Ferritin Nanocage: A Versatile Nanocarrier Utilized in the Field of Food, Nutrition, and Medicine
Abstract
1. Introduction
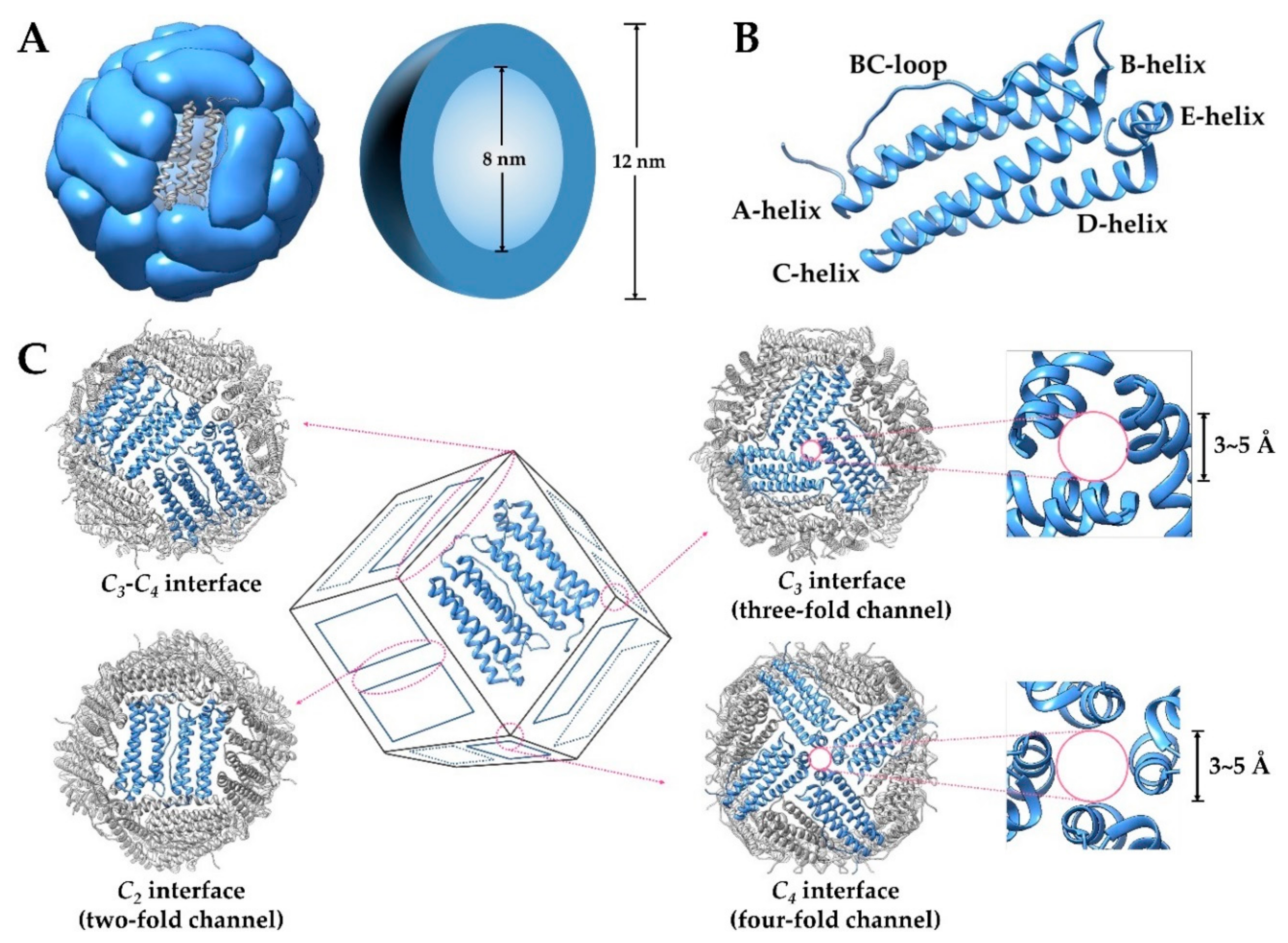
2. Structure and Property of Ferritin
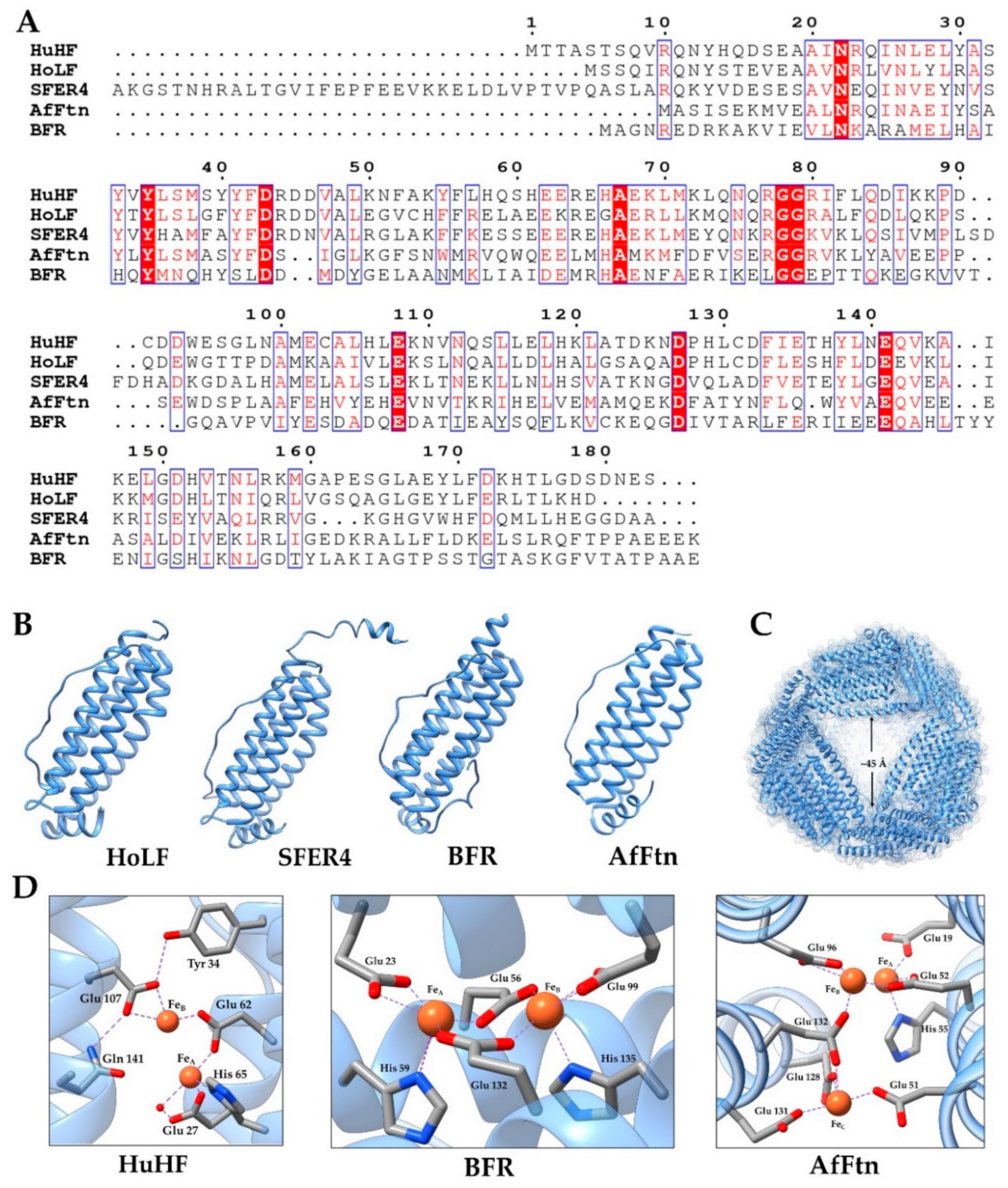
2.1. General Aspects of Ferritin Structure
2.2. Animal Ferritin
2.3. Phytoferritin
2.4. Bacterial Ferritin
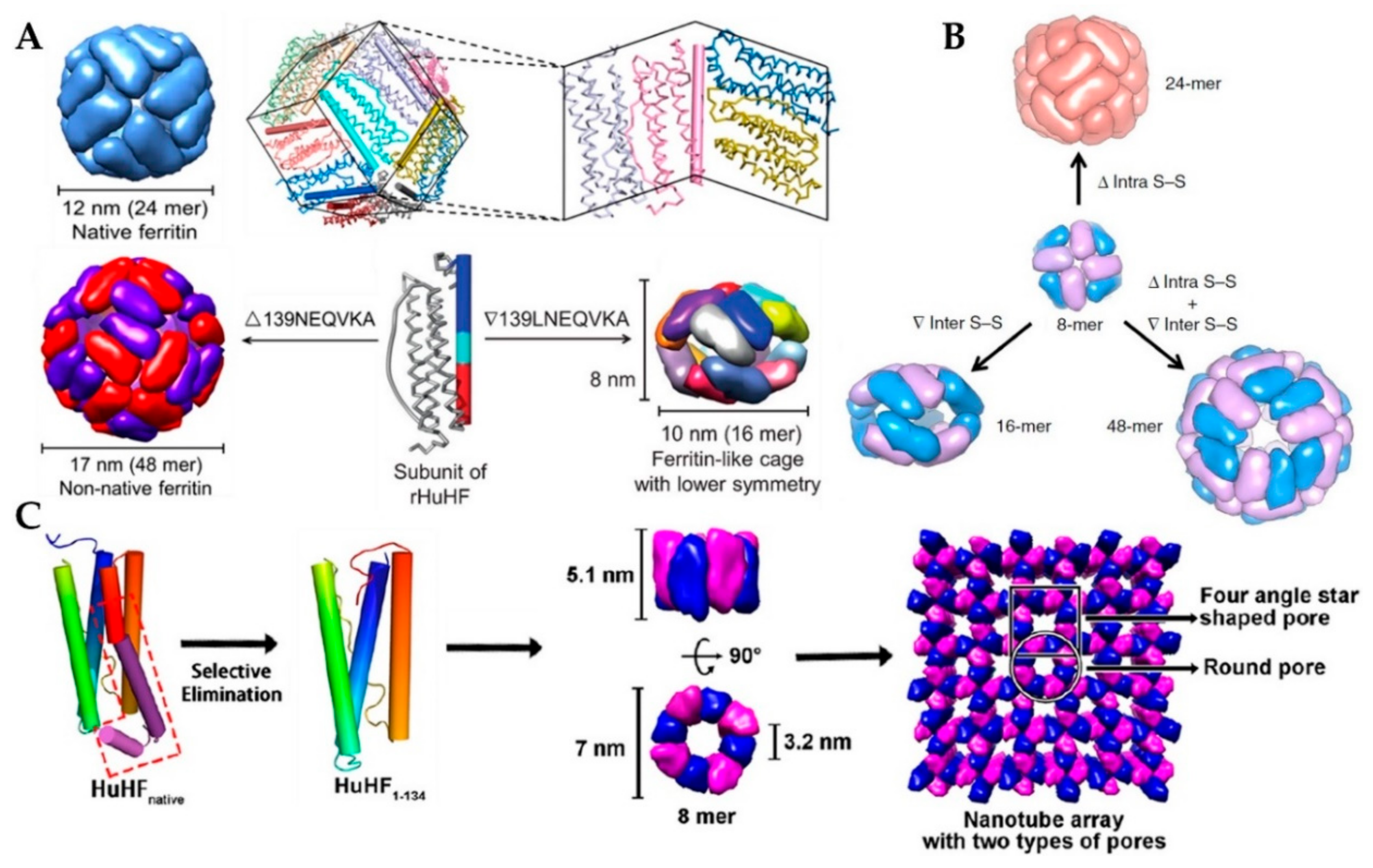
2.5. Non-Native Ferritin
3. Preparation of Ferritin-Hybrid Nanoparticles
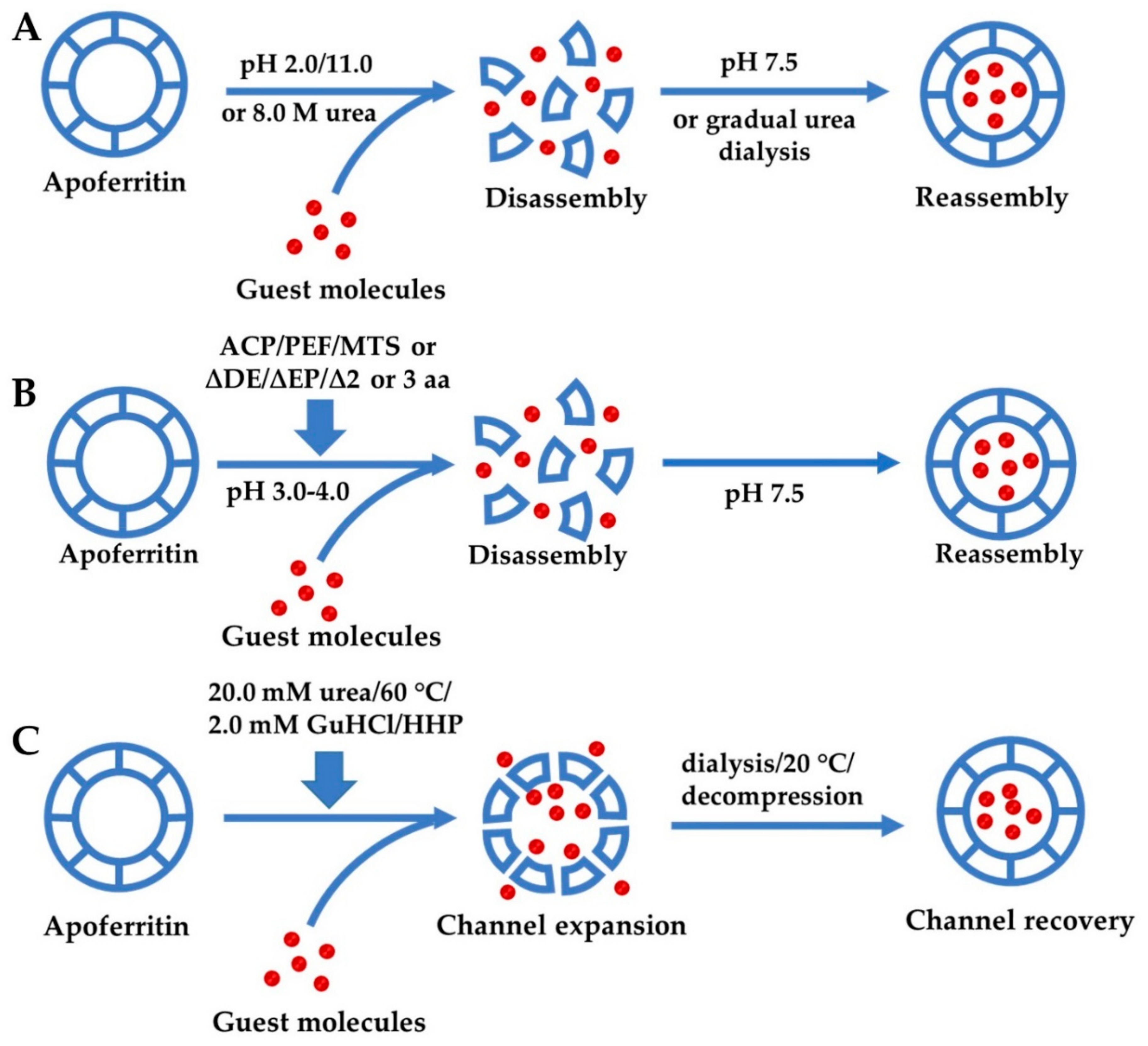
3.1. Reversible Self-Assembly Property of Ferritin
3.1.1. Self-Assembly of Ferritin Controlled by Different Chemicals
3.1.2. Regulation of Ferritin Self-Assembly by Physical Methods
3.1.3. Genetic Modification for Controlling Ferritin Self-Assembly
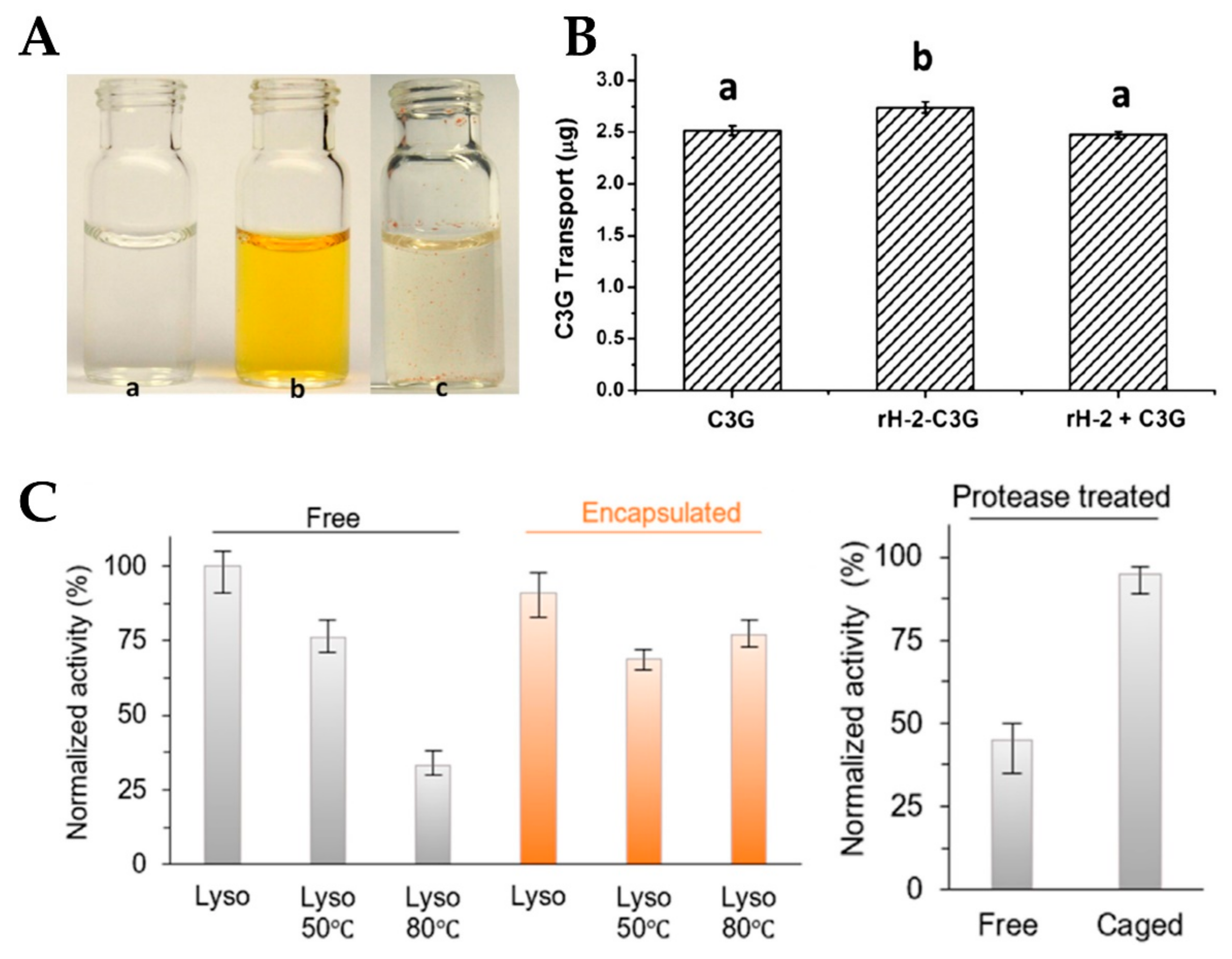
3.2. Expansion of Ferritin Channels by Different Methods for Encapsulation
| Encapsulation Methods | Guest Molecules | Strengths | Weaknesses | Encapsulation Ratio (w/w) | Loading Efficiency | References | ||
|---|---|---|---|---|---|---|---|---|
| Self-assembly | Addition of chemicals | HCl/NaOH | curcumin; β-carotene; C3G; metallodrugs | suitable for a variety of molecules | harsh condition; low protein recovery | 15–32% | 1–3% | [14,17,18,80] |
| 8 M urea | DOX | suitable for pH-sensitive molecules | protein precipitation; guest molecules waste | - | - | [21] | ||
| Physical methods | ACP/PEF | curcumin/rutin | encapsulation under moderate pH conditions | require sophisticated equipment | 12.7–13.7% | - | [65,66] | |
| MTS | EGCG | 25.29% | [68] | |||||
| Genetic modification | ΔDE | curcumin/DOX | large pores up to 18 Å at fourfold channel; incomplete protein recovery | - | ~1% | [69,70] | ||
| ΔEP | EGCG | 11.6% | - | [71] | ||||
| Δ45DD46/Δ44RDD46 | DOX/curcumin | disturb biocompatibility and in vivo performance | - | 1.67% | [72] | |||
| Channel expansion | 20.0 mM urea | EGCG; chlorogenic acid; anthocyanin | little damage to protein; encapsulation without pH adjustment and genetic modification | urea/GuHCl can also be trapped within ferritin cavity; not suitable for larger molecules | 17.6% | 2.1% | [20] | |
| 2.0 mM GuHCl | rutin | 10.1% | - | [75] | ||||
| 60 °C treatment | rutin/EGCG | may cause guest molecules degradation | 8.08/12.8% | - | [19] | |||
| HHP | DOX | long processing time | - | - | [77] | |||
3.3. Biomineralization for Preparing Ferritin-Hybrid Nanoparticles
4. Applications of Ferritin-Directed Nanoparticles
4.1. Applications in Food Science and Nutrition
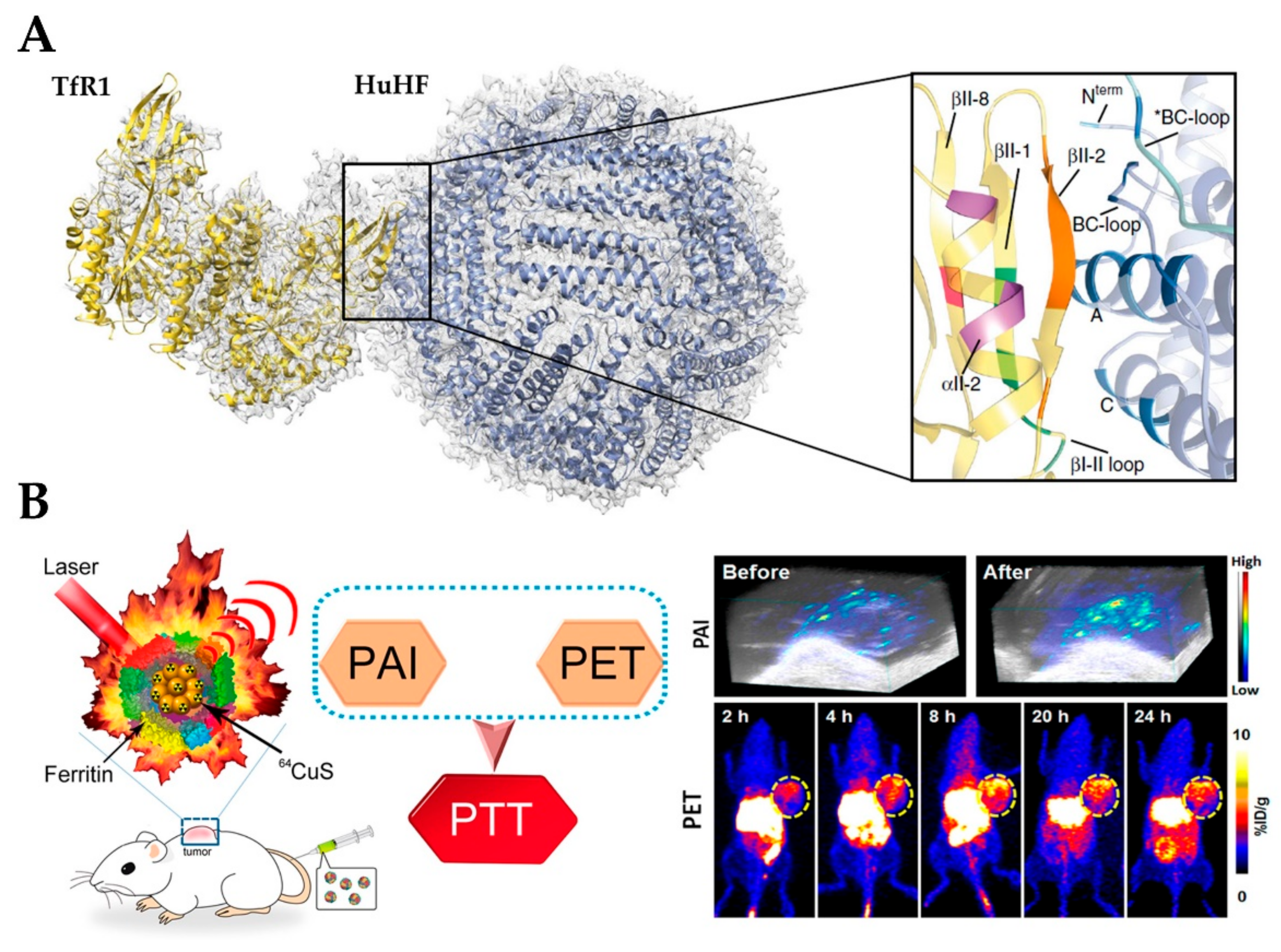
4.2. Applications in Medicine and Diagnostics
5. Challenges of Ferritin as a Nanocarrier
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bhaskar, S.; Lim, S. Engineering protein nanocages as carriers for biomedical applications. NPG Asia Mater. 2017, 9, e371. [Google Scholar] [CrossRef]
- Ferrer-Miralles, N.; Rodríguez-Carmona, E.; Corchero, J.L.; García-Fruitós, E.; Vázquez, E.; Villaverde, A. Engineering protein self-assembling in protein-based nanomedicines for drug delivery and gene therapy. Crit. Rev. Biotechnol. 2015, 35, 209–221. [Google Scholar] [CrossRef]
- Maham, A.; Tang, Z.; Wu, H.; Wang, J.; Lin, Y. Protein-based nanomedicine platforms for drug delivery. Small 2009, 5, 1706–1721. [Google Scholar] [CrossRef] [PubMed]
- Theil, E. Iron, ferritin, and nutrition. Annu. Rev. Nutr. 2004, 24, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Levi, S. Ferritin, iron homeostasis, and oxidative damage. Free Radic. Biol. Med. 2002, 33, 457–463. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- Zhao, G. Phytoferritin and its implications for human health and nutrition. Biochim. Biophys. Acta 2010, 1800, 815–823. [Google Scholar] [CrossRef]
- Stefanini, S.; Cavallo, S.; Wang, C.Q.; Tataseo, P.; Vecchini, P.; Giartosio, A.; Chiancone, E. Thermal stability of horse spleen apoferritin and human recombinant H apoferritin. Arch. Biochem. Biophys. 1996, 325, 58–64. [Google Scholar] [CrossRef]
- Liu, X.; Jin, W.; Theil, E.C. Opening protein pores with chaotropes enhances Fe reduction and chelation of Fe from the ferritin biomineral. Proc. Natl. Acad. Sci. USA 2003, 100, 3653–3658. [Google Scholar] [CrossRef]
- Klem, M.; Mosolf, J.; Young, M.; Douglas, T. Photochemical mineralization of europium, titanium, and iron oxyhydroxide nanoparticles in the ferritin protein cage. Inorg. Chem. 2009, 47, 2237–2239. [Google Scholar] [CrossRef]
- Fan, K.; Cao, C.; Pan, Y.; Lu, D.; Yang, D.; Feng, J.; Song, L.; Liang, M.; Yan, X. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 2012, 7, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. Immunological tolerance and autoimmunity. Intern. Emerg. Med. 2006, 1, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, J.; Lea, S.A.; Lin, Y. Bioassay labels based on apoferritin nanovehicles. ChemBioChem 2006, 7, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lv, C.; Chen, L.; Bai, G.; Zhao, G.; Xu, C. Encapsulation of anthocyanin molecules within a ferritin nanocage increases their stability and cell uptake efficiency. Food Res. Int. 2014, 62, 183–192. [Google Scholar] [CrossRef]
- Chasteen, N.D.; Harrison, P.M. Mineralization in ferritin: An efficient means of iron storage. J. Struct. Biol. 1999, 126, 182–194. [Google Scholar] [CrossRef]
- Jutz, G.; van Rijn, P.; Santos Miranda, B.; Boker, A. Ferritin: A versatile building block for bionanotechnology. Chem. Rev. 2015, 115, 1653–1701. [Google Scholar] [CrossRef]
- Chen, L.; Bai, G.; Yang, R.; Zang, J.; Zhou, T.; Zhao, G. Encapsulation of β-carotene within ferritin nanocages greatly increases its water-solubility and thermal stability. Food Chem. 2014, 149, 307–312. [Google Scholar] [CrossRef]
- Chen, L.; Bai, G.; Yang, S.; Yang, R.; Zhao, G.; Xu, C.; Leung, W. Encapsulation of curcumin in recombinant human H-chain ferritin increases its water-solubility and stability. Food Res. Int. 2014, 62, 1147–1153. [Google Scholar] [CrossRef]
- Yang, R.; Tian, J.; Liu, Y.; Yang, Z.; Wu, D.; Zhou, Z. Thermally induced encapsulation of food nutrients into phytoferritin through the flexible channels without additives. J. Agric. Food Chem. 2017, 65, 9950–9955. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Meng, D.; Chen, Z.; Blanchard, C.L.; Zhou, Z. Urea-driven epigallocatechin gallate (EGCG) permeation into the ferritin cage, an innovative method for fabrication of protein-polyphenol co-assemblies. J. Agric. Food Chem. 2017, 65, 1410–1419. [Google Scholar] [CrossRef]
- Liang, M.; Fan, K.; Zhou, M.; Duan, D.; Zheng, J.; Yang, D.; Feng, J.; Yan, X. H-ferritin-nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc. Natl. Acad. Sci. USA 2014, 111, 14900–14905. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Rong, P.; Jin, A.; Yan, X.; Zhang, M.G.; Lin, J.; Hu, H.; Wang, Z.; Yue, X.; Li, W.; et al. Dye-loaded ferritin nanocages for multimodal imaging and photothermal therapy. Adv. Mater. 2014, 26, 6401–6408. [Google Scholar] [CrossRef] [PubMed]
- Crichton, R.R.; Declercq, J.P. X-ray structures of ferritins and related proteins. Biochim. Biophys. Acta 2010, 1800, 706–718. [Google Scholar] [CrossRef]
- Masuda, T.; Goto, F.; Yoshihara, T.; Mikami, B. Crystal structure of plant ferritin reveals a novel metal binding site that functions as a transit site for metal transfer in ferritin. J. Biol. Chem. 2010, 285, 4049–4059. [Google Scholar] [CrossRef]
- Zang, J.; Chen, H.; Zhao, G.; Wang, F.; Ren, F. Ferritin cage for encapsulation and delivery of bioactive nutrients: From structure, property to applications. Crit. Rev. Food Sci. Nutr. 2016, 57, 3673–3683. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, L.; Bertrand, L.; Hue, L.; Crichton, R.R.; Declercq, J.P. High-resolution X-ray structures of human apoferritin H-chain mutants correlated with their activity and metal-binding sites. J. Mol. Biol. 2007, 365, 440–452. [Google Scholar] [CrossRef]
- Crichton, R.R.; Herbas, A.; Chavez-Alba, O.; Roland, F. Identification of catalytic residues involved in iron uptake by L-chain ferritins. J. Biol. Inorg. Chem. 1996, 1, 567–574. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Z.; Sun, G.; Gao, Y.; Xu, J. Ferritin, a novel vehicle for iron supplementation and food nutritional factors encapsulation. Trends Food Sci. Technol. 2015, 44, 189–200. [Google Scholar] [CrossRef]
- Dickey, L.F.; Sreedharan, S.; Theil, E.C.; Didsbury, J.R.; Wang, Y.H.; Kaufman, R.E. Differences in the regulation of messenger RNA for housekeeping and specialized-cell ferritin. A comparison of three distinct ferritin complementary DNAs, the corresponding subunits, and identification of the first processed in amphibia. J. Biol. Chem. 1987, 262, 7901–7907. [Google Scholar]
- Nie, G.; Sheftel, A.D.; Kim, S.F.; Ponka, P. Overexpression of mitochondrial ferritin causes cytosolic iron depletion and changes cellular iron homeostasis. Blood 2005, 105, 2161–2167. [Google Scholar] [CrossRef]
- Levi, S.; Corsi, B.; Bosisio, M.; Invernizzi, R.; Volz, A.; Sanford, D.; Arosio, P.; Drysdale, J. A human mitochondrial ferritin encoded by an intronless gene. J. Biol. Chem. 2001, 276, 24437–24440. [Google Scholar] [CrossRef]
- Treffry, A.; Harrison, P.M.; Luzzago, A.; Cesareni, G. Recombinant H-chain ferritins: Effects of changes in the 3-fold channels. FEBS Lett. 1989, 247, 268–272. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
- De Val, N.; Declercq, J.-P.; Lim, C.K.; Crichton, R.R. Structural analysis of haemin demetallation by L-chain apoferritins. J. Inorg. Biochem. 2012, 112, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Macedo, S.; Romão, C.V.; Mitchell, E.; Matias, P.M.; Liu, M.Y.; Xavier, A.V.; LeGall, J.; Teixeira, M.; Lindley, P.; Carrondo, M.A. The nature of the di-iron site in the bacterioferritin from Desulfovibrio desulfuricans. Nat. Struct. Mol. Biol. 2003, 10, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.; Cascio, D.; Sawaya, M.R.; Gingery, M.; Schroder, I. Crystal structures of a tetrahedral open pore ferritin from the hyperthermophilic archaeon Archaeoglobus fulgidus. Structure 2005, 13, 637–648. [Google Scholar] [CrossRef]
- Waldo, G.S.; Wright, E.; Whang, Z.H.; Briat, J.F.; Theil, E.C.; Sayers, D.E. Formation of the ferritin iron mineral occurs in plastids. Plant. Physiol. 1995, 109, 797–802. [Google Scholar] [CrossRef]
- Lescure, A.M.; Proudhon, D.; Pesey, H.; Ragland, M.; Theil, E.C.; Briat, J.F. Ferritin gene transcription is regulated by iron in soybean cell cultures. Proc. Natl. Acad. Sci. USA 1991, 88, 8222–8226. [Google Scholar] [CrossRef]
- Laulhere, J.P.; Briat, J.F. Iron release and uptake by plant ferritin: Effects of pH, reduction and chelation. Biochem. J. 1993, 290, 693–699. [Google Scholar] [CrossRef]
- Masuda, T.; Goto, F.; Yoshihara, T. A novel plant ferritin subunit from soybean that is related to a mechanism in iron release. J. Biol. Chem. 2001, 276, 19575–19579. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Deng, J.; Yang, H.; Masuda, T.; Goto, F.; Yoshihara, T. A novel EP-involved pathway for iron release from soya bean seed ferritin. Biochem. J. 2010, 427, 313–321. [Google Scholar] [CrossRef]
- Li, C.; Fu, X.; Qi, X.; Hu, X.; Chasteen, N.D.; Zhao, G. Protein association and dissociation regulated by ferric ion: A novel pathway for oxidative deposition of iron in pea seed ferritin. J. Biol. Chem. 2009, 284, 16743–16751. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, S.; Zang, J.; Zhao, G.; Xu, C. Four-fold channels are involved in iron diffusion into the inner cavity of plant ferritin. Biochemistry 2014, 53, 2232–2241. [Google Scholar] [CrossRef]
- Le Brun, N.E.; Crow, A.; Murphy, M.E.; Mauk, A.G.; Moore, G.R. Iron core mineralisation in prokaryotic ferritins. Biochim. Biophys. Acta 2010, 1800, 732–744. [Google Scholar] [CrossRef]
- Tatur, J.; Hagen, W.R.; Matias, P.M. Crystal structure of the ferritin from the hyperthermophilic archaeal anaerobe Pyrococcus furiosus. J. Biol. Inorg. Chem. 2007, 12, 615–630. [Google Scholar] [CrossRef]
- Ceci, P.; Forte, E.; Di Cecca, G.; Fornara, M.; Chiancone, E. The characterization of Thermotoga maritima ferritin reveals an unusual subunit dissociation behavior and efficient DNA protection from iron-mediated oxidative stress. Extremophiles 2011, 15, 431–439. [Google Scholar] [CrossRef]
- Andrews, S.C.; Le Brun, N.E.; Barynin, V.; Thomson, A.J.; Moore, G.R.; Guest, J.R.; Harrison, P.M. Site-directed replacement of the coaxial heme ligands of bacterioferritin generates heme-free variants. J. Biol. Chem. 1995, 270, 23268–23274. [Google Scholar] [CrossRef]
- Zhang, S.; Zang, J.; Zhang, X.; Chen, H.; Mikami, B.; Zhao, G. “Silent” amino acid residues at key subunit interfaces regulate the geometry of protein nanocages. ACS Nano 2016, 10, 10382–10388. [Google Scholar] [CrossRef]
- Zhang, S.; Zang, J.; Wang, W.; Chen, H.; Zhang, X.; Wang, F.; Wang, H.; Zhao, G. Conversion of the native 24-mer ferritin nanocage into its non-native 16-mer analogue by insertion of extra amino acid residues. Angew. Chem. Int. Ed. 2016, 55, 16064–16070. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Chen, H.; Zhang, X.; Zhang, C.; Guo, J.; Du, M.; Zhao, G. Disulfide-mediated conversion of 8-mer bowl-like protein architecture into three different nanocages. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Chen, H.; Zang, J.; Zhao, X.; Zhao, G.; Wang, H. Selective elimination of the key subunit interfaces facilitates conversion of native 24-mer protein nanocage into 8-mer nanorings. J. Am. Chem. Soc. 2018, 140, 14078–14081. [Google Scholar] [CrossRef]
- Sato, D.; Ohtomo, H.; Yamada, Y.; Hikima, T.; Kurobe, A.; Fujiwara, K.; Ikeguchi, M. Ferritin assembly revisited: A time-resolved small-angle X-ray scattering study. Biochemistry 2016, 55, 287–293. [Google Scholar] [CrossRef]
- Gerl, M.; Jaenicke, R. Mechanism of the self-assembly of apoferritin from horse spleen-cross-linking and spectroscopic analysis. Eur. Biophys. J. 1987, 15, 103–109. [Google Scholar] [CrossRef]
- Sato, D.; Takebe, S.; Kurobe, A.; Ohtomo, H.; Fujiwara, K.; Ikeguchi, M. Electrostatic repulsion during ferritin assembly and its screening by ions. Biochemistry 2016, 55, 482–488. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, X.; Diao, H.; Zhang, J.; Li, H.; Sun, H.; Guo, Z. Encapsulation of platinum anticancer drugs by apoferritin. Chem. Commun. 2007, 33, 3453–3455. [Google Scholar] [CrossRef]
- Yang, R.; Sun, G.; Zhang, M.; Zhou, Z.; Li, Q.; Strappe, P.; Blanchard, C. Epigallocatechin gallate (EGCG) decorating soybean seed ferritin as a rutin nanocarrier with prolonged release property in the gastrointestinal tract. Plant. Foods Hum. Nutr. 2016, 71, 277–285. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, P.; Jacobson, O.; Wang, Z.; Liu, Y.; Lin, L.; Lin, J.; Lu, N.; Zhang, H.; Tian, R.; et al. Biomineralization-inspired synthesis of copper sulfide-ferritin nanocages as cancer theranostics. ACS Nano 2016, 10, 3453–3460. [Google Scholar] [CrossRef]
- Kim, M.; Rho, Y.; Jin, K.S.; Ahn, B.; Jung, S.; Kim, H.; Ree, M. pH-dependent structures of ferritin and apoferritin in solution: Disassembly and reassembly. Biomacromolecules 2011, 12, 1629–1640. [Google Scholar] [CrossRef]
- Kilic, M.A.; Ozlu, E.; Calis, S. A novel protein-based anticancer drug encapsulating nanosphere: Apoferritin-doxorubicin complex. J. Biomed. Nanotechnol. 2012, 8, 508–514. [Google Scholar] [CrossRef]
- Chakraborti, S.; Korpi, A.; Kumar, M.; Stępień, P.; Kostiainen, M.A.; Heddle, J.G. Three-dimensional protein cage array capable of active enzyme capture and artificial chaperone activity. Nano Lett. 2019, 19, 3918–3924. [Google Scholar] [CrossRef] [PubMed]
- Tetter, S.; Hilvert, D. Enzyme encapsulation by a ferritin cage. Angew. Chem. Int. Ed. Engl. 2017, 56, 14933–14936. [Google Scholar] [CrossRef] [PubMed]
- Macone, A.; Masciarelli, S.; Palombarini, F.; Quaglio, D.; Boffi, A.; Trabuco, M.C.; Baiocco, P.; Fazi, F.; Bonamore, A. Ferritin nanovehicle for targeted delivery of cytochrome C to cancer cells. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Santambrogio, P.; Levi, S.; Arosio, P.; Palagi, L.; Vecchio, G.; Lawson, D.M.; Yewdall, S.J.; Artymiuk, P.J.; Harrison, P.M.; Jappelli, R.; et al. Evidence that a salt bridge in the light chain contributes to the physical stability difference between heavy and light human ferritins. J. Biol. Chem. 1992, 267, 14077–14083. [Google Scholar] [PubMed]
- Zhou, B.; Wan, J.; Wang, J.; Cao, X. Effect of chaotropes in reverse micellar extraction of kallikrein. Process. Biochem. 2012, 47, 229–233. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Meng, D.; Wang, D.; Blanchard, C.L.; Zhou, Z. Effect of atmospheric cold plasma on structure, activity, and reversible assembly of the phytoferritin. Food Chem. 2018, 264, 41–48. [Google Scholar] [CrossRef]
- Meng, D.; Wang, B.; Zhen, T.; Zhang, M.; Yang, R. Pulsed electric fields-modified ferritin realizes loading of rutin by a moderate pH transition. J. Agric. Food Chem. 2018, 66, 12404–12411. [Google Scholar] [CrossRef]
- Yildiz, G.; Andrade, J.; Engeseth, N.E.; Feng, H. Functionalizing soy protein nano-aggregates with pH-shifting and mano-thermo-sonication. J. Colloid Interface Sci. 2017, 505, 836–846. [Google Scholar] [CrossRef]
- Meng, D.; Zuo, P.; Song, H.; Yang, R. Influence of Manothermosonication on the Physicochemical and Functional Properties of Ferritin as a Nanocarrier of Iron or Bioactive Compounds. J. Agric. Food Chem. 2019, 67, 6633–6641. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Xu, C.; Zhao, G. Engineering protein interfaces yields ferritin disassembly and reassembly under benign experimental conditions. Chem. Commun. 2016, 52, 7402–7405. [Google Scholar] [CrossRef]
- Ahn, B.; Lee, S.G.; Yoon, H.R.; Lee, J.M.; Oh, H.J.; Kim, H.M.; Jung, Y. Four-fold channel-nicked human ferritin nanocages for active drug loading and pH-responsive drug release. Angew. Chem. Int. Ed. Engl. 2018, 57, 2909–2913. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Meng, D.; Blanchard, C.L.; Zhou, Z. Alcalase enzymolysis of red bean (adzuki) ferritin achieves nanoencapsulation of food nutrients in a mild condition. J. Agric. Food Chem. 2018, 66, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, L.; Li, G.; Zhao, G.; Zhao, X.; Wang, H. AB loop engineered ferritin nanocages for drug loading under benign experimental conditions. Chem. Commun. 2019, 55, 12344–12347. [Google Scholar] [CrossRef] [PubMed]
- Huard, D.J.; Kane, K.M.; Tezcan, F.A. Re-engineering protein interfaces yields copper-inducible ferritin cage assembly. Nat. Chem. Biol. 2013, 9, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, R.; Liu, J.; Meng, D.; Zhou, Z.; Zhang, Y.; Blanchard, C. Fabrication, structure, and function evaluation of the ferritin based nano-carrier for food bioactive compounds. Food Chem. 2019, 299, 125097. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Blanchard, C.; Zhou, Z. Channel directed rutin nano-encapsulation in phytoferritin induced by guanidine hydrochloride. Food Chem. 2018, 240, 935–939. [Google Scholar] [CrossRef]
- Theil, E.C.; Liu, X.S.; Tosha, T. Gated pores in the ferritin protein nanocage. Inorg. Chim. Acta 2008, 361, 868–874. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Liu, L.; Li, Z.; Guo, F.; Li, X.; Luo, J.; Zhao, D.; Liu, Y.; Su, Z. High hydrostatic pressure encapsulation of doxorubicin in ferritin nanocages with enhanced efficiency. J. Biotechnol. 2017, 254, 34–42. [Google Scholar] [CrossRef]
- Zhang, T.; Lv, C.; Yun, S.; Liao, X.; Zhao, G.; Leng, X. Effect of high hydrostatic pressure (HHP) on structure and activity of phytoferritin. Food Chem. 2012, 130, 273–278. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yamamoto, E.; Mannen, T.; Nagamune, T.; Nagamune, T. Protein refolding using chemical refolding additives. Biotechnol. J. 2013, 8, 17–31. [Google Scholar] [CrossRef]
- Monti, D.M.; Ferraro, G.; Merlino, A. Ferritin-based anticancer metallodrug delivery: Crystallographic, analytical and cytotoxicity studies. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 101997. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Walsh, D.; Yao, S.; Kou, Y.; Ma, D. Caged-protein-confined bimetallic structural assemblies with mimetic peroxidase activity. Small 2012, 8, 2948–2953. [Google Scholar] [CrossRef]
- Ueno, T.; Suzuki, M.; Goto, T.; Matsumoto, T.; Nagayama, K.; Watanabe, Y. Size-selective olefin hydrogenation by a Pd nanocluster provided in an apo-ferritin cage. Angew. Chem. Int. Ed. Engl. 2004, 43, 2527–2530. [Google Scholar] [CrossRef] [PubMed]
- Kasyutich, O.; Ilari, A.; Fiorillo, A.; Tatchev, D.; Hoell, A.; Ceci, P. Silver ion incorporation and nanoparticle formation inside the cavity of Pyrococcus furiosus ferritin: Structural and size-distribution analyses. J. Am. Chem. Soc. 2010, 132, 3621–3627. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Abe, M.; Ueno, T.; Abe, S.; Goto, T.; Toda, Y.; Akita, T.; Yamada, Y.; Watanabe, Y. Preparation and catalytic reaction of Au/Pd bimetallic nanoparticles in Apo-ferritin. Chem. Commun. 2009, 32, 4871–4873. [Google Scholar] [CrossRef] [PubMed]
- Maity, B.; Fukumori, K.; Abe, S.; Ueno, T. Immobilization of two organometallic complexes into a single cage to construct protein-based microcompartments. Chem. Commun. 2016, 52, 5463–5466. [Google Scholar] [CrossRef]
- Niemeyer, J.; Abe, S.; Hikage, T.; Ueno, T.; Erker, G.; Watanabe, Y. Noncovalent insertion of ferrocenes into the protein shell of apo-ferritin. Chem. Commun. 2008, 48, 6519–6521. [Google Scholar] [CrossRef]
- Abe, S.; Niemeyer, J.; Abe, M.; Takezawa, Y.; Ueno, T.; Hikage, T.; Erker, G.; Watanabe, Y. Control of the coordination structure of organometallic palladium complexes in an apo-ferritin cage. J. Am. Chem. Soc. 2008, 130, 10512–10514. [Google Scholar] [CrossRef]
- Abe, S.; Hirata, K.; Ueno, T.; Morino, K.; Shimizu, N.; Yamamoto, M.; Takata, M.; Yashima, E.; Watanabe, Y. Polymerization of phenylacetylene by rhodium complexes within a discrete space of apo-ferritin. J. Am. Chem. Soc. 2009, 131, 6958–6960. [Google Scholar] [CrossRef]
- Ciambellotti, S.; Pratesi, A.; Severi, M.; Ferraro, G.; Alessio, E.; Merlino, A.; Messori, L. The NAMI A-human ferritin system: A biophysical characterization. Dalton Trans. 2018, 47, 11429–11437. [Google Scholar] [CrossRef]
- Takezawa, Y.; Böckmann, P.; Sugi, N.; Wang, Z.; Abe, S.; Murakami, T.; Hikage, T.; Erker, G.; Watanabe, Y.; Kitagawa, S.; et al. Incorporation of organometallic Ru complexes into apo-ferritin cage. Dalton Trans. 2011, 40, 2190–2195. [Google Scholar] [CrossRef]
- Fujita, K.; Tanaka, Y.; Sho, T.; Ozeki, S.; Abe, S.; Hikage, T.; Kuchimaru, T.; Kizaka-Kondoh, S.; Ueno, T. Intracellular CO Release from Composite of Ferritin and Ruthenium Carbonyl Complexes. J. Am. Chem. Soc. 2014, 136, 16902–16908. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.; Monti, D.M.; Amoresano, A.; Pontillo, N.; Petruk, G.; Pane, F.; Cinellu, M.A.; Merlino, A. Gold-based drug encapsulation within a ferritin nanocage: X-ray structure and biological evaluation as a potential anticancer agent of the Auoxo3-loaded protein. Chem. Commun. 2016, 52, 9518–9521. [Google Scholar] [CrossRef]
- Monti, D.M.; Ferraro, G.; Petruk, G.; Maiore, L.; Pane, F.; Amoresano, A.; Cinellu, M.A.; Merlino, A. Ferritin nanocages loaded with gold ions induce oxidative stress and apoptosis in MCF-7 human breast cancer cells. Dalton Trans. 2017, 46, 15354–15362. [Google Scholar] [CrossRef]
- Ferraro, G.; Petruk, G.; Maiore, L.; Pane, F.; Amoresano, A.; Cinellu, M.A.; Monti, D.M.; Merlino, A. Caged noble metals: Encapsulation of a cytotoxic platinum(II)-gold(I) compound within the ferritin nanocage. Int. J. Biol. Macromol. 2018, 115, 1116–1121. [Google Scholar] [CrossRef]
- Petruk, G.; Monti, D.M.; Ferraro, G.; Pica, A.; D’Elia, L.; Pane, F.; Amoresano, A.; Furrer, J.; Kowalski, K.; Merlino, A. Encapsulation of the dinuclear trithiolato-bridged arene ruthenium complex diruthenium-1 in an apoferritin nanocage: Structure and cytotoxicity. ChemMedChem 2019, 14, 594–602. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Chen, H.; Sun, J.; Feng, F. Protein nanocages for delivery and release of luminescent ruthenium(II) polypyridyl complexes. ACS Appl. Mater. Interfaces 2016, 8, 22756–22761. [Google Scholar] [CrossRef]
- Pontillo, N.; Pane, F.; Messori, L.; Amoresano, A.; Merlino, A. Cisplatin encapsulation within a ferritin nanocage: A high-resolution crystallographic study. Chem. Commun. 2016, 52, 4136–4139. [Google Scholar] [CrossRef]
- Maity, B.; Abe, S.; Ueno, T. Observation of gold sub-nanocluster nucleation within a crystalline protein cage. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Yun, S.; Zhang, T.; Li, M.; Chen, B.; Zhao, G. Proanthocyanidins inhibit iron absorption from soybean (glycine max) seed ferritin in rats with iron deficiency anemia. Plant. Foods Hum. Nutr. 2011, 66, 212–217. [Google Scholar] [CrossRef]
- Liao, X.; Yun, S.; Zhao, G. Structure, function, and nutrition of phytoferritin: A newly functional factor for iron supplement. Crit. Rev. Food. Sci. Nutr. 2014, 54, 1342–1352. [Google Scholar] [CrossRef]
- Theil, E.C.; Chen, H.; Miranda, C.; Janser, H.; Elsenhans, B.; Núñez, M.T.; Pizarro, F.; Schümann, K. Absorption of iron from ferritin is independent of heme iron and ferrous salts in women and rat intestinal segments. J. Nutr. 2012, 142, 478–483. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Bryant, A.; Liu, X.; Theil, E.C. Iron absorption from soybean ferritin in nonanemic women. Am. J. Clin. Nutr. 2006, 83, 103–107. [Google Scholar] [CrossRef]
- Heaney, R.P. Effect of calcium on skeletal development, bone loss, and risk of fractures. Am. J. Med. 1991, 91, S23–S28. [Google Scholar] [CrossRef]
- Weaver, C.M. Closing the gap between calcium intake and requirements. J. Am. Diet. Assoc. 2009, 109, 812–813. [Google Scholar] [CrossRef]
- Perales, S.; Barberá, R.; Lagarda, M.J.; Farré, R. Bioavailability of calcium from milk-based formulas and fruit juices containing milk and cereals estimated by in vitro methods (solubility, dialyzability, and uptake and transport by caco-2 cells). J. Agric. Food Chem. 2005, 53, 3721–3726. [Google Scholar] [CrossRef]
- Lynch, S.R. The effect of calcium on iron absorption. Nutr. Res. Rev. 2000, 13, 141–158. [Google Scholar] [CrossRef]
- Cámara, F.; Amaro, M.A. Nutritional aspect of zinc availability. Int. J. Food Sci. Nutr. 2003, 54, 143–151. [Google Scholar] [CrossRef]
- Li, M.; Zhang, T.; Yang, H.; Zhao, G.; Xu, C. A novel calcium supplement prepared by phytoferritin nanocages protects against absorption inhibitors through a unique pathway. Bone 2014, 64, 115–123. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Z.; Sun, G.; Gao, Y.; Xu, J.; Strappe, P.; Blanchard, C.; Cheng, Y.; Ding, X. Synthesis of homogeneous protein-stabilized rutin nanodispersions by reversible assembly of soybean (glycine max) seed ferritin. RSC Adv. 2015, 5, 31533–31540. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, G.; Liu, Y.; Gao, Y.; Xu, J.; Meng, D.; Strappe, P.; Blanchard, C.; Yang, R. A novel approach to prepare protein-proanthocyanidins nano-complexes by the reversible assembly of ferritin cage. Food Sci. Technol. Res. 2017, 23, 329–337. [Google Scholar] [CrossRef]
- Yang, R.; Gao, Y.; Zhou, Z.; Meng, D.; Sun, G.; Liu, Y.; Chen, Z.; Strappe, P.; Blanchard, C. Fabrication and characterization of ferritin-chitosan-lutein shell-core nanocomposites and lutein stability and release evaluation in vitro. RSC Adv. 2016, 6, 35267–35279. [Google Scholar] [CrossRef]
- Ogawa, H.; Miyazaki, H.; Kimura, M. Isolation and characterization of human skin lysozyme. J. Invest. Dermatol. 1971, 57, 111–116. [Google Scholar] [CrossRef]
- Wu, T.; Huang, J.; Jiang, Y.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Formation of hydrogels based on chitosan/alginate for the delivery of lysozyme and their antibacterial activity. Food Chem. 2018, 240, 361–369. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, Q.; Wu, D.; Hu, Y.; Chen, S.; Ding, T.; Ye, X.; Liu, D.; Chen, J. What is new in lysozyme research and its application in food industry? A review. Food Chem. 2019, 274, 698–709. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, K.; Zhao, G. Gold nanoparticles: From synthesis, properties to their potential application as colorimetric sensors in food safety screening. Trends Food Sci. Technol. 2018, 78, 83–94. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Tseng, W.-L. Ultrasensitive sensing of Hg2+ and CH3Hg+ based on the fluorescence quenching of lysozyme type VI-stabilized gold nanoclusters. Anal. Chem. 2010, 82, 9194–9200. [Google Scholar] [CrossRef]
- Zang, J.; Li, C.; Zhou, K.; Dong, H.; Chen, B.; Wang, F.; Zhao, G. Nanomolar Hg2+ detection using β-lactoglobulin-stabilized fluorescent gold nanoclusters in beverage and biological media. Anal. Chem. 2016, 88, 10275–10283. [Google Scholar] [CrossRef]
- Lv, C.; Yin, S.; Zhang, X.; Hu, J.; Zhang, T.; Zhao, G. 16-Mer ferritin-like protein templated gold nanoclusters for bioimaging detection of methylmercury in the brain of living mice. Anal. Chim. Acta 2020, 1127, 149–155. [Google Scholar] [CrossRef]
- Truffi, M.; Fiandra, L.; Sorrentino, L.; Monieri, M.; Corsi, F.; Mazzucchelli, S. Ferritin nanocages: A biological platform for drug delivery, imaging and theranostics in cancer. Pharmacol. Res. 2016, 107, 57–65. [Google Scholar] [CrossRef]
- Montemiglio, L.C.; Testi, C.; Ceci, P.; Falvo, E.; Pitea, M.; Savino, C.; Arcovito, A.; Peruzzi, G.; Baiocco, P.; Mancia, F.; et al. Cryo-EM structure of the human ferritin-transferrin receptor 1 complex. Nat. Commun. 2019, 10, 1121. [Google Scholar] [CrossRef]
- Geninatti Crich, S.; Cadenazzi, M.; Lanzardo, S.; Conti, L.; Ruiu, R.; Alberti, D.; Cavallo, F.; Cutrin, J.C.; Aime, S. Targeting ferritin receptors for the selective delivery of imaging and therapeutic agents to breast cancer cells. Nanoscale 2015, 7, 6527–6533. [Google Scholar] [CrossRef]
- Lin, C.Y.; Yang, S.J.; Peng, C.L.; Shieh, M.J. Panitumumab-conjugated and platinum-cored pH-sensitive apoferritin nanocages for colorectal cancer-targeted therapy. ACS Appl. Mater. Interfaces 2018, 10, 6096–6106. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Wang, X.; Zhang, C.; Zhang, Y.; Wang, Q.; Yang, Z.; Guo, Z. Characterization and cellular uptake of platinum anticancer drugs encapsulated in apoferritin. J. Inorg. Biochem. 2009, 103, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, L.; Bellini, M.; Vanna, R.; Morasso, C.; Zago, A.; Carcano, S.; Avvakumova, S.; Bertolini, J.A.; Rizzuto, M.A.; Colombo, M.; et al. H-ferritin enriches the curcumin uptake and improves the therapeutic efficacy in triple negative breast cancer cells. Biomacromolecules 2017, 18, 3318–3330. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wang, X.; Cai, Y.; Sun, L.; Tian, L.; Wu, H.; He, X.; Lei, H.; Liu, W.; Chen, G.; et al. Targeted in vivo imaging of microscopic tumors with ferritin-based nanoprobes across biological barriers. Adv. Mater. 2014, 26, 2566–2571. [Google Scholar] [CrossRef]
- Sánchez, P.; Valero, E.; Gálvez, N.; Domínguez-Vera, J.M.; Marinone, M.; Poletti, G.; Corti, M.; Lascialfari, A. MRI relaxation properties of water-soluble apoferritin-encapsulated gadolinium oxide-hydroxide nanoparticles. Dalton Trans. 2009, 800–804. [Google Scholar] [CrossRef]
- Kálmán, F.K.; Geninatti-Crich, S.; Aime, S. Reduction/dissolution of a β-MnOOH nanophase in the ferritin cavity to yield a highly sensitive, biologically compatible magnetic resonance imaging agent. Angew. Chem. Int. Ed. Engl. 2010, 49, 612–615. [Google Scholar] [CrossRef]
- Geninatti Crich, S.; Bussolati, B.; Tei, L.; Grange, C.; Esposito, G.; Lanzardo, S.; Camussi, G.; Aime, S. Magnetic resonance visualization of tumor angiogenesis by targeting neural cell adhesion molecules with the highly sensitive gadolinium-loaded apoferritin probe. Cancer Res. 2006, 66, 9196–9201. [Google Scholar] [CrossRef]
- Hoppler, M.; Schönbächler, A.; Meile, L.; Hurrell, R.F.; Walczyk, T. Ferritin-iron is released during boiling and in vitro gastric digestion. J. Nutr. 2008, 138, 878–884. [Google Scholar] [CrossRef]
- Kalantzi, L.; Goumas, K.; Kalioras, V.; Abrahamsson, B.; Dressman, J.B.; Reppas, C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm. Res. 2006, 23, 165–176. [Google Scholar] [CrossRef]
- Engle-Stone, R.; Yeung, A.; Welch, R.; Glahn, R. Meat and ascorbic acid can promote Fe availability from Fe-phytate but not from Fe-tannic acid complexes. J. Agric. Food Chem. 2005, 53, 10276–10284. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhou, K.; Qi, X. Phytoferritin association induced by EGCG inhibits protein degradation by proteases. Plant. Foods Hum. Nutr. 2014, 69, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, K.; Ning, Y.; Zhao, G. Effect of the structure of gallic acid and its derivatives on their interaction with plant ferritin. Food Chem. 2016, 213, 260–267. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Gao, Y.; Wang, Y.; Blanchard, C.; Zhou, Z. Ferritin glycosylated by chitosan as a novel EGCG nano-carrier: Structure, stability, and absorption analysis. Int. J. Biol. Macromol. 2017, 105, 252–261. [Google Scholar] [CrossRef]
- Lv, C.; Zhao, G.; Lönnerdal, B. Bioavailability of iron from plant and animal ferritins. J. Nutr. Biochem. 2015, 26, 532–540. [Google Scholar] [CrossRef]
- Yang, R.; Tian, J.; Wang, D.; Blanchard, C.; Zhou, Z. Chitosan binding onto the epigallocatechin-loaded ferritin nanocage enhances its transport across Caco-2 cells. Food Funct. 2018, 9, 2015–2024. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, X.; Zhao, G. Ferritin Nanocage: A Versatile Nanocarrier Utilized in the Field of Food, Nutrition, and Medicine. Nanomaterials 2020, 10, 1894. https://doi.org/10.3390/nano10091894
Zhang C, Zhang X, Zhao G. Ferritin Nanocage: A Versatile Nanocarrier Utilized in the Field of Food, Nutrition, and Medicine. Nanomaterials. 2020; 10(9):1894. https://doi.org/10.3390/nano10091894
Chicago/Turabian StyleZhang, Chenxi, Xiaorong Zhang, and Guanghua Zhao. 2020. "Ferritin Nanocage: A Versatile Nanocarrier Utilized in the Field of Food, Nutrition, and Medicine" Nanomaterials 10, no. 9: 1894. https://doi.org/10.3390/nano10091894
APA StyleZhang, C., Zhang, X., & Zhao, G. (2020). Ferritin Nanocage: A Versatile Nanocarrier Utilized in the Field of Food, Nutrition, and Medicine. Nanomaterials, 10(9), 1894. https://doi.org/10.3390/nano10091894






