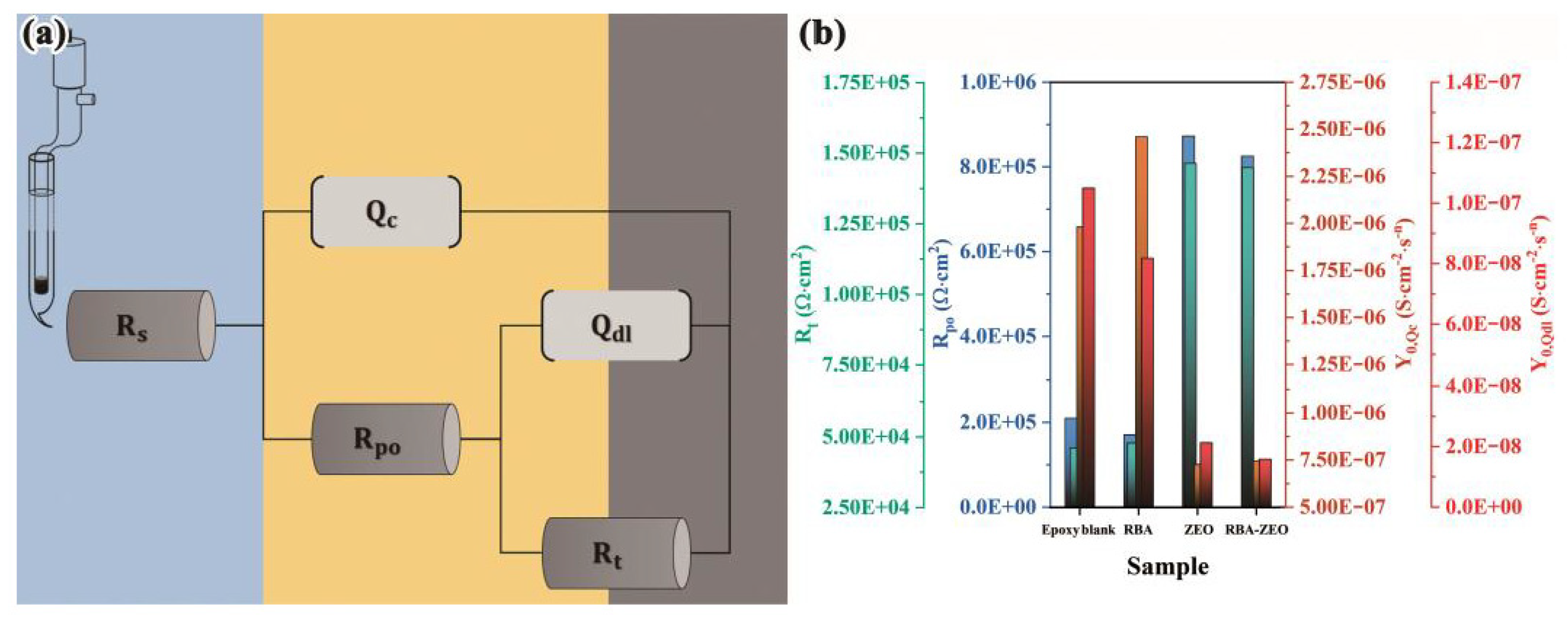
3.3. Anti-Corrosion Performance of RBA/ZEO Coatings
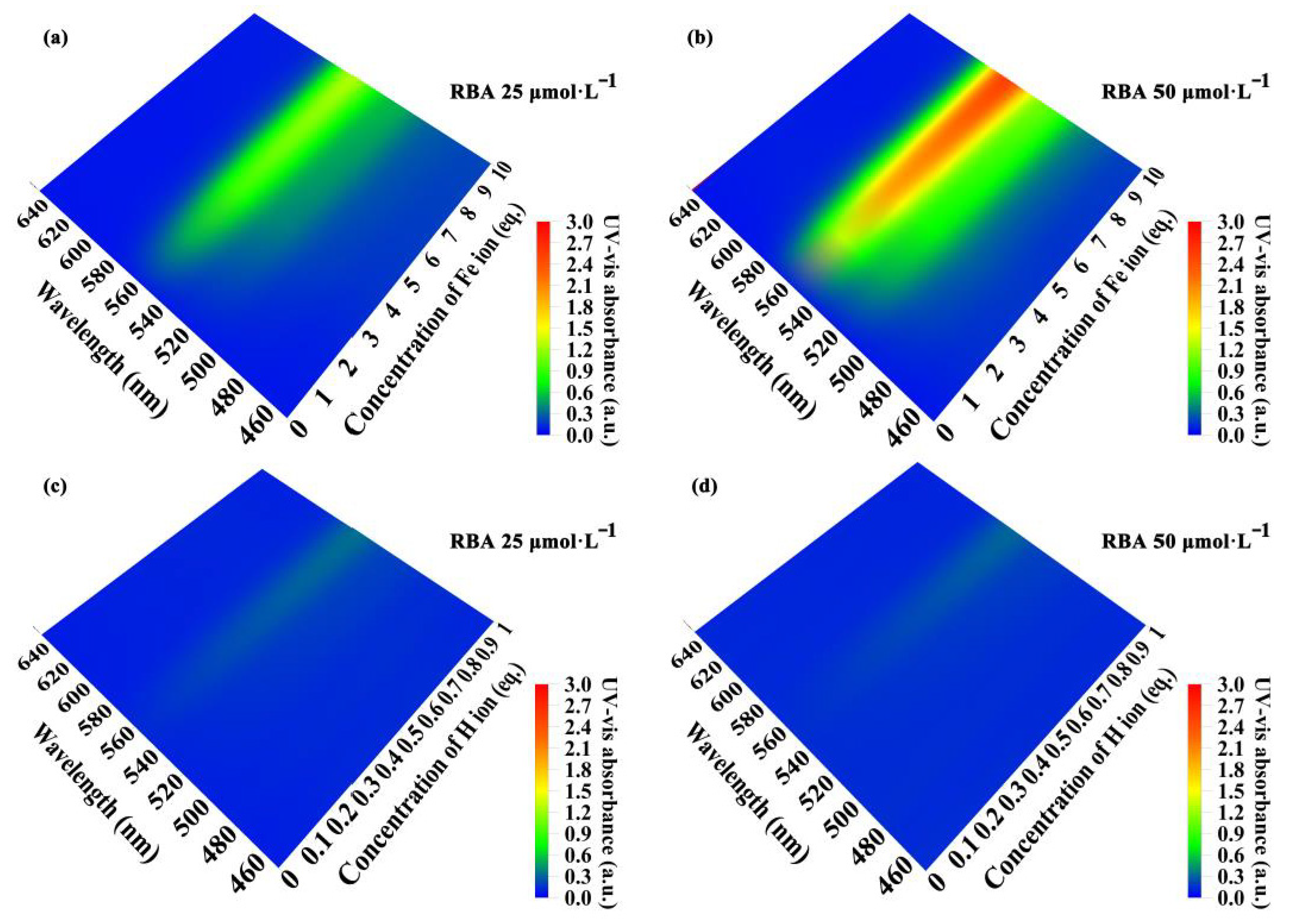
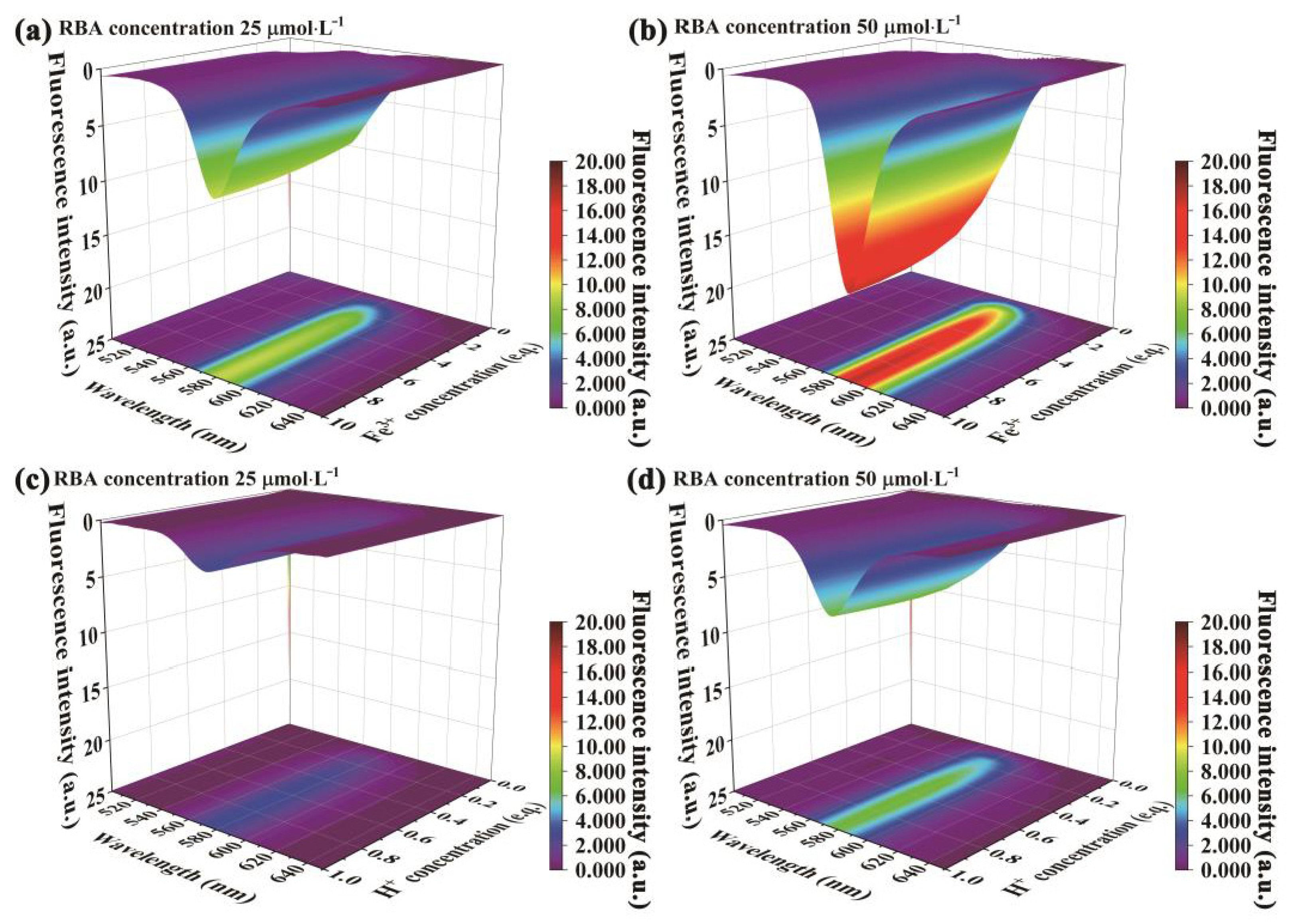
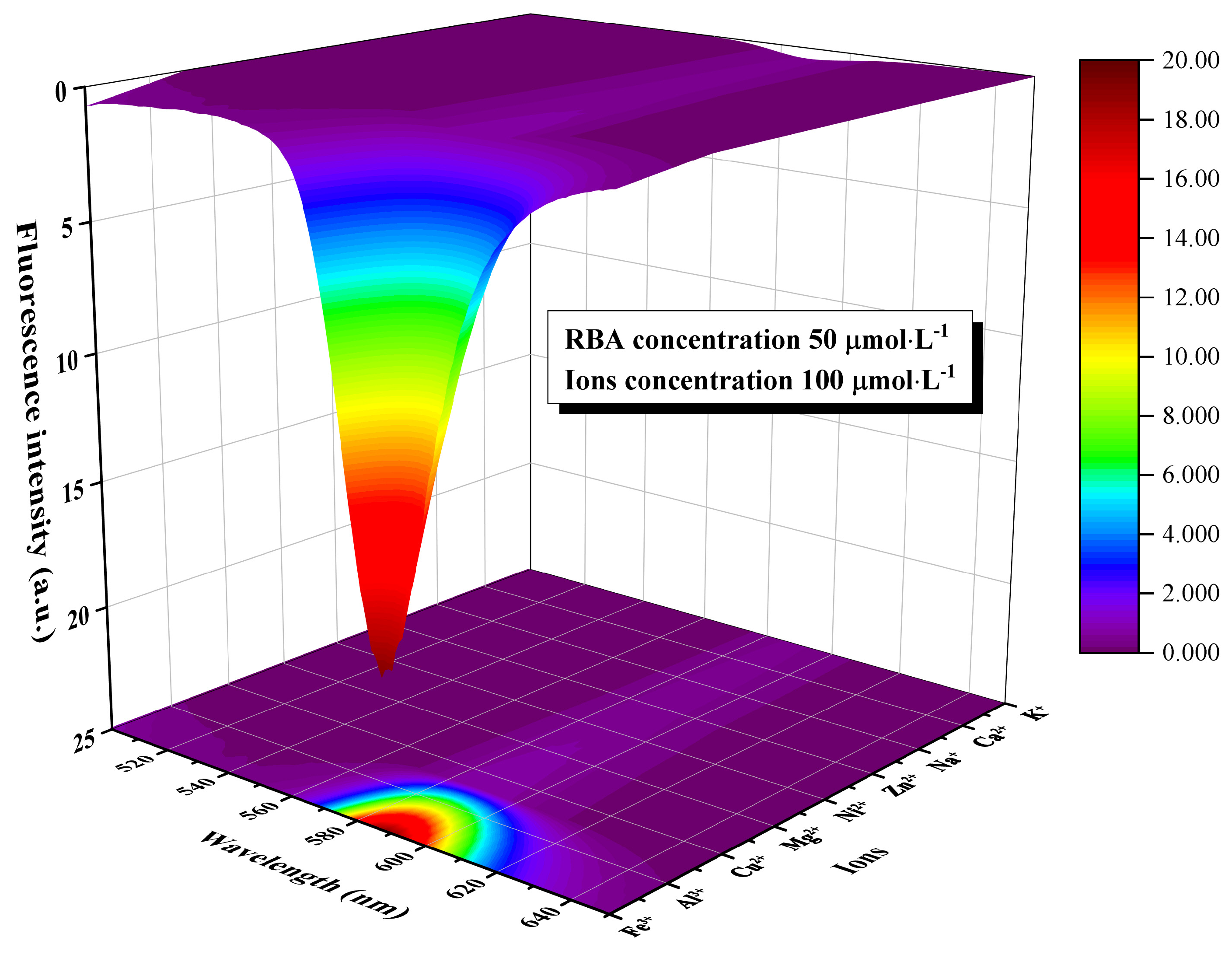
3.4. Corrosion Factor Responsive Fluorescence Switching Behavior of RBA
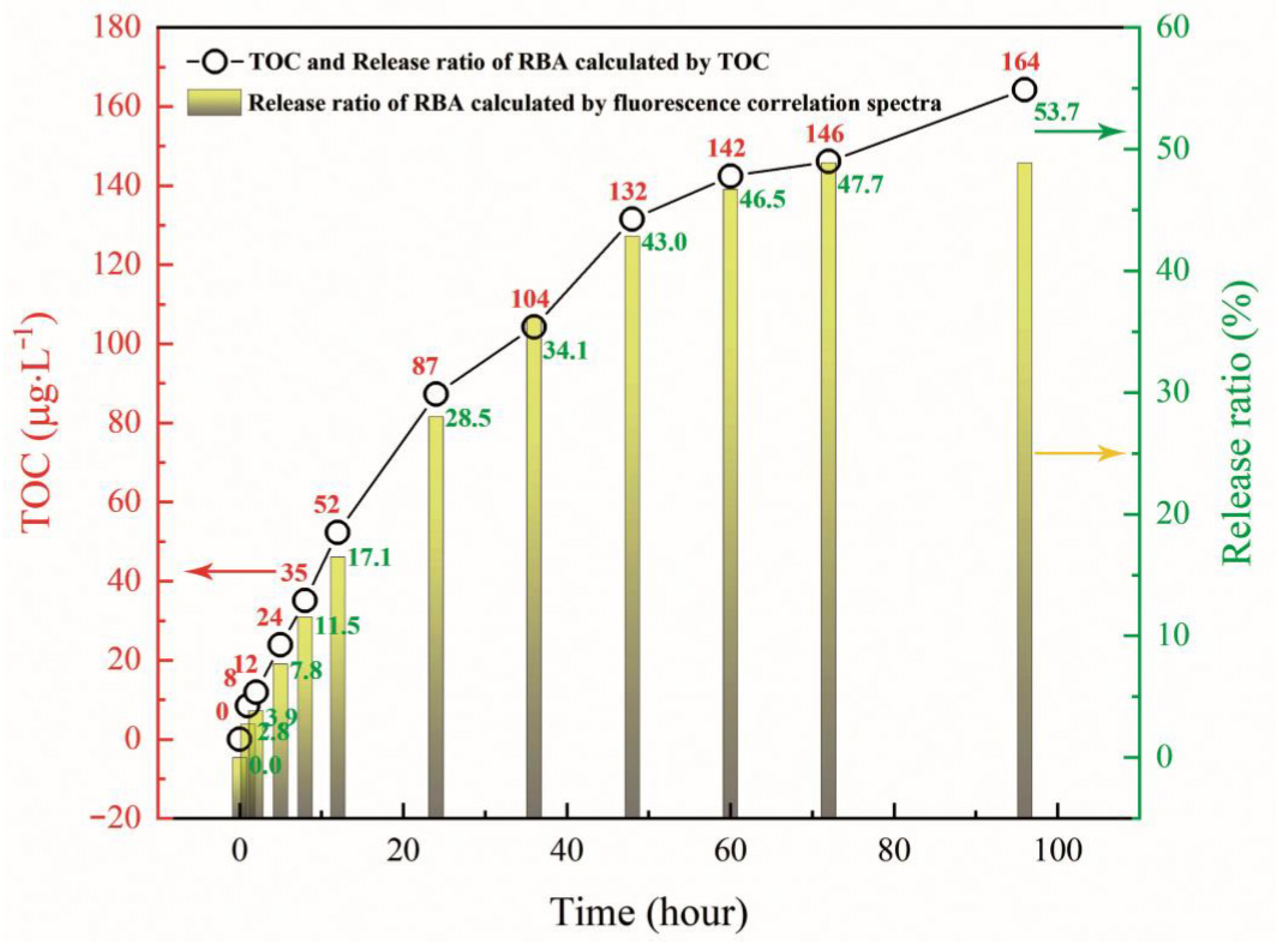
3.5. Release Behavior of RBA/ZEO
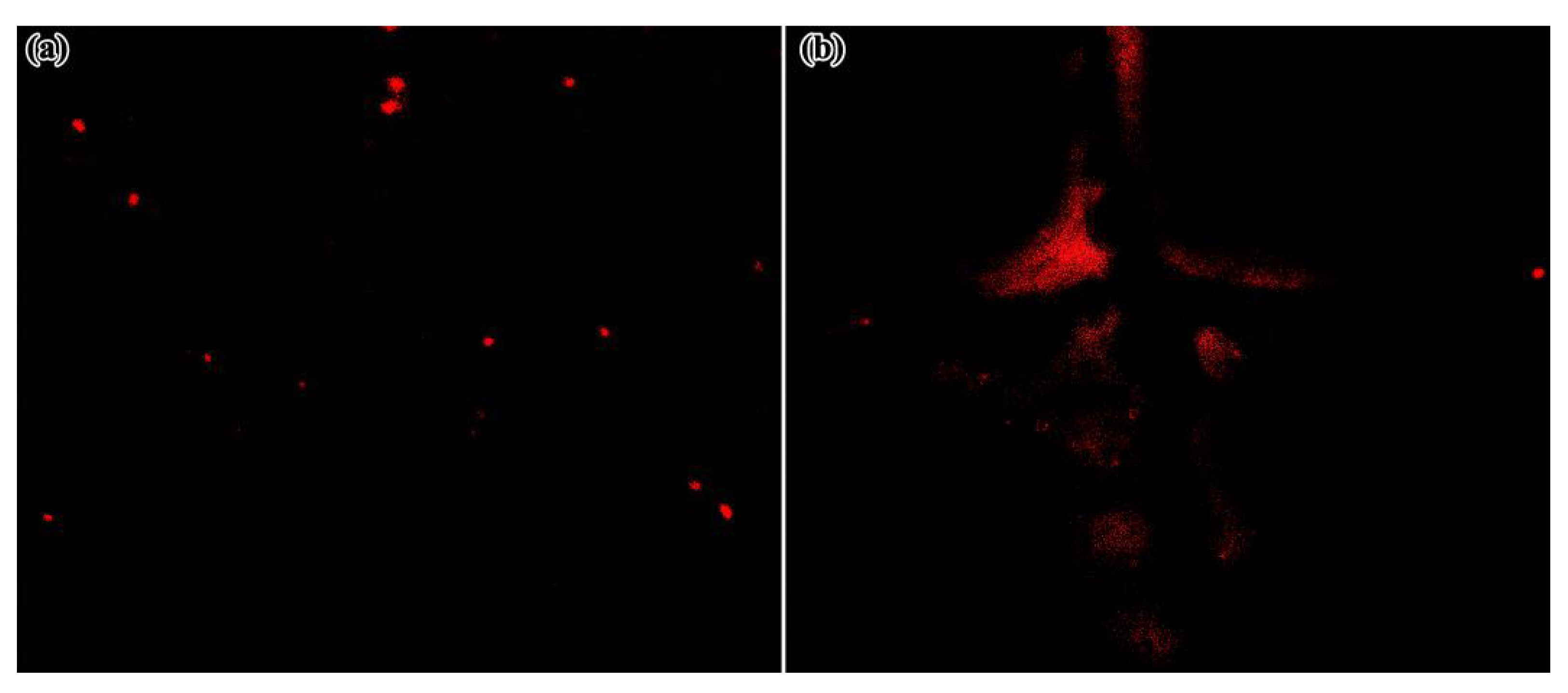
3.6. Corrosion Detection Performance of RBA/ZEO in Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Francis, K.; Mitchell, S.; Matari, N. Use of Corrosion-Mitigating Thixotropic Gel for Riser Abandonment Inside Pull-Tube. In Proceedings of the 27th International Ocean and Polar Engineering Conference, San Francisco, CA, USA, 25–30 June 2017; International Society of Offshore and Polar Engineers: Mountain View, CA, USA, 2017. [Google Scholar]
- Choi, H.; Kim, K.Y.; Park, J.M. Encapsulation of aliphatic amines into nanoparticles for self-healing corrosion protection of steel sheets. Prog. Org. Coat. 2013, 76, 1316–1324. [Google Scholar] [CrossRef]
- Zaki, A.; Chai, H.K.; Behnia, A.; Aggelis, D.G.; Tan, J.Y.; Ibrahim, Z. Monitoring fracture of steel corroded reinforced concrete members under flexure by acoustic emission technique. Constr. Build. Mater. 2017, 136, 609–618. [Google Scholar] [CrossRef]
- Suchato, N.; Kalashnikov, A.; Light, R.; Sharples, S. Experimental setup of continuous ultrasonic monitoring for corrosion assessment. In Proceedings of the 2017 IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017. [Google Scholar]
- Zhu, W.; Cai, X.; Yang, L.; Xia, J.; Zhou, Y.; Pi, Z. The evolution of pores in thermal barrier coatings under volcanic ash corrosion using X-ray computed tomography. Surf. Coat. Nanotechnol. 2019, 357, 372–378. [Google Scholar] [CrossRef]
- Jones, R.; Lo, M.; Dorman, M.; Bowler, A.; Roles, D.; Wade, S. Lock-in Thermography for the Wide Area Detection of Paint Degradation and Incipient Corrosion. In Non-Destructive Evaluation of Corrosion and Corrosion-Assisted Cracking; Wiley: Hoboken, NJ, USA, 2019; pp. 122–123. [Google Scholar]
- Ren, Y.; Pan, M.; Chen, D.; Tian, W. An Electromagnetic/Capacitive Composite Sensor for Testing of Thermal Barrier Coatings. Sensors 2018, 18, 1630. [Google Scholar] [CrossRef] [PubMed]
- Denenberg, S.; Dunford, T.; Sheiretov, Y.; Haque, S.; Manning, B.; Washabaugh, A.; Goldfine, N. Advancements in imaging corrosion under insulation for piping and vessels. Mater. Eval. 2015, 73, 987–995. [Google Scholar]
- Dieleman, C.D.; Denissen, P.J.; Garcia, S.J. Long-term active corrosion protection of damaged Coated-AA2024-T3 by embedded electrospun inhibiting nanonetworks. Adv. Mater. Interfaces 2018, 5, 1800176. [Google Scholar] [CrossRef]
- Taheri, S. A review on five key sensors for monitoring of concrete structures. Constr. Build. Mater. 2019, 204, 492–509. [Google Scholar] [CrossRef]
- Huang, M.; Yang, J. Facile microencapsulation of HDI for self-healing anticorrosion coatings. J. Mater. Chem. 2011, 21, 11123–11130. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Venugopala, T.; Chen, S.; Sun, T.; Grattan, K.T.; Taylor, S.E.; Basheer, P.M.; Long, A.E. Fluorescence based fibre optic pH sensor for the pH 10–13 range suitable for corrosion monitoring in concrete structures. Sens. Actuators B Chem. 2014, 191, 498–507. [Google Scholar] [CrossRef]
- Warren-Smith, S.C.; Heng, S.; Ebendorff-Heidepriem, H.; Abell, A.D.; Monro, T.M. Fluorescence-based aluminum ion sensing using a surface-functionalized microstructured optical fiber. Langmuir 2011, 27, 5680–5685. [Google Scholar] [CrossRef] [PubMed]
- Roshan, S.; Dariani, A.A.S.; Mokhtari, J. Monitoring underlying epoxy-coated St-37 corrosion via 8-hydroxyquinoline as a fluorescent indicator. Appl. Surf. Sci. 2018, 440, 880–888. [Google Scholar] [CrossRef]
- Augustyniak, A.; Tsavalas, J.; Ming, W. Early detection of steel corrosion via “turn-on” fluorescence in smart epoxy coatings. ACS Appl. Mater. Interfaces 2009, 1, 2618–2623. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Song, Y.K.; Park, S.H.; Park, Y.I.; Noh, S.M.; Kim, J.C. Dual stimuli responsive self-reporting material for chemical reservoir coating. Appl. Surf. Sci. 2018, 434, 1327–1335. [Google Scholar] [CrossRef]
- Maesen, T. Chapter 1 The zeolite scene—An overview. Cheminform 2001, 168, 1–12. [Google Scholar]
- Karapinar, N. Application of natural zeolite for phosphorus and ammonium removal from aqueous solutions. J. Hazard. Mater. 2009, 170, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; An, Y.; Wang, Z.; Yang, S.; Chen, H.; Zhou, Z.; Mai, S. Study on zeolite enhanced contact-adsorption regeneration-stabilization process for nitrogen removal. J. Hazard. Mater. 2008, 156, 317–326. [Google Scholar] [CrossRef] [PubMed]
- El Hanache, L.; Lebeau, B.; Nouali, H.; Toufaily, J.; Hamieh, T.; Daou, T.J. Performance of surfactant-modified *BEA-type zeolite nanosponges for the removal of nitrate in contaminated water: Effect of the external surface. J. Hazard. Mater. 2019, 364, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Kovalevskiy, N.S.; Lyulyukin, M.N.; Selishchev, D.S.; Kozlov, D.V. Analysis of air photocatalytic purification using a total hazard index: Effect of the composite TiO2/zeolite photocatalyst. J. Hazard. Mater. 2018, 358, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, E.L.; Rollon, A.P.; Mendoza, H.D.; Lafont, U.; Garcia, S.J. Ugo Double-doped zeolites for corrosion protection of aluminium alloys. Microporous Mesoporous Mater. 2014, 188, 8–15. [Google Scholar] [CrossRef]

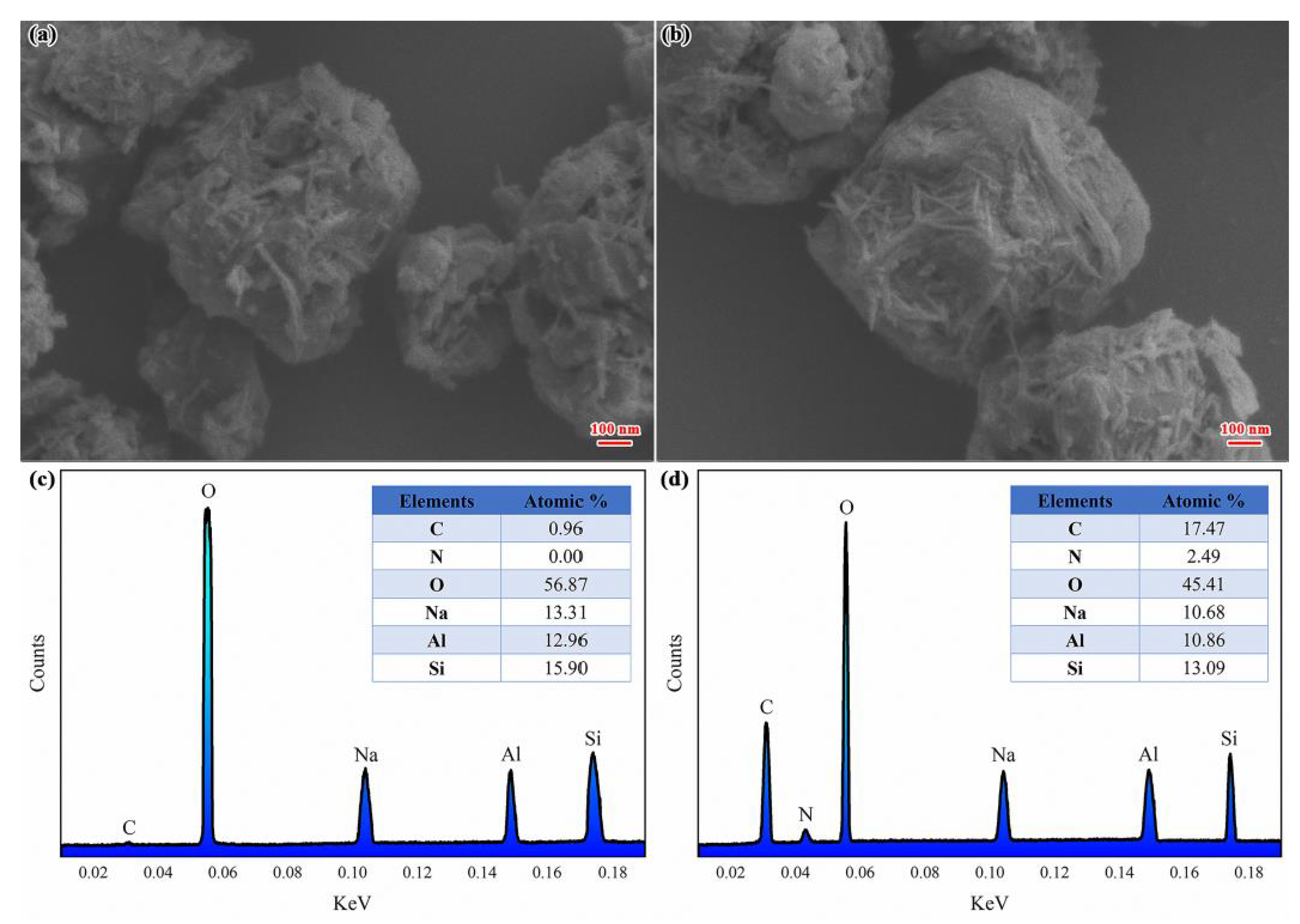
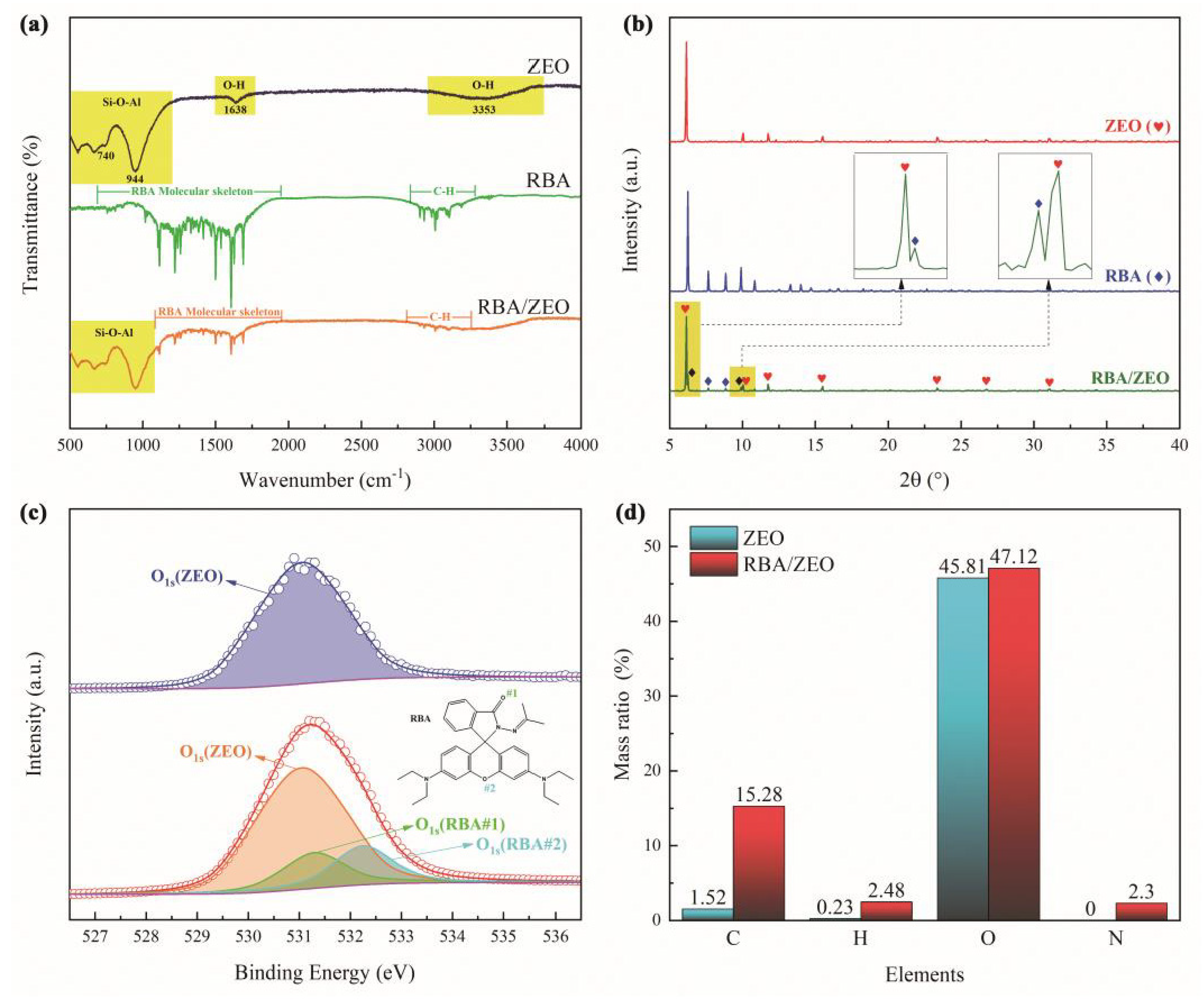


| Elements | Element Mass Ratio in ZEO | Element Mass Ratio in RBA/ZEO | RBA Load Ratio |
|---|---|---|---|
| N | 0 | 2.30 | 20.38 |
| C | 1.52 | 15.28 | 18.35 |
| H | 0.23 | 2.48 | 31.00 |
| O | 45.81 | 47.12 | 20.31 |
| Coatings | Epoxy Blank | RBA | ZEO | RBA/ZEO |
|---|---|---|---|---|
| 11.31 | 15.34 | 8.56 | 10.70 | |
| 1.98 × 10−6 | 2.59 × 10−6 | 7.04 × 10−7 | 7.67 × 10−7 | |
| 0.86 | 0.93 | 0.89 | 0.92 | |
| 2.16 × 105 | 1.83 × 105 | 8.35 × 105 | 8.31 × 105 | |
| 1.18 × 10−7 | 8.66 × 10−8 | 2.24 × 10−8 | 1.59 × 10−8 | |
| 0.85 | 0.92 | 0.83 | 0.86 | |
| 5.05 × 104 | 4.63 × 104 | 1.56 × 105 | 1.43 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.; Yue, Q.-X.; Ding, R.; Han, Q.; Liu, X.; Liu, J.-L.; Yu, H.-J.; An, K.; Yu, H.-B.; Zhao, X.-D. Construction of Zeolite-Loaded Fluorescent Supramolecular on-off Probes for Corrosion Detection Based on a Cation Exchange Mechanism. Nanomaterials 2021, 11, 169. https://doi.org/10.3390/nano11010169
Lv J, Yue Q-X, Ding R, Han Q, Liu X, Liu J-L, Yu H-J, An K, Yu H-B, Zhao X-D. Construction of Zeolite-Loaded Fluorescent Supramolecular on-off Probes for Corrosion Detection Based on a Cation Exchange Mechanism. Nanomaterials. 2021; 11(1):169. https://doi.org/10.3390/nano11010169
Chicago/Turabian StyleLv, Jing, Qing-Xian Yue, Rui Ding, Qi Han, Xin Liu, Jia-Long Liu, Hui-Jie Yu, Kang An, Hai-Bin Yu, and Xiao-Dong Zhao. 2021. "Construction of Zeolite-Loaded Fluorescent Supramolecular on-off Probes for Corrosion Detection Based on a Cation Exchange Mechanism" Nanomaterials 11, no. 1: 169. https://doi.org/10.3390/nano11010169
APA StyleLv, J., Yue, Q.-X., Ding, R., Han, Q., Liu, X., Liu, J.-L., Yu, H.-J., An, K., Yu, H.-B., & Zhao, X.-D. (2021). Construction of Zeolite-Loaded Fluorescent Supramolecular on-off Probes for Corrosion Detection Based on a Cation Exchange Mechanism. Nanomaterials, 11(1), 169. https://doi.org/10.3390/nano11010169






