The Adsorption and Sensing Performances of Ir-modified MoS2 Monolayer toward SF6 Decomposition Products: A DFT Study
Abstract
1. Introduction
2. Computational Detail
3. Results and Discussions
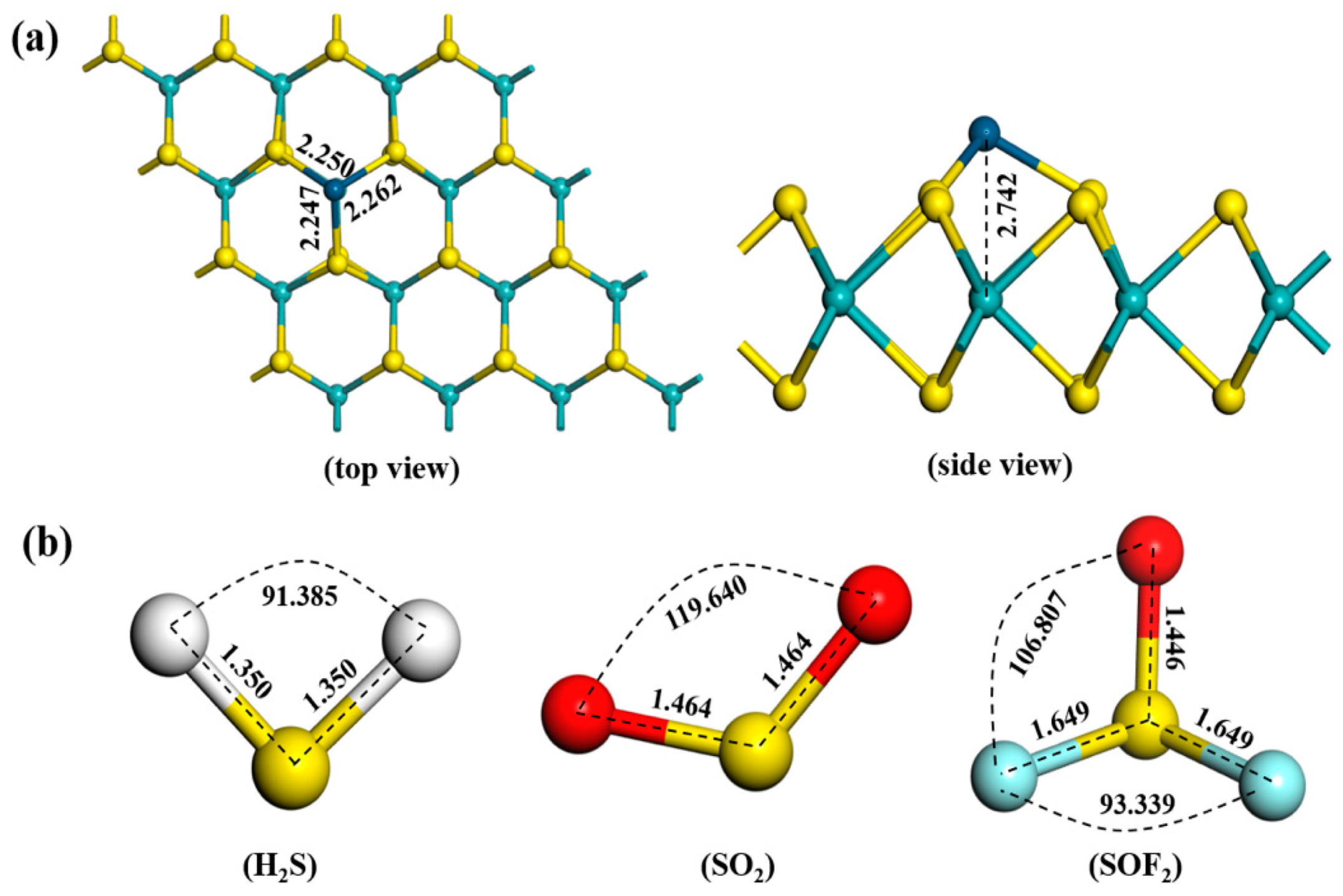
3.1. Structure and Properties of the Ir-modified MoS2 Monolayer
3.2. Gas Molecules Adsorption on the Ir-modified MoS2 Surface
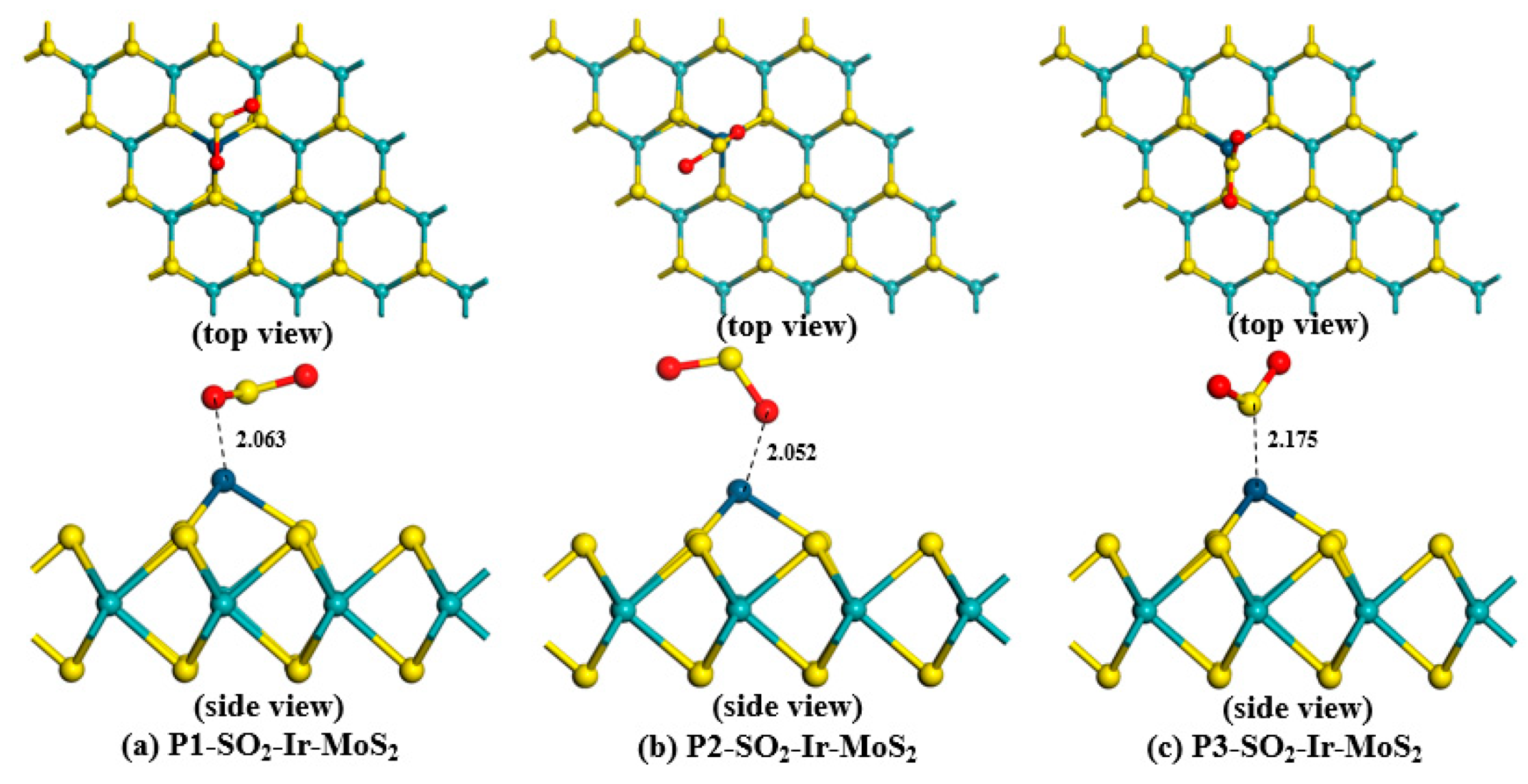
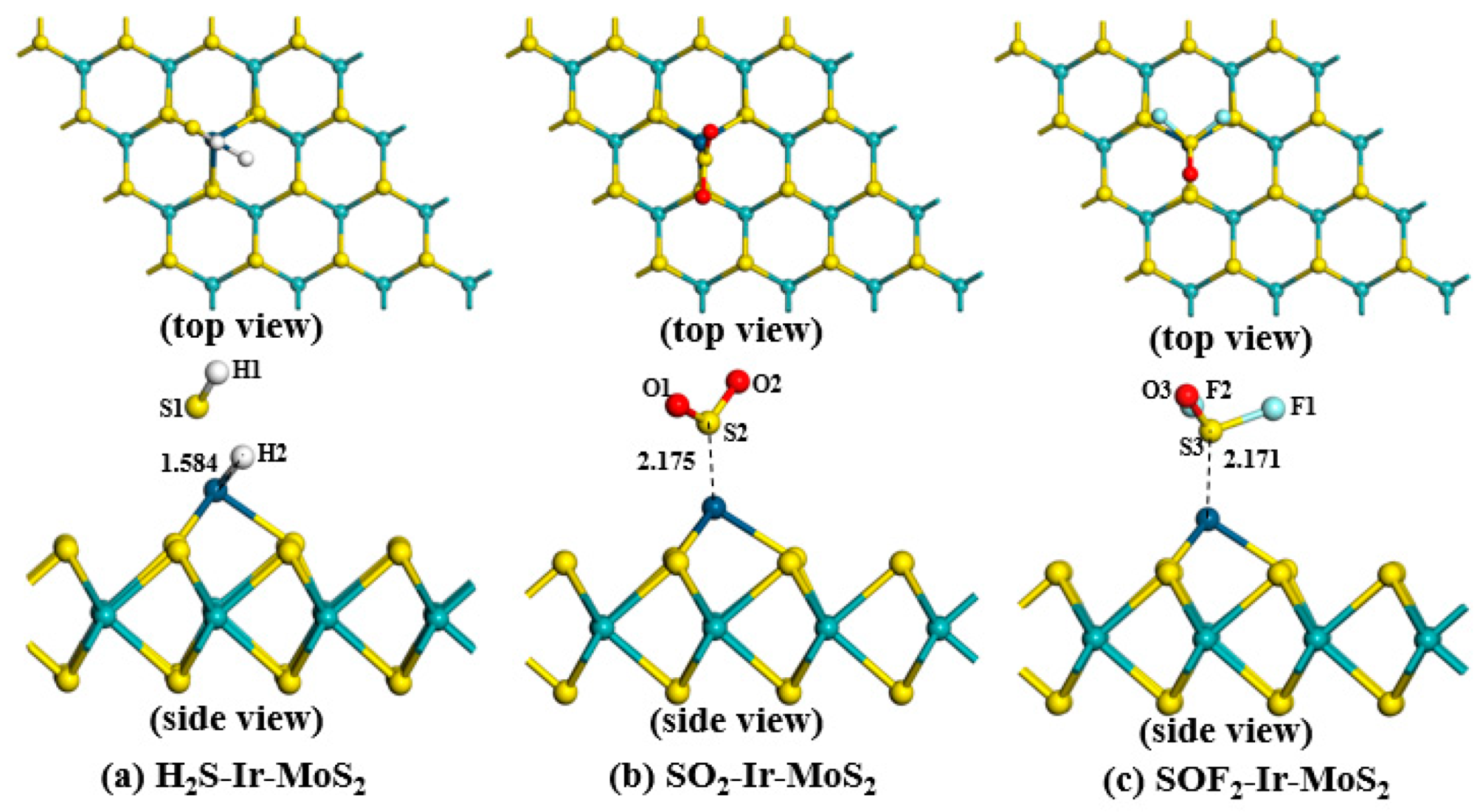
3.2.1. Structures of Different Adsorption Systems
3.2.2. DOS Analysis of Different Adsorption Systems
3.2.3. DCD Analysis of Various Adsorption Systems
3.2.4. Frontier Molecular Orbital Analysis of Different Systems
3.3. Gas-Sensing Prediction of Ir-modified MoS2 to SF6 Decomposition Products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beslija, D.; Gorenc, D.; Muratovic, M.; Kapetanovic, M. Enhanced Method for Pressure Rise Calculation in SF6 GIS Due to Fault Arcs. IEEE Trans. Power Deliv. 2020, 35, 1619–1624. [Google Scholar] [CrossRef]
- Wu, S.Y.; Zeng, F.P.; Tang, J.; Yao, Q.; Miao, Y.L. Triangle Fault Diagnosis Method for SF6 Gas-Insulated Equipment. IEEE Trans. Power Deliv. 2019, 34, 1470–1477. [Google Scholar] [CrossRef]
- Chen, D.C.; Zhang, X.X.; Tang, J.; Cui, Z.L.; Cui, H.; Pi, S.M. Theoretical Study of Monolayer PtSe2 as Outstanding Gas Sensor to Detect SF6 Decompositions. IEEE Electron Device Lett. 2018, 39, 1405–1408. [Google Scholar] [CrossRef]
- Pepi, F.; Ricci, A.; Di Stefano, M.; Rosi, M. Sulfur hexafluoride corona discharge decomposition: Gas-phase ion chemistry of SOFx+ (x = 1–3) ions. Chem. Phys. Lett. 2003, 381, 168–176. [Google Scholar] [CrossRef]
- Liu, D.K.; Gui, Y.G.; Ji, C.; Tang, C.; Zhou, Q.; Li, J.; Zhang, X.X. Adsorption of SF6 decomposition components over Pd (111): A density functional theory study. Appl. Surf. Sci. 2019, 465, 172–179. [Google Scholar] [CrossRef]
- Wang, D.W.; Lan, T.S.; Yang, A.J.; Pan, J.B.; Chu, J.F.; Yuan, H.; Li, Y.J.; Wang, X.H.; Rong, M.Z. SF6 Decomposition Gas Sensor Based on GeP Monolayer: A First-Principle Study. IEEE Sens. J. 2020, 20, 8997–9003. [Google Scholar] [CrossRef]
- Cui, H.P.; Zheng, K.; Tao, L.Q.; Yu, J.B.; Zhu, X.Y.; Li, X.D.; Chen, X.P. Monolayer Tellurene-Based Gas Sensor to Detect SF6 Decompositions: A First Principles Study. IEEE Electron Device Lett. 2019, 40, 1522–1525. [Google Scholar] [CrossRef]
- Qian, H.; Deng, J.; Xie, Z.C.; Pan, Z.C.; Zhang, J.Y.; Zhou, H.B. Adsorption and Gas Sensing Properties of the Pt3-MoSe2 Monolayer to SOF2 and SO2F2. ACS Omega 2020, 5, 7722–7728. [Google Scholar] [CrossRef]
- Lu, Z.R.; Zhou, Q.; Wang, C.S.; Wei, Z.J.; Xu, L.N.; Gui, Y.G. Electrospun ZnO-SnO2 Composite Nanofibers and Enhanced Sensing Properties to SF6 Decomposition Byproduct H2S. Front. Chem. 2018, 6, 9. [Google Scholar] [CrossRef]
- Zeng, W.W.; Liu, Y.Z.; Mei, J.; Tang, C.Y.; Luo, K.; Li, S.M.; Zhan, H.R.; He, Z.K. Hierarchical SnO2-Sn3O4 heterostructural gas sensor with high sensitivity and selectivity to NO2. Sens. Actuator B Chem. 2019, 301, 9. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. Development of Pd/TiO2 Porous Layers by Pulsed Laser Deposition for Surface Acoustic Wave H2 Gas Sensor. Nanomaterials 2020, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Luxa, J.; Sofer, Z. Emerging pnictogen-based 2D semiconductors: Sensing and electronic devices. Nanoscale 2020, 12, 10430–10446. [Google Scholar] [CrossRef] [PubMed]
- Rui, H.S.; Li, L.; Zhang, N.; Lin, X.; Hua, Y.L.; Wu, X.M.; Wang, D.; Yin, S.G. Tunable Deep-Red Electroluminescence From Flexible Quasi-2D Perovskites Light-Emitting Diodes. IEEE Electron Device Lett. 2019, 40, 59–62. [Google Scholar] [CrossRef]
- Gui, Y.G.; Li, T.; He, X.; Ding, Z.Y.; Yang, P.G. Pt Cluster Modified h-BN for Gas Sensing and Adsorption of Dissolved Gases in Transformer Oil: A Density Functional Theory Study. Nanomaterials 2019, 9, 1746. [Google Scholar] [CrossRef]
- Yang, Z.M.; Zhang, D.Z.; Chen, H.N. MOF-derived indium oxide hollow microtubes/MoS2 nanoparticles for NO2 gas sensing. Sens. Actuator B Chem. 2019, 300, 10. [Google Scholar] [CrossRef]
- Rathi, K.; Kumar, A.N.; Pal, K. Fabrication of flexible La-MoS2 hybrid-heterostructure based sensor for NO2 gas sensing at room temperature. Nanotechnology 2020, 31, 13. [Google Scholar] [CrossRef]
- Urs, K.M.B.; Katiyar, N.K.; Kumar, R.; Biswas, K.; Singh, A.K.; Tiwary, C.S.; Kamble, V. Multi-component (Ag-Au-Cu-Pd-Pt) alloy nanoparticle-decorated p-type 2D-molybdenum disulfide (MoS2) for enhanced hydrogen sensing. Nanoscale 2020. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhou, Q.; Lu, Z.R.; Gui, Y.G.; Zeng, W. Adsorption of H2O molecule on TM (Au, Ag) doped-MoS2 monolayer: A first-principles study. Physica E 2019, 113, 72–78. [Google Scholar] [CrossRef]
- Lin, L.; Shi, Z.G.; Huang, J.T.; Wang, P.T.; Yu, W.Y.; He, C.Z.; Zhang, Z.Y. Molecular adsorption properties of CH4 with noble metals doped onto oxygen vacancy defect of anatase TiO2(101) surface: First-principles calculations. Appl. Surf. Sci. 2020, 514, 7. [Google Scholar] [CrossRef]
- Basharnavaz, H.; Habibi-Yangjeh, A.; Kamali, S.H. A first-principle investigation of NO2 adsorption behavior on Co, Rh, and Ir-embedded graphitic carbon nitride: Looking for highly sensitive gas sensor. Phys. Lett. A 2020, 384, 8. [Google Scholar] [CrossRef]
- Zhang, X.X.; Chen, Z.W.; Chen, D.C.; Cui, H.; Tang, J. Adsorption behaviour of SO2 and SOF2 gas on Rh-doped BNNT: A DFT study. Mol. Phys. 2020, 118, 9. [Google Scholar] [CrossRef]
- Gui, Y.G.; Liu, D.K.; Li, X.D.; Tang, C.; Zhou, Q. DFT-based study on H2S and SOF2 adsorption on Si-MoS2 monolayer. Results Phys. 2019, 13, 8. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhou, Q.; Lu, Z.R.; Wei, Z.J.; Zeng, W. Gas sensing performances and mechanism at atomic level of Au-MoS2 microspheres. Appl. Surf. Sci. 2019, 490, 124–136. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 6. [Google Scholar] [CrossRef]
- Zhang, X.X.; Fang, R.X.; Chen, D.C.; Zhang, G.Z. Using Pd-Doped gamma-Graphyne to Detect Dissolved Gases in Transformer Oil: A Density Functional Theory Investigation. Nanomaterials 2019, 9, 1490. [Google Scholar] [CrossRef]
- Peng, S.; Cho, K.J.; Qi, P.F.; Dai, H.J. Ab initio study of CNT NO2 gas sensor. Chem. Phys. Lett. 2004, 387, 271–276. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.X.; Zhang, G.Z.; Tang, J. Pd-doped MoS2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. [Google Scholar] [CrossRef]
- Zhang, X.X.; Yu, L.; Wu, X.Q.; Hu, W.H. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 10. [Google Scholar] [CrossRef]
- Li, J.; Pang, L.; Cai, F.; Yuan, X.; Kong, F. Adsorption Properties of Pd3-Modified Double-Vacancy Defect Graphene toward SF6 Decomposition Products. Sensors 2020, 20, 4188. [Google Scholar] [CrossRef]
- Ma, S.X.; Li, D.J.; Rao, X.J.; Xia, X.F.; Su, Y.; Lu, Y.F. Pd-doped h-BN monolayer: A promising gas scavenger for SF6 insulation devices. Adsorpt. J. Int. Adsorpt. Soc. 2020, 26, 619–626. [Google Scholar] [CrossRef]
- Cui, H.; Yan, C.; Jia, P.F.; Cao, W. Adsorption and sensing behaviors of SF6 decomposed species on Ni-doped C3N monolayer: A first-principles study. Appl. Surf. Sci. 2020, 512, 7. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.X.; Chen, D.C.; Tang, J. Adsorption mechanism of SF6 decomposed species on pyridine-like PtN3 embedded CNT: A DFT study. Appl. Surf. Sci. 2018, 447, 594–598. [Google Scholar] [CrossRef]
- Wang, F.P.; Liu, H.C.; Hu, K.L.; Li, Y.Q.; Zeng, W.; Zeng, L. Hierarchical composites of MoS2 nanoflower anchored on SnO2 nanofiber for methane sensing. Ceram. Int. 2019, 45, 22981–22986. [Google Scholar] [CrossRef]









| System | Adsorption Position | Ead (eV) | D (Å) | Qt (e) |
|---|---|---|---|---|
| H2S-Ir-MoS2 | Position 1 (Figure 4a) | −1.578 | 2.317 | 0.341 |
| Position 2 (Figure 4b) | −2.310 | 1.594 | 0.292 | |
| Position 3 (Figure 4c) | −2.323 | 1.584 | 0.286 | |
| SO2-Ir-MoS2 | Position 1 (Figure 5a) | −1.656 | 2.063 | −0.158 |
| Position 2 (Figure 5b) | −1.053 | 2.052 | −0.190 | |
| Position 3 (Figure 5c) | −1.757 | 2.175 | 0.114 | |
| SOF2-Ir-MoS2 | Position 1 (Figure 6a) | −0.411 | 2.216 | 0.069 |
| Position 2 (Figure 6b) | −0.104 | 2.873 | 0.048 | |
| Position 3 (Figure 6c) | −1.492 | 2.171 | 0.154 |
| System | Ead (eV) | D (Å) | Qt (e) |
|---|---|---|---|
| H2S-Ir-MoS2 | −2.323 | 1.584 | 0.286 |
| SO2-Ir-MoS2 | −1.757 | 2.175 | 0.114 |
| SOF2-Ir-MoS2 | −1.492 | 2.171 | 0.154 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, F.; Hu, K.; Li, T.; Yan, Y.; Li, J. The Adsorption and Sensing Performances of Ir-modified MoS2 Monolayer toward SF6 Decomposition Products: A DFT Study. Nanomaterials 2021, 11, 100. https://doi.org/10.3390/nano11010100
Liu H, Wang F, Hu K, Li T, Yan Y, Li J. The Adsorption and Sensing Performances of Ir-modified MoS2 Monolayer toward SF6 Decomposition Products: A DFT Study. Nanomaterials. 2021; 11(1):100. https://doi.org/10.3390/nano11010100
Chicago/Turabian StyleLiu, Hongcheng, Feipeng Wang, Kelin Hu, Tao Li, Yuyang Yan, and Jian Li. 2021. "The Adsorption and Sensing Performances of Ir-modified MoS2 Monolayer toward SF6 Decomposition Products: A DFT Study" Nanomaterials 11, no. 1: 100. https://doi.org/10.3390/nano11010100
APA StyleLiu, H., Wang, F., Hu, K., Li, T., Yan, Y., & Li, J. (2021). The Adsorption and Sensing Performances of Ir-modified MoS2 Monolayer toward SF6 Decomposition Products: A DFT Study. Nanomaterials, 11(1), 100. https://doi.org/10.3390/nano11010100






