Kinetics of Carbon Nanotubes and Graphene Growth on Iron and Steel: Evidencing the Mechanisms of Carbon Formation
Abstract
1. Introduction
2. Recent Experimental Work
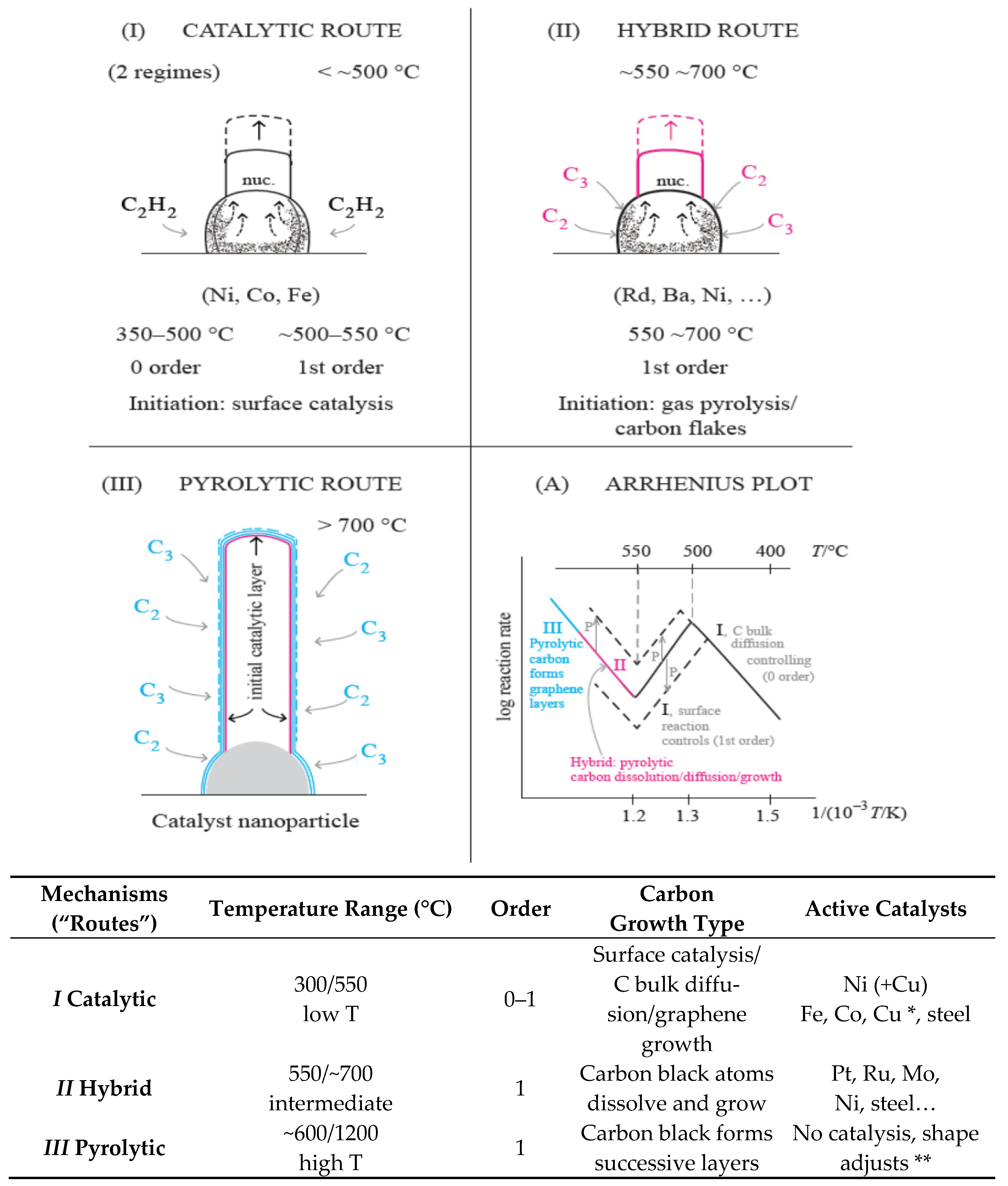
3. Kinetic Routes/Mechanisms of CNTs and Graphene Formation
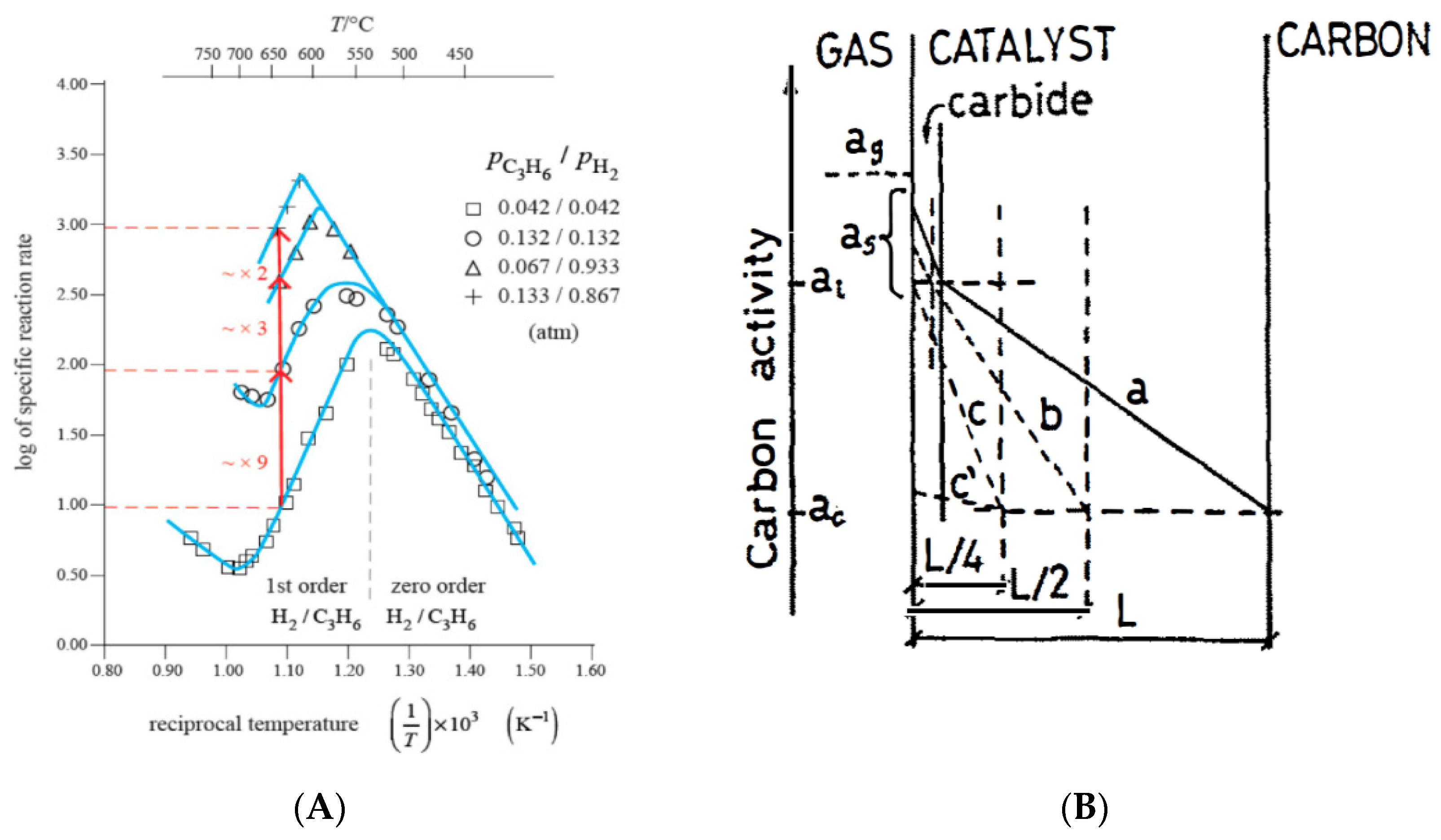
4. Role of Solid-State Chemistry in CNTs Growth Mechanism
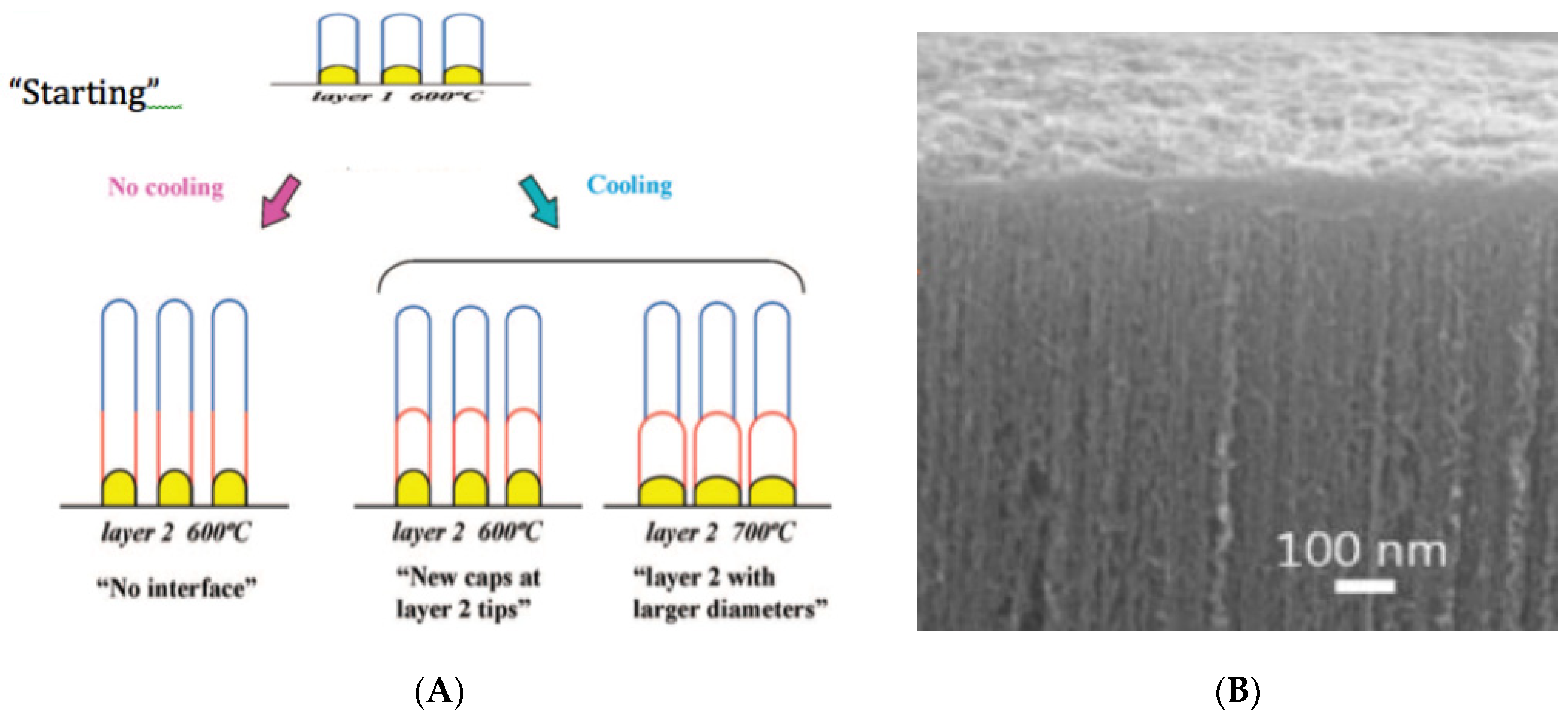
5. CNT Forests Growth Optimization vs. Kinetics and Mechanisms
6. CNTs Application Areas
7. Conclusions
- Detailed kinetic studies of catalytic carbon formation enabled an important scientific progress proving the modes of growth-established in 1971 for Ni, in 1980 for Co, and in 1990 for Fe and steel. The kinetics and transitions of the alternative mechanisms have recently been studied in more detail. The approach based in “rational recipes” [98] is much better than atomic scale simulations [99], which ignore experimental behavior and basic rules of solid-state chemistry.
- The kinetic studies of CNTs formation and the knowledge of which mechanism is operating (catalytic, hybrid, or pyrolytic) saves much time in optimizing the experimental conditions and in adjusting the CNTs’ properties to a desired use.
- Without the detailed kinetic analysis, the “main change” of rate, corresponding to the volcano-shape Arrhenius plot, is usually seen as a change of mechanism. However, it is, in fact, just a change of rate determining step. The change of mechanism from Route I to Route II is not usually understood. The restriction to Fe, Co, Ni, and C2H2 and low olefins is mandatory for Route I to operate, but Route II is operative with a C containing gas and many transition metals.
- The rates of diffusion of C through Fe and steel are much slower than through Ni or Co. The transition of rate determining step (“volcano”) occurs at ~700 °C (Figure 1) instead of ~500 °C for that reason.
- Additionally, the linear increase of rate from 600 °C to 1200 °C includes a major change of mechanism from Route II (hybrid) to pyrolytic external C layers deposition (Route III, no catalysis). This transition is invisible in the Arrhenius plot, just showing continuous straight-line temperature dependence.
- The structure and properties of CNTs are easier to adjust when the particular growth mechanism operating is known, which is now the case. Iron has applications in coatings, protective layers, antifouling substrates for metallic pipelines and blades, rails, etc. Optimizing the production and properties is easier when the growth mechanism is well understood.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palmer, H.B.; Cullis, C.I. The formation of carbon from gases. In Chemistry and Physics of Carbon; Peter, A., Ed.; CRC Press: Boca Raton, FL, USA, 1965; Volume 1, pp. 265–325. [Google Scholar]
- Singer, J.M.; Grumer, J. Carbon formation in very rich hydrocarbon-air flames—I. Studies of chemical content, temperature, ionization and particulate matter. Symp. Int. Combust. 1958, 7, 559–569. [Google Scholar]
- Harris, P.J.F. Carbon Nanotubes Science. Synthesis, Properties and Applications; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Physical Properties of Carbon Nanotubes; Imperial College Press: London, UK, 1998. [Google Scholar]
- Lobo, L.S. Catalytic carbon formation: Clarifying the alternative kinetic routes and defining a kinetic linearity for sustained growth concept. React. Kinet. Mech. Catal. 2016, 118, 393–414. [Google Scholar] [CrossRef]
- Jost, W. Diffusion in Solids, Liquids and Gases; Academic Press: New York, NY, USA, 1952. [Google Scholar]
- Lobo, L.S.; Trimm, D.L. Complex temperature dependencies of the rate of carbon deposition on nickel. Nat. Phys. Sci. 1971, 234, 15–16. [Google Scholar]
- Lobo, L.S.; Trimm, D.L. Studies of carbon formation on metals using a vacuum microbalance. In Progress in Vacuum Microbalance Techniques; Heyden and Son: London, UK, 1972; Volume 2. [Google Scholar]
- Lobo, L.S.; Franco, M.D. Kinetics of catalytic carbon formation on steel surfaces from light hydrocarbons. Catal. Today 1990, 7, 247–256. [Google Scholar]
- Bernardo, C.A.; Lobo, L.S. Kinetics of carbon formation from acetylene and 1-butene on cobalt. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1980; Volume 6, pp. 409–420. [Google Scholar]
- Jourdain, V.; Bichara, C. Current understanding of the growth of carbon nanotubes in catalytic chemical vapour deposition. Carbon 2013, 58, 2–39. [Google Scholar]
- Emmenegger, C.; Bonard, J.-M.; Mauron, P.; Sudan, P.; Lepora, A.; Grobety, B.; Züttel, A.; Schlapbach, L. Synthesis of carbon nanotubes over Fe catalyst on aluminum and suggested growth mechanism. Carbon 2003, 41, 539–547. [Google Scholar] [CrossRef]
- Carneiro, O.C.; Kim, M.S.; Yim, J.B.; Rodriguez, N.M.; Baker, R.T.K. Growth of graphite nanofibers from iron-copper catalyzed decomposition of CO/H2 mixtures. J. Phys. Chem. B 2003, 107, 4237. [Google Scholar] [CrossRef]
- Puretzky, A.A.; Geohegan, D.B.; Jesse, S.; Ivanov, I.N.; Eres, G. In situ measurements and modeling of CNTs arrays growth kinetics during CVD. Appl. Phys. A 2005, 81, 223–240. [Google Scholar]
- Karwa, M.; Iqbal, Z.; Mitra, S. Selective self-assembly of single walled CNTs in long steel tubing for chemical separations. J. Mater. Chem. 2006, 16, 2890–2895. [Google Scholar]
- Masarapu, C.; Bingqing, W. Direct growth of aligned multiwalled carbon nanotubes on treated stainless steel substrates. Langmuir 2007, 23, 9046–9049. [Google Scholar] [CrossRef]
- Zhong, T.I.; Robertson, J.; Kawarada, H. Growth kinetics of 0.5 cm vertically aligned SWCNTs. J. Phys. Chem. B 2007, 111, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Robertson, J.; Kawarada, H. Mechanism analysis of interrupted growth of SWCNTs arrays. Nano Lett. 2008, 8, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Takeda, S.; Uchiyama, T.; Khono, H.; Homma, Y. Atomic-scale in-situ observation of CNT growth from solid state iron carbide nanoparticles. Nano Lett. 2008, 8, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Baddour, C.E.; Fadlallah, F.; Nasuhoglu, D.; Mitra, R.; Vandsburger Meunier, J.-L. A simple thermal CVD method for carbon nanotube synthesis on stainless steel 304 without the addition of an external catalyst. Carbon 2008, 47, 313–347. [Google Scholar] [CrossRef]
- Sengupta, S.; Jacob, C. Growth temperature dependence of partially Fe filled MWCNTs using CVD. J. Cryst. Growth 2009, 311, 2642–2697. [Google Scholar] [CrossRef]
- Li, D.-A.; Pan, L.-J.; Liu, D.-P.; Yu, N.-S. Relationship between geometric structures of catalyst particles and growth of carbon nanocoils. Chem. Vap. Depos. 2010, 16, 166–169. [Google Scholar] [CrossRef]
- Nessim, G.D.; Seita, M.; Plata, D.L.; O’Brien, K.P.; Hart, A.J. Precursor gas chemistry determines the crystallinity of carbon nanotubes synthesized at low temperature. Carbon 2011, 49, 804–810. [Google Scholar] [CrossRef]
- Dasgupta, K.; Joshi, J.B.; Banerjee, S. Fluidized bed synthesis of CNTs—A review. Chem. Eng. J. 2011, 171, 841–869. [Google Scholar]
- Carneiro, O.C.; Anderson, P.E.; Rodriguez, N.M.; Baker, R.T.K. Synthesis of high purity narrow-width CNTs. Carbon 2012, 50, 3200–3209. [Google Scholar] [CrossRef]
- Robertson, J.; Zhong, G.; Esconjauregui, S.; Zhang, C.; Fouquet, M.; Hofmann, S. Chemical vapor deposition of CNT forests. Phys. Status Solidi B 2012, 249, 2315–2322. [Google Scholar] [CrossRef]
- Hashempour, M.; Vicenzo, A.; Zhao, F.; Bestetti, M. Direct growth of MWC mesh monoliths. NTs on stainless steel by chemical vapor deposition: Effect of surface nano-features on CNT growth and structure. Carbon 2013, 63, 330–347. [Google Scholar] [CrossRef]
- Hordy, N.; Mendoza-Gonzalez, N.-Y.; Coulombe, S.; Meunier, J.-L. The effect of carbon input on the morphology and attachment of carbon nanotubes grown directly from stainless steel. Carbon 2013, 63, 348–357. [Google Scholar] [CrossRef]
- Patel, R.B.; Liu, J.; Scicolone, J.V.; Roy, S.; Mitra, S.; Dave, R.N.; Iqbal, Z. Formation of stainless steel-carbon nanotubes composites using a scalable chemical vapor infiltration process. J. Mater. Sci. 2013, 48, 1387–1395. [Google Scholar] [CrossRef]
- Zhong, G.; Xie, R.; Yang, J.; Robertson, J. Single-step of high-density carbon nanotube forests on metallic Ti coatings through catalyst engineering. Carbon 2014, 67, 680. [Google Scholar] [CrossRef]
- Hashempour, M.; Vicenzo, A.; Zhao, F.; Bestetti, M. Effects of direct growth of carbon nanotubes and nanofibers on microstructure and electrochemical corrosion behavior of 316 stainless steel. Mater. Charact. 2014, 92, 64–76. [Google Scholar] [CrossRef]
- Bayer, B.C.; Baehtz, C.; Kidambi, P.R.; Weatherup, R.S.; Mangler, C.; Kotakoski, J.; Goddard, C.L.J.; Caneva, S.; Cabrero-Vilatela, A.; Hofmann, S.; et al. Nitrogen controlled iron catalyst phase during carbon nanotube growth. Appl. Phys. Lett. 2014, 105, 143111. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, X.; Zhang, K.; Yuen, M.M.F. Growth of VACNT arrays on Al substrates through controlled diffusion of catalyst. J. Phys. Chem. C 2015, 119, 15636–15642. [Google Scholar] [CrossRef]
- Romero, P.; Oro, R.; Campos, M.; Torralba, J.M.; Villoria, R.G. Simultaneous synthesis of VACNTs and amorphous carbon thin films on stainless steel. Carbon 2015, 82, 31–38. [Google Scholar] [CrossRef]
- Wang, H.; Na, C. Chemical bath deposition of aluminum oxide buffer on curved surfaces for growing aligned carbon nanotube arrays. Langmuir 2015, 31, 7401–7409. [Google Scholar] [CrossRef]
- Latorre, N.; Cazana, F.; Sebastian, V.; Royo, C.; Romeo, E.; Centeno, M.A.; Monzón, A. Growth of carbonaceous nanomaterials over stainless foams. Effect of activation temperature. Catal. Today 2016, 273, 41–49. [Google Scholar] [CrossRef]
- Pakdee, U.; Chianga, S.; Suwannatus, S.; Limsuwan, P. Groth of MWCNTs on flexible stainless steels without additional catalysts. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef]
- Latorre, N.; Cazana, F.; Sebastian, V.; Royo, C.; Romeo, E.; Monzón, A. Effect of the operating conditions on the growth of carbonaceous nanomaterials over stainless steel foams. Kinetic and characterization studies. Int. J. Chem. React. Eng. 2016, 15, 6. [Google Scholar] [CrossRef]
- Thapa, A.; Neupane, S.; Guo, R.; Jungjohahnn, K.L.; Pete, D.; Li, W. Direct growth of vertically aligned CNTs on stainless steel by plasma enhanced CVD. Diam. Relat. Mater. 2018, 90, 144–153. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Sun, B.-M.; Wu, C.-Y. Direct growth of MWCNTs on stainless steel V-type flame: Mechanism of carbon nanotube growth induced by surface reconstruction. Chem. Pap. 2019, 73, 2143–2151. [Google Scholar] [CrossRef]
- Xin, B.; Sun, G.; Lao, C.; Shang, D.; Zhang, X.; Wen, Z.; He, M. Chemical vapor deposition synthesis of carbon nanosprouts on calcined staineless steel. Mater. Lett. 2019, 238, 290–291. [Google Scholar] [CrossRef]
- Roumeli, E.; Diamantopolos, M.; Serra-Garcia, M.; Johanns, P.; Parcianello, G.; Daraio, C. Characterization of vertically aligned carbon nanotube forests grown on stainless steel surfaces. Nanomaterials 2019, 9, 444. [Google Scholar] [CrossRef]
- Panahi, A.; Wei, Z.; Song, G.; Levendis, Y.A. Influence of stainless-steel catalyst substrate type and pretreatment on growing CNTs from waste postconsumer plastics. Ind. Eng. Chem. Res. 2019, 58, 3009–3023. [Google Scholar] [CrossRef]
- Hasanzadeh, I.; Eskandari, J. Direct growth of multiwall carbon nanotube on metal catalyst by CVD: In situ nucleation. Surf. Coat. Technol. 2020, 381, 125109. [Google Scholar] [CrossRef]
- Lobo, L.S. Carbon Formation from Hydrocarbons on Metals. Ph.D. Thesis, Imperial College, London, UK, 1971. [Google Scholar]
- Venables, J.A. Introduction to Surface and Thin Film Processes; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Lobo, L.S.; Carabineiro, S.A.C. Review: Explaining bamboo-like carbon fiber growth mechanism: Catalyst shape adjustments above tammann temperature. J. Carbon Res. 2020, 6, 18. [Google Scholar] [CrossRef]
- Barrer, R.M. Diffusion in and Through Solids; Cambridge University Press: Cambridge, UK, 1941. [Google Scholar]
- Budnikov, P.P.; Ginstling, A.M. Principles of Solid-State Chemistry. Reactions in Solids; Gordon Breach: New York NY, USA, 1968. [Google Scholar]
- De Jong, K.; Geus, J.W. Carbon nanofibers: Catalytic synthesis and applications. Catal. Rev. Sci. Eng. 2000, 42, 481–510. [Google Scholar]
- Lobo, L.S. Mechanism of catalytic CNTs Growth in 400–650 °C Range: Explaining volcano shape Arrhenius plot and catalytic synergism using both Pt/Pd and Ni, Co or Fe. J. Carbon Res. 2019, 5, 42. [Google Scholar] [CrossRef]
- Baker, R.T.K.; Barber, M.A.; Harris, P.S.; Feats, F.S.; Waite, R.J. Nucleation and growth of carbon deposits from nickel catalyzed decomposition of acetylene. J. Catal. 1972, 26, 51–62. [Google Scholar] [CrossRef]
- Lobo, L.S.; Figueiredo, J.L.; Bernardo, C.A. Carbon formation and gasification on metals. Bulk diffusion mechanism: A reassessment. Catal. Today 2011, 178, 110–116. [Google Scholar] [CrossRef]
- Latorre Romeo, E.; Canzana, F.; Ubieto, T.; Royo, C.; Villacampa, J.I.; Monzón, A. CNT Growth by CVD: A phenomenological kinetic model. J. Phys. Chem. C 2010, 114, 4773–4782. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y.; Chuvilin, A.L.; Kuvshinov, G. Decomposition of methane over iron catalysts at the range of moderate temperatures: The influence of structure of the catalytic systems and the reaction conditions on the yield of carbon and morphology of carbon filaments. J. Catal. 2001, 2, 183–197. [Google Scholar] [CrossRef]
- Lobo, L.S.; Carabineiro, S.A.C. Mechanism of CNTs and graphene growth: Kinetics versus thermodynamics. J. Carbon Res. 2020, 6, 67. [Google Scholar] [CrossRef]
- Kayastha, V.K.; Yap, Y.K.; Pan, Z.; Ivanov, I.N.; Puretzky, A.A.; Geohegan, D.B. High-density vertically aligned MWCNTs with tubular structures. Appl. Phys. Lett. 2005, 86, 253105. [Google Scholar] [CrossRef]
- Lobo, L.S.; Sonia, S.A.C. Carbon formation at high temperatures (550–1400 °C): Kinetics, alternative mechanisms and growth modes. Catalysts 2020, 10, 465. [Google Scholar]
- Shaikjee, A.; Franklyn, P.J.; Coville, N.J. The use of electron microscopy tomography to correlate copper catalyst particle morphology with carbon fibered catalytic CVD. Carbon 2011, 49, 2950–2959. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; de Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Bethune Co-catalyzed growth of CNTs with single-atomic-layer walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Koyama, T. Formation of carbon fibers from benzene. Carbon 1972, 10, 757. [Google Scholar]
- Katsuki, H.; Matsunaga, K.; Egashira, M.; Kawasumi, S. Formation of carbon fibers from naphthalene on some sulfur-containing substrates. Carbon 1981, 19, 148. [Google Scholar] [CrossRef]
- Tibbetts, G.G. Carbon fibers produced by pyrolysis of natural gas in stainless steel tubes. Appl. Phys. Lett. 1983, 42, 666–668. [Google Scholar] [CrossRef]
- Mendez, A.; Freitas, M.M.A.; Figueiredo, J.L.F. Synthesis of carbon filaments and nanotubes on graphitic substrate: Optimization studies. Carbon 2006, 44, 2330–2356. [Google Scholar] [CrossRef]
- Cunha, A.F.; Órfão, J.J.M.; Figueiredo, J.L. Methane decomposition on Fe-Cu Raney-type catalysts. Fuel Proc. Technol. 2009, 90, 1234–1240. [Google Scholar] [CrossRef]
- Boyes, E.D.; LaGrow, A.P.; Ward, M.R.; Mitchel, R.W.; Gai, P.L. Single atom dynamics in chemical reactions. Acc. Chem. Res. 2020, 53, 390–399. [Google Scholar] [CrossRef]
- Shewmon, P.G. Diffusion in Solids; McGrow-Hill Book: New York, NY, USA, 1963. [Google Scholar]
- Budnikov, P.P.; Ginstling, A.M. Principles of Solid-State Chemistry. Reactions in Solids; McLaren and Sons Ltd.: London, OH, USA, 1968. [Google Scholar]
- Schmalzried, H. Solid State Reactions; Springer: Boston, MA, USA, 1981. [Google Scholar]
- Zhong, G.; Warner, J.H.; Fouquet, M.; Robertson, A.W.; Chen, B.; Robertson, J. Growth of ultrahigh density SWCNT forests by improved catalyst design. ACS Nano 2012, 6, 2893–2903. [Google Scholar]
- Robertson, J. Heterogeneous catalysis model of growth mechanisms of CNTs, graphene and silicon nanowires. J. Mater. Chem. 2012, 22, 19858. [Google Scholar] [CrossRef]
- Bayer, B.C.; Fouquet, M.; Blume, R.; Wirth, C.T.; Wetherup, R.S.; Ogata, K.; Knop-Gericke, A.; Schlogl, R.; Hofmann, S.; Robertson, J. Co-catalytic solid-state reduction applied to CNT growth. J. Phys. Chem. C 2012, 116, 1107–1113. [Google Scholar] [CrossRef]
- Zhong, G.; Yang, J.; Sugime, H.; Rao, R.; Zhao, J.; Liu, D.; Harutyunyan, A.; Robertson, J. Growth of high quality, high density single walled CNT forests on copper foils. Carbon 2016, 98, 624–632. [Google Scholar] [CrossRef]
- Nessim, G.D.; Acquaviva, D.; Seita, M.; O’Brien, K.P. Thickness in growing vertically aligned CNTs and nanofibers on metallic substrates by CVD. Adv. Funct. Mater. 2010, 20, 1306–1312. [Google Scholar] [CrossRef]
- Nessim, G.D. Review: Properties, synthesis, and growth mechanisms of carbon nanotubes with special focus on thermal chemical vapor deposition. Nanoscale 2010, 2, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Delzeit, L.; Chen, B.; Cassel, A.; Stevens, R.; Nguyen, C.; Meyyappan, M. Multilayered metal catalyst for controlling the density of single-walled CNT growth. Cem. Phys. Lett. 2001, 348, 368–374. [Google Scholar] [CrossRef]
- Burt, D.P.; Whyte, M.; Weaver, J.M.R.; Glide, A.; Edgeworth, J.P.; Maspherson, J.V.; Dobson, P.S. Effects of metal underlayer grain size on carbon nanotube growth. J. Phys. Chem. C 2009, 113, 34. [Google Scholar] [CrossRef]
- Nessim, G.D.; Acquaviva Seita, M.; O’Brien Thompson, C.V. The critical role of the underlayer material and thickness in growing VACNTS and nanofibers on metallic substrates by chemical vapour deposition. Adv. Funct. Mater. 2010, 20, 1306–1312. [Google Scholar] [CrossRef]
- Garcia-Lekue, A.; Ollé, M.; Sanchez-Portal, D.; Palacios, J.J.; Mugarza, A.; Ceballos, G.; Gambardellla, P. Substrate induced stabilization and reconstruction of zigzag edges in graphene nano islands on Ni(111). J. Phys. Chem. C 2015, 119, 4072–4078. [Google Scholar] [CrossRef]
- Gao, F.; Tian, W.; Wang, Z.; Wang, F. Effect of MWCNTs on mechanical properties and microstructure of the cement-based materials. Constr. Build. Mater. 2020, 260, 120452. [Google Scholar]
- Yao, D.; Wang, C.-H. Pyrolysis and in-line catalytic decomposition of polypropylene to carbon nanomaterials and H2 over Fe- and Ni-based catalysts. Appl. Energy 2020, 265, 114819. [Google Scholar]
- Yamazaki, Y.; Katagiri Sakuma, N.; Suzuki, M. Synthesis of a closed packed CNT Forest by a multi-step growth method using plasma-based CVD. Appl. Phys. Express 2010, 3, 5. [Google Scholar] [CrossRef]
- Lee, J.; Abdulhafez, M.; Bedewy, M. Decoupling catalyst dewetting, gas decomposition, and surface reactions in CNT forests growth reveals dependence of density on nucleation temperature. J. Phys. Chem. C 2019, 123, 28726–28738. [Google Scholar] [CrossRef]
- Bedewy, M.; Meshot, E.R.; Hart, A.J. Diameter-dependent kinetics of activation and deactivation in carbon nanotube population growth. Carbon 2012, 50, 5106–5116. [Google Scholar] [CrossRef]
- Park, J.S.; Schmidt, A.J.; Bedewy, M.; Hart, A.J. Measurement of carbon nanotube microstructure relative density by optical attenuation and observation of size-dependent variations. Phys. Chem. Chem. Phys. 2013, 15, 11511–11519. [Google Scholar] [CrossRef] [PubMed]
- Meshot, E.R.; Bedewy, M.; Lyons, K.M.; Woll, A.R.; Juggernauth, K.A.; Tawfick, S.; Hart, A.J. Measuring the lengthening kinetics of aligned nanostructures by spatiotemporal correlation of height and orientation. Nanoscale 2010, 2, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Nessim, G.D.; Hart, J.; Kim, J.S.; Acquaviva, D.; Oh, J.; Morgan, C.D.; Seita, M.; Leib, J.S.; Thompson, C.V. Tunning of vertically-aligned CNT diameter and areal density through catalyst pre-treatment. Nano Lett. 2008, 11, 3587–3593. [Google Scholar]
- Baker, R.T.K. Catalytic growth of carbon filaments. Carbon 1989, 27, 315–323. [Google Scholar] [CrossRef]
- Sarno, M.; Cirilo, C.; Piscitelli, R.; Ciambelli, P. A study of the key parameters, including the crucial role of H2 for uniform graphene growth on Ni foil. J. Mol. Catal. A 2013, 366, 303–314. [Google Scholar]
- Um, J.W.; Kim, S.-Y.; Lee, B.-H.; Park, J.B.; Jeong, S. Direct writing of graphite thin film by laser-assisted CVD. Carbon 2020, 169, 163–171. [Google Scholar]
- Treacy, M.; Ebbesen, T.; Gibson, J. Exceptional high young modulus observed for individual CNTs. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Adhikary, S.K.; Rudzionis, Z.; Rajapriya, R. The effect of carbon nanotubes on the floeability, mechanical, microstructural and durability properties of cementite composite: An overview. Sustainability 2020, 12, 8362. [Google Scholar] [CrossRef]
- Guceri, S.; Gogotsi, Y.G. Nanoengineered Nanofibrous Materials; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Ebbesen, T.W.; Lesec, H.J.; Hiura, H.; Bennet, J.W.; Chemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Earp, B.; Dunn, D.; Phillips, J.; Agrawal, R.; Ansell, T.; Aceves, P.; De Rosa, I.; Xin, W.; Luhrsa, C. Enhancement of electrical conductivity of CNT sheets through Cu addition using reduction expansion synthesis. Mater. Res. Bull. 2020, 131, 110969. [Google Scholar] [CrossRef]
- Chen, T.-C.; Zhang, Q.; Zhao, M.-Q.; Huang, J.-Q.; Tang, C.; Wei, F. Rational recipe for bulk growth of graphene/carbon nanotube hybrids: New insights from in-situ characterization on working catalysts. Carbon 2015, 95, 292–301. [Google Scholar] [CrossRef]
- Khalilov, U.; Bagaerts, A.; Neyts, E.C. Atomic scale simulation of carbon nanotubes nucleation from hydrocarbon precursors. Nat. Commun. 2015, 6, 10306. [Google Scholar]

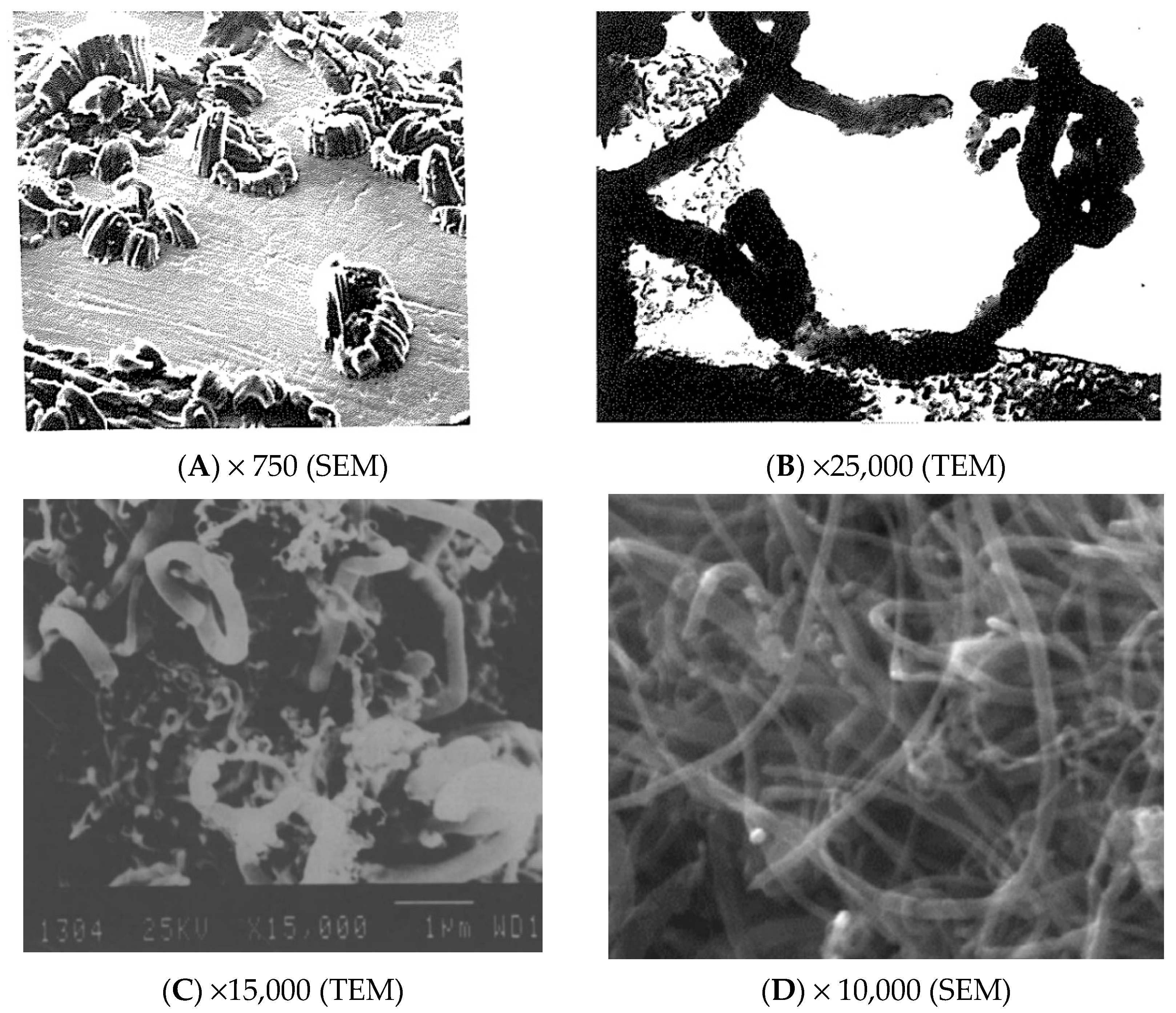
| Gas | Kinetics | Ni | Co | Fe | Steel | AISI 302 |
|---|---|---|---|---|---|---|
| C2H2 | rw | 85 | 20 | 2 | 1 | - |
| Ea | 130 | 134 | 88–100 | 180 | - | |
| C4H8 | rw | 50 | 15 | 2 | 1.5 | 0.017 |
| Ea | 121 | 142 | 188 | 171 | - | |
| Reference | [7,8] | [10] | [10] | [9] | - | |
| Year | 1st Author | Ref. | Metal | Gas | T, °C | Study |
|---|---|---|---|---|---|---|
| 2003 | Emmenegger | [12] | Fe/Al | C2H2 | 650 | Nature of C |
| 2003 | Carneiro | [13] | Fe-Cu | CO/H2 | 500–700 | Flow reactor |
| 2005 | Puretzky | [14] | Fe/Mo | C2H2 | 535–900 | Mechanism(s) |
| 2006 | Karwa | [15] | Steel | Benzene… | 725 | Self assemble |
| 2007 | Masarapu | [16] | Steel 304 | Xylene/Ar/H2 | 700 | Aligned MWCNT (multi-walled carbon nanotubes) |
| 2007 | Zhong | [17] | Fe | CH4 | 600 | Mechanism |
| 2007 | Iawasaki | [18] | Fe | CH4/H2 | 600 | Mechanism |
| 2008 | Yoshida | [19] | Fe3C | C2H2 | 600 | Mechanism |
| 2008 | Baddour | [20] | Steel 304 | C2H2/N2 | 700 | Simple procedure |
| 2009 | Sengupta | [21] | Fe | Propane/H2 | 650–950 | Optimize growth |
| 2010 | Li | [22] | Fe, Sn | C2H2 | 700 | Nanocoils |
| 2011 | Nessim | [23] | Fe | C2H4/H2 | 730–770 | Hot-wall reactor |
| 2011 | Dasgupta | [24] | Fe,Co,Ni,Cu | Various/CO | 550–750 | Review (>1996) |
| 2012 | Carneiro | [25] | Fe-Ni | CO/H2 | 670 | Shape: TEM, XRD |
| 2012 | Robertson | [26] | Fe | C2H2/H2 | 680 | CNT (carbon nanotubes) Forests |
| 2013 | Hashempour | [27] | Steel | C2H4 | 760 | Surface treating |
| 2013 | Hordy | [28] | Steel | C2H2 | 700 | H2/NH3 |
| 2013 | Patel | [29] | Steel/Fe | C2H2/CO | 800 | H2/Cr/SS mesh |
| 2014 | Zhong | [30] | Fe-Ti | C2H2/H2 | 700 | CNT HD forests |
| 2014 | Hashempour | [31] | Steel | N2/C2H4/H2 | 760 | Hybrid rate… |
| 2014 | Bayer | [32] | Fe/Fe3C | C2H2/NH3 | 750 | Fe-C-N solid phase |
| 2015 | Gao | [33] | Fe | C2H2/H2/Ar | 600 | Catalyst lifetime |
| 2015 | Romero | [34] | Steel/Fe | Ar, H2. C2H4 | 716+ | VACNTs (vertically aligned CNTs) |
| 2015 | Wang | [35] | Steel mesh | C2H4/H2 | 750 | AOB curve layer |
| 2016 | Latorre | [36] | Steel foam | N2, C2H6, H2 | 800 | Max. 8000/FLG |
| 2017 | Pakdee | [37] | Steel | C2H2/H2 | 700~ | Amorphous C test |
| 2017 | Latorre | [38] | Steel foam | N2/C2H6/H2 | 900 | Operation adjust |
| 2018 | Thapa | [39] | Steel | C2H2 | 650+ | Temp.-ramp/NH3 |
| 2019 | Sun | [40] | Steel | C2H2 | 760 | Substrate surface |
| 2019 | Xin | [41] | Steel | CO/H2 | 600 | CNTs shape |
| 2019 | Roumeli | [42] | Steel | C7H8/Ferroc. | 827 | Properties |
| 2019 | Panahi | [43] | SS 304,316 | Plastic: PE,PP | 800 | Waste plastics |
| 2020 | Hasanzadeh | [44] | Fe,Co,Ni | C2H2 | 500+ | T: yield + diameter |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobo, L.S.; Carabineiro, S.A.C. Kinetics of Carbon Nanotubes and Graphene Growth on Iron and Steel: Evidencing the Mechanisms of Carbon Formation. Nanomaterials 2021, 11, 143. https://doi.org/10.3390/nano11010143
Lobo LS, Carabineiro SAC. Kinetics of Carbon Nanotubes and Graphene Growth on Iron and Steel: Evidencing the Mechanisms of Carbon Formation. Nanomaterials. 2021; 11(1):143. https://doi.org/10.3390/nano11010143
Chicago/Turabian StyleLobo, Luís Sousa, and Sónia A. C. Carabineiro. 2021. "Kinetics of Carbon Nanotubes and Graphene Growth on Iron and Steel: Evidencing the Mechanisms of Carbon Formation" Nanomaterials 11, no. 1: 143. https://doi.org/10.3390/nano11010143
APA StyleLobo, L. S., & Carabineiro, S. A. C. (2021). Kinetics of Carbon Nanotubes and Graphene Growth on Iron and Steel: Evidencing the Mechanisms of Carbon Formation. Nanomaterials, 11(1), 143. https://doi.org/10.3390/nano11010143






