Photocatalytic Properties of Eco-Friendly ZnO Nanostructures on 3D-Printed Polylactic Acid Scaffolds
Abstract
1. Introduction
2. Experimental Details
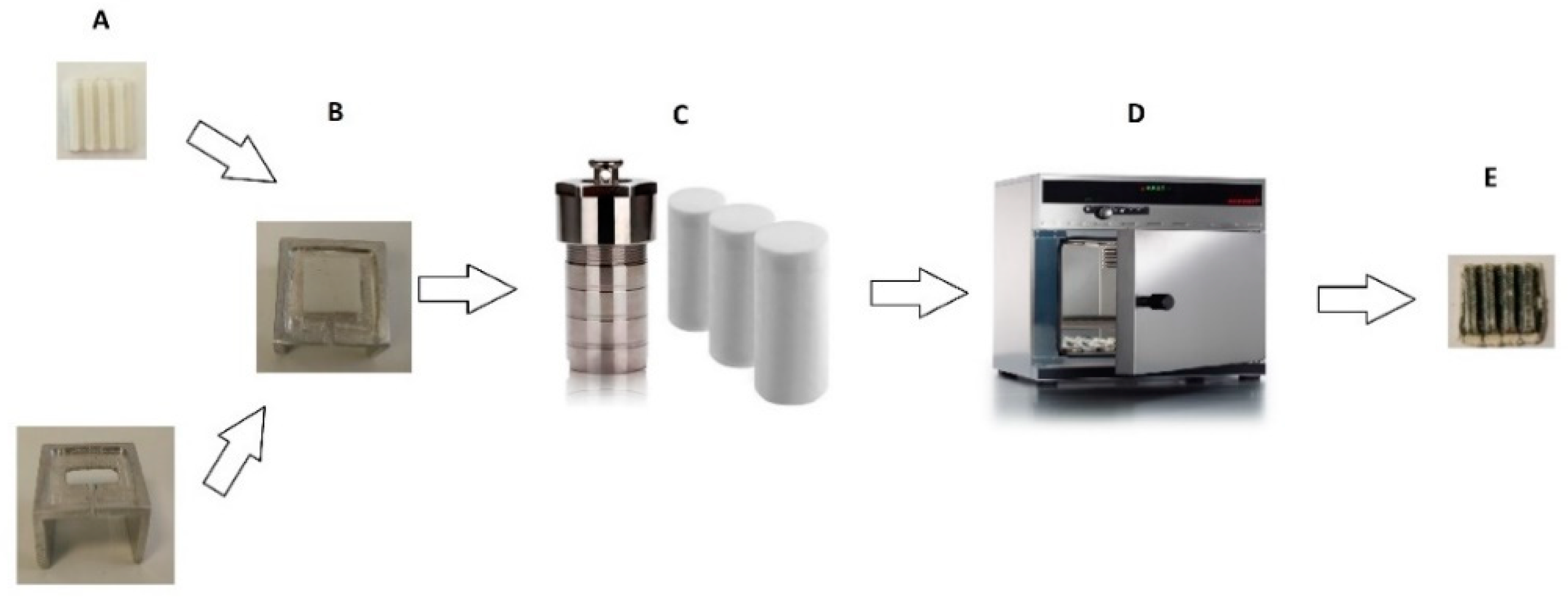
2.1. Production of 3D-Printed Scaffolds
2.2. Synthesis of the Photocatalytic Nanostructure
2.3. Characterization and Photocatalytic Experiments
2.3.1. Scanning Electron Microscopy–Energy-Dispersive X-Ray Spectroscopy
2.3.2. X-ray Diffraction
2.3.3. Raman Spectroscopy Studies
2.3.4. Photocatalytic Efficiency Measurements
3. Results and Discussion
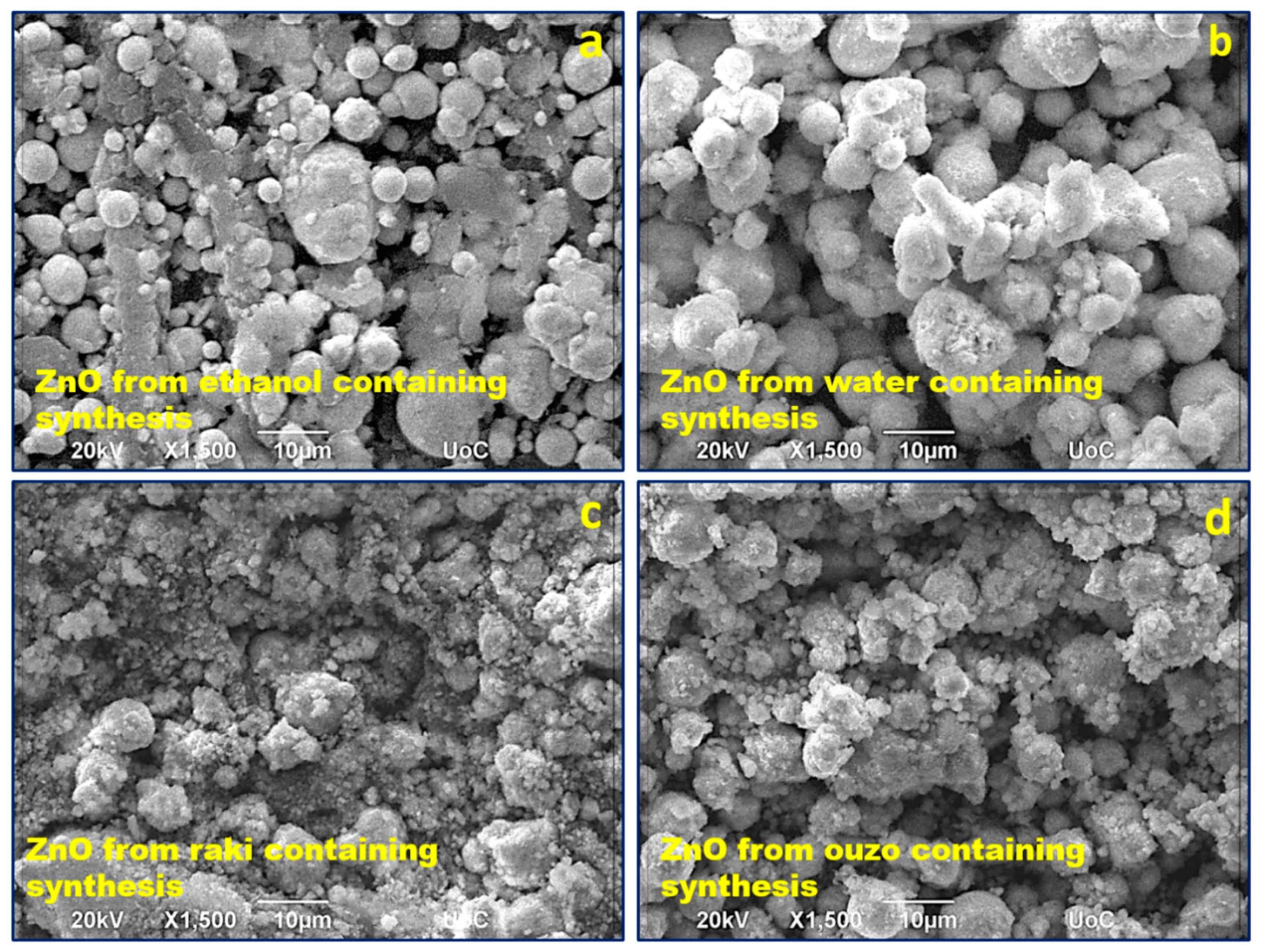
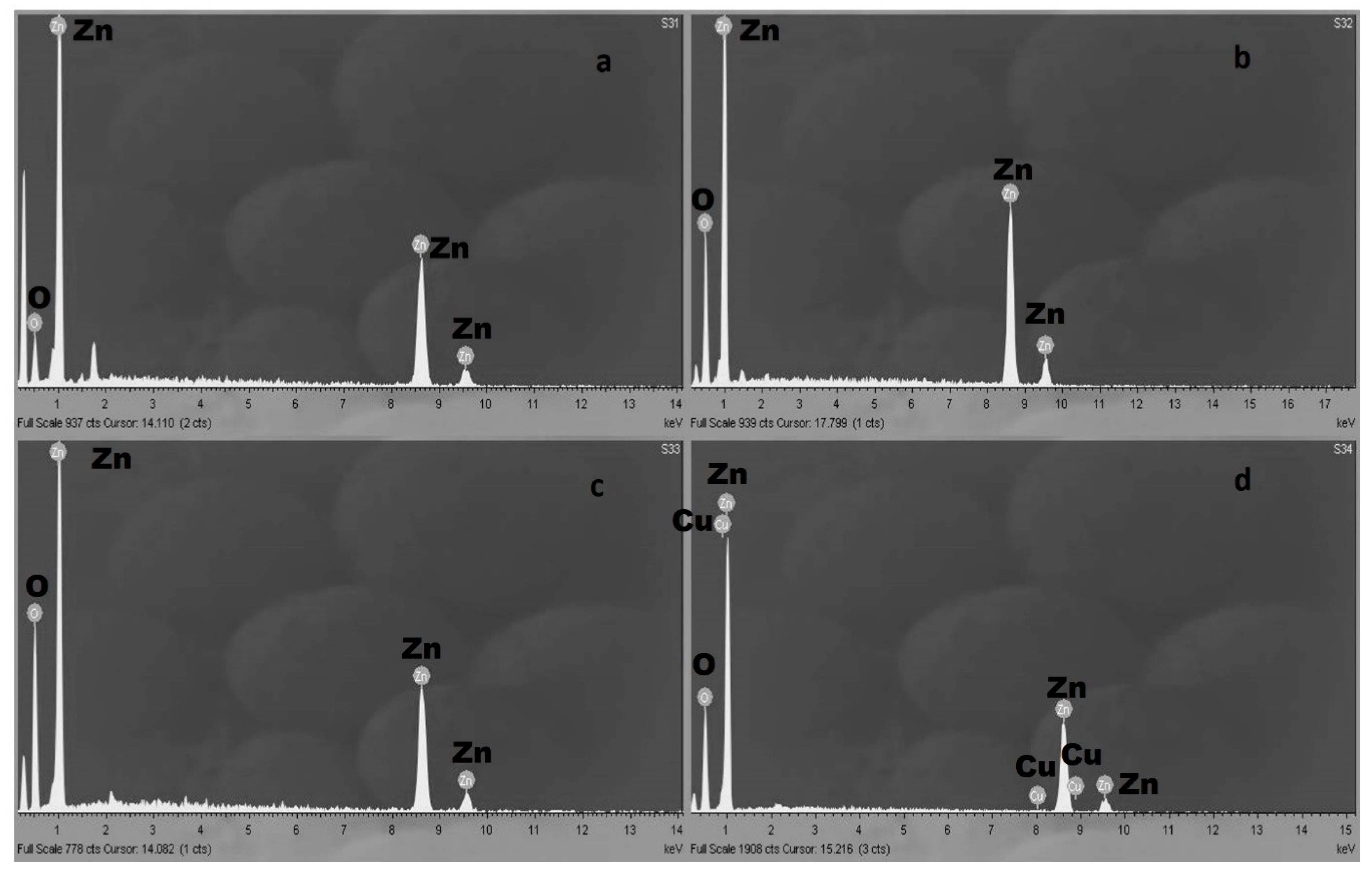
3.1. SEM and EDX Analyses
3.2. XRD Analysis
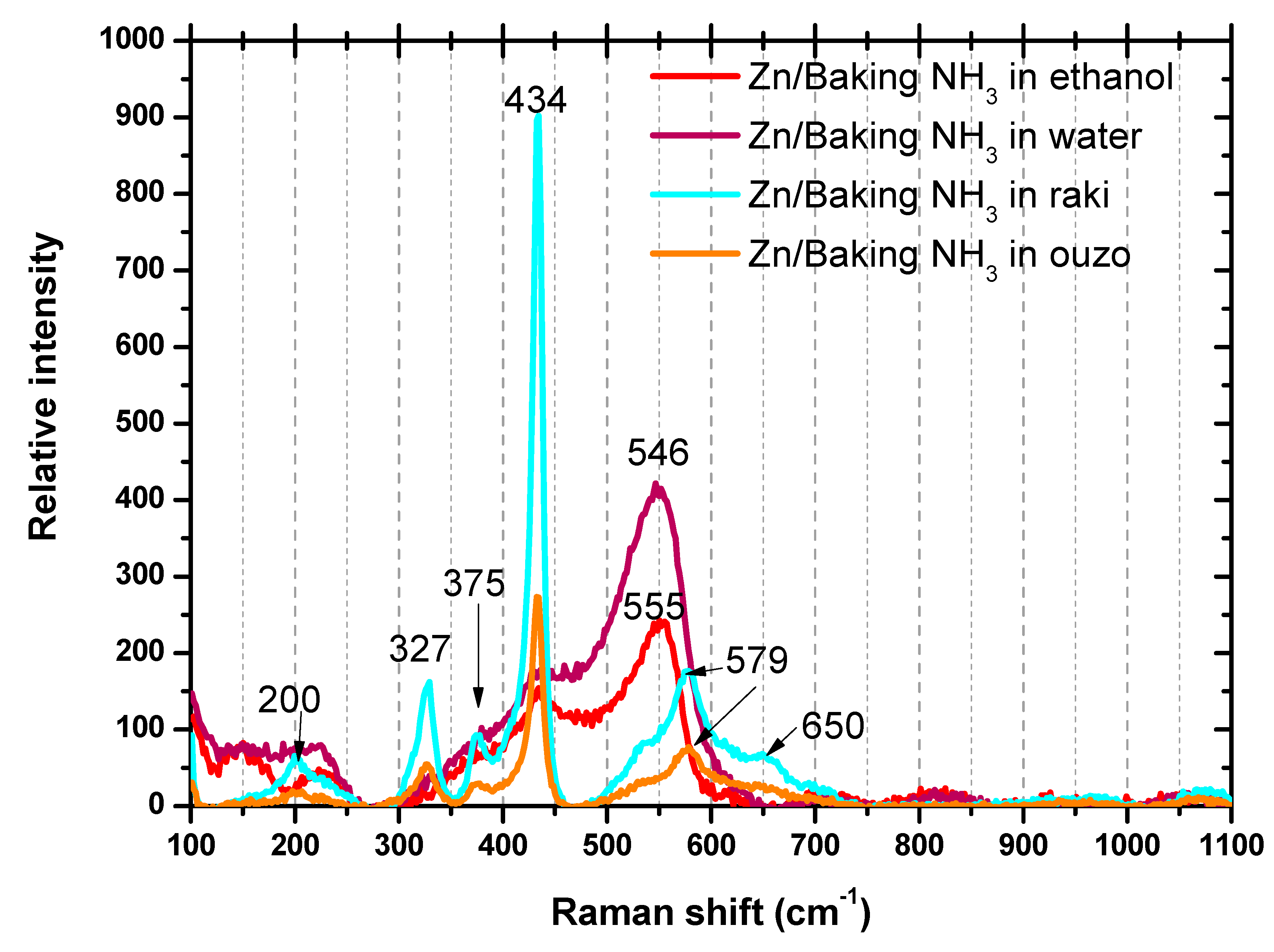
3.3. Raman Spectroscopy Analysis
3.4. Photocatalysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lijuan, J.; Yajun, W.; Changgen, F. Application of photocatalytic technology in environmental safety. Procedia Eng. 2012, 45, 993–997. [Google Scholar]
- Yanga, L.; Yu, L.E.; Ray, M.B. Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Thi, V.H.-T.; Lee, B.-K. Effective photocatalytic degradation of paracetamol using La-doped ZnO photocatalyst under visible light irradiation. Mater. Res. Bull. 2017, 96, 171–182. [Google Scholar] [CrossRef]
- Cifci, D.I.; Tuncal, T.; Pala, A.; Uslu, O. Determination of optimum extinction wavelength for paracetamolremoval through energy efficient thin film reactor. J. Photochem. Photobiol. A 2016, 322–323, 102–109. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Arasu, M.V. Environmentally-Friendly Green Approach for the Production of Zinc Oxide Nanoparticles and Their Anti-Fungal, Ovicidal, and Larvicidal Properties. Nanomaterials 2018, 8, 500. [Google Scholar] [CrossRef]
- Giakoumaki, A.N.; Kenanakis, G.; Klini, A.; Androulidaki, M.; Viskadourakis, Z.; Farsari, M.; Selimis, A. 3D micro-structured arrays of ZnO nanorods. Sci. Rep. 2017, 7, 2100. [Google Scholar] [CrossRef]
- Shakir, M.; Faraz, M.; Sherwani, M.A.; Al-Resayes, S.I. Photocatalytic degradation of the Paracetamol drug using Lanthanum doped ZnO nanoparticles and their in-vitro cytotoxicity assay. J. Lumin. 2016, 176, 159–167. [Google Scholar] [CrossRef]
- Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. Formation of ZnO Nanoparticles by the Reaction of Zinc Metal with Aliphatic Alcohols. J. Clust. Sci. 2007, 18, 660–670. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles–An eco-friendly approach. Resour.-Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Viskadourakis, Z.; Sevastaki, M.; Kenanakis, G. 3D structured nanocomposites by FDM process: A novel approach for large-scale photocatalytic applications. Appl. Phys. A 2018, 124, 585. [Google Scholar] [CrossRef]
- Sun, X.M.; Chen, X.; Deng, Z.X.; Li, Y.D. A CTAB-Assisted Hydrothermal Orientation Growth of ZnO Nanorods. Mater. Chem. Phys. 2003, 78, 99–104. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, L.; Yin, J.; Su, H.; Liao, C.; Yan, C. Control of ZnO morphology via a simple solution route. Chem. Mater. 2002, 14, 4172–4177. [Google Scholar] [CrossRef]
- Xu, F.; Yuan, Z.-Y.; Du, G.-H.; Ren, T.-Z.; Volcke, C.; Thiry, P.; Su, B.-L. A low-temperature aqueous solutionroute to large-scale growth of ZnO nanowire arrays. J. Non-Cryst. Solids 2006, 352, 2569–2574. [Google Scholar] [CrossRef]
- Pan, A.L.; Liu, R.B.; Wang, S.Q.; Wu, Z.Y.; Cao, L.; Xie, S.S.; Zou, B.S. Controllable growth and optical properties of large scale ZnO arrays. J. Cryst. Growth 2005, 282, 125–130. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Jie, W.; Zhang, X.; Yu, D. Preparation of ZnO cluster and rod-like whiskers through hydrothermal methods. Mater. Lett. 2006, 60, 1394–1398. [Google Scholar] [CrossRef]
- Chandrinou, C.; Boukos, N.; Giannakopoulos, K.; Lusson, A.; Travlos, A. Growth and optical study of ZnO nanorods. Superlattice Microstruct. 2007, 42, 431–437. [Google Scholar] [CrossRef]
- Li, Z.; Huang, X.; Liu, J.; Li, Y.; Li, G. Morphology control and transition of ZnO nanorod arrays by a simple hydrothermal method. Mater. Lett. 2008, 62, 1503–1506. [Google Scholar] [CrossRef]
- Zhao, A.; Luo, T.; Chen, L.; Liu, Y.; Li, X.; Tang, Q.; Cai, P.; Qian, Y. Synthesis of ordered ZnO nanorods film on zinc-coated Si substrate and their photoluminescence property. Mater. Chem. Phys. 2006, 99, 50–53. [Google Scholar] [CrossRef]
- Fang, Y.G.; Pang, Q.; Wen, X.; Wang, J.; Yang, S. Synthesis of Ultrathin ZnO Nanofibers Aligned on a Zinc Substrate. Small 2006, 5, 612–615. [Google Scholar] [CrossRef]
- Yan, C.; Xue, D. Solution growth of nano- to microscopic ZnO on Zn. J. Cryst. Growth 2008, 310, 1836–1840. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, S.; Jamzad, M.; Kabiri Fard, H. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef]
- Davar, F.; Majedi, A.; Mirzaei, A. Green Synthesis of ZnO Nanoparticles and Its Application in the Degradation of Some Dyes. J. Am. Ceram. Soc. 2015, 98, 1739–1746. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Hussein, M.Z.B.; Yun, H.-Y. Hydrothermal synthesis of zinc oxide nanoparticles using rice as soft biotemplate. Chem. Cent. J. 2013, 7, 136. [Google Scholar] [CrossRef]
- Shah, M.A.; Al-Shahry, M. Zinc oxide nanoparticles prepared by the reaction of zinc metal with ethanol. JKAU Sci. 2009, 21, 61–67. [Google Scholar] [CrossRef]
- Vetter, J.L. Leavening Agents. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Jeddah, Saudi Arabia, 2003. [Google Scholar]
- Komy, Z.R. Determination of zinc, cadmium, lead and copper in kakad, Anise, Cumin, Caraway and black pepper extracted using differential pluse anodic stripping Voltametry with hanging mercury drop electrode. Am. J. Appl. Sci. 2005, 2, 961–968. [Google Scholar]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2019, 3, 27–35. [Google Scholar] [CrossRef]
- Gueven, A. Chemical fingerprints of Raki: A traditional distilled alcoholic beverage. J. Inst. Brew. 2013, 119, 126–132. [Google Scholar] [CrossRef]
- Anli, R.E.; Bayram, M. Traditional Aniseed-Flavored Spirit Drinks. Food Rev. Int. 2010, 26, 246–269. [Google Scholar] [CrossRef]
- Jurado, J.M.; Ballesteros, O.; Alcázar, A.; Pablos, F.; Martín, M.J.; Vílchez, J.L.; Navalón, A. Characterization of aniseed-flavoured spirit drinks by headspace solid-phase microextraction gas chromatography–mass spectrometry and chemometrics. Talanta 2007, 72, 506–511. [Google Scholar] [CrossRef]
- Floyd, E.L.; Wang, J.; Regens, J.L. Fume emissions from alow-cost 3-D printer with various filament. J. Occup. Environ. Hyg. 2017, 14, 523–533. [Google Scholar] [CrossRef]
- Davis, A.-Y.; Zhang, Q.; Wong, J.P.S.; Weber, R.J.; Black, M.S. Characterization of volatile organic compound emissions from consumer level material extrusion 3D printers. Build. Environ. 2019, 160, 106209. [Google Scholar] [CrossRef]
- Loudon, R. Theory of the first-order Raman effect in crystals. Proc. R. Soc. London Ser. A 1963, 275, 218. [Google Scholar]
- Bowen, K.; Tanner, B. High Resolution X-ray Diffractometer and Topography; Taylor & Francis: London, UK, 1998; ISBN 0850667585. [Google Scholar]
- Birkholz, M.; Birkholz, M.; Fewster, P.F.; Genzel, C. Thin Film Analysis by X-ray Scattering; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9783527310524. [Google Scholar]
- Yu, P.Y.; Cardona, M. Fundamentals of Semiconductors; Springer: Berlin, Germany, 1996; ISBN 978-3-540-26475-0. [Google Scholar]
- Damen, T.C.; Porto, S.P.S.; Tell, B. Raman Effect in Zinc Oxide. Phys. Rev. 1966, 142, 570. [Google Scholar] [CrossRef]
- Jang, M.S.; Ryu, M.K.; Yoon, M.H.; Lee, S.H.; Kim, H.K.; Onodera, A.; Kojima, S. A study on the Raman spectra of Al-doped and Ga-doped ZnO ceramics. Curr. Appl. Phys. 2009, 9, 651–657. [Google Scholar] [CrossRef]
- Cusco, R.; Alarcon-Llado, E.; Ibanez, J.; Artus, L.; Jimenez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Ozgur, U.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Dogan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevastaki, M.; Papadakis, V.M.; Romanitan, C.; Suchea, M.P.; Kenanakis, G. Photocatalytic Properties of Eco-Friendly ZnO Nanostructures on 3D-Printed Polylactic Acid Scaffolds. Nanomaterials 2021, 11, 168. https://doi.org/10.3390/nano11010168
Sevastaki M, Papadakis VM, Romanitan C, Suchea MP, Kenanakis G. Photocatalytic Properties of Eco-Friendly ZnO Nanostructures on 3D-Printed Polylactic Acid Scaffolds. Nanomaterials. 2021; 11(1):168. https://doi.org/10.3390/nano11010168
Chicago/Turabian StyleSevastaki, Maria, Vassilis M. Papadakis, Cosmin Romanitan, Mirela Petruta Suchea, and George Kenanakis. 2021. "Photocatalytic Properties of Eco-Friendly ZnO Nanostructures on 3D-Printed Polylactic Acid Scaffolds" Nanomaterials 11, no. 1: 168. https://doi.org/10.3390/nano11010168
APA StyleSevastaki, M., Papadakis, V. M., Romanitan, C., Suchea, M. P., & Kenanakis, G. (2021). Photocatalytic Properties of Eco-Friendly ZnO Nanostructures on 3D-Printed Polylactic Acid Scaffolds. Nanomaterials, 11(1), 168. https://doi.org/10.3390/nano11010168








