Effect of Al2O3 Dot Patterning on CZTSSe Solar Cell Characteristics
Abstract
:1. Introduction
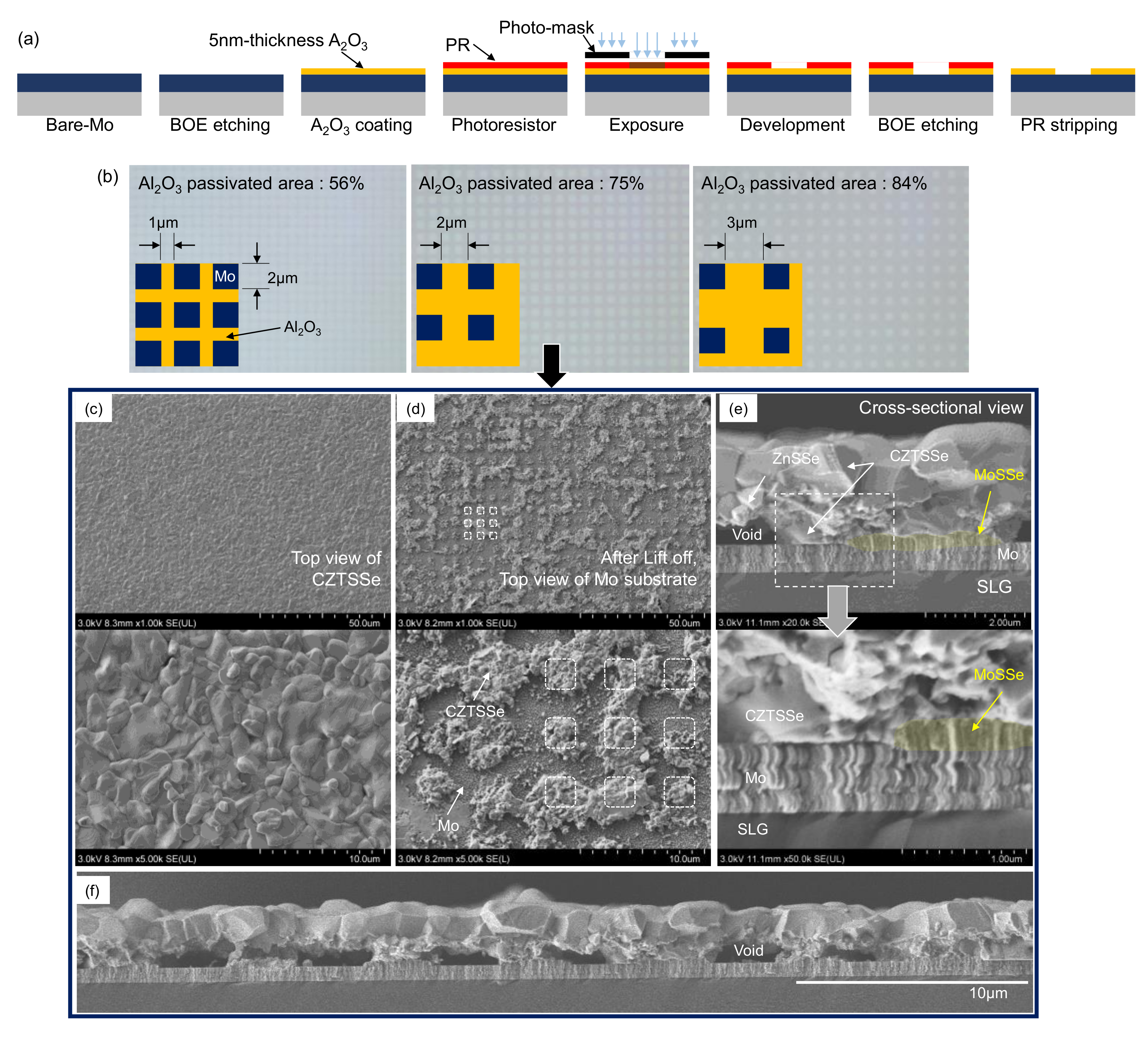
2. Materials and Methods
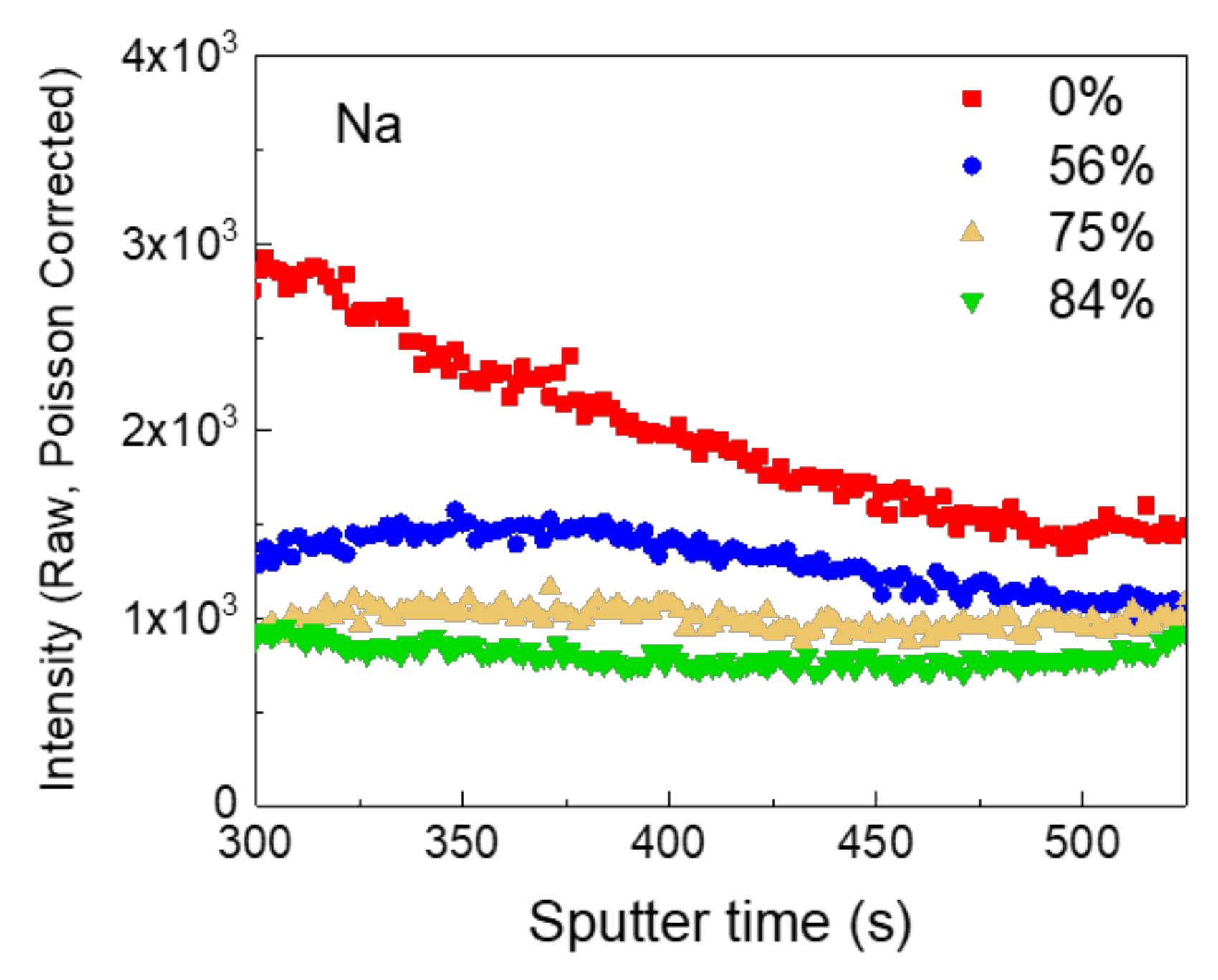
3. Results
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Green, M.A.; Hishikawa, Y.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Yoshita, M.; Ho-Baillie, A.W.Y. Solar cell efficiency tables (version 53). Prog. Photovolt. 2019, 27, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Powalla, M.; Paetel, S.; Ahlswede, E.; Wuerz, R.; Wessendorf, C.D.; Friedlmeier, T.M. Thin-film solar cells exceeding 22% solar cell efficiency: An overview on CuTe, Cu(In,Ga)Se2, and perovskite based materials. Appl. Phys. Rev. 2018, 5, 041602. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Chae, W.-S.; Na, Y.-J.; Kim, S.-H.; Lee, S.; Lee, J.-H.; Heo, Y.-W. Excitation dynamics of MAPb(I1-xBrx)3 during phase separation by photoirradiation: Evidence of sink, band filling, and Br-rich phase coarsening. J. Alloys Compd. 2019, 806, 1180–1187. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Lee, S.; Chae, W.-S.; Lee, J.-H.; Heo, Y.-W. Real time observation of photo-instability of ternary-halide mixed CH3NH3Pb(Br1-x-yClxIy)3 perovskite: Preferential diffusion of small halide ions. J. Alloys Compd. 2019, 808, 151716. [Google Scholar] [CrossRef]
- Cho, H.; Kim, Y.-H.; Wolf, C.; Lee, H.-D.; Lee, T.-W. Improving the stability of Metal Halide perovskite Materials and Light-Emitting Diodes. Adv. Mater. 2018, 30, 1704587. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.J.; Svadlenak, S.R.; Goulas, K.A.; Labram, J.G. Thermal stability of mobility in methylammonium lead iodide. J. Phys. Mater. 2020, 3, 014003. [Google Scholar] [CrossRef] [Green Version]
- Ava, T.T.; Mamun, A.A.; Marsillac, S.; Namkoong, G. A Review: Thermal Stability of Methylammonium Lead Halide Based Perovskite Solar Cells. Appl. Sci. 2019, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Seol, J.-S.; Lee, S.-Y.; Lee, J.-C.; Nam, H.-D.; Kim, K.-H. Electrical and optical properties of Cu2ZnSnS4 thin films prepared by rf magnetron sputtering process. Sol. Energy Mater. Sol. Cells 2003, 75, 155–162. [Google Scholar] [CrossRef]
- Walsh, A.; Chen, S.; Wei, S.-H.; Gong, X.-G. Kesterite Thin-Film Solar Cells: Advances in Materials Modelling of Cu2ZnSnS4. Adv. Energy Mater. 2012, 2, 400–409. [Google Scholar] [CrossRef]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device Characteristics of CZTSSe Thin-Film Solar Cells with 12.6% Efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Chen, S.; Walsh, A.; Yang, J.-H.; Gong, X.G.; Sun, L.; Yang, P.-X.; Chu, J.-H.; Wei, S.-H. Compositional dependence of structural and electronic properties of Cu2ZnSn(S,Se)4 alloys for thin film solar cells. Phys. Rev. B 2011, 83, 125201. [Google Scholar] [CrossRef] [Green Version]
- Gokmen, T.; Gunawan, O.; Todorov, T.K.; Mitzi, D.B. Band tailing and efficiency limitation in kesterite solar cells. Appl. Phys. Lett. 2013, 103, 103506. [Google Scholar] [CrossRef]
- Scragg, J.J.S.; Larsen, J.K.; Kumar, M.; Persson, C.; Sendler, J.; Siebentritt, S.; Bjorkman, C.P. Cu-Zn disorder and band gap fluctuations in Cu2ZnSn(S,Se)4: Theoretical and experimental investigations. Phys. Status Solidi B 2016, 253, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Nam, D.; Cho, S.; Sim, J.-H.; Yang, K.-J.; Son, D.-H.; Kim, D.-H.; Kang, J.-K.; Kwon, M.-S.; Jeon, C.-W.; Cheong, H. Solar conversion efficiency and distribution of ZnS secondary phase in Cu2ZnSnS4 solar cells. Sol. Energy Mater. Sol. Cells 2016, 149, 226–231. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Son, D.-H.; Kim, Y.-I.; Kim, S.-H.; Kim, S.; Ahn, K.; Sung, S.-J.; Hwang, D.-K.; Yang, K.-J.; Kang, J.-K.; et al. Void and Secondary phase formation mechanisms of CZTSSe using Sn/Cu/Zn/Mo stacked elemental precursors. Nano Energy 2019, 59, 399–411. [Google Scholar] [CrossRef]
- Hernández-Martínez, A.; Placidi, M.; Arqués, L.; Giraldo, S.; Sánchez, Y.; Izquierdo-Roca, V.; Pistor, P.; Valentini, M.; Malerba, C.; Saucedo, E. Insights into the Formation Pathways of Cu2ZnSnSe4 Using Rapid Thermal Processes. ACS Appl. Energy Mater. 2018, 1, 1981–1989. [Google Scholar] [CrossRef]
- Schorr, S.; Gurieva, G.; Guc, M.; Dimitrievska, M.; Pérez-Rodríguez, A.; Izquierdo-Roca, V.; Schnohr, C.S.; Kim, J.; Jo, W.; Merino, J.M. Point defects, compositional fluctuations, and secondary phases in non-stoichiometric kesterites. J. Phys. Energy 2020, 2, 012002. [Google Scholar] [CrossRef] [Green Version]
- Giraldo, S.; Jehl, Z.; Placidi, M.; Izquierdo-Roca, V.; Pérez-Rodríguez, A.; Saucedo, E. Progress and Perspectives of Thin Film Kesterite Photovoltaic Technology: A Critical Review. Adv. Mater. 2019, 31, 1806692. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yuan, Z.-K.; Chen, S.; Gong, X.-G.; Wei, S.-H. Effective and Noneffective Recombination Center Defects in Cu2ZnSnS4: Significant Difference in Carrier Capture Cross Sections. Chem. Mater. 2019, 31, 826–833. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.-S.; Walsh, A. Identification of Killer Defects in Kesterite Thin-Film Solar Cells. ACS Energy Lett. 2018, 3, 496–500. [Google Scholar] [CrossRef] [Green Version]
- Weber, W.; Mainz, R.; Schock, H.W. On the Sn loss from this films of the material system Cu-Zn-Sn-S in high vacuum. J. Appl. Phys. 2010, 107, 013516. [Google Scholar] [CrossRef] [Green Version]
- Redinger, A.; Berg, D.M.; Dale, P.J.; Siebentritt, S. The consequences of Kesterite Equilibria for Efficient Solar Cells. J. Am. Chem. Soc. 2011, 133, 3320–3323. [Google Scholar] [CrossRef] [PubMed]
- Son, D.-H.; Kim, S.-H.; Kim, S.-Y.; Kim, Y.-I.; Sim, J.-H.; Park, S.-N.; Jeon, D.-H.; Hwang, D.-K.; Sung, S.-J.; Kang, J.-K.; et al. Effect of soild-H2S gas reaction on CZTSSe thin film growth and photovoltaic properties of a 12.62% efficiency device. J. Mater. Chem. A 2019, 44, 25279–258289. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.; Kim, S.-Y.; Kim, S.; Son, D.-H.; Kim, S.-H.; Kim, S.Y.; Kim, J.; Sung, S.-J.; Kim, D.-H.; Kang, J.-K. Flexible high-efficiency CZTSSe solar cells on stainless steel substrates. J. Mater. Chem. A 2019, 43, 24891–24899. [Google Scholar] [CrossRef]
- Yang, K.-J.; Kim, S.; Kim, S.-Y.; Ahn, K.; Son, D.-H.; Kim, S.-H.; Lee, S.-J.; Kim, Y.-I.; Park, S.-N.; Sung, S.-J.; et al. Flexible Cu2ZnSn(S,Se)4 solar cells with over 10% efficiency and methods of enlarging the cell area. Nat. Commun. 2019, 10, 2959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Kim, S.-Y.; Son, D.-H.; Kim, S.-H.; Kim, Y.-I.; Yang, K.-J.; Heo, Y.-W.; Kang, J.-K.; Kim, D.-H. Self-Alignment of Bottom CZTSSe by Patterning of an Al2O3 Intermediate Layer. Nanomaterials 2020, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Huang, J.; Sun, K.; Johnston, S.; Zhang, Y.; Sun, H.; Pu, A.; He, M.; Liu, F.; Eder, K.; et al. Cu2ZnSnS4 solar cells with over 10% power conversion efficiency enabled by heterojunction heat treatment. Nat. Energy 2018, 3, 764–772. [Google Scholar] [CrossRef]
- Giraldo, S.; Saucedo, E.; Neuschitzer, M.; Oliva, F.; Placidi, M.; Alcobé, X.; Izquierdo-Roca, V.; Kim, S.; Tampo, H.; Shibata, H.; et al. How small amounts of Ge modify the formation pathways and crystallization of kesterites. Energy Environ. Sci. 2018, 11, 582. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-Y.; Kim, S.-H.; Hong, S.; Son, D.-H.; Kim, Y.-I.; Kim, S.; Ahn, K.; Yang, K.-J.; Kim, D.-H.; Kang, J.-K. Secondary Phase Formation Mechanism in the Mo-Back Contact Region during Sulfo-Selenization Using a Metal Precursor: Effect of Wettability between a Liquid Metal and Substrate on Secondary Phase Formation. ACS Appl. Mater. Interfaces 2019, 11, 23160–23167. [Google Scholar] [CrossRef]
- Vermang, B.; Fjällström, V.; Pettersson, J.; Salomé, P.; Edorff, M. Develpoment of rear surface passivated Cu(In,Ga)Se2 thin film solar cells with nano-sized local rear point contacts. Sol. Energy Mater. Sol. Cells 2013, 117, 505–511. [Google Scholar] [CrossRef]
- Vermang, B.; Wätjen, J.T.; Fjällström, V.; Rostvall, F.; Edoff, M.; Kotipalli, R.; Henry, F.; Flandre, D. Employing Si solar cell technology to increase efficiency of ultra-thin Cu(In,Ga)Se2 solar cells. Prog. Photovolt. 2014, 22, 1023–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Park, S.; Ryu, S.; Oh, J.; Shin, B. Improving the open-circuit voltage of Cu2ZnSnSe4 thin film solar cells via interface passivation. Prog. Photovolt. Res. Appl. 2017, 25, 308–317. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Son, D.-H.; Kim, S.-H.; Kim, Y.-I.; Kim, S.; Ahn, K.; Yang, K.-J.; Kang, J.-K.; Kim, D.-H. Effect of Cu–Sn–Se Liquid Phase on Grain Growth and Efficiency of CZTSSe Solar Cells. Adv. Energy. Mater. 2020, 10, 1903173. [Google Scholar] [CrossRef]
- Vermang, B.; Fjällström, V.; Gao, X.; Edoff, M. Improved Rear Surface Passivation of Cu (In,Ga)Se2 Solar Cells: A Combination of an Al2O3 Rear Surface Passivation Layer and Nanosized Local Rear Point Contacts. IEEE J. Photovolt. 2014, 4, 486–492. [Google Scholar] [CrossRef]
- Johnson, M.; Baryshev, S.V.; Thimsen, E.; Manno, M.; Zhang, X.; Veryovkin, I.V.; Leighton, C.; Aydil, E.S. Alkali-metal-enhanced grain growth in Cu2ZnSnS4 thin films. Energy Environ. Sci. 2014, 7, 1931. [Google Scholar] [CrossRef]
- Gershon, T.; Shin, B.; Bojarczuk, N.; Hopstaken, M.; Mitzi, D.B.; Guha, S. The Role of Sodium as a Surfactant and Suppressor of Non-Radiative Recombination at Internal Surfaces in Cu2ZnSnS4. Adv. Energy Mater. 2015, 5, 1400849. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.; Wu, M.; Gao, S.; Wu, L.; Ao, J.; Sun, Y.; Zhang, Y. Synergistic effect of Na and Se on CZTSe solar cells through a soft chemical process. Sol. Energy Mater. Sol. Cells 2019, 198, 35–43. [Google Scholar] [CrossRef]
- Yang, K.-J.; Sim, J.-H.; Jeon, B.; Son, D.-H.; Kim, D.-H.; Sung, S.-J.; Hwang, D.-K.; Song, S.; Khadka, D.B.; Kim, J.; et al. Effects of Na and MoS2 on Cu2ZnSnS4 thin-film solar cell. Prog. Photovolt. 2015, 23, 862–873. [Google Scholar] [CrossRef]
- Li, J.B.; Chawla, V.; Clemens, B.M. Investigating the Role of Grain Boundaries in CZTS and CZTSSe Thin Film Solar Cells with Scanning Probe Microscopy. Adv. Mater. 2012, 24, 720–723. [Google Scholar] [CrossRef]
- Liu, C.Y.; Li, Z.M.; Gu, H.Y.; Chen, S.Y.; Xiang, H.; Gong, X.G. Sodium Passivation of the Grain Boundaries in CuInSe2 and Cu2ZnSnS4 for High-Efficiency Solar Cells. Adv. Energy Mater. 2017, 7, 1601457. [Google Scholar] [CrossRef]
- Prabhakar, T.; Jampan, N. Effect of sodium diffusion on the structural and electrical properties of Cu2ZnSnS4 thin films. Sol. Energy Mater. Sol. Cells 2011, 95, 1001–1004. [Google Scholar] [CrossRef]
- Tampo, H.; Kim, K.M.; Kim, S.; Shibata, H.; Niki, S. Improvement of minority carrier lifetime and conversion efficiency by Na incorporation in Cu2ZnSnSe4 solar cells. J. Appl. Phys. 2017, 122, 023106. [Google Scholar] [CrossRef]
- Gershon, T.; Lee, Y.S.; Mankad, R.; Gunawan, O.; Gokmen, T.; Bishop, D.; McCandless, B.; Guha, S. The impact of sodium on the sub-bandgap states in CZTSe and CZTS. Appl. Phys. Lett. 2015, 106, 123905. [Google Scholar] [CrossRef]
- Ledinek, D.; Keller, J.; Hägglund, C.; Chen, W.-C.; Edoff, M. Effect of NaF pre-cursor on alumina and hafnia rear contact passivation layers in ultra-thin Cu (In,Ga)Se2 solar cells. Thin Solid Films 2019, 683, 156–164. [Google Scholar] [CrossRef]
- Casper, P.; Hünig, R.; Gomard, G.; Kiowski, O.; Reitz, C.; Lemmer, U.; Powalla, M.; Hetterich, M. Optoelectrical improvement of ultra-thin Cu(In,Ga)Se2 solar cells through microstructured MgF2 and Al2O3 back contact passivation layer. Phys. Status Solidi RRL 2016, 10, 376–380. [Google Scholar] [CrossRef]
- Hages, C.J.; Redinger, A.; Levcenko, S.; Hempel, H.; Koeper, M.J.; Agrawal, R.; Greiner, D.; Kaufmann, C.A.; Unold, T. Identifying the Real Minority Carrier Lifetime in Nonideal Semiconductors: A Case Study of Kesterite Materials. Adv. Energy Mater. 2017, 7, 1700167. [Google Scholar] [CrossRef]
- Courel, M.; Andrade-Arvizu, J.A.; Vigil-Galán, O. The role of buffer/kesterite interface recombination and minority carrier lifetime on kesterite thin film solar cells. Mater. Res. Express 2016, 3, 095501. [Google Scholar] [CrossRef]
- Gunawan, O.; Pae, S.R.; Bishop, D.M.; Virgus, Y.; Noh, J.H.; Jeon, N.J.; Lee, Y.S.; Shao, X.; Todorov, T.; Mitzi, D.B.; et al. Carrier-resolved photo-Hall effect. Nature 2019, 575, 151–155. [Google Scholar] [CrossRef]
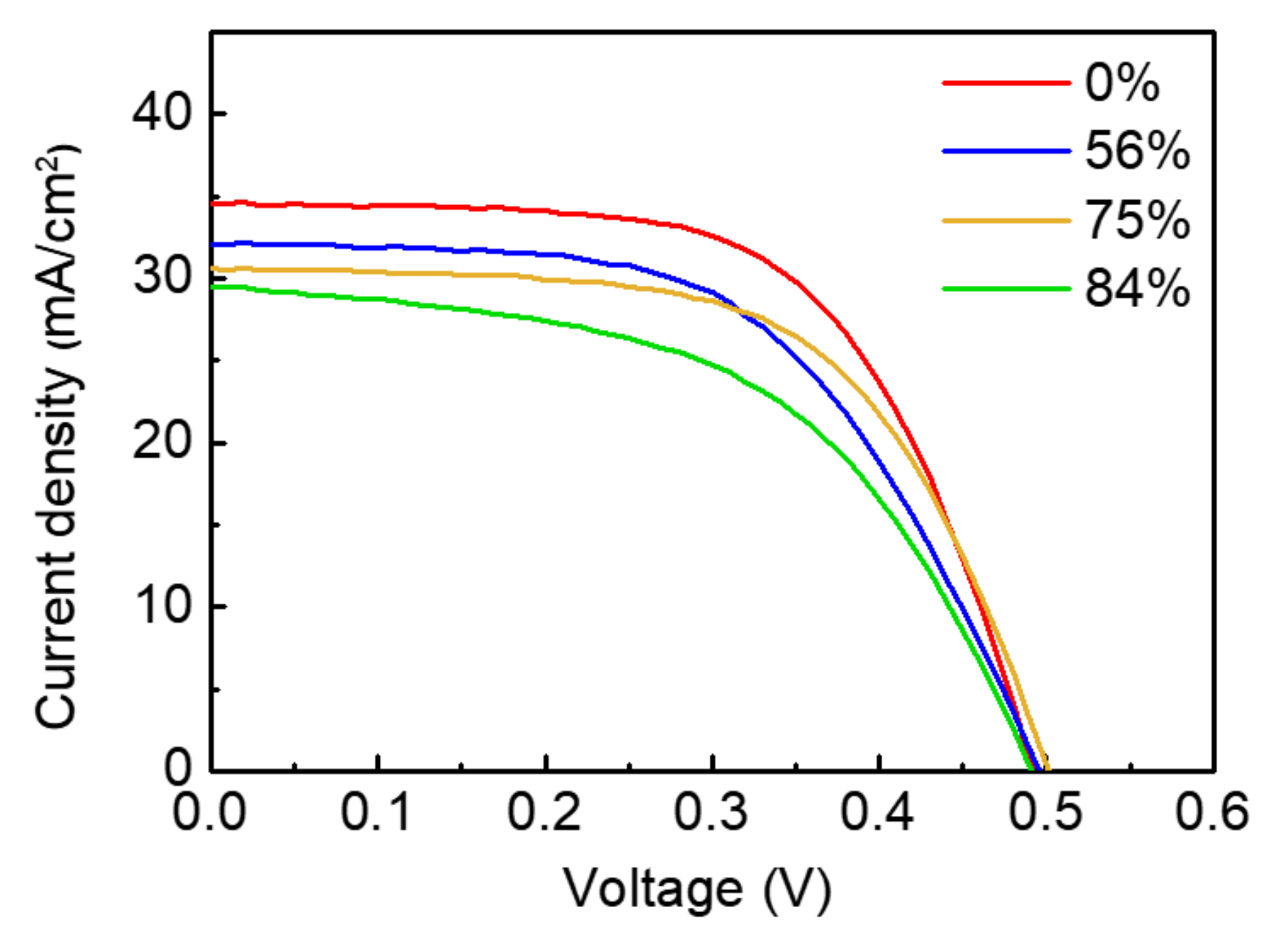
| Al2O3 Passivated Area (%) | VOC [V] | JSC [mA/cm2] | FF [%] | Eff [%] |
|---|---|---|---|---|
| 0 | 0.493 | 34.58 | 61.22 | 10.43 |
| 56 | 0.496 | 32.09 | 56.16 | 8.94 |
| 75 | 0.502 | 30.61 | 60.37 | 9.27 |
| 84 | 0.492 | 29.54 | 52.82 | 7.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Hong, S.; Kim, S.-H.; Son, D.-H.; Kim, Y.-I.; Kim, S.; Heo, Y.-W.; Kang, J.-K.; Kim, D.-H. Effect of Al2O3 Dot Patterning on CZTSSe Solar Cell Characteristics. Nanomaterials 2020, 10, 1874. https://doi.org/10.3390/nano10091874
Kim S-Y, Hong S, Kim S-H, Son D-H, Kim Y-I, Kim S, Heo Y-W, Kang J-K, Kim D-H. Effect of Al2O3 Dot Patterning on CZTSSe Solar Cell Characteristics. Nanomaterials. 2020; 10(9):1874. https://doi.org/10.3390/nano10091874
Chicago/Turabian StyleKim, Se-Yun, Sanghun Hong, Seung-Hyun Kim, Dae-Ho Son, Young-Ill Kim, Sammi Kim, Young-Woo Heo, Jin-Kyu Kang, and Dae-Hwan Kim. 2020. "Effect of Al2O3 Dot Patterning on CZTSSe Solar Cell Characteristics" Nanomaterials 10, no. 9: 1874. https://doi.org/10.3390/nano10091874
APA StyleKim, S.-Y., Hong, S., Kim, S.-H., Son, D.-H., Kim, Y.-I., Kim, S., Heo, Y.-W., Kang, J.-K., & Kim, D.-H. (2020). Effect of Al2O3 Dot Patterning on CZTSSe Solar Cell Characteristics. Nanomaterials, 10(9), 1874. https://doi.org/10.3390/nano10091874