A Human-Relevant 3D In Vitro Platform for an Effective and Rapid Simulation of Workplace Exposure to Nanoparticles: Silica Nanoparticles as Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Silica Nanoparticles
2.3. Aerosol Characterization
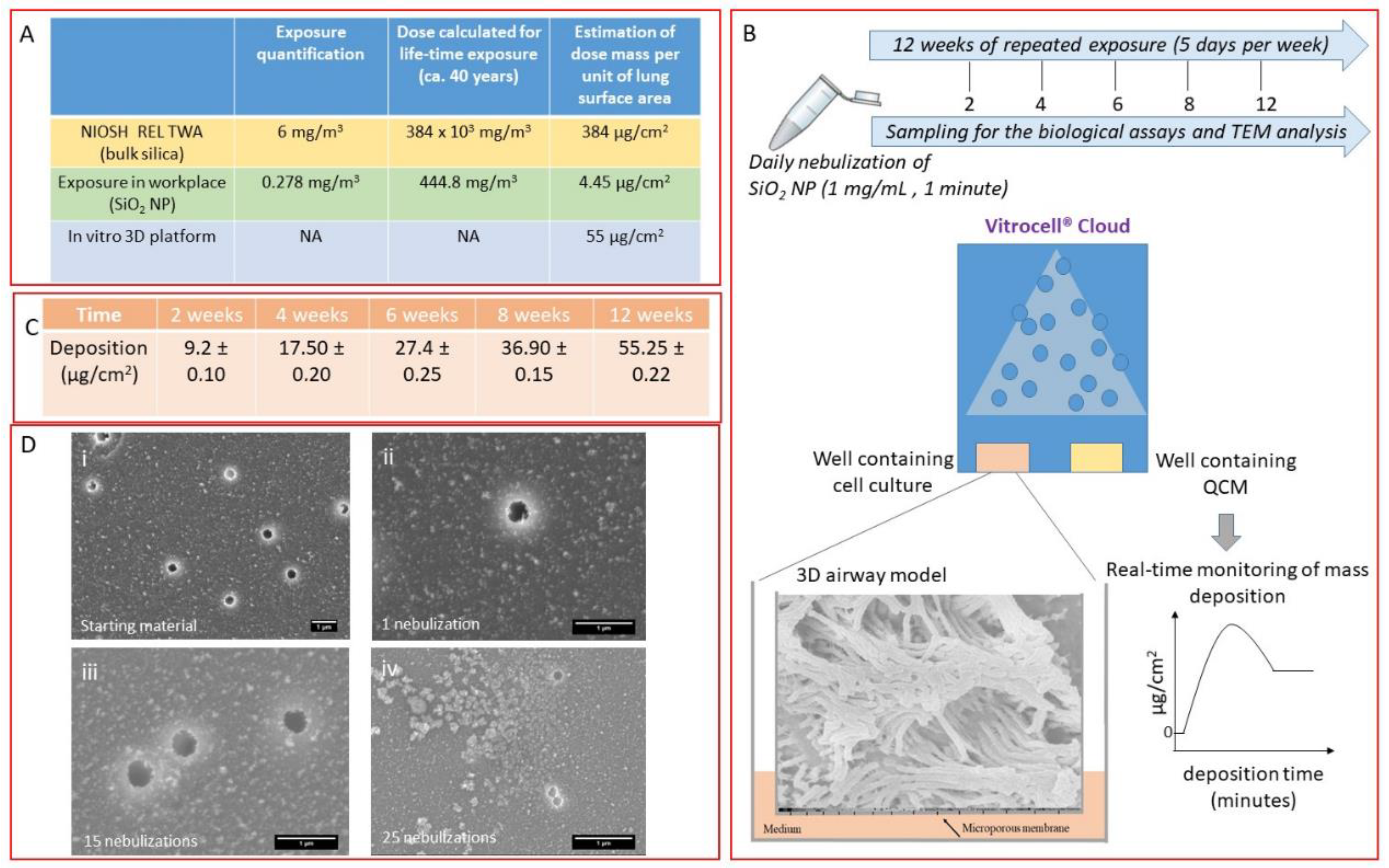
2.4. Exposure Conditions
2.5. 3D Airway Model
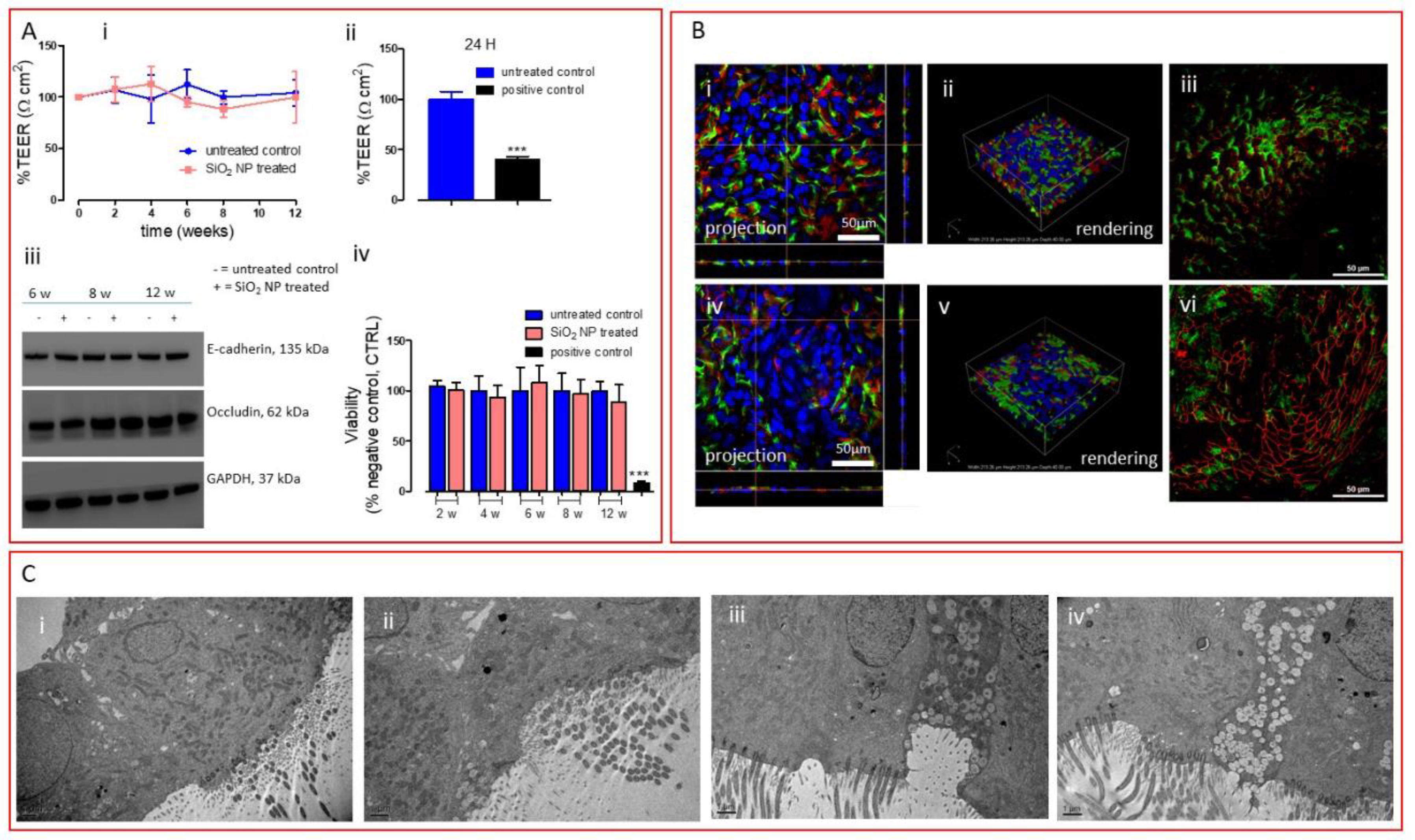
2.6. Cell Viability
2.7. Barrier Integrity Characterization
2.8. Measurements of Mass Concentration of SiO2 NPs in the Workplace
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fischman, M.; Murashov, V.; Borak, J.; Seward, J.; Fischmann, M. Nanotechnology and Health. J. Occup. Environ. Med. 2019, 61, e95–e98. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.T. Occupational health risk to nanoparticulate exposure. Environ. Sci. Process Impacts 2013, 15, 49–62. [Google Scholar] [CrossRef]
- Schneider, T.; Brouwer, D.H.; Koponen, I.K.; Jensen, K.A.; Fransman, W.; Van Duuren-Stuurman, B.; Van Tongeren, M.; Tielemans, E. Conceptual model for assessment of inhalation exposure to manufactured nanoparticles. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 450–463. [Google Scholar] [CrossRef]
- Shepard, M.N.; Brenner, S. An occupational exposure assessment for engineered nanoparticles used in semiconductor fabrication. Ann. Occup. Hyg. 2014, 58, 251–265. [Google Scholar]
- Bakand, S.; Hayes, A. Toxicological Considerations, Toxicity Assessment, and Risk Management of Inhaled Nanoparticles. Int. J. Mol. Sci. 2016, 17, 929. [Google Scholar] [CrossRef] [PubMed]
- Bakand, S.; Hayes, A.; Dechsakulthorn, F. Nanoparticles: A review of particle toxicology following inhalation exposure. Inhal Toxicol. 2012, 24, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Bessa, M.J.; Brandão, F.; Viana, M.; Gomes, J.F.P.; Monfort, E.; Cassee, F.R.; Fraga, S.; Teixeira, J. Nanoparticle exposure and hazard in the ceramic industry: An overview of potential sources, toxicity and health effects. Environ. Res. 2020, 184, 109297. [Google Scholar] [CrossRef] [PubMed]
- Di Cristo, L.; Movia, D.; Bianchi, M.G.; Allegri, M.; Mohamed, B.M.; Bell, A.P.; Moore, C.; Pinelli, S.; Rasmussen, K.; Riego-Sintes, J.; et al. Proinflammatory Effects of Pyrogenic and Precipitated Amorphous Silica Nanoparticles in Innate Immunity Cells. Toxicol. Sci. 2016, 150, 40–53. [Google Scholar] [CrossRef]
- Pavan, C.; Piane, M.D.; Gullo, M.; Filippi, F.; Fubini, B.; Hoet, P.; Horwell, C.; Huaux, F.; Lison, D.; Giudice, C.L.; et al. The puzzling issue of silica toxicity: Are silanols bridging the gaps between surface states and pathogenicity? Part Fibre Toxicol. 2019, 16, 32. [Google Scholar] [CrossRef]
- A Schulte, P.; Leso, V.; Niang, M.; Iavicoli, I. Current state of knowledge on the health effects of engineered nanomaterials in workers: A systematic review of human studies and epidemiological investigations. Scand J. Work Environ. Health 2019, 45, 217–238. [Google Scholar] [CrossRef]
- Ursini, C.L.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Del Frate, V.; Folesani, G.; Galetti, M.; Poli, D.; Buresti, G.; Di Cristo, L.; et al. Occupational exposure to graphene and silica nanoparticles. Part II: Pilot study to identify a panel of sensitive biomarkers of genotoxic/oxidative and inflammatory effects on suitable biological matrices. Nanotoxicology 2020. submitted. [Google Scholar]
- Boccuni, F.; Ferrante, R.; Tombolini, F.; Natale, C.; Gordini, A.; Sabella, S.; Iavicoli, S. Occupational exposure to graphene and silica nanoparticles: Part I – Workplace measurements and samplings. Nanotoxicology 2020. submitted. [Google Scholar]
- Arts, J.H.; Muijser, H.; Duistermaat, E.; Junker, K.; Kuper, C.F.; Kuper, F. Five-day inhalation toxicity study of three types of synthetic amorphous silicas in Wistar rats and post-exposure evaluations for up to 3 months. Food Chem. Toxicol. 2007, 45, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Reed, K.L.; Glover, K.P.; Swain, K.A.; Ostraat, M.L.; Donner, E.M.; Warheit, D.B. Changing the dose metric for inhalation toxicity studies: Short-term study in rats with engineered aerosolized amorphous silica nanoparticles. Inhal Toxicol. 2010, 22, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Jeon, K.; Kim, J.K.; Kim, Y.; Jo, M.S.; Lee, J.S.; Baek, J.E.; Park, H.S.; An, H.J.; Park, J.D.; et al. Subacute inhalation toxicity study of synthetic amorphous silica nanoparticles in Sprague-Dawley rats. Inhal Toxicol. 2017, 29, 567–576. [Google Scholar] [CrossRef]
- Sutunkova, M.P.; Solovyeva, S.N.; Katsnelson, B.A.; Gurvich, V.B.; Privalova, L.I.; Minigalieva, I.A.; Slyshkina, T.V.; Valamina, I.E.; Makeyev, O.H.; Shur, V.; et al. A paradoxical response of the rat organism to long-term inhalation of silica-containing submicron (predominantly nanoscale) particles of a collected industrial aerosol at realistic exposure levels. Toxicology 2017, 384, 59–68. [Google Scholar] [CrossRef]
- Hosseinkhani, H. 3D in vitro technology for drug discovery. Curr. Drug Saf. 2012, 7, 37–43. [Google Scholar] [CrossRef]
- Movia, D.; Bruni-Favier, S.; Prina-Mello, A. In vitro Alternatives to Acute Inhalation Toxicity Studies in Animal Models-A Perspective. Front Bioeng. Biotechnol. 2020, 8, 549. [Google Scholar] [CrossRef]
- Kabadi, P.K.; Rodd, A.L.; Simmons, A.E.; Messier, N.J.; Hurt, R.H.; Kane, A.B. A novel human 3D lung microtissue model for nanoparticle-induced cell-matrix alterations. Part Fibre Toxicol. 2019, 16, 15. [Google Scholar] [CrossRef]
- Barosova, H.; Maione, A.G.; Septiadi, D.; Sharma, M.; Haeni, L.; Balog, S.; O’Connell, O.; Jackson, G.R.; Brown, D.; Clippinger, A.J.; et al. Use of EpiAlveolar Lung Model to Predict Fibrotic Potential of Multiwalled Carbon Nanotubes. ACS Nano 2020, 14, 3941–3956. [Google Scholar] [CrossRef]
- Chortarea, S.; Barosova, H.; Clift, M.J.; Wick, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Human Asthmatic Bronchial Cells Are More Susceptible to Subchronic Repeated Exposures of Aerosolized Carbon Nanotubes At Occupationally Relevant Doses Than Healthy Cells. ACS Nano 2017, 11, 7615–7625. [Google Scholar] [CrossRef] [PubMed]
- Di Cristo, L.; Grimaldi, B.; Catelani, T.; Vázquez, E.; Pompa, P.P.; Sabella, S. Repeated exposure to aerosolized graphene oxide mediates autophagy inhibition and inflammation in a three-dimensional human airway model. Mater. Today Bio. 2020, 6, 100050. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry: Pyrogen and Endotoxins Testing. 2018. Available online: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm314718.htm (accessed on 3 August 2018).
- Di Cristo, L.; Maguire, C.M.; McQuillan, K.; Aleardi, M.; Volkov, Y.; Movia, D.; Prina-Mello, A. Towards the Identification of an In Vitro Tool for Assessing the Biological Behavior of Aerosol Supplied Nanomaterials. Int. J. Environ. Res. Public Health 2018, 15, 563. [Google Scholar] [CrossRef] [PubMed]
- Di Cristo, L.; Bianchi, M.G.; Chiu, M.; Taurino, G.; Donato, F.; Garzaro, G.; Bussolati, O.; Bergamaschi, E.; Di Cristo, L. Comparative in Vitro Cytotoxicity of Realistic Doses of Benchmark Multi-Walled Carbon Nanotubes towards Macrophages and Airway Epithelial Cells. Nanomaterials (Basel) 2019, 9, 982. [Google Scholar] [CrossRef]
- Farcal, L.; Andon, F.T.; Di Cristo, L.; Rotoli, B.M.; Bussolati, O.; Bergamaschi, E.; Mech, A.; Hartmann, N.B.; Rasmussen, K.; Riego-Sintes, J.; et al. Comprehensive In Vitro Toxicity Testing of a Panel of Representative Oxide Nanomaterials: First Steps towards an Intelligent Testing Strategy. PLoS ONE 2015, 10, e0127174. [Google Scholar] [CrossRef]
- Rotoli, B.M.; Bussolati, O.; Costa, A.L.; Blosi, M.; Di Cristo, L.; Zanello, P.P.; Bianchi, M.G.; Visigalli, R.; Bergamaschi, E. Comparative effects of metal oxide nanoparticles on human airway epithelial cells and macrophages. J. Nanoparticle Res. 2012, 14, 14. [Google Scholar] [CrossRef]
- Rotoli, B.M.; Gatti, R.; Movia, D.; Bianchi, M.G.; Di Cristo, L.; Fenoglio, I.; Sonvico, F.; Bergamaschi, E.; Prina-Mello, A.; Bussolati, O. Identifying contact-mediated, localized toxic effects of MWCNT aggregates on epithelial monolayers: A single-cell monitoring toxicity assay. Nanotoxicology 2015, 9, 230–241. [Google Scholar] [CrossRef]
- Malvindi, M.A.; De Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering. PLoS ONE 2014, 9, e85835. [Google Scholar] [CrossRef]
- Malvindi, M.A.; Di Corato, R.; Curcio, A.; Melisi, D.; Rimoli, M.G.; Tortiglione, C.; Tino, A.; George, C.; Brunetti, V.; Cingolani, R.; et al. Multiple functionalization of fluorescent nanoparticles for specific biolabeling and drug delivery of dopamine. Nanoscale 2011, 3, 5110–5119. [Google Scholar] [CrossRef]
- Murugadoss, S.; Lison, D.; Godderis, L.; Brûle, S.V.D.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P. Toxicology of silica nanoparticles: An update. Arch Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef]
- Barsan, M. NIOSH Pocket Guide to Chemical Hazards. Available online: https://www.cdc.gov/niosh/npg/default.html (accessed on 18 February 2020).
- Derk, R.; Davidson, D.C.; Manke, A.; Stueckle, T.A.; Rojanasakul, Y.; Wang, L. Potential in vitro model for testing the effect of exposure to nanoparticles on the lung alveolar epithelial barrier. Sens. Biosensing Res. 2015, 3, 38–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ando-Akatsuka, Y.; Yonemura, S.; Itoh, M.; Furuse, M.; Tsukita, S. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J. Cell Physiol. 1999, 179, 115–125. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect Biol. 2017, 9, a028241. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Cristo, L.; Boccuni, F.; Iavicoli, S.; Sabella, S. A Human-Relevant 3D In Vitro Platform for an Effective and Rapid Simulation of Workplace Exposure to Nanoparticles: Silica Nanoparticles as Case Study. Nanomaterials 2020, 10, 1761. https://doi.org/10.3390/nano10091761
Di Cristo L, Boccuni F, Iavicoli S, Sabella S. A Human-Relevant 3D In Vitro Platform for an Effective and Rapid Simulation of Workplace Exposure to Nanoparticles: Silica Nanoparticles as Case Study. Nanomaterials. 2020; 10(9):1761. https://doi.org/10.3390/nano10091761
Chicago/Turabian StyleDi Cristo, Luisana, Fabio Boccuni, Sergio Iavicoli, and Stefania Sabella. 2020. "A Human-Relevant 3D In Vitro Platform for an Effective and Rapid Simulation of Workplace Exposure to Nanoparticles: Silica Nanoparticles as Case Study" Nanomaterials 10, no. 9: 1761. https://doi.org/10.3390/nano10091761
APA StyleDi Cristo, L., Boccuni, F., Iavicoli, S., & Sabella, S. (2020). A Human-Relevant 3D In Vitro Platform for an Effective and Rapid Simulation of Workplace Exposure to Nanoparticles: Silica Nanoparticles as Case Study. Nanomaterials, 10(9), 1761. https://doi.org/10.3390/nano10091761







