Black Phosphorus as Multifaceted Advanced Material Nanoplatforms for Potential Biomedical Applications
Abstract
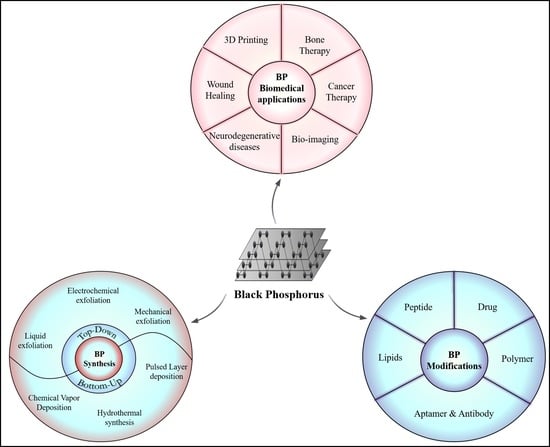
1. Introduction
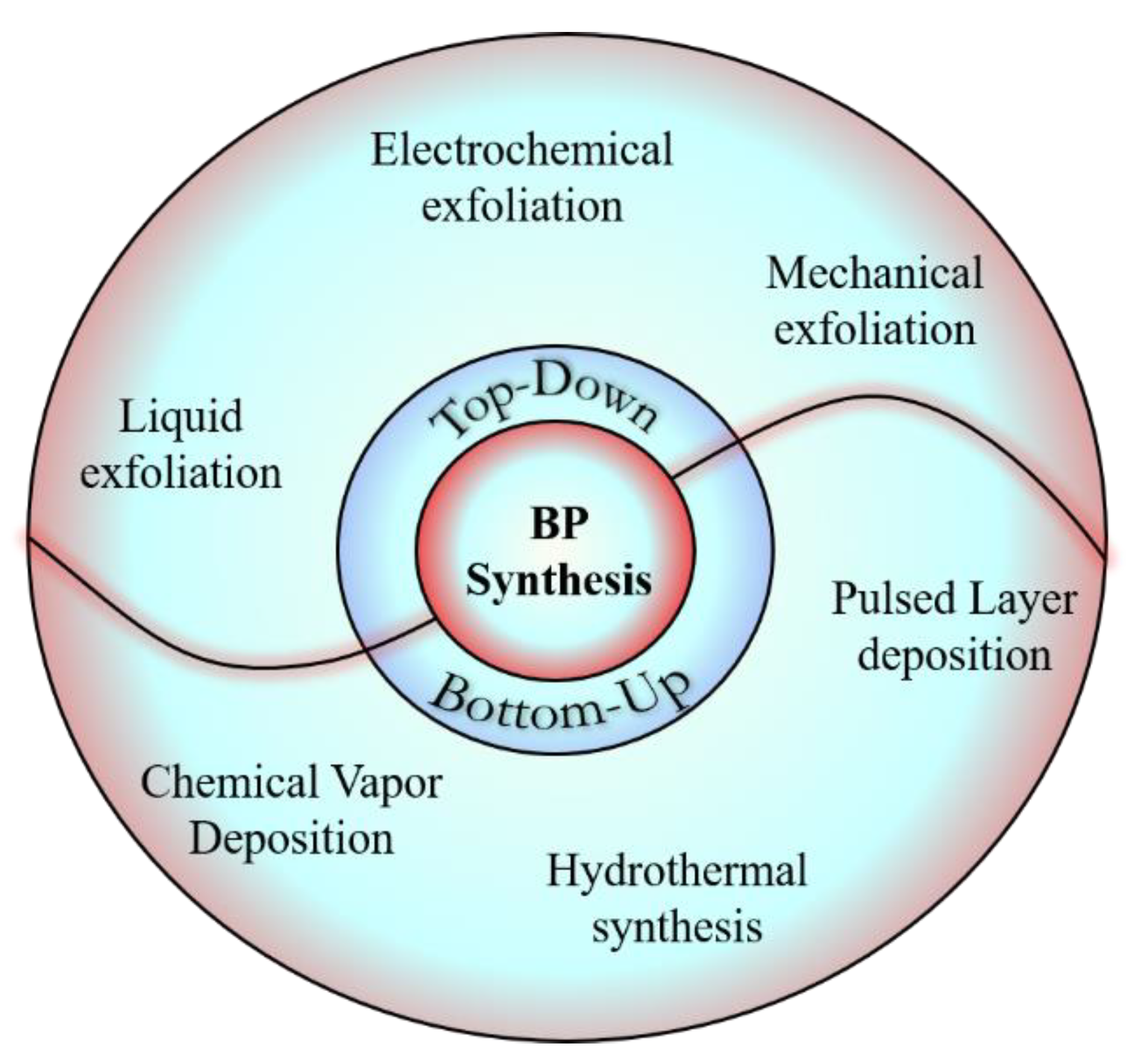
2. Synthesis
3. Optical and Electronic Properties
4. Surface Modification of Black Phosphorus
4.1. Modification Using Peptides
4.2. Modification Using Drugs
4.3. Modification Using Polymers
4.4. Modification Using Aptamers and Antibodies
4.5. Modification Using Lipids
5. Characterizations Techniques for BP
5.1. Spectroscopic Techniques
5.2. Thermal Techniques
5.3. Optical Techniques
5.4. Electron Microscopic Techniques
5.5. Plasma-Protein Adsorption Study
6. Biomedical Applications of Black Phosphorus
6.1. Application in Drug Delivery
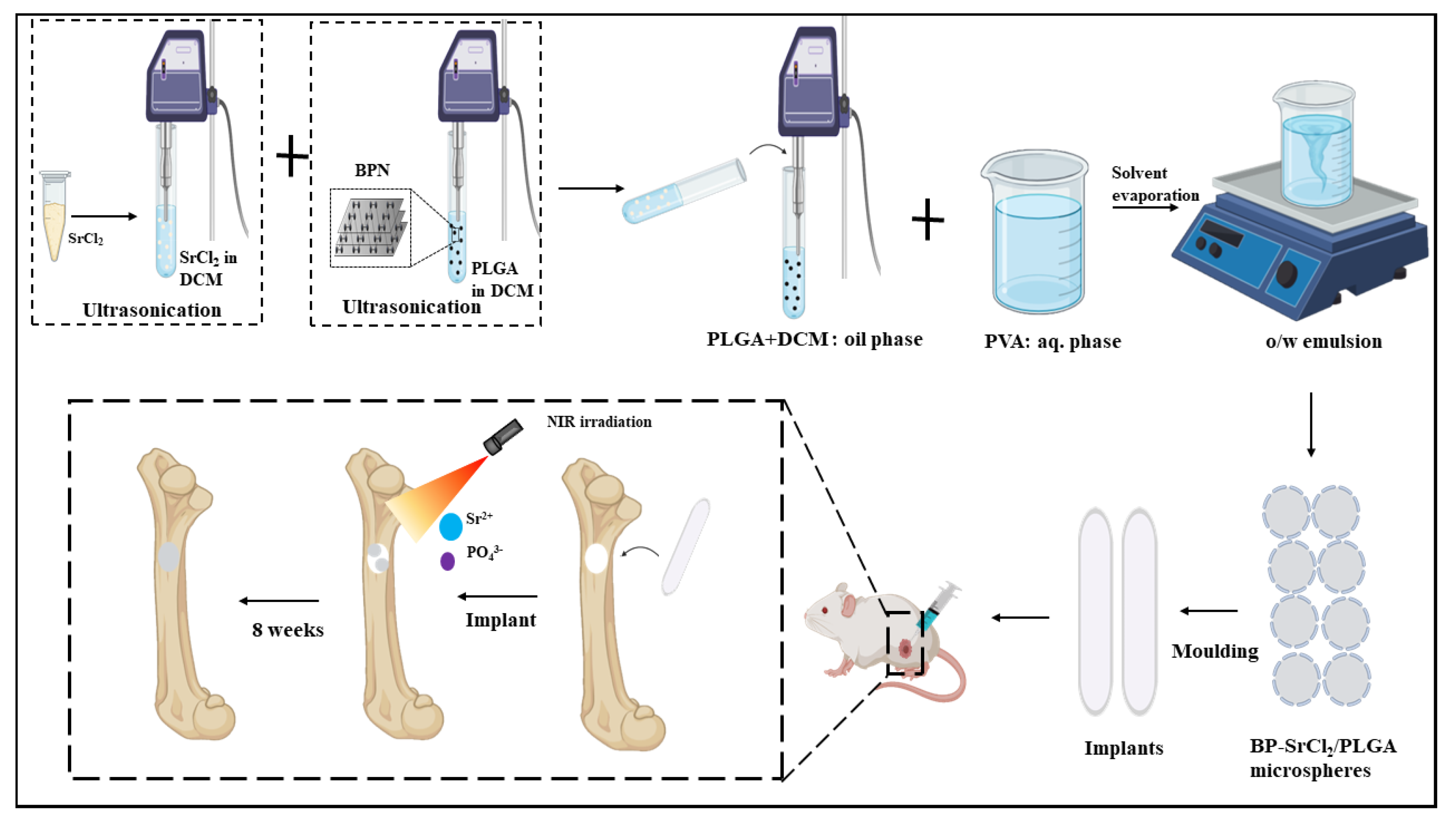
6.1.1. Bone Therapy
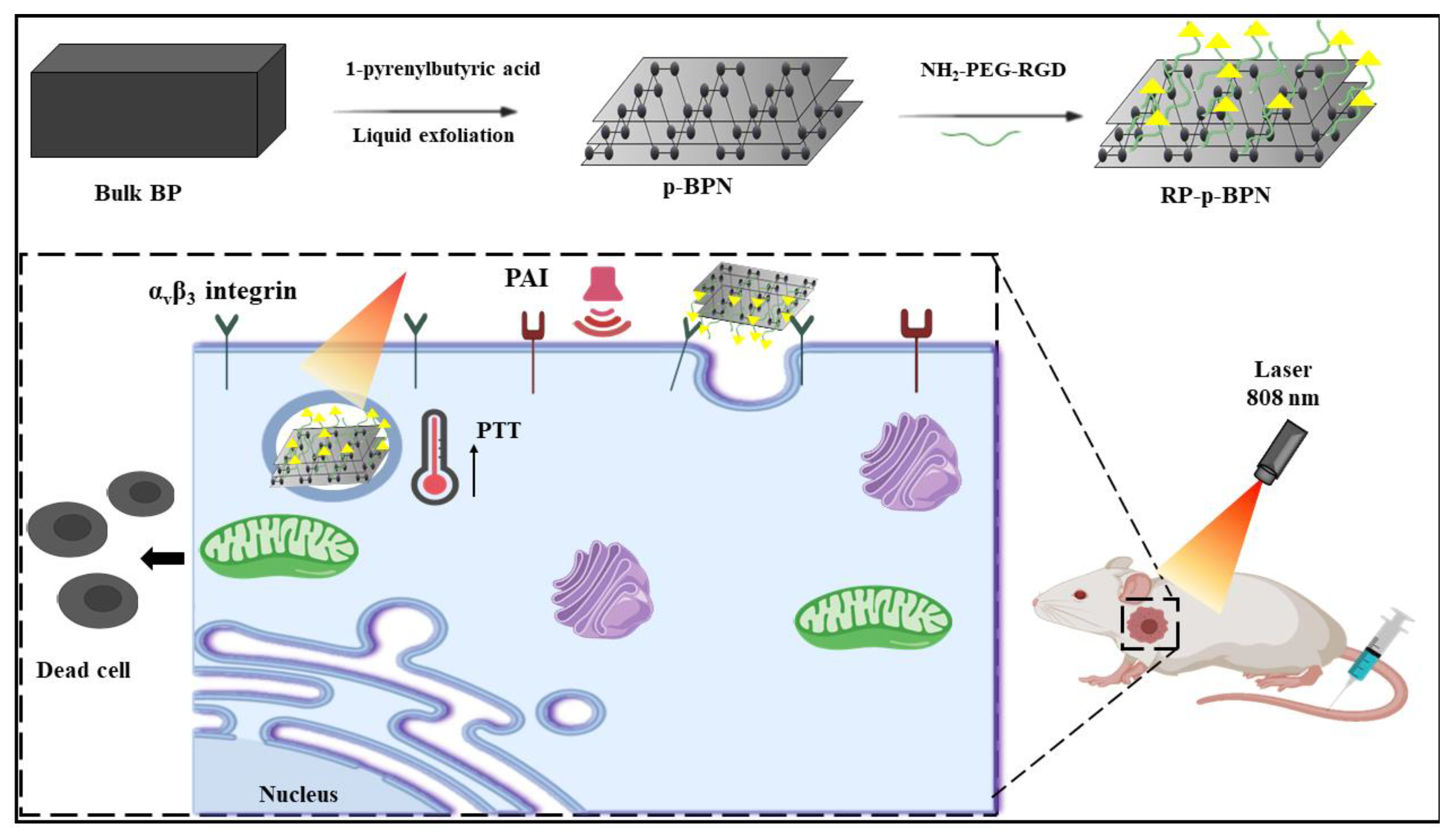
6.1.2. Cancer Therapy
6.1.3. Wound Healing
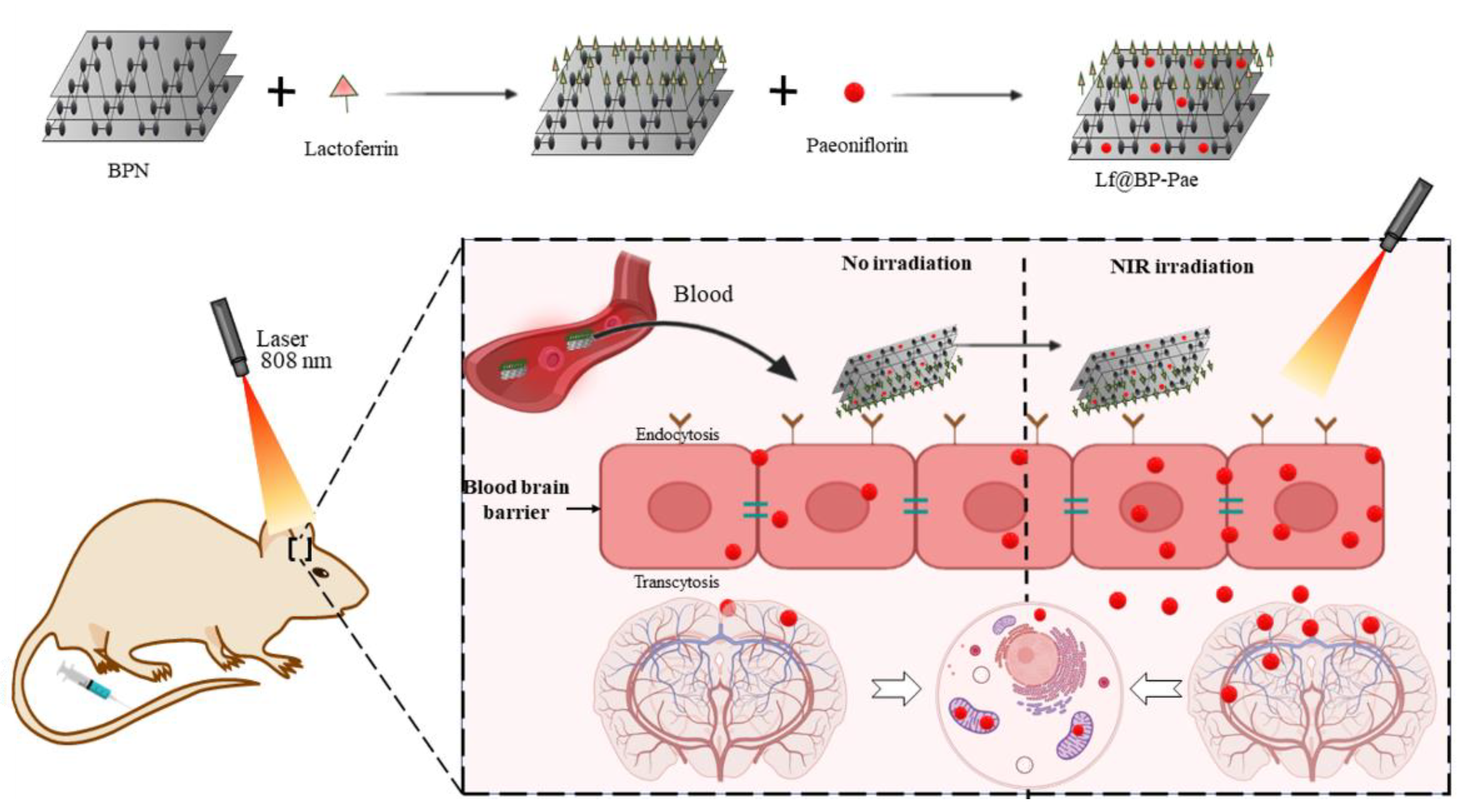
6.1.4. Neurodegenerative Disease
6.2. BP and 3D Printing
6.3. Bioimaging
7. Biodegradation and Toxicity of BP
8. Future Prospective
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Bridgman, P.W. Two New Modifications of Phosphorus. J. Am. Chem. Soc. 1914, 36, 1344–1363. [Google Scholar] [CrossRef]
- Hultgren, R.; Gingrich, N.S.; Warren, B.E. The Atomic Distribution in Red and Black Phosphorus and the Crystal Structure of Black Phosphorus. J. Chem. Phys. 1935, 3, 351–355. [Google Scholar] [CrossRef]
- Nikam, A.N.; More, M.P.; Pandey, A.P.; Patil, P.O.; Patil, A.G.; Deshmukh, P.K. Design and Development of Thiolated Graphene Oxide Nanosheets for Brain Tumor Targeting. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 611–621. [Google Scholar] [CrossRef]
- Qiu, M.; Ren, W.X.; Jeong, T.; Won, M.; Park, G.Y.; Sang, D.K.; Liu, L.-P.; Zhang, H.; Kim, J.S. Omnipotent Phosphorene: A next-Generation, Two-Dimensional Nanoplatform for Multidisciplinary Biomedical Applications. Chem. Soc. Rev. 2018, 47, 5588–5601. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, D.; Backes, C.; Doherty, E.; Cucinotta, C.S.; Berner, N.C.; Boland, C.; Lee, K.; Harvey, A.; Lynch, P.; Gholamvand, Z. Liquid Exfoliation of Solvent-Stabilized Few-Layer Black Phosphorus for Applications beyond Electronics. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y. Black Phosphorus Field-Effect Transistors. Nat. Nanotechnol. 2014, 9, 372. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, J.; Yi, X.; Xu, Y.; Niu, C.; Zhang, W.; Wang, L.; Sheng, J.; Deng, L.; Liu, Y.; et al. Black Phosphorus Nanosheets as a Neuroprotective Nanomedicine for Neurodegenerative Disorder Therapy. Adv. Mater. 2018, 30, 1703458. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Lu, W.; Chui, Y.S.; Rogée, L.; Bao, Q.; Lau, S.P. In Situ Observation of the Thermal Stability of Black Phosphorus. 2D Mater. 2017, 4. [Google Scholar] [CrossRef]
- Maruyama, Y.; Suzuki, S.; Kobayashi, K.; Tanuma, S. Synthesis and Some Properties of Black Phosphorus Single Crystals. Phys. BC 1981, 105, 99–102. [Google Scholar] [CrossRef]
- Lee, T.H.; Kim, S.Y.; Jang, H.W. Black Phosphorus: Critical Review and Potential for Water Splitting Photocatalyst. Nanomaterials 2016, 6, 194. [Google Scholar] [CrossRef]
- Lange, S.; Schmidt, P.; Nilges, T. Au3SnP7@Black Phosphorus: An Easy Access to Black Phosphorus. Inorg. Chem. 2007, 46, 4028–4035. [Google Scholar] [CrossRef] [PubMed]
- Köpf, M.; Eckstein, N.; Pfister, D.; Grotz, C.; Krüger, I.; Greiwe, M.; Hansen, T.; Kohlmann, H.; Nilges, T. Access and in Situ Growth of Phosphorene-Precursor Black Phosphorus. J. Cryst. Growth 2014, 405, 6–10. [Google Scholar] [CrossRef]
- Anju, S.; Ashtami, J.; Mohanan, P.V. Black Phosphorus, a Prospective Graphene Substitute for Biomedical Applications. Mater. Sci. Eng. C 2019, 97, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Inamuddin; Boddula, R.; Asiri, A.M. (Eds.) Black Phosphorus: Synthesis, Properties and Applications; Engineering Materials; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-030-29554-7. [Google Scholar]
- Woomer, A.H.; Farnsworth, T.W.; Hu, J.; Wells, R.A.; Donley, C.L.; Warren, S.C. Phosphorene: Synthesis, Scale-up, and Quantitative Optical Spectroscopy. ACS Nano 2015, 9, 8869–8884. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid Exfoliation of Layered Materials. Science 2013, 340. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Bourlinos, A.B.; Georgakilas, V.; Zboril, R.; Steriotis, T.A.; Stubos, A.K.; Trapalis, C. Aqueous-Phase Exfoliation of Graphite in the Presence of Polyvinylpyrrolidone for the Production of Water-Soluble Graphenes. Solid State Commun. 2009, 149, 2172–2176. [Google Scholar] [CrossRef]
- Vadukumpully, S.; Paul, J.; Valiyaveettil, S. Cationic Surfactant Mediated Exfoliation of Graphite into Graphene Flakes. Carbon 2009, 47, 3288–3294. [Google Scholar] [CrossRef]
- Hughes, J.M.; Aherne, D.; Coleman, J.N. Generalizing Solubility Parameter Theory to Apply to One-and Two-dimensional Solutes and to Incorporate Dipolar Interactions. J. Appl. Polym. Sci. 2013, 127, 4483–4491. [Google Scholar] [CrossRef]
- Coleman, J.N. Liquid Exfoliation of Defect-Free Graphene. Acc. Chem. Res. 2013, 46, 14–22. [Google Scholar] [CrossRef]
- Lin, S.; Chui, Y.; Li, Y.; Lau, S.P. Liquid-Phase Exfoliation of Black Phosphorus and Its Applications. FlatChem 2017, 2, 15–37. [Google Scholar] [CrossRef]
- Achee, T.C.; Sun, W.; Hope, J.T.; Quitzau, S.G.; Sweeney, C.B.; Shah, S.A.; Habib, T.; Green, M.J. High-Yield Scalable Graphene Nanosheet Production from Compressed Graphite Using Electrochemical Exfoliation. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Shen, Z. A Review on Mechanical Exfoliation for the Scalable Production of Graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Lu, W.; Nan, H.; Hong, J.; Chen, Y.; Zhu, C.; Liang, Z.; Ma, X.; Ni, Z.; Jin, C.; Zhang, Z. Plasma-Assisted Fabrication of Monolayer Phosphorene and Its Raman Characterization. Nano Res. 2014, 7, 853–859. [Google Scholar] [CrossRef]
- Bhoria, R.S. Enhancing Liquid Phase Exfoliation of Graphene in Organic Solvents with Additives. In Graphene and Its Derivatives-Synthesis and Applications; IntechOpen: London, UK, 2019. [Google Scholar]
- Xia, F.; Wang, H.; Jia, Y. Rediscovering Black Phosphorus as an Anisotropic Layered Material for Optoelectronics and Electronics. Nat. Commun. 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Mao, N.; Tang, J.; Xie, L.; Wu, J.; Han, B.; Lin, J.; Deng, S.; Ji, W.; Xu, H.; Liu, K. Optical Anisotropy of Black Phosphorus in the Visible Regime. J. Am. Chem. Soc. 2016, 138, 300–305. [Google Scholar] [CrossRef]
- Lan, S.; Rodrigues, S.; Kang, L.; Cai, W. Visualizing Optical Phase Anisotropy in Black Phosphorus. Acs Photonics 2016, 3, 1176–1181. [Google Scholar] [CrossRef]
- Tran, V.; Soklaski, R.; Liang, Y.; Yang, L. Tunable Band Gap and Anisotropic Optical Response in Few-Layer Black Phosphorus. arXiv 2014, arXiv:1402.4192. [Google Scholar]
- Çakır, D.; Sahin, H.; Peeters, F.M. Tuning of the Electronic and Optical Properties of Single-Layer Black Phosphorus by Strain. Phys. Rev. B 2014, 90, 205421. [Google Scholar] [CrossRef]
- Tran, V.; Soklaski, R.; Liang, Y.; Yang, L. Layer-Controlled Band Gap and Anisotropic Excitons in Few-Layer Black Phosphorus. Phys. Rev. B 2014, 89, 235319. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, J.; Xu, R.; Wang, F.; Li, W.; Ghufran, M.; Zhang, Y.-W.; Yu, Z.; Zhang, G.; Qin, Q. Extraordinary Photoluminescence and Strong Temperature/Angle-Dependent Raman Responses in Few-Layer Phosphorene. ACS Nano 2014, 8, 9590–9596. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P.D. Phosphorene: An Unexplored 2D Semiconductor with a High Hole Mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, B.; Zhang, H.-J.; Wang, J.; Xu, G.; Tang, P.; Duan, W.; Zhang, S.-C. Large-Gap Quantum Spin Hall Insulators in Tin Films. Phys. Rev. Lett. 2013, 111, 136804. [Google Scholar] [CrossRef] [PubMed]
- Ospina, D.A.; Duque, C.A.; Correa, J.D.; Morell, E.S. Twisted Bilayer Blue Phosphorene: A Direct Band Gap Semiconductor. Superlattices Microstruct. 2016, 97, 562–568. [Google Scholar] [CrossRef]
- Zhu, Z.; Tománek, D. Semiconducting Layered Blue Phosphorus: A Computational Study. Phys. Rev. Lett. 2014, 112, 176802. [Google Scholar] [CrossRef]
- Guan, J.; Zhu, Z.; Tománek, D. Tiling Phosphorene. ACS Nano 2014, 8, 12763–12768. [Google Scholar] [CrossRef]
- Boulfelfel, S.E.; Seifert, G.; Grin, Y.; Leoni, S. Squeezing Lone Pairs: The A 17 to A 7 Pressure-Induced Phase Transition in Black Phosphorus. Phys. Rev. B 2012, 85, 014110. [Google Scholar] [CrossRef]
- Guan, J.; Zhu, Z.; Tománek, D. Phase Coexistence and Metal-Insulator Transition in Few-Layer Phosphorene: A Computational Study. Phys. Rev. Lett. 2014, 113, 046804. [Google Scholar] [CrossRef]
- Khandelwal, A.; Mani, K.; Karigerasi, M.H.; Lahiri, I. Phosphorene–The Two-Dimensional Black Phosphorous: Properties, Synthesis and Applications. Mater. Sci. Eng. B 2017, 221, 17–34. [Google Scholar] [CrossRef]
- Wang, H.; Hu, K.; Li, Z.; Wang, C.; Yu, M.; Li, Z.; Li, Z. Black Phosphorus Nanosheets Passivation Using a Tripeptide. Small 2018, 14, 1801701. [Google Scholar] [CrossRef]
- Huang, W.-Q.; Wang, F.; Nie, X.; Zhang, Z.; Chen, G.; Xia, L.; Wang, L.-H.; Ding, S.-G.; Hao, Z.-Y.; Zhang, W.-J.; et al. Stable Black Phosphorus Nanosheets Exhibiting High Tumor-Accumulating and Mitochondria-Targeting for Efficient Photothermal Therapy via Double Functionalization. ACS Appl. Bio Mater. 2020, 3, 1176–1186. [Google Scholar] [CrossRef]
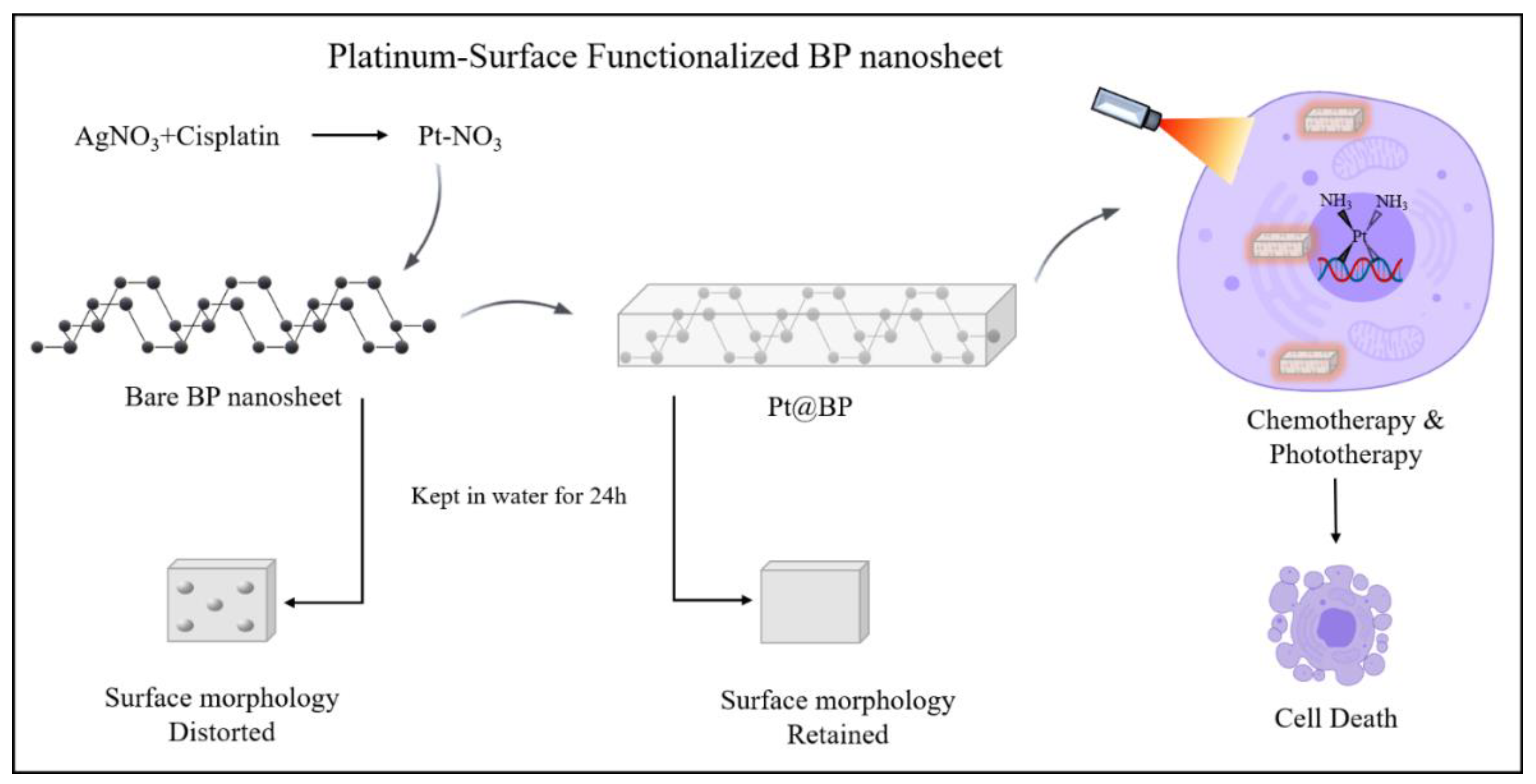
- Zhang, J.; Ma, Y.; Hu, K.; Feng, Y.; Chen, S.; Yang, X.; Fong-Chuen Loo, J.; Zhang, H.; Yin, F.; Li, Z. Surface Coordination of Black Phosphorus with Modified Cisplatin. Bioconjug. Chem. 2019, 30, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xie, H.; Tang, S.; Yu, X.-F.; Guo, Z.; Shao, J.; Zhang, H.; Huang, H.; Wang, H.; Chu, P.K. Ultrasmall Black Phosphorus Quantum Dots: Synthesis and Use as Photothermal Agents. Angew. Chem. Int. Ed. 2015, 54, 11526–11530. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, D.; Qu, Z.; Kislyakov, I.M.; Kiselev, V.M.; Liu, J. PEGylated-Folic Acid–Modified Black Phosphorus Quantum Dots as near-Infrared Agents for Dual-Modality Imaging-Guided Selective Cancer Cell Destruction. Nanophotonics 2020, 9, 2425–2435. [Google Scholar] [CrossRef]
- Gao, N.; Nie, J.; Wang, H.; Xing, C.; Mei, L.; Xiong, W.; Zeng, X.; Peng, Z. A Versatile Platform Based on Black Phosphorus Nanosheets with Enhanced Stability for Cancer Synergistic Therapy. J. Biomed. Nanotechnol. 2018, 14, 1883–1897. [Google Scholar] [CrossRef]
- Kumar, V.; Brent, J.R.; Shorie, M.; Kaur, H.; Chadha, G.; Thomas, A.G.; Lewis, E.A.; Rooney, A.P.; Nguyen, L.; Zhong, X.L.; et al. Nanostructured Aptamer-Functionalized Black Phosphorus Sensing Platform for Label-Free Detection of Myoglobin, a Cardiovascular Disease Biomarker. ACS Appl. Mater. Interfaces 2016, 8, 22860–22868. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, F.; Liu, H.; Zhang, H.; Han, Y.; Liu, Z.; Wang, W.; Sun, Q.; Zhao, C.; Li, Z. Cholesterol-Modified Black Phosphorus Nanospheres for the First NIR-II Fluorescence Bioimaging. ACS Appl. Mater. Interfaces 2019, 11, 21399–21407. [Google Scholar] [CrossRef]
- Lukyanov, A.N.; Torchilin, V.P. Micelles from Lipid Derivatives of Water-Soluble Polymers as Delivery Systems for Poorly Soluble Drugs. Adv. Drug Deliv. Rev. 2004, 56, 1273–1289. [Google Scholar] [CrossRef]
- Professor, S.F.C.D. Polymeric Stabilization of Colloidal Dispersions. Donald H. Napper. Academic Press, New York, 1984. Pp. Xvi & plus; 428. $65.00. J. Dispers. Sci. Technol. 1985, 6, 497. [Google Scholar] [CrossRef]
- Sun, C.; Xu, Y.; Deng, L.; Zhang, H.; Sun, Q.; Zhao, C.; Li, Z. Blood Circulation, Biodistribution, and Pharmacokinetics of Dextran-Modified Black Phosphorus Nanoparticles. ACS Appl. Bio Mater. 2018, 1, 673–682. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, M.; Chu, X.; Zhang, Q.; Su, Y.; Sun, B.; Lu, T.; Zhou, N.; Zhang, J.; Wang, J.; et al. Black Phosphorus Nanosheets-Based Nanocarriers for Enhancing Chemotherapy Drug Sensitiveness via Depleting Mutant P53 and Resistant Cancer Multimodal Therapy. Chem. Eng. J. 2019, 370, 387–399. [Google Scholar] [CrossRef]
- PRP-Chitosan Thermoresponsive Hydrogel Combined with Black Phosphorus Nanosheets as Injectable Biomaterial for Biotherapy and Phototherapy Treatment of Rheumatoid Arthritis. Biomaterials 2020, 239, 119851. [CrossRef] [PubMed]
- Liu, S.; Luo, J.; Jiang, X.; Li, X.; Yang, M. Gold Nanoparticle–Modified Black Phosphorus Nanosheets with Improved Stability for Detection of Circulating Tumor Cells. Microchim. Acta 2020, 187, 397. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, X.; Zhang, L.; Zhu, C.; Wang, J.; Li, Y.; Wang, Y.; Wang, C.; Zhang, Y.; Yuan, Q. Bioinspired Extracellular Vesicles Embedded with Black Phosphorus for Molecular Recognition-Guided Biomineralization. Nat. Commun. 2019, 10, 2829. [Google Scholar] [CrossRef]
- Wang, D.; Ge, C.; Liang, W.; Yang, Q.; Liu, Q.; Ma, W.; Shi, L.; Wu, H.; Zhang, Y.; Wu, Z.; et al. In Vivo Enrichment and Elimination of Circulating Tumor Cells by Using a Black Phosphorus and Antibody Functionalized Intravenous Catheter. Adv. Sci. 2020, 7, 2000940. [Google Scholar] [CrossRef] [PubMed]
- Eswaraiah, V.; Zeng, Q.; Long, Y.; Liu, Z. Black Phosphorus Nanosheets: Synthesis, Characterization and Applications. Small 2016, 12, 3480–3502. [Google Scholar] [CrossRef]
- Surrente, A.; Mitioglu, A.A.; Galkowski, K.; Tabis, W.; Maude, D.K.; Plochocka, P. Excitons in Atomically Thin Black Phosphorus. Phys. Rev. B 2016, 93, 121405. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Qian, J.; Lau, S.P. Emerging Opportunities for Black Phosphorus in Energy Applications. Mater. Today Energy 2019, 12, 1–25. [Google Scholar] [CrossRef]
- Qiao, J.; Kong, X.; Hu, Z.-X.; Yang, F.; Ji, W. High-Mobility Transport Anisotropy and Linear Dichroism in Few-Layer Black Phosphorus. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Castellanos-Gomez, A.; Vicarelli, L.; Prada, E.; Island, J.O.; Narasimha-Acharya, K.L.; Blanter, S.I.; Groenendijk, D.J.; Buscema, M.; Steele, G.A.; Alvarez, J.V. Isolation and Characterization of Few-Layer Black Phosphorus. 2D Mater. 2014, 1, 025001. [Google Scholar] [CrossRef]
- Li, L.; Ye, G.J.; Tran, V.; Fei, R.; Chen, G.; Wang, H.; Wang, J.; Watanabe, K.; Taniguchi, T.; Yang, L. Quantum Oscillations in a Two-Dimensional Electron Gas in Black Phosphorus Thin Films. Nat. Nanotechnol. 2015, 10, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, J.; Li, B. Phonon Thermal Conduction in Novel 2D Materials. J. Phys. Condens. Matter 2016, 28, 483001. [Google Scholar] [CrossRef] [PubMed]
- Akahama, Y.; Utsumi, W.; Endo, S.; Kikegawa, T.; Iwasaki, H.; Shimomura, O.; Yagi, T.; Akimoto, S. Melting Curve of Black Phosphorous. Phys. Lett. A 1987, 122, 129–131. [Google Scholar] [CrossRef]
- Su, L.; Zhang, Y. Temperature Coefficients of Phonon Frequencies and Thermal Conductivity in Thin Black Phosphorus Layers. Appl. Phys. Lett. 2015, 107, 071905. [Google Scholar] [CrossRef]
- Jeon, S.G.; Shin, H.; Jaung, Y.H.; Ahn, J.; Song, J.Y. Thickness-Dependent and Anisotropic Thermal Conductivity of Black Phosphorus Nanosheets. Nanoscale 2018, 10, 5985–5989. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.; Svitlyk, V.; Mezouar, M.; Sifré, D.; Garbarino, G.; Ceppatelli, M.; Serrano-Ruiz, M.; Peruzzini, M.; Datchi, F. Anisotropic Thermal Expansion of Black Phosphorus from Nanoscale Dynamics of Phosphorene Layers. Nanoscale 2020, 12, 4491–4497. [Google Scholar] [CrossRef] [PubMed]
- Judek, J.; Gertych, A.P.; Świniarski, M.; Łapińska, A.; Dużyńska, A.; Zdrojek, M. High Accuracy Determination of the Thermal Properties of Supported 2D Materials. Sci. Rep. 2015, 5, 12422. [Google Scholar] [CrossRef]
- Luo, Z.; Maassen, J.; Deng, Y.; Du, Y.; Garrelts, R.P.; Lundstrom, M.S.; Peide, D.Y.; Xu, X. Anisotropic In-Plane Thermal Conductivity Observed in Few-Layer Black Phosphorus. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Guo, Z.; Miao, L.; Han, S.-T.; Wang, Z.; Zhang, X.; Zhang, H.; Peng, Z. Recent Advances in Black Phosphorus-Based Photonics, Electronics, Sensors and Energy Devices. Mater. Horiz. 2017, 4, 997–1019. [Google Scholar] [CrossRef]
- Chaves, A.; Low, T.; Avouris, P.; Çakır, D.; Peeters, F.M. Anisotropic Exciton Stark Shift in Black Phosphorus. Phys. Rev. B 2015, 91, 155311. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, X.; Afshinmanesh, F.; Li, W.; Xu, G.; Sun, J.; Lian, B.; Curto, A.G.; Ye, G.; Hikita, Y.; et al. Polarization-Sensitive Broadband Photodetector Using a Black Phosphorus Vertical p-n Junction. Nat. Nanotechnol. 2015, 10, 707–713. [Google Scholar] [CrossRef]
- Liu, S.; Huo, N.; Gan, S.; Li, Y.; Wei, Z.; Huang, B.; Liu, J.; Li, J.; Chen, H. Thickness-Dependent Raman Spectra, Transport Properties and Infrared Photoresponse of Few-Layer Black Phosphorus. J. Mater. Chem. C 2015, 3, 10974–10980. [Google Scholar] [CrossRef]
- Fei, R.; Yang, L. Lattice Vibrational Modes and Raman Scattering Spectra of Strained Phosphorene. Appl. Phys. Lett. 2014, 105, 083120. [Google Scholar] [CrossRef]
- Wu, J.; Mao, N.; Xie, L.; Xu, H.; Zhang, J. Identifying the Crystalline Orientation of Black Phosphorus Using Angle-resolved Polarized Raman Spectroscopy. Angew. Chem. Int. Ed. 2015, 54, 2366–2369. [Google Scholar] [CrossRef]
- Pawbake, A.S.; Erande, M.B.; Jadkar, S.R.; Late, D.J. Temperature Dependent Raman Spectroscopy of Electrochemically Exfoliated Few Layer Black Phosphorus Nanosheets. RSC Adv. 2016, 6, 76551–76555. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, H.; Lu, S.; Wang, Z.; Tang, S.; Shao, J.; Sun, Z.; Xie, H.; Wang, H.; Yu, X.-F.; et al. From Black Phosphorus to Phosphorene: Basic Solvent Exfoliation, Evolution of Raman Scattering, and Applications to Ultrafast Photonics. Adv. Funct. Mater. 2015, 25, 6996–7002. [Google Scholar] [CrossRef]
- Mutalik, S.; Pandey, A.; Mutalik, S. Nanoarchitectronics: A Versatile Tool for Deciphering Nanoparticle Interaction with Cellular Proteins, Nucleic Acids and Phospholipids at Biological Interfaces. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Raychaudhuri, R.; Pandey, A.; Hegde, A.; Abdul Fayaz, S.M.; Chellappan, D.K.; Dua, K.; Mutalik, S. Factors Affecting the Morphology of Some Organic and Inorganic Nanostructures for Drug Delivery: Characterization, Modifications and Toxicological Perspectives. Expert Opin. Drug Deliv. 2020. [Google Scholar] [CrossRef]
- Chen, L.; Qian, M.; Jiang, H.; Zhou, Y.; Du, Y.; Yang, Y.; Huo, T.; Huang, R.; Wang, Y. Multifunctional Mesoporous Black Phosphorus-Based Nanosheet for Enhanced Tumor-Targeted Combined Therapy with Biodegradation-Mediated Metastasis Inhibition. Biomaterials 2020, 236, 119770. [Google Scholar] [CrossRef]
- Raucci, M.G.; Fasolino, I.; Caporali, M.; Serrano-Ruiz, M.; Soriente, A.; Peruzzini, M.; Ambrosio, L. Exfoliated Black Phosphorus Promotes in Vitro Bone Regeneration and Suppresses Osteosarcoma Progression through Cancer-Related Inflammation Inhibition. ACS Appl. Mater. Interfaces 2019, 11, 9333–9342. [Google Scholar] [CrossRef]
- Phakatkar, A.H.; Firlar, E.; Alzate, L.; Song, B.; Narayanan, S.; Rojaee, R.; Foroozan, T.; Deivanayagam, R.; Banner, D.J.; Shahbazian-Yassar, R. TEM Studies on Antibacterial Mechanisms of Black Phosphorous Nanosheets. Int. J. Nanomedicine 2020, 15, 3071. [Google Scholar] [CrossRef] [PubMed]
- Böhmert, L.; Voß, L.; Stock, V.; Braeuning, A.; Lampen, A.; Sieg, H. Isolation Methods for Particle Protein Corona Complexes from Protein-Rich Matrices. Nanoscale Adv. 2020, 2, 563–582. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the Nanoparticle–Protein Corona Using Methods to Quantify Exchange Rates and Affinities of Proteins for Nanoparticles. Proc. Natl. Acad. Sci. 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Hansen, C.; Martin, F.; Redemann, C.; Yau-Young, A. Liposomes Containing Synthetic Lipid Derivatives of Poly (Ethylene Glycol) Show Prolonged Circulation Half-Lives in Vivo. Biochim. Biophys. Acta BBA-Biomembr. 1991, 1066, 29–36. [Google Scholar] [CrossRef]
- Ânia, M.; Emilio, R.; Angel, M.; Rafael, G.; Manuel, F. Protein Interactions and Nanomaterials: A Key Role of the Protein Corona in Nanobiocompatibility. Protein-Protein Interact. Assays 2018, 29. [Google Scholar]
- Mo, J.; Xu, Y.; Wang, X.; Wei, W.; Zhao, J. Exploiting the Protein Corona: Coating of Black Phosphorus Nanosheets Enables Macrophage Polarization via Calcium Influx. Nanoscale 2020, 12, 1742–1748. [Google Scholar] [CrossRef]
- Mo, J.; Xie, Q.; Wei, W.; Zhao, J. Revealing the Immune Perturbation of Black Phosphorus Nanomaterials to Macrophages by Understanding the Protein Corona. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Han, M.; Zhu, L.; Mo, J.; Wei, W.; Yuan, B.; Zhao, J.; Cao, C. Protein Corona and Immune Responses of Borophene: A Comparison of Nanosheet–Plasma Interface with Graphene and Phosphorene. ACS Appl. Bio Mater. 2020. [Google Scholar] [CrossRef]
- Ray, S.J. First-Principles Study of MoS2, Phosphorene and Graphene Based Single Electron Transistor for Gas Sensing Applications. Sens. Actuators B Chem. 2016, 222, 492–498. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Shao, W.; Chen, S.; Xie, J.; Zhang, X.; Wang, J.; Xie, Y. Ultrathin Black Phosphorus Nanosheets for Efficient Singlet Oxygen Generation. J. Am. Chem. Soc. 2015, 137, 11376–11382. [Google Scholar] [CrossRef]
- Tayari, V.; Hemsworth, N.; Fakih, I.; Favron, A.; Gaufrès, E.; Gervais, G.; Martel, R.; Szkopek, T. Two-Dimensional Magnetotransport in a Black Phosphorus Naked Quantum Well. Nat. Commun. 2015, 6, 7702. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Zhu, X.; Yu, X.; Zeng, X.; Xiao, Q.; Zhang, X.; Ji, X.; Wang, X.; Shi, J.; Zhang, H.; et al. Black Phosphorus Nanosheets as a Robust Delivery Platform for Cancer Theranostics. Adv. Mater. Deerfield Beach Fla 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wu, J.; Gu, Z. Black Phosphorus Hydrogel Scaffolds Enhance Bone Regeneration via a Sustained Supply of Calcium-Free Phosphorus. ACS Appl. Mater. Interfaces 2019, 11, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Weng, J.; Fu, X.; Lin, J.; Fan, W.; Lu, N.; Qu, J.; Chen, S.; Wang, T.; Huang, P. Black Phosphorus Nanosheets for Mild Hyperthermia-Enhanced Chemotherapy and Chemo-Photothermal Combination Therapy. Nanotheranostics 2017, 1, 208–216. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; Liu, Y.; Sun, L.; Sun, L.; Zhao, Y. Black Phosphorus-Loaded Separable Microneedles as Responsive Oxygen Delivery Carriers for Wound Healing. ACS Nano 2020, 14, 5901–5908. [Google Scholar] [CrossRef]
- Xie, H.; Shao, J.; Ma, Y.; Wang, J.; Huang, H.; Yang, N.; Wang, H.; Ruan, C.; Luo, Y.; Wang, Q.-Q.; et al. Biodegradable Near-Infrared-Photoresponsive Shape Memory Implants Based on Black Phosphorus Nanofillers. Biomaterials 2018, 164, 11–21. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Naskar, D.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Investigating the Potential of Combined Growth Factors Delivery, from Non-Mulberry Silk Fibroin Grafted Poly(ɛ-Caprolactone)/Hydroxyapatite Nanofibrous Scaffold, in Bone Tissue Engineering. Appl. Mater. Today 2016, 5, 52–67. [Google Scholar] [CrossRef]
- Tang, D.; Tare, R.S.; Yang, L.-Y.; Williams, D.F.; Ou, K.-L.; Oreffo, R.O.C. Biofabrication of Bone Tissue: Approaches, Challenges and Translation for Bone Regeneration. Biomaterials 2016, 83, 363–382. [Google Scholar] [CrossRef]
- Lopes, D.; Martins-Cruz, C.; Oliveira, M.B.; Mano, J.F. Bone Physiology as Inspiration for Tissue Regenerative Therapies. Biomaterials 2018, 185, 240–275. [Google Scholar] [CrossRef]
- Augusto Oshiro, J.; Rillo Sato, M.; Rocha Scardueli, C.; Jose Pimentel Lopes de Oliveira, G.; Paiva Abucafy, M.; Chorilli, M. Bioactive Molecule-Loaded Drug Delivery Systems to Optimize Bone Tissue Repair. Curr. Protein Pept. Sci. 2017, 18, 850–863. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Nuzzo, M.; Yang, X.; Yang, Y.; Zhang, X. Layer-by-Layer Nanofiber-Enabled Engineering of Biomimetic Periosteum for Bone Repair and Reconstruction. Biomaterials 2018, 182, 279–288. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.-J.; Guo, Y.; Weir, M.D.; Reynolds, M.A.; Gu, N.; Xu, H.H.K. Magnetic Field and Nano-Scaffolds with Stem Cells to Enhance Bone Regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Childers, D.L.; Corman, J.; Edwards, M.; Elser, J.J. Sustainability Challenges of Phosphorus and Food: Solutions from Closing the Human Phosphorus Cycle. BioScience 2011, 61, 117–124. [Google Scholar] [CrossRef]
- Pravst, I. Risking Public Health by Approving Some Health Claims?—The Case of Phosphorus. Food Policy 2011, 36, 726–728. [Google Scholar] [CrossRef]
- Cui, L.; Houston, D.A.; Farquharson, C.; MacRae, V.E. Characterisation of Matrix Vesicles in Skeletal and Soft Tissue Mineralisation. Bone 2016, 87, 147–158. [Google Scholar] [CrossRef]
- González Díaz, E.C.; Shih, Y.-R.V.; Nakasaki, M.; Liu, M.; Varghese, S. Mineralized Biomaterials Mediated Repair of Bone Defects Through Endogenous Cells. Tissue Eng. Part A 2018, 24, 1148–1156. [Google Scholar] [CrossRef]
- Island, J.O.; Steele, G.A.; van der Zant, H.S.J.; Castellanos-Gomez, A. Environmental Instability of Few-Layer Black Phosphorus. 2D Mater. 2015, 2, 011002. [Google Scholar] [CrossRef]
- Rashdan, N.A.; Rutsch, F.; Kempf, H.; Váradi, A.; Lefthériotis, G.; MacRae, V.E. New Perspectives on Rare Connective Tissue Calcifying Diseases. Curr. Opin. Pharmacol. 2016, 28, 14–23. [Google Scholar] [CrossRef]
- Nørgaard, R.; Kassem, M.; Rattan, S. Heat Shock–Induced Enhancement of Osteoblastic Differentiation of HTERT-Immortalized Mesenchymal Stem Cells. Ann. N. Y. Acad. Sci. 2006. [Google Scholar] [CrossRef]
- Miao, Y.; Shi, X.; Li, Q.; Hao, L.; Liu, L.; Liu, X.; Chen, Y.; Wang, Y. Engineering Natural Matrices with Black Phosphorus Nanosheets to Generate Multi-Functional Therapeutic Nanocomposite Hydrogels. Biomater. Sci. 2019, 7, 4046–4059. [Google Scholar] [CrossRef]
- Wang, X.; Shao, J.; Abd El Raouf, M.; Xie, H.; Huang, H.; Wang, H.; Chu, P.K.; Yu, X.-F.; Yang, Y.; AbdEl-Aal, A.M. Near-Infrared Light-Triggered Drug Delivery System Based on Black Phosphorus for in Vivo Bone Regeneration. Biomaterials 2018, 179, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.; Liu, Z.; Han, Y.; Wang, L.; Li, J.; et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv. Mater. 2017, 29, 1603864. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Xie, H.; Huang, H.; Li, Z.; Sun, Z.; Xu, Y.; Xiao, Q.; Yu, X.-F.; Zhao, Y.; Zhang, H.; et al. Biodegradable Black Phosphorus-Based Nanospheres for in Vivo Photothermal Cancer Therapy. Nat. Commun. 2016, 7, 12967. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, D.; Zhu, X.; Cai, G.; Wu, J.; Chen, M.; Du, P.; Chen, Y.; Liu, W.; Yang, S. Enhancing the Photodynamic Therapy Efficacy of Black Phosphorus Nanosheets by Covalently Grafting Fullerene C60. Chem. Sci. 2020. [Google Scholar] [CrossRef]
- Liu, G.; Tsai, H.I.; Zeng, X.; Qi, J.; Luo, M.; Wang, X.; Mei, L.; Deng, W. Black Phosphorus Nanosheets-Based Stable Drug Delivery System via Drug-Self-Stabilization for Combined Photothermal and Chemo Cancer Therapy. Chem. Eng. J. 2019, 121917. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Hou, Y.; Yang, G.; Fei, X.; Zhao, H.; Guo, Y.; Su, C.; Wang, Z.; Zhong, H.; et al. Multifunctional Nanoplatform Based on Black Phosphorus Quantum Dots for Bioimaging and Photodynamic/Photothermal Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 25098–25106. [Google Scholar] [CrossRef]
- Xia, F.; Wang, H.; Xiao, D.; Dubey, M.; Ramasubramaniam, A. Two-Dimensional Material Nanophotonics. Nat. Photonics 2014, 8, 899–907. [Google Scholar] [CrossRef]
- Ling, X.; Wang, H.; Huang, S.; Xia, F.; Dresselhaus, M.S. The Renaissance of Black Phosphorus. Proc. Natl. Acad. Sci. 2015, 112, 4523–4530. [Google Scholar] [CrossRef]
- Shao, J.; Ruan, C.; Xie, H.; Li, Z.; Wang, H.; Chu, P.K.; Yu, X.-F. Black-Phosphorus-Incorporated Hydrogel as a Sprayable and Biodegradable Photothermal Platform for Postsurgical Treatment of Cancer. Adv. Sci. 2018, 5, 1700848. [Google Scholar] [CrossRef]
- Li, Z.; Guo, T.; Hu, Y.; Qiu, Y.; Liu, Y.; Wang, H.; Li, Y.; Chen, X.; Song, J.; Yang, H. A Highly Effective π–π Stacking Strategy To Modify Black Phosphorus with Aromatic Molecules for Cancer Theranostics. ACS Appl. Mater. Interfaces 2019, 11, 9860–9871. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Z.; Li, Y.; Hou, Y.; Fei, X.; Su, C.; Wang, S.; Zhuang, Z.; Guo, Z. Facile Synthesis of Black Phosphorus–Au Nanocomposites for Enhanced Photothermal Cancer Therapy and Surface-Enhanced Raman Scattering Analysis. Biomater. Sci. 2017, 5, 2048–2055. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Pan, T.; Cui, H.; Zhao, Z.; Chu, P.K.; Yu, X.-F. Black Phosphorus: Bioactive Nanomaterials with Inherent and Selective Chemotherapeutic Effects. Angew. Chem. Int. Ed Engl. 2019, 58, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Zhang, B.; Li, S.; He, B.; Pu, Y. Combination of PEG-Decorated Black Phosphorus Nanosheets and Immunoadjuvant for Photoimmunotherapy of Melanoma. J. Mater. Chem. B 2020, 8, 2805–2813. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, G.; Yang, P.; Lv, R.; Gai, S.; Li, C.; He, F.; Lin, J. Assembly of Au Plasmonic Photothermal Agent and Iron Oxide Nanoparticles on Ultrathin Black Phosphorus for Targeted Photothermal and Photodynamic Cancer Therapy. Adv. Funct. Mater. 2017. [Google Scholar] [CrossRef]
- Luo, M.; Cheng, W.; Zeng, X.; Mei, L.; Liu, G.; Deng, W. Folic Acid-Functionalized Black Phosphorus Quantum Dots for Targeted Chemo-Photothermal Combination Cancer Therapy. Pharmaceutics 2019, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, D.; Zhu, J.; Xue, L.; Ou, C.; Wang, W.; Lu, M.; Song, X.; Dong, X. Functional Black Phosphorus Nanosheets for Mitochondria-Targeting Photothermal/Photodynamic Synergistic Cancer Therapy. Chem. Sci. 2019, 10, 3779–3785. [Google Scholar] [CrossRef]
- Liu, Q.; Fan, T.; Zheng, Y.; Yang, S.; Yu, Z.; Duo, Y.; Zhang, Y.; Adah, D.; Shi, L.; Sun, Z.; et al. Immunogenic Exosome-Encapsulated Black Phosphorus Nanoparticles as an Effective Anticancer Photo-Nanovaccine. Nanoscale 2020, 12, 19939–19952. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, Y.; Gao, N.; Cheng, W.; Wang, X.; Cao, J.; Zeng, X.; Liu, G.; Mei, L. Mesenchymal Stem Cells Transporting Black Phosphorus-Based Biocompatible Nanospheres: Active Trojan Horse for Enhanced Photothermal Cancer Therapy. Chem. Eng. J. 2020, 385, 123942. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B.; et al. Novel Concept of the Smart NIR-Light–Controlled Drug Release of Black Phosphorus Nanostructure for Cancer Therapy. Proc. Natl. Acad. Sci. 2018, 115, 501–506. [Google Scholar] [CrossRef]
- Liang, X.; Ye, X.; Wang, C.; Xing, C.; Miao, Q.; Xie, Z.; Chen, X.; Zhang, X.; Zhang, H.; Mei, L. Photothermal Cancer Immunotherapy by Erythrocyte Membrane-Coated Black Phosphorus Formulation. J. Controlled Release 2019, 296, 150–161. [Google Scholar] [CrossRef]
- Xing, C.; Chen, S.; Qiu, M.; Liang, X.; Liu, Q.; Zou, Q.; Li, Z.; Xie, Z.; Wang, D.; Dong, B.; et al. Conceptually Novel Black Phosphorus/Cellulose Hydrogels as Promising Photothermal Agents for Effective Cancer Therapy. Adv. Healthc. Mater. 2018, 7, 1701510. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, C.; Chen, W.; Li, K.; Chen, X.; Tang, X.; Xie, G.; Luo, X.; Wang, X.; Liang, H.; et al. Biodegradable Black Phosphorus Nanosheets Mediate Specific Delivery of HTERT SiRNA for Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21137–21148. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, H.; Shao, J.; Jiang, C.; Zhang, F.; Lin, J.; Zhang, H.; Li, J.; Huang, P. Polydopamine-Functionalized Black Phosphorus Quantum Dots for Cancer Theranostics. Appl. Mater. Today 2019, 15, 297–304. [Google Scholar] [CrossRef]
- Jana, D.; Jia, S.; Bindra, A.K.; Xing, P.; Ding, D.; Zhao, Y. Clearable Black Phosphorus Nanoconjugate for Targeted Cancer Phototheranostics. ACS Appl. Mater. Interfaces 2020, 12, 18342–18351. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, D.; Shi, Y.; Zou, J.; Zhao, Q.; Zhang, Q.; Huang, W.; Shao, J.; Xie, X.; Dong, X. Black Phosphorus Nanosheets Immobilizing Ce6 for Imaging-Guided Photothermal/Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 12431–12440. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, H.; Guo, Z.; Zhang, W.; Yu, H.; Zhuang, Z.; Zhong, H.; Liu, Z. In Situ Photothermal Activation of Necroptosis Potentiates Black Phosphorus-Mediated Cancer Photo-Immunotherapy. Chem. Eng. J. 2020, 394, 124314. [Google Scholar] [CrossRef]
- Su, Y.; Wang, T.; Su, Y.; Li, M.; Zhou, J.; Zhang, W.; Wang, W. A Neutrophil Membrane-Functionalized Black Phosphorus Riding Inflammatory Signal for Positive Feedback and Multimode Cancer Therapy. Mater. Horiz. 2020, 7, 574–585. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.-M.; Prantl, L.; Landthaler, M.; Babilas, P. Wound Healing in the 21st Century. J. Am. Acad. Dermatol. 2010, 63, 866–881. [Google Scholar] [CrossRef]
- Martin, P.; Nunan, R. Cellular and Molecular Mechanisms of Repair in Acute and Chronic Wound Healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef]
- Ahmadi, M.; Adibhesami, M. The Effect of Silver Nanoparticles on Wounds Contaminated with Pseudomonas Aeruginosa in Mice: An Experimental Study. Iran. J. Pharm. Res. IJPR 2017, 16, 661. [Google Scholar]
- Biondi-Zoccai, G.G.; Lotrionte, M.; Agostoni, P.; Abbate, A.; Fusaro, M.; Burzotta, F.; Testa, L.; Sheiban, I.; Sangiorgi, G. A Systematic Review and Meta-Analysis on the Hazards of Discontinuing or Not Adhering to Aspirin among 50 279 Patients at Risk for Coronary Artery Disease. Eur. Heart J. 2006, 27, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- Parani, M.; Lokhande, G.; Singh, A.; Gaharwar, A.K. Engineered Nanomaterials for Infection Control and Healing Acute and Chronic Wounds. ACS Appl. Mater. Interfaces 2016, 8, 10049–10069. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-W.; Wei, J.-J.; Zhang, M.-Y.; Zhang, X.-L.; Yin, X.-F.; Lu, C.-H.; Song, J.-B.; Bai, S.-M.; Yang, H.-H. Water-Based Black Phosphorus Hybrid Nanosheets as a Moldable Platform for Wound Healing Applications. ACS Appl. Mater. Interfaces 2018, 10, 35495–35502. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef]
- Baquero, M.; Martín, N. Depressive Symptoms in Neurodegenerative Diseases. World J. Clin. Cases WJCC 2015, 3, 682–693. [Google Scholar] [CrossRef]
- Hurley, L.L.; Tizabi, Y. Neuroinflammation, Neurodegeneration and Depression. Neurotox. Res. 2013, 23, 131–144. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Ageing, Neurodegeneration and Brain Rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef]
- Furtado, D.; Björnmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the Blood–Brain Barrier: The Role of Nanomaterials in Treating Neurological Diseases. Adv. Mater. 2018, 30, 1801362. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Xiong, S.; Li, Z.; Liu, Y.; Wang, Q.; Luo, J.; Chen, X.; Xie, Z.; Zhang, Y.; Zhang, H.; Chen, T. Brain-Targeted Delivery Shuttled by Black Phosphorus Nanostructure to Treat Parkinson’s Disease. Biomaterials 2020, 260, 120339. [Google Scholar] [CrossRef]
- Jin, L.; Hu, P.; Wang, Y.; Wu, L.; Qin, K.; Cheng, H.; Wang, S.; Pan, B.; Xin, H.; Zhang, W.; et al. Fast-Acting Black-Phosphorus-Assisted Depression Therapy with Low Toxicity. Adv. Mater. 2020, 32, 1906050. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J.T.; Chen, S.W.; Borg, C.B.; Ness, S.; Bahl, J.M.; Heegaard, N.H.H.; Dobson, C.M.; Hemmingsen, L.; Cremades, N.; Teilum, K. Amyloid-β and α-Synuclein Decrease the Level of Metal-Catalyzed Reactive Oxygen Species by Radical Scavenging and Redox Silencing. J. Am. Chem. Soc. 2016, 138, 3966–3969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhai, D.; Xu, M.; Yao, Q.; Zhu, H.; Chang, J.; Wu, C. 3D-Printed Bioceramic Scaffolds with Antibacterial and Osteogenic Activity. Biofabrication 2017, 9, 025037. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H. Structurally and Functionally Optimized Silk-fibroin–Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury in Vitro and in Vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C. Nanomaterial-Based Bone Regeneration. Nanoscale 2017, 9, 4862–4874. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yin, J.; Chen, Y.; Pan, S.; Yao, H.; Gao, Y.; Shi, J. 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds:A Stepwise Countermeasure for Osteosarcoma. Adv. Mater. 2018, 30, 1705611. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Miller, A.L.; Park, S.; George, M.N.; Waletzki, B.E.; Xu, H.; Terzic, A.; Lu, L. Two-Dimensional Black Phosphorus and Graphene Oxide Nanosheets Synergistically Enhance Cell Proliferation and Osteogenesis on 3D Printed Scaffolds. ACS Appl. Mater. Interfaces 2019, 11, 23558–23572. [Google Scholar] [CrossRef]
- Wang, C.; Ye, X.; Zhao, Y.; Bai, L.; He, Z.; Tong, Q.; Xie, X.; Zhu, H.; Cai, D.; Zhou, Y. Cryogenic 3D Printing of Porous Scaffolds for in Situ Delivery of 2D Black Phosphorus Nanosheets, Doxorubicin Hydrochloride and Osteogenic Peptide for Treating Tumor Resection-Induced Bone Defects. Biofabrication 2020, 12, 035004. [Google Scholar] [CrossRef]
- Kunjachan, S.; Ehling, J.; Storm, G.; Kiessling, F.; Lammers, T. Noninvasive Imaging of Nanomedicines and Nanotheranostics: Principles, Progress, and Prospects. Chem. Rev. 2015, 115, 10907–10937. [Google Scholar] [CrossRef]
- Ding, K.; Zeng, J.; Jing, L.; Qiao, R.; Liu, C.; Jiao, M.; Li, Z.; Gao, M. Aqueous Synthesis of PEGylated Copper Sulfide Nanoparticles for Photoacoustic Imaging of Tumors. Nanoscale 2015, 7, 11075–11081. [Google Scholar] [CrossRef]
- Luke, G.P.; Yeager, D.; Emelianov, S.Y. Biomedical Applications of Photoacoustic Imaging with Exogenous Contrast Agents. Ann. Biomed. Eng. 2012, 40, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.F.; de la Zerda, A.; Jokerst, J.V.; Zavaleta, C.L.; Kempen, P.J.; Mittra, E.; Pitter, K.; Huang, R.; Campos, C.; Habte, F.; et al. A Brain Tumor Molecular Imaging Strategy Using A New Triple-Modality MRI-Photoacoustic-Raman Nanoparticle. Nat. Med. 2012, 18, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Chen, C.; Smith, S.C. Phosphorene: Fabrication, Properties, and Applications. J. Phys. Chem. Lett. 2015, 6, 2794–2805. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wen, L.; Zeng, J.; Wang, Y.; Sun, Q.; Deng, L.; Zhao, C.; Li, Z. One-Pot Solventless Preparation of PEGylated Black Phosphorus Nanoparticles for Photoacoustic Imaging and Photothermal Therapy of Cancer. Biomaterials 2016, 91, 81–89. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Y.; Li, Z.; Cui, H.; Zhou, Y.; Li, W.; Tao, W.; Zhang, H.; Wang, H.; Chu, P.K.; et al. TiL4 -Coordinated Black Phosphorus Quantum Dots as an Efficient Contrast Agent for In Vivo Photoacoustic Imaging of Cancer. Small Weinh. Bergstr. Ger. 2017, 13. [Google Scholar] [CrossRef]
- Engel, M.; Steiner, M.; Avouris, P. Black Phosphorus Photodetector for Multispectral, High-Resolution Imaging. Nano Lett. 2014, 14, 6414–6417. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X. Black Phosphorus and Its Biomedical Applications. Theranostics 2018, 8, 1005–1026. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, L.; Li, Z.; Yang, N.; Fu, H.; Wu, L.; Cui, H.; Zhou, W.; Wang, J.; Wang, H.; et al. Stable and Multifunctional Dye-Modified Black Phosphorus Nanosheets for Near-Infrared Imaging-Guided Photothermal Therapy. Chem. Mater. 2017, 29, 7131–7139. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhang, S.; Li, D.; Ma, W.; Ma, C.; Wu, F.; Zhao, Q.; Yan, Q.; Xing, B. Size Effect on the Cytotoxicity of Layered Black Phosphorus and Underlying Mechanisms. Small 2017, 13, 1701210. [Google Scholar] [CrossRef]
- Lim, C.T. Biocompatibility and Nanotoxicity of Layered Two-Dimensional Nanomaterials. ChemNanoMat 2017, 3, 5–16. [Google Scholar] [CrossRef]
- Song, S.-J.; Shin, Y.C.; Lee, H.U.; Kim, B.; Han, D.-W.; Lim, D. Dose- and Time-Dependent Cytotoxicity of Layered Black Phosphorus in Fibroblastic Cells. Nanomaterials 2018, 8, 408. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Park, S.Y.; Lee, S.C.; Choi, S.; Seo, S.; Kim, H.; Won, J.; Choi, K.; Kang, K.S.; Park, H.G.; et al. Black Phosphorus (BP) Nanodots for Potential Biomedical Applications. Small 2016, 12, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Latiff, N.M.; Teo, W.Z.; Sofer, Z.; Fisher, A.C.; Pumera, M. The Cytotoxicity of Layered Black Phosphorus. Chem. – Eur. J. 2015, 21, 13991–13995. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fan, S.; Fan, S.; Li, C.; Shang, Z.; Gu, M.; Liang, S.; Tian, X. In Vitro and In Vivo Toxicity of Black Phosphorus Nanosheets. J. Nanosci. Nanotechnol. 2020, 20, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Gobaa, S.; Hoehnel, S.; Lutolf, M.P. Substrate Elasticity Modulates the Responsiveness of Mesenchymal Stem Cells to Commitment Cues. Integr. Biol. 2015, 7, 1135–1142. [Google Scholar] [CrossRef]
- Tseng, Q.; Wang, I.; Duchemin-Pelletier, E.; Azioune, A.; Carpi, N.; Gao, J.; Filhol, O.; Piel, M.; Théry, M.; Balland, M. A New Micropatterning Method of Soft Substrates Reveals That Different Tumorigenic Signals Can Promote or Reduce Cell Contraction Levels. Lab. Chip 2011, 11, 2231. [Google Scholar] [CrossRef]
- Shin, S.R.; Zihlmann, C.; Akbari, M.; Assawes, P.; Cheung, L.; Zhang, K.; Manoharan, V.; Zhang, Y.S.; Yüksekkaya, M.; Wan, K.; et al. Reduced Graphene Oxide-GelMA Hybrid Hydrogels as Scaffolds for Cardiac Tissue Engineering. Small 2016, 12, 3677–3689. [Google Scholar] [CrossRef]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.b.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J.; et al. Carbon-Nanotube-Embedded Hydrogel Sheets for Engineering Cardiac Constructs and Bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef]
- Sresht, V.; Pádua, A.A.H.; Blankschtein, D. Liquid-Phase Exfoliation of Phosphorene: Design Rules from Molecular Dynamics Simulations. ACS Nano 2015, 9, 8255–8268. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132. [Google Scholar] [CrossRef]
- Marom, N.; Tkatchenko, A.; Rossi, M.; Gobre, V.V.; Hod, O.; Scheffler, M.; Kronik, L. Dispersion Interactions with Density-Functional Theory: Benchmarking Semiempirical and Interatomic Pairwise Corrected Density Functionals. J. Chem. Theory Comput. 2011, 7, 3944–3951. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.J.; Lin, S.; Strano, M.S.; Blankschtein, D. Understanding the Stabilization of Liquid-Phase-Exfoliated Graphene in Polar Solvents: Molecular Dynamics Simulations and Kinetic Theory of Colloid Aggregation. J. Am. Chem. Soc. 2010, 132, 14638–14648. [Google Scholar] [CrossRef] [PubMed]
- Kamath, G.; Baker, G.A. Are Ionic Liquids Suitable Media for Boron Nitride Exfoliation and Dispersion? Insight via Molecular Dynamics. RSC Adv. 2013, 3, 8197–8202. [Google Scholar] [CrossRef]
- Kamath, G.; Baker, G.A. In Silico Free Energy Predictions for Ionic Liquid-Assisted Exfoliation of a Graphene Bilayer into Individual Graphene Nanosheets. Phys. Chem. Chem. Phys. 2012, 14, 7929–7933. [Google Scholar] [CrossRef]
- Ludwig, T.; Guo, L.; Mccrary, P.; Zhang, Z.; Gordon, H.; Quan, H.; Stanton, M.; Frazier, R.M.; Rogers, R.D.; Wang, H.T.; et al. Mechanism of Bismuth Telluride Exfoliation in an Ionic Liquid Solvent. Langmuir 2015, 31, 3644–3652. [Google Scholar] [CrossRef]
- Luo, M.; Fan, T.; Zhou, Y.; Zhang, H.; Mei, L. 2D Black Phosphorus–Based Biomedical Applications. Adv. Funct. Mater. 2019, 29, 1808306. [Google Scholar] [CrossRef]
- Konvalina, G.; Haick, H. Sensors for Breath Testing: From Nanomaterials to Comprehensive Disease Detection. Acc. Chem. Res. 2014, 47, 66–76. [Google Scholar] [CrossRef]



| Method | Mechanism | Application Materials | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| Liquid exfoliation | Cleavage of multilayered crystals dispersed in a solvent in the presence of ultrasonic energy | Transistors, optical devices, super capacitors, sensors, photocatalysts, bioimaging etc. | Quick, easier, and sturdy to environmental conditions and flexible to scale up. | Difficult to control the thickness of the layered crystals | [22] |
| Electrochemical Method | Exfoliation of layers occur by applying voltage between anode and cathode that are usually dipped in an acidic solution | Most commonly used for synthesis of graphene from graphite | Various metal 2D nanosheets can be prepared. | Harmful and toxic chemicals are used | [23] |
| Mechanical Exfoliation Method | A scotch tape process that repeatedly peels off various layers from multilayer crystals/sheets | Imaging, photonic devices, coating, electronic devices, advanced composites, energy storage, metrology, paint, sensors | No chemical is required and eco-friendly | Time consuming, no uniform thickness of the product, low yield, and is not scalable | [24] |
| Plasma Etching Method | An oxidation process where monolayer is placed on a silica substrate which is exposed to oxygen plasma, due to which the surface layer gets oxidized | Optoelectronics and nanoelectronics | Water soluble, Most commonly used in multiple layered hexagonal carbides and nitrides (MAX) materials | Toxic chemicals such as hydrofluoric acid is used; time consuming | [25] |
| Chemical Vapour deposition (CVD) Method | A coating process where the precursors, gas, or vapor, can react or decompose on the preselected substrate at high temperature and vacuum in a chamber | For synthesis of 2D nanomaterials and thin films on solid substrates | High-quality product, large surface area | Sensitive and expensive method that involves toxic gases | [26] |
| Functionalized Material | Modification Using | Advantages | Reference |
|---|---|---|---|
| BP@FKK Complex | Tripeptide Fmoc-Lys-Lys-Phe (Fmoc-KKF) | improved stability; improved cellular uptake; favorable cell compatibility. | [42] |
| DFBP nanosheets (MTP-BP-al-PEG) | Mitochondrial Targeting Peptide (MTP) and acid-labile polymer shell | improved stability; ability to accumulate in tumor tissue; target mitochondria. | [43] |
| Pt@BP | Cisplatin | preserves surface morphology; significant cellular uptake rate; improved cisplatin-resistant cancer cell lines (A2780 and HepG2) drug response. | [44] |
| PEG-modified BPQDs | PEG | Increased stability in the physiological medium; no observable toxicity. | [45] |
| FA-PEG@BPQD@DOX | PEG-NH2-FA | Better PTT effect; precise targeting capability for tumor ablation. | [46] |
| BP@PDA-PEG-FA | HS-PEG-FA; Polydopamine | Synergistic therapy combining chemotherapy with photothermal therapy; enhanced stability; targeting ability for cancer cells. | [47] |
| PLL-BP-Apt | anti-Mb DNA aptamer | Electrochemical-based sensing for the qualitative and quantitative recognition of the cardiac disease biomarker, Mb; high sensitivity and specificity for Mb; potential in POC diagnosis for cardiac disease management. | [48] |
| BP@lipid-PEG | Cholesterol | Exhibit broad emissions; sharp contrast and may be utilized to in situ quantify the measurement of blood vessels; potential in photoacoustic (PA) imaging. | [49] |
| Nanocomposite Material | Application | Synthesis of BPNS/BPQD | Cancer type/Cell Lines | Research Outcome | Reference |
|---|---|---|---|---|---|
| BPNS | PDT | Liquid-phase exfoliation | MDA-MB-231 cells | BPNS inhibited tumor growth with short exposure of light irradiation due to effective photosensing efficiency | [92] |
| BP Quantum Dots Resealed erythrocyte nanovesicle (BPQD@RM) | PTT/Immunotherapy | Sonication exfoliation | 4T1-breast tumor cells | The combination of PTT with programmed cell death protein delayed the metastatic growth in vivo by increasing activity of CD8+ T cells activity in tumor | [132] |
| BPNS cellulose hydrogels | PTT | Liquid-phase exfoliation | SMMC-7721 hepatocellular cancer cell lines | The BPNS hydrogel composites displayed excellent PTT efficiency against cancer and was also biocompatible | [133] |
| PEGylated BPQD | PDT/PTT | Sonication exfoliation | Hep G2 cells | The PEGylated BPQDs showed strong NIR absorption to generate ROS and inhibit tumor thereby showing a theranostic activity in cancer therapy | [118] |
| BPN/MnO2 | PDT/PTT/Gene therapy | Modified Liquid-phase exfoliation | HeLa and A549 lung cancer cells | Combinatorial therapy resulted in the release of siRNA to targeted tumor cells by specific nanocomposite degradation and ROS produced by irradiation, led to the suppression of tumor growth. | [134] |
| PDA@BPQD (Polydopamine functionalized BPQDs) | Photoacoustic imaging (PAI) PAI/PTT | Sonication exfoliation | Nude mice bearing A375 human melanoma tumor | Along with the stability, PDA@BPQDs possessed good photothermal performance on cancer cells | [135] |
| BPN-CuS-FA (Folic acid anchored CuS nanodot-modified BPN) | PDT/PTT/PAI | Modified mechanical exfoliation | 4T1-breast tumor cells | BPN-CuS-FA displayed good PAI activity, enabling in vivo monitoring. The FA targeted folate receptors on the tumor cells enabled the uptake of BPN-CuS complex which showed PDT-PTT-mediated antitumor activity | [136] |
| PEG@BPN-Ce6 (Chlorin e6 BPN) | PDT/PTT | Liquid-phase exfoliation | HeLa cells | The complex showed good PDT/PTT activity. The in vivo fluorescence imaging displayed that the complex accumulated in tumor cells and synergetic antitumor efficacy was observed due to incorporation of Ce6 | [137] |
| BPNS-bPEI-PEG | PTT/Immunotherapy | Liquid-phase exfoliation | HepG2, RAW264.7 and 4T1 cell lines | The BPNS grafted CpG (immunologic adjuvant) provided as an efficient necroptosis modulator to mediate PTT and anticancer immunotherapy by activating the immunogenic cell death and initiating the immune response. | [138] |
| NE-BP Neutrophil-coated BP nanoflakes adsorbed on PEI and TGF-β inhibitor | PDT/PTT/Immunotherapy | Liquid-phase exfoliation | 4T1 lung cancer cell line | The complex showed enhanced stability, high tumor accumulation, and superior PDT/PTT efficiency in comparison with bare BP resulting in suppressed tumors metastasis. | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, A.; Nikam, A.N.; Fernandes, G.; Kulkarni, S.; Padya, B.S.; Prassl, R.; Das, S.; Joseph, A.; Deshmukh, P.K.; Patil, P.O.; et al. Black Phosphorus as Multifaceted Advanced Material Nanoplatforms for Potential Biomedical Applications. Nanomaterials 2021, 11, 13. https://doi.org/10.3390/nano11010013
Pandey A, Nikam AN, Fernandes G, Kulkarni S, Padya BS, Prassl R, Das S, Joseph A, Deshmukh PK, Patil PO, et al. Black Phosphorus as Multifaceted Advanced Material Nanoplatforms for Potential Biomedical Applications. Nanomaterials. 2021; 11(1):13. https://doi.org/10.3390/nano11010013
Chicago/Turabian StylePandey, Abhijeet, Ajinkya N. Nikam, Gasper Fernandes, Sanjay Kulkarni, Bharath Singh Padya, Ruth Prassl, Subham Das, Alex Joseph, Prashant K. Deshmukh, Pravin O. Patil, and et al. 2021. "Black Phosphorus as Multifaceted Advanced Material Nanoplatforms for Potential Biomedical Applications" Nanomaterials 11, no. 1: 13. https://doi.org/10.3390/nano11010013
APA StylePandey, A., Nikam, A. N., Fernandes, G., Kulkarni, S., Padya, B. S., Prassl, R., Das, S., Joseph, A., Deshmukh, P. K., Patil, P. O., & Mutalik, S. (2021). Black Phosphorus as Multifaceted Advanced Material Nanoplatforms for Potential Biomedical Applications. Nanomaterials, 11(1), 13. https://doi.org/10.3390/nano11010013