Enhanced Electrochemical Properties and OER Performances by Cu Substitution in NiCo2O4 Spinel Structure
Abstract
1. Introduction
2. Materials and Methods
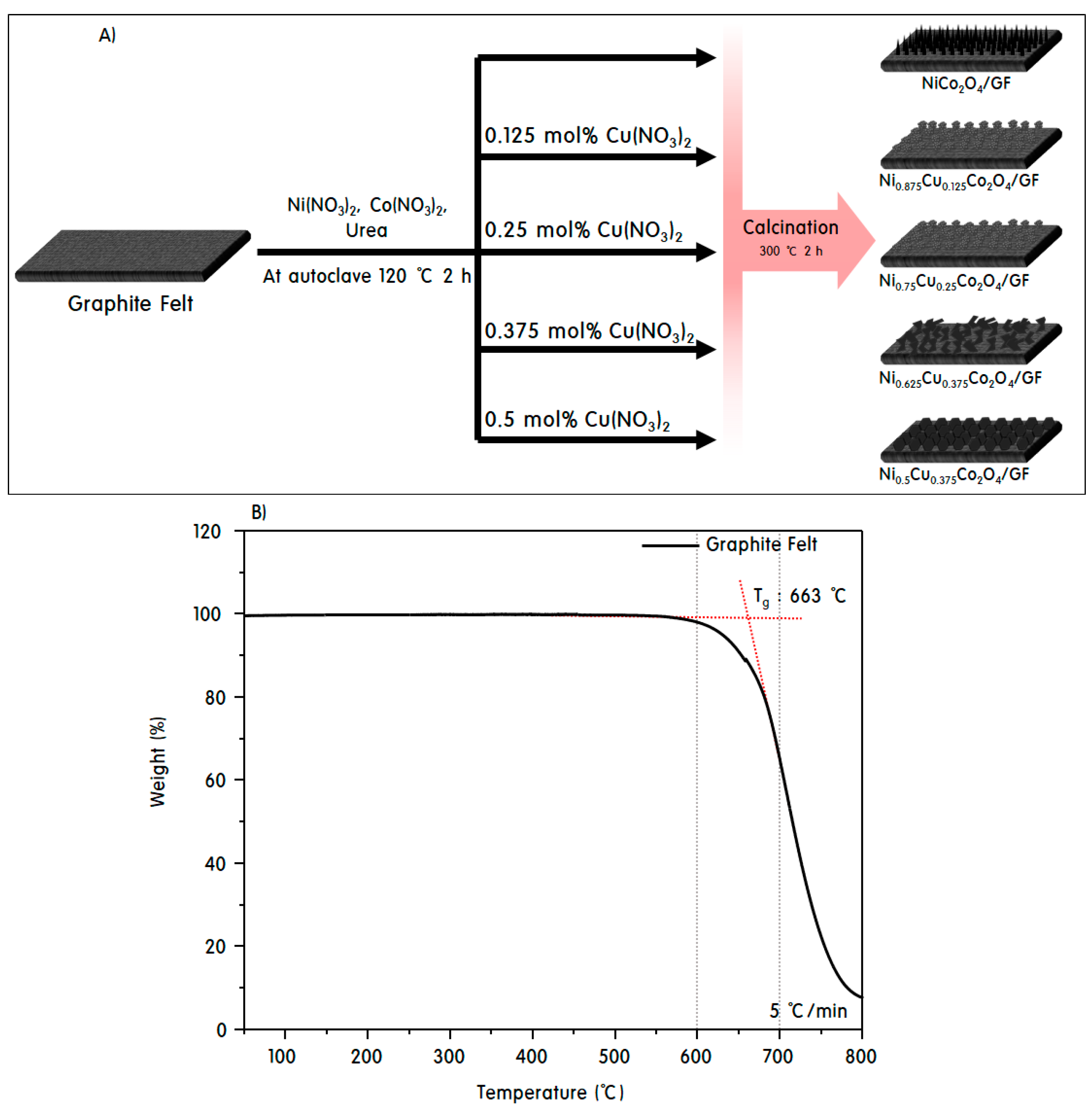
2.1. Fabrications of NiCo2O4/GF and Ni1−xCuxCo2O4/GF Electrodes
2.2. Evaluation of Physicochemical Properties of Electrodes
2.3. Electrochemical Characterizations of Electrodes
3. Results and Discussion
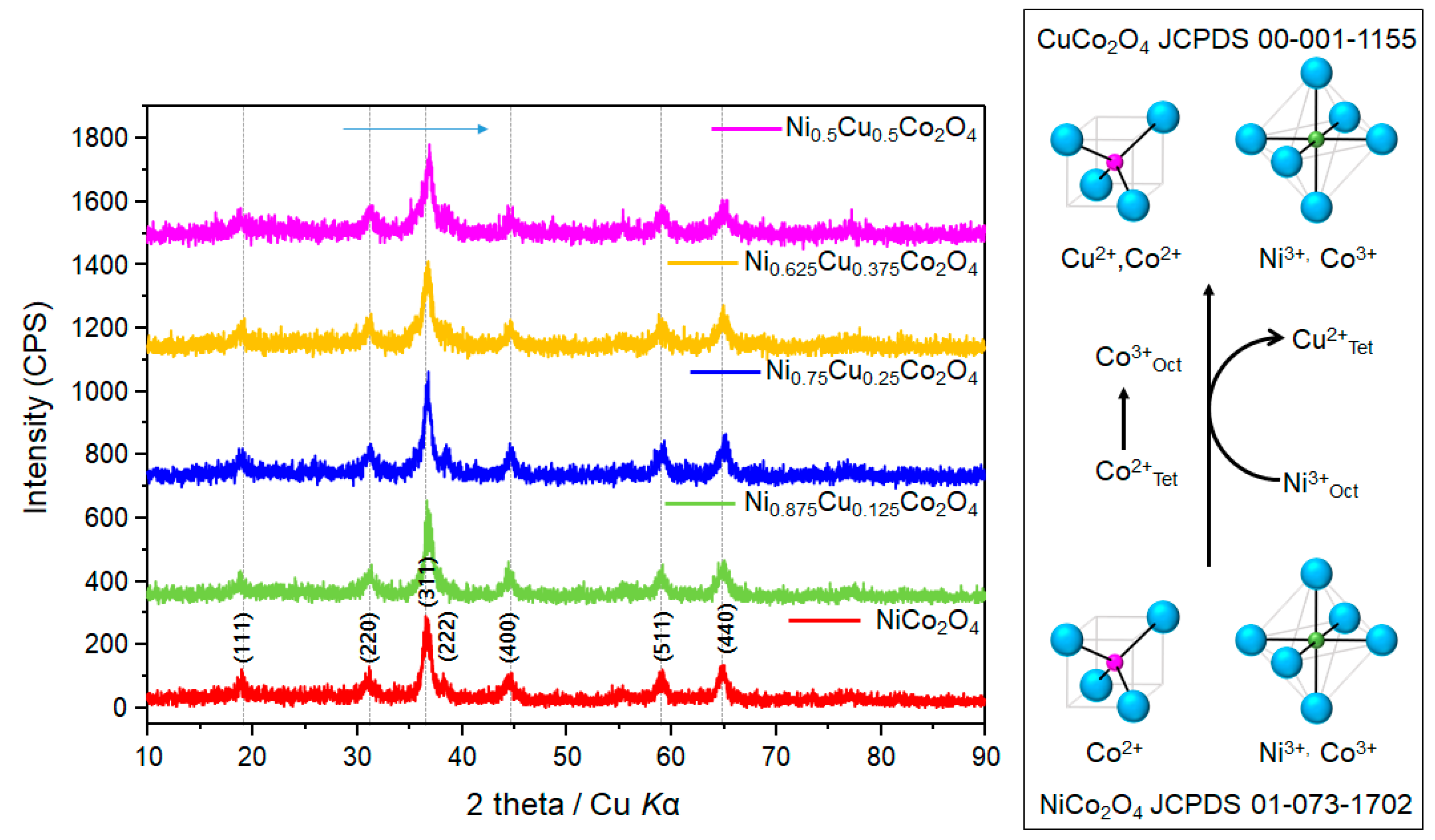
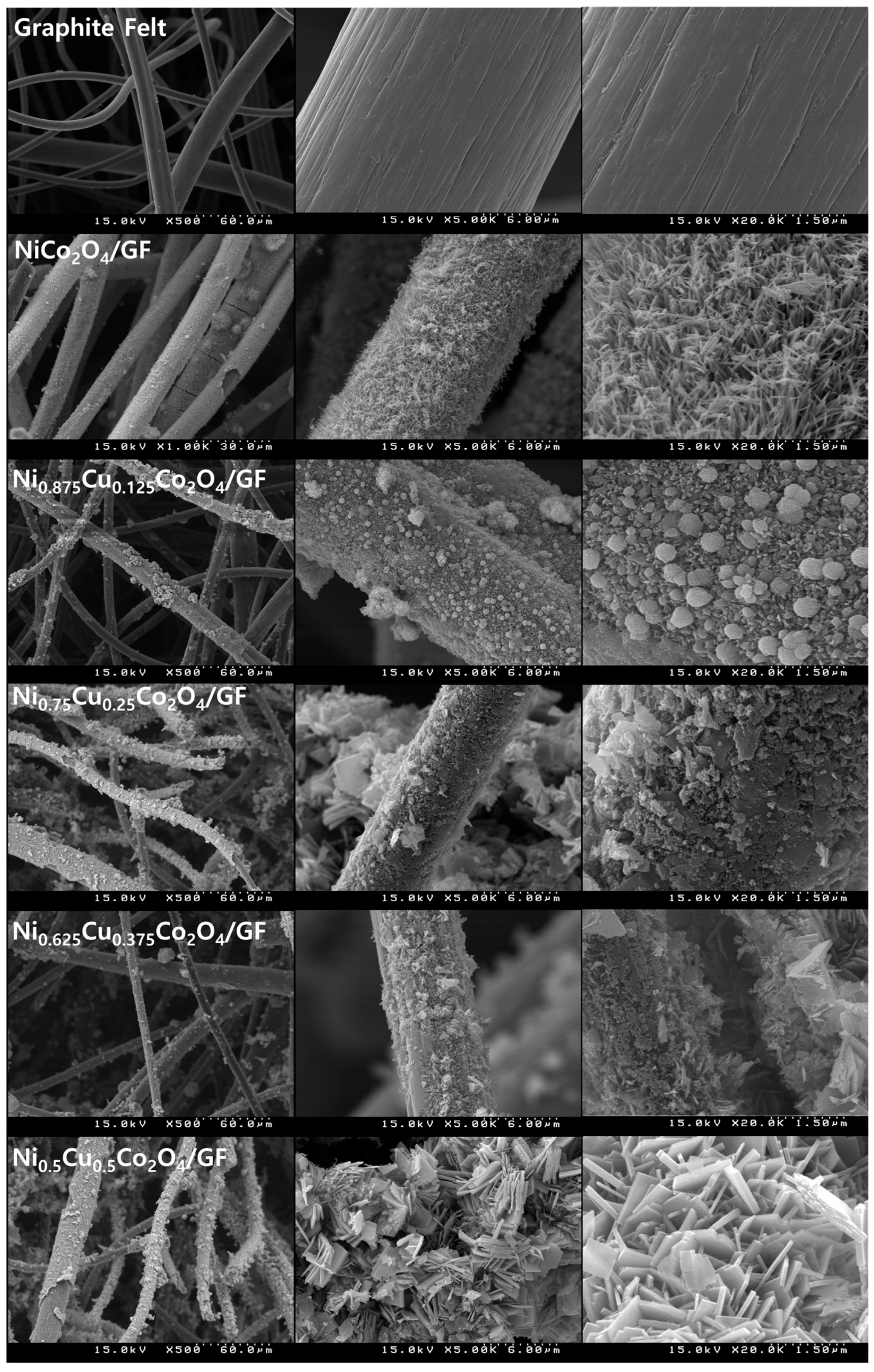
3.1. Physicochemical Properties of Ni1−xCuxCo2O4 Electrode Active Materials Grown on GF
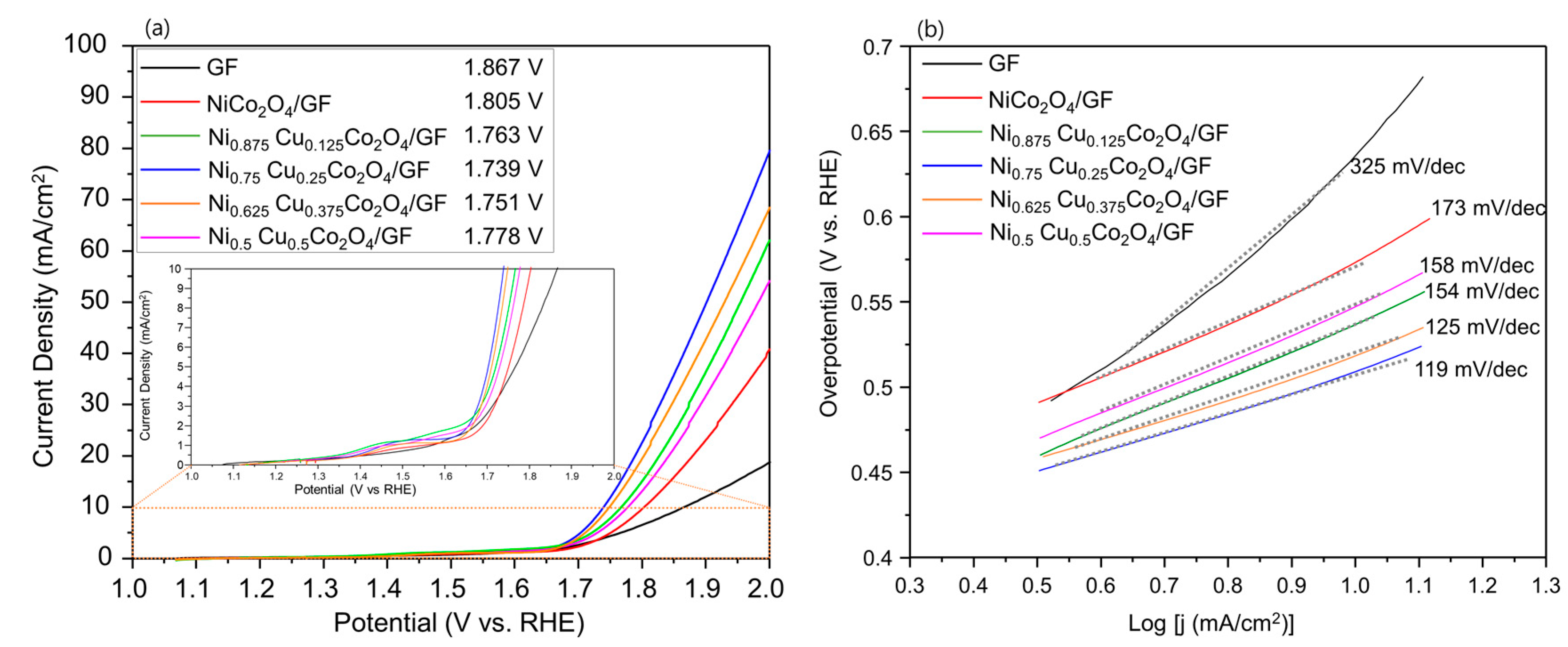
3.2. Electrochemical Properties of Ni1−xCuxCo2O4 Electrode Active Materials Grown on GF
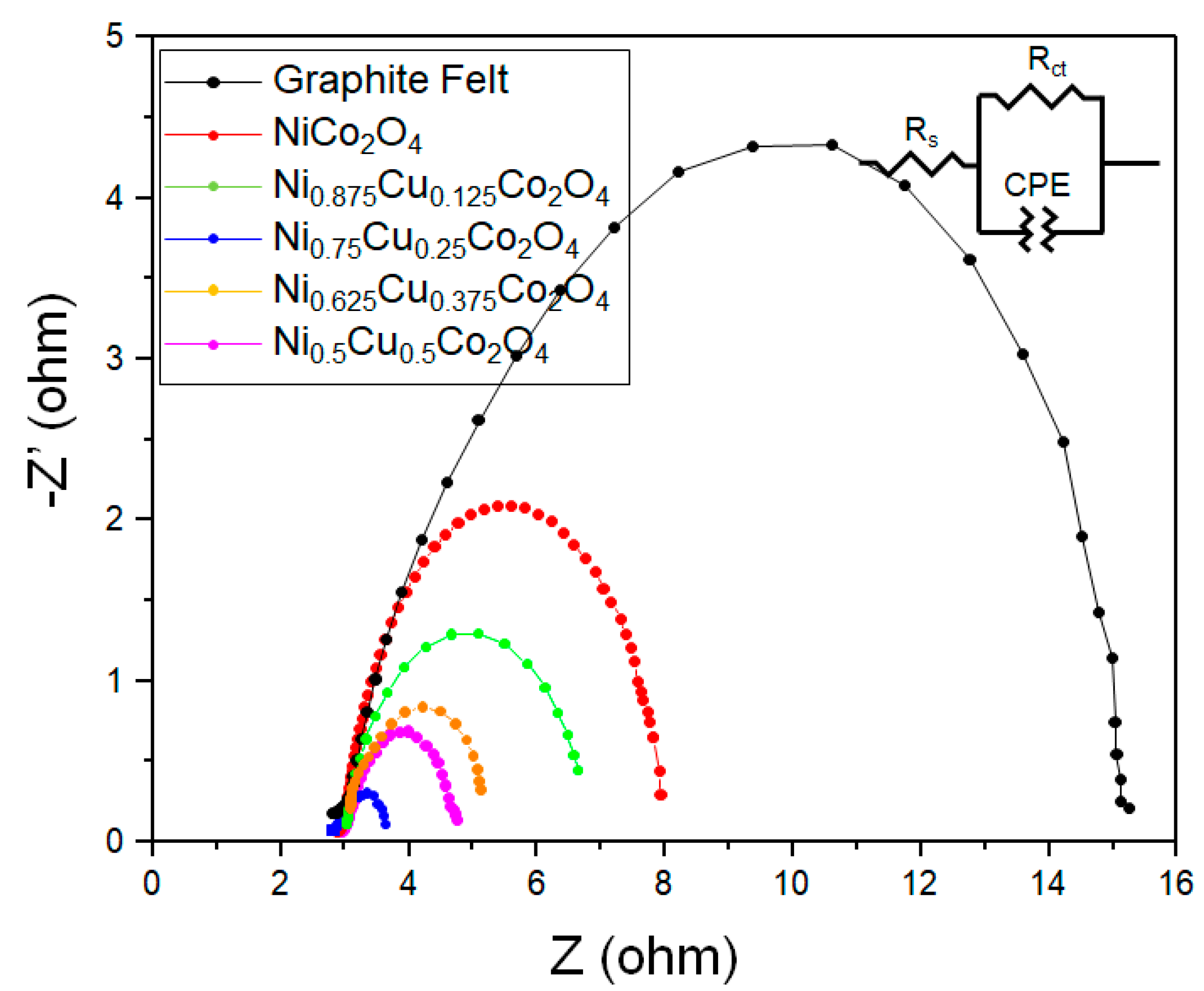
3.3. Evaluation of the Durability of the Electrode
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cobo, S.; Heidkamp, J.; Jacques, P.A.; Fize, J.; Fourmond, V.; Guetaz, L.; Jousselme, B.; Ivanova, V.; Dau, H.; Palacin, S.; et al. A Janus cobalt-based catalytic material for electro-splitting of water. Nat. Mater. 2012, 11, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yu, F.; Zhu, Q.; Sun, J.; Qin, F.; Yu, L.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Water splitting by electrolysis at high current densities under 1.6 volts. Energy Environ. Sci. 2018, 11, 2858–2864. [Google Scholar] [CrossRef]
- Bizzotto, F.; Quinson, J.; Zana, A.; Kirkensgaard, J.J.K.; Dworzak, A.; Oezaslan, M.; Arenz, M. Ir nanoparticles with ultrahigh dispersion as oxygen evolution reaction (OER) catalysts: Synthesis and activity benchmarking. Catal. Sci. Technol. 2019, 9, 6345–6356. [Google Scholar] [CrossRef]
- Yi, J.; Lee, W.H.; Choi, C.H.; Lee, Y.; Park, K.S.; Min, B.K.; Hwang, Y.J.; Oh, H.S. Effect of Pt introduced on Ru-based electrocatalyst for oxygen evolution activity and stability. Electrochem Commun. 2019, 104, 106469. [Google Scholar] [CrossRef]
- Markoulaki, V.I.; Papadas, I.T.; Kornarakis, I.; Armatas, G.S. Synthesis of Ordered Mesoporous CuO/CeO2 Composite Frameworks as Anode Catalysts for Water Oxidation. Nanomaterials 2015, 5, 1971–1984. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, Y.; Liu, D.; Zou, Y.; Wang, S. Water-Plasma-Enabled Exfliation of Ultrathin Layered Double Hydroxide Nanosheets with Multivacancies for Water Oxidation. Adv. Mater. 2017, 29, 1701546. [Google Scholar] [CrossRef] [PubMed]
- Man, H.W.; Tsang, C.S.; Li, M.M.J.; Mo, J.; Huang, B.; Lee, L.Y.S.; Leung, Y.C.; Wong, K.Y.; Tsang, S.C.E. Tailored transition metal-doped nickel phosphide nanoparticles for the electrochemical oxygen evolution reaction (OER). Chem. Commun. 2018, 54, 8630–8633. [Google Scholar] [CrossRef]
- He, F.; Wu, L.; Wei, G.; Zhang, H.; Yan, J. Electrical conductivity and ion transfer behavior of NiCo2O4 with different micro structures. Results Phys. 2020, 16, 102875. [Google Scholar] [CrossRef]
- Finger, L.W.; Hazen, R.M.; Hofmeister, A.M. High-Pressure Crystal Chemistry of Spinel (MgAl2O4) and Magnetite (Fe3O4): Conparisons with Silicate Spinels. Phys. Chem. Miner. 1986, 13, 215–220. [Google Scholar] [CrossRef]
- Kumari, P.; Sharma, K.; Pal, P.; Kumar, M.; Ichikawa, T.; Jain, A. highly efficient & stable Bi & Sb anodes using lithium borohydride as solid electrolyte in Li-ion batteries. RSC Adv. 2019, 9, 13077–13081. [Google Scholar]
- Chen, Y.; Ji, S.; Zhao, S.; Chen, W.; Dong, J.; Cheong, W.C.; Shen, R.; Wen, X.; Zheng, L.; Rykov, A.I.; et al. Enhanced oxygen reduction with single-atomic-site iron catalysts for a zinc-air vattery and hydrogen-air fuel cell. Nat. Commun. 2018, 9, 5422. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.G.; Hassoun, J.; Park, J.B.; Sun, Y.K.; Scrosati, B. An improved high-performance lithium-air battery. Nat. Chem. 2012, 4, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, H.Y.; Miao, J.; Yang, H.; Liu, B. A flexible high-performance oxygen evolution electrode with three-dimensional NiCo2O4 core-shell nanowires. Nano Energy 2015, 11, 333–340. [Google Scholar] [CrossRef]
- An, L.; Huang, L.; Zhou, P.; Yin, J.; Liu, H.; Xi, P. A Self-Standing High-Performance Hydrogen Evolution Electrode with Nanostructured NiCo2O4/CuS Heterostructures. Adv. Funct. Mater. 2015, 25, 6814–6822. [Google Scholar] [CrossRef]
- Hassan, D.; El-safty, S.; Khalil, K.A.; Dewidar, M.; El-magd, G.A. Carbon Supported Engineering NiCo2O4 Hybrid Nanofibers with Enhanced Electrocatalytic Activity for Oxygen Reduction Reaction. Materials 2016, 9, 759. [Google Scholar] [CrossRef]
- Das, A.K.; Layek, R.K.; Kim, N.H.; Jung, D.; Lee, J.H. Reduced graphene oxide (RGO)-supported NiCo2O4 nanoparticles: An electrocatalyst for methanol oxidation. Nanoscale 2014, 6, 10657–10665. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, B.; Fang, B.; Feng, L. A comparative study of NiCo2O4 catalyst supported on Ni foam and from solution residuals fabricated by a hydrothermal approach for electrochemical oxygen evolution reaction. Chem. Commun. 2018, 54, 13151–13154. [Google Scholar] [CrossRef]
- Tavares, A.C.; Cartaxo, M.A.M.; da Silva Pereira, M.I.; Costa, F.M. Effect of the partial replacement of Ni or Co by Cu on the electrocatalytic activity of the NiCo2O4 spinel oxide. J. Electroanal. Chem. 1999, 464, 187–197. [Google Scholar] [CrossRef]
- Mugheri, A.Q.; Tahira, A.; Aftab, U.; Bhatti, A.L.; Memon, N.N.; Memon, J.R.; Abro, M.I.; Shah, A.A.; Willander, M.; Hullioa, A.A.; et al. Efficient tri-metallic oxides NiCo2O4/CuO for the oxygen evolution reaction. RSC Adv. 2019, 9, 42387–42394. [Google Scholar] [CrossRef]
- Ezeigwe, E.R.; Khiew, P.S.; Siong, C.W.; Tan, M.T.T. Solvothermal synthesis of NiCo2O4 nanocomposites on liquid-phase exfoliated graphene as an electrode material for electrochemical capacitors. J. Alloys Compd. 2017, 693, 1133–1142. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Xu, J.; Jaber, F.; Musharavati, F.; Zalnezhad, E.; Bae, S.; Hui, K.S.; Hui, K.N.; Liu, J. Synthesis and Characterization of a NiCo2O4@NiCo2O4 Hierarchical Mesoporous Nanoflake Electrode for Supercapacitor Applications. Nanomaterials 2020, 10, 1292. [Google Scholar] [CrossRef]
- Yan, A.L.; Wang, W.D.; Chen, W.Q.; Wang, X.C.; Liu, F.; Cheng, J.P. The Synthesis of NiCo2O4-MnO2 Core-shell nanowires by Electrodeposition and Its Supercapacitive Properties. Nanomaterials 2019, 9, 1398. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gui, X.; Yi, T.F.; Li, Y.; Yue, C. Recent progress of NiCo2O4-based anodes for high-performance lithium-ion batteries. Curr. Opin. Solid State Mater. Sci. 2018, 22, 109–126. [Google Scholar] [CrossRef]
- Laziz, N.A.; Rjeily, J.A.; Darwiche, A.; Toufaily, J.; Outzourhit, A.; Ghanouss, F.; Sougrati, M.T. Li- and Na-ion Storage Performance of Natural Graphite via Simple Flotation Process. J. Electrochem. Sci. Technol. 2018, 9, 320–329. [Google Scholar] [CrossRef]
- Choi, S.K.; Choi, W.; Park, H. Solar water oxidation using nickel-borate coupled BiVO4 photoelectrodes. Phys. Chem. Chem. Phys. 2013, 15, 6499–6507. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, J.; Yang, W.; Zhuang, Z.; Zhao, Y.; He, L.; Xu, L.; Liao, X.; Zhu, R.; Mai, L. Oxygen Vacancy-Determined Highly Efficient Oxygen Reduction in NiCo2O4/Hollow Carbon Spheres. ACS Appl. Mater. Interfaces 2018, 10, 16410–16417. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ai, L.; Jiang, J.; Liu, S. Spinel-type oxygen-incorporated Ni3+ self-doped Ni3S4 ultrathin nanosheets for highly efficient and stable oxygen evolution electrocatalysis. J. Colloid Interf. Sci. 2020, 564, 418–427. [Google Scholar] [CrossRef]
- Ivanenko, I.; Coronova, A.; Astrelin, I.; Romanenko, Y. Structural and catalytic properties of Ni-Co spinel and its conposites. Bull. Mater. Sci. 2019, 42, 172. [Google Scholar] [CrossRef]
- Marco, J.F.; Gancedo, J.R.; Gracia, M. Characterization of the Nickel Cobaltite, NiCo2O4, Prepared by Several Methods: An XRD, XANES, EXAFS, and XPS Study. J. Solid State Chem. 2000, 153, 74–81. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, J.; Wub, D.; Zhou, Z.; Deng, Y.; Zhang, T.; Shih, K. Efficient degradation of sulfamethazine with CuCo2O4 spinel nanocatalysts for peroxymonosulfate activation. Chem. Eng. J. 2015, 280, 514–524. [Google Scholar] [CrossRef]
- Do, J.Y.; Son, N.; Park, N.K.; Kwak, B.S.; Beak, J.I.; Ryu, H.J.; Kang, M. Reliable oxygen transfer in MgAl2O4 spinel through the reversible formation of oxygen vacancies by Cu2+/Fe3+ anchoring. Appl. Energy 2018, 219, 138–150. [Google Scholar] [CrossRef]
- Avaji, P.G.; Patil, S.A.; Badami, P.S. Synthesis, spectral, thermal, solid state d.c. electrical conductivity and biological studies of Co(II), Ni(II) and Cu(II) complexes with 3-substituted-4-amino (indole-3-aldehydo)-5-mercapto-1,2,4-triazole Schiff bases. J. Coord. Chem. 2008, 61, 1884–1896. [Google Scholar] [CrossRef]
- Tsai, C.L.; Chou, B.; Cheng, S.; Lee, J.F. Synthesis of TMBQ using Cu(II)-substituted MCM-41 as the catalyst. Appl. Catal. A-Gen. 2001, 208, 279–289. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, D.; Ren, Z.; Huo, M.; Dang, G.; Min, F.; Zhang, Q.; Xie, J. Mesoporous NiCo2O4 Micro/nanospheres with Hierarchical Structures for Supercapacitors and Methanol Electro–Oxidation. Chem. Electro. Chem. 2017, 4, 441–449. [Google Scholar]
- Shanmugavel, T.; Raj, S.G.; Kumar, G.R.; Rajarajan, G.; Saravanan, D. Cost effective preparation and characterization of nanocrystalline nickel ferrites (NiFe2O4) in low temperature regime. J. King Saud Univ. Sci. 2015, 27, 176–181. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Lin, X.; Tian, M.; An, X.; Fu, Y.; Li, R.; Jin, J.; Ma, J. MOF derived Co3O4 nanoparticles embedded in N-doped mesoporous carbon layer/MWCNT hybrids: Extraordinary bi-functional electrocatalysts for OER and ORR. J. Mater. Chem. A 2015, 3, 17392–17402. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Karthick, K.; Sankar, S.S.; Sangeetha, K.; Karthick, P.E.; Kundu, S. Precision and correctness in the evaluation of electrocatalytic water splitting: Revisiting activity parameters with a critical assessment. Energy Environ. Sci. 2018, 11, 744–771. [Google Scholar] [CrossRef]
- He, J.; Guan, L.; Zhou, Y.; Shao, P.; Yao, Y.; Lu, S.; Kong, L.; Liao, X. One-pot preparation of mesoporous KxPMo12O40 (x = 1, 2, 3, 4) materials for oxidative desulfurization: Electrochemically-active surface area (ECSA) determines their activity. React. Chem. Eng. 2020, in press. [Google Scholar] [CrossRef]
- Liu, T.; Asirib, A.M.; Sun, X. Electrodeposited Co-doped NiSe2 nanoparticles film: A good electrocatalyst for efficient water splitting. Nanoscale 2016, 8, 3911–3915. [Google Scholar] [CrossRef]
- Wu, P.; Wu, J.; Si, H.; Zhang, Z.; Liao, Q.; Wang, X.; Dai, F.; Ammarah, K.; Kang, Z.; Zhang, Y. 3D Holey-Graphene Architecture Expedites Ion Transport Kinetics to Push the OER Performance. Adv. Energy Mater. 2020, 10, 2001005. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, D.; Duan, D.; Li, Y.; Yuan, Q.; Chen, L.; Liu, S. In situ fabrication of 3D self-supporting cobalt phosphate-modified graphite felt electrocatalysts for oxygen evolution reaction in neutral solution. J. Electroanal. Chem. 2020, 862, 114031. [Google Scholar] [CrossRef]
- Zang, N.; Wu, Z.; Wang, J.; Jin, W. Rational design of Cu-Co thiospinel ternary sheet arrays for highly efficient electrocatalytic water splitting. J. Mater. Chem. A 2020, 8, 1799–1807. [Google Scholar] [CrossRef]
- Xie, Y.S.; Wang, Z.; Ju, M.; Long, X.; Yang, S. Dispersing transition metal vacancies in layered double hydroxides by ionic reductive complexation extraction for efficient water oxidation. Chem. Sci. 2019, 10, 8354–8359. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiong, Y.; Liu, X.; Bo, X.; Zhang, Y.; Han, C.; Guo, L. Facile synthesis of electrospun MFe2O4 (M = Co, Ni, Cu, Mn) spinel nanofibers with excellent electrocatalytic properties for oxygen evolution and hydrogen peroxide reduction. Nanoscale 2015, 7, 8920–8930. [Google Scholar] [CrossRef]
- Cao, B.; Luo, C.; Lao, J.; Chen, H.; Qi, R.; Lin, H.; Peng, H. Facile Synthesis of 3d Transition-Metal-Doped α-Co(OH)2 Nanomaterials in Water−Methanol Mediated with Ammonia for Oxygen Evolution Reaction. ACS Omega 2019, 4, 16612–16618. [Google Scholar] [CrossRef]
- Shuai, C.; Mo, Z.; Niu, X.; Yang, X.; Liu, G.; Wang, J.; Liu, N.; Guo, R. Hierarchical NiCo2S4 nanosheets grown on graphene to catalyze the oxygen evolution reaction. J. Mater. Sci. 2020, 55, 1627–1636. [Google Scholar] [CrossRef]
- Qiao, M.F.; Wang, Y.; Li, L.; Hu, G.Z.; Zou, G.A.; Mamat, X.; Dong, Y.M.; Hu, X. Self-templated nitrogen-doped mesoporous carbon decorated with double transition-metal active sites for enhanced oxygen electrode catalysis. Rare Met. 2020, 39, 824–833. [Google Scholar] [CrossRef]
- Cao, X.; Johnson, E.; Nath, M. Identifying high-efficiency oxygen evolution electrocatalysts from Co–Ni–Cu based selenides through combinatorial electrodeposition. J. Mater. Chem. A 2019, 7, 9877–9889. [Google Scholar] [CrossRef]
- Shi, Q.; Fu, S.; Zhu, C.; Song, J.; Du, D.; Lin, Y. Metal-organic frameworks-based catalysts for electrochemical oxygen evolution. Mater. Horiz. 2019, 6, 684–702. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.Y.; Lee, S.Y.; Hong, J.A.; Kim, N.; Baik, J.; Hwang, Y.J. Comparative study of catalytic activities among transition metal-doped IrO2 nanoparticles. Sci. Rep. 2018, 8, 16777. [Google Scholar] [CrossRef]
- Samant, S.; Bhuni, K.; Prandhan, D.; Satpati, B.; Srivastava, R. NiCuCo2O4 Supported Ni−Cu Ion-Exchanged Mesoporous Zeolite Heteronano Architecture: An Efficient, Stable, and Economical Nonprecious Electrocatalyst for Methanol Oxidation. ACS Sustain. Chem. Eng. 2018, 6, 2023–2036. [Google Scholar] [CrossRef]
- Feng, B.; Liu, C.; Yan, W.; Geng, J.; Wang, G. MoS2 nanotuves loaded with TiO2 nanoparticles for enhanced electrocatalytic hydrogen evolution. RSC Adv. 2019, 9, 26487–26494. [Google Scholar] [CrossRef]
- Yi, Y.; Weinberg, G.; Prenzel, M.; Greiner, M.; Heumann, S.; Becker, S.; Schlögl, R. Electrochemical corrosion of a glassy carbon electrode. Catal. Today 2017, 295, 32–40. [Google Scholar] [CrossRef]
- Kusmierek, E. Evaluating the Effect of WO3 on Electrochemical and Corrosion Properties of TiO2-RuO2-Coated Titanium Anodes with Low Content of RuO2. Electrocatalysis 2020, 11, 555–566. [Google Scholar] [CrossRef]
- Mosa, I.M.; Biswas, S.; El-Sawy, A.M.; Botu, V.; Guild, C.; Song, W.; Ramprasad, R.; Ruslingace, J.F.; Suib, S.L. Tunable mesoporous manganese oxide for high performance oxygen reduction and evolution reactions. J. Mater. Chem. A 2016, 4, 620–631. [Google Scholar] [CrossRef]
- Monte, M.; Munuera, G.; Costa, D.; Conesa, J.C.; Arias, A.M. Near-ambient XPS characterization of interfacial copper species in ceria-supported copper catalysts. Phys. Chem. Chem. Phys. 2015, 17, 29995–30004. [Google Scholar] [CrossRef]
- Amri, A.; Jiang, Z.T.; Zhao, X.; Xie, Z.; Yin, C.Y.; Ali, N.; Mondinos, N.; Rahman, M.M.; Habibi, D. Tailoring the physicochemical and mechanical properties of optical copper–cobalt oxide thin films through annealing treatment. Surf. Coat. Technol. 2014, 239, 212–221. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Vieker, H.; Kouotou, P.M.; Beyer, A. In situ characterization of Cu–Co oxides for catalytic application. Faraday Discuss. 2015, 177, 249–262. [Google Scholar] [CrossRef]
- Li, X.; Xin, M.; Guo, S.; Cai, T.; Du, D.; Xing, W.; Zhao, L.; Guo, W.; Xue, Q.; Yan, Z. Insight of synergistic effect of different active metal ions in layered double hydroxides on their electrochemical behaviors. Electrochim. Acta 2017, 253, 302–310. [Google Scholar] [CrossRef]
- Lenglet, M.; D’huysser, A.; Bonelle, J.P.; Dürr, J.; Jørgensen, C.K. Analysis of X-Ray Ni Kβ Emission, XANES, XPS, Ni 2p, and Optical Spectra of Nickel(II) Spinels and Structure Inference. Chem. Phys. Lett. 1987, 136, 478–482. [Google Scholar] [CrossRef]
- Huang, J.; Qian, W.; Ma, H.; Zhang, H.; Ying, W. Highly selective production of heavy hydrocarbons over cobalt–graphene–silica nanocomposite catalysts. RSC Adv. 2017, 7, 33441–33449. [Google Scholar] [CrossRef]
- Amri, A.; Duan, X.F.; Yin, C.Y.; Jiang, Z.T.; Rahman, M.M.; Pryor, T. Solar absorphance of copper-cobalt oxide thin film coatings with nano-size, grain-like morphology: Optimization and synchrotron radiation XPS studies. Appl. Surf. Sci. 2013, 275, 127–135. [Google Scholar] [CrossRef]
- Li, D.; Gong, Y.; Zhang, Y.; Luo, C.; Li, W.; Fu, Q.; Pan, C. Facile Synthesis of Carbon Nanosphere/NiCo2O4 Coreshell Sub-microspheres for High Performance Supercapacitor. Sci. Rep. 2015, 5, 12903. [Google Scholar] [CrossRef] [PubMed]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
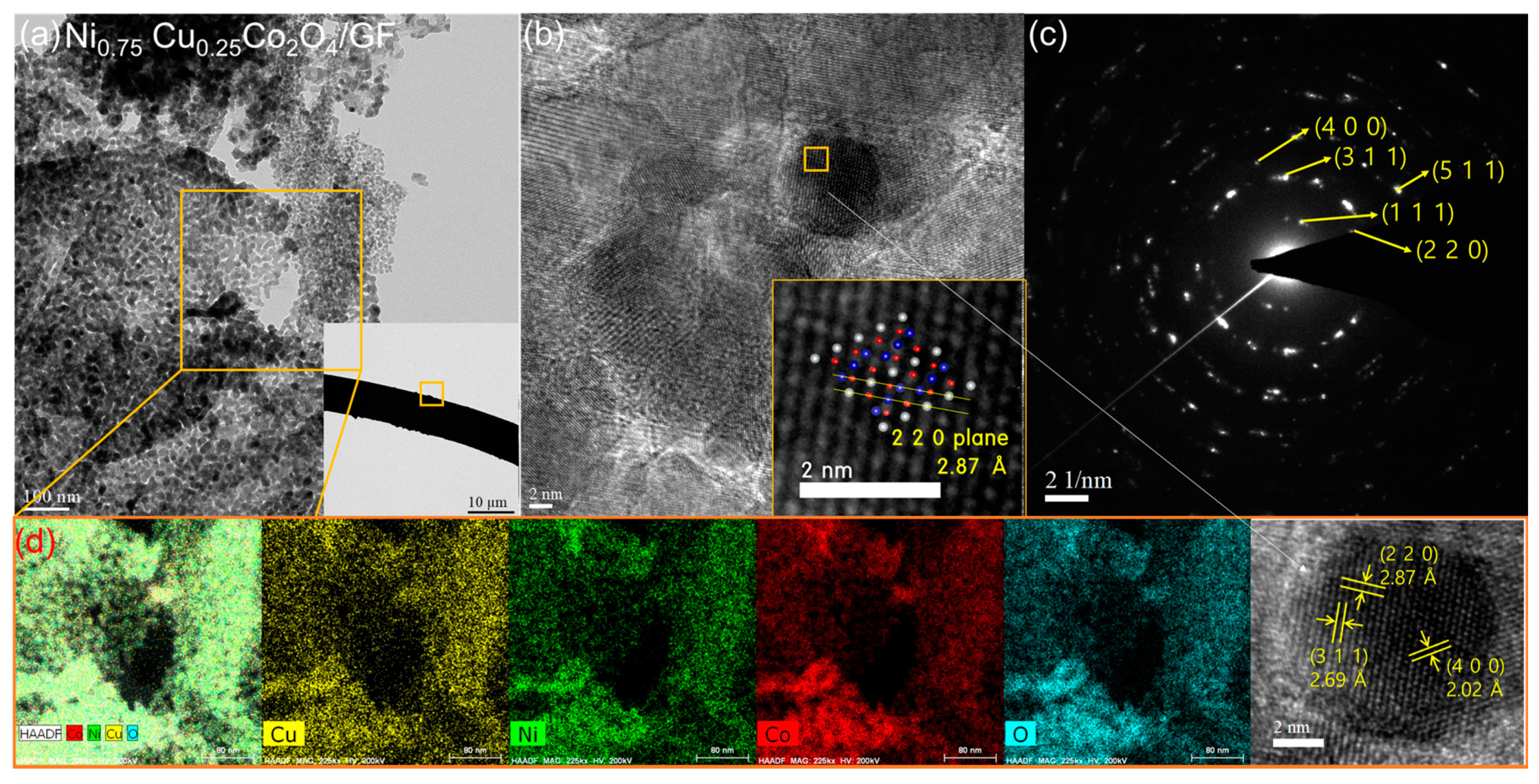

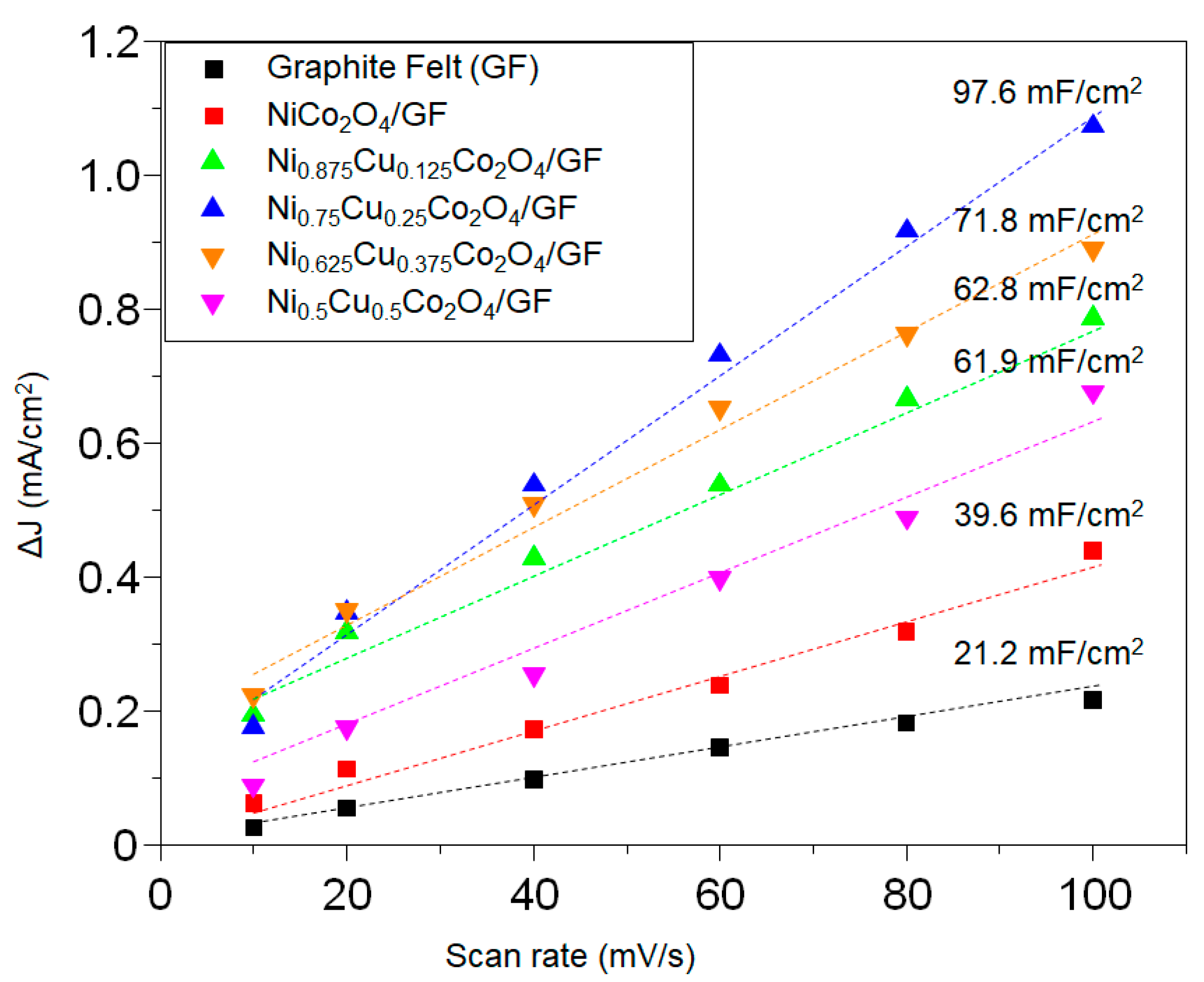
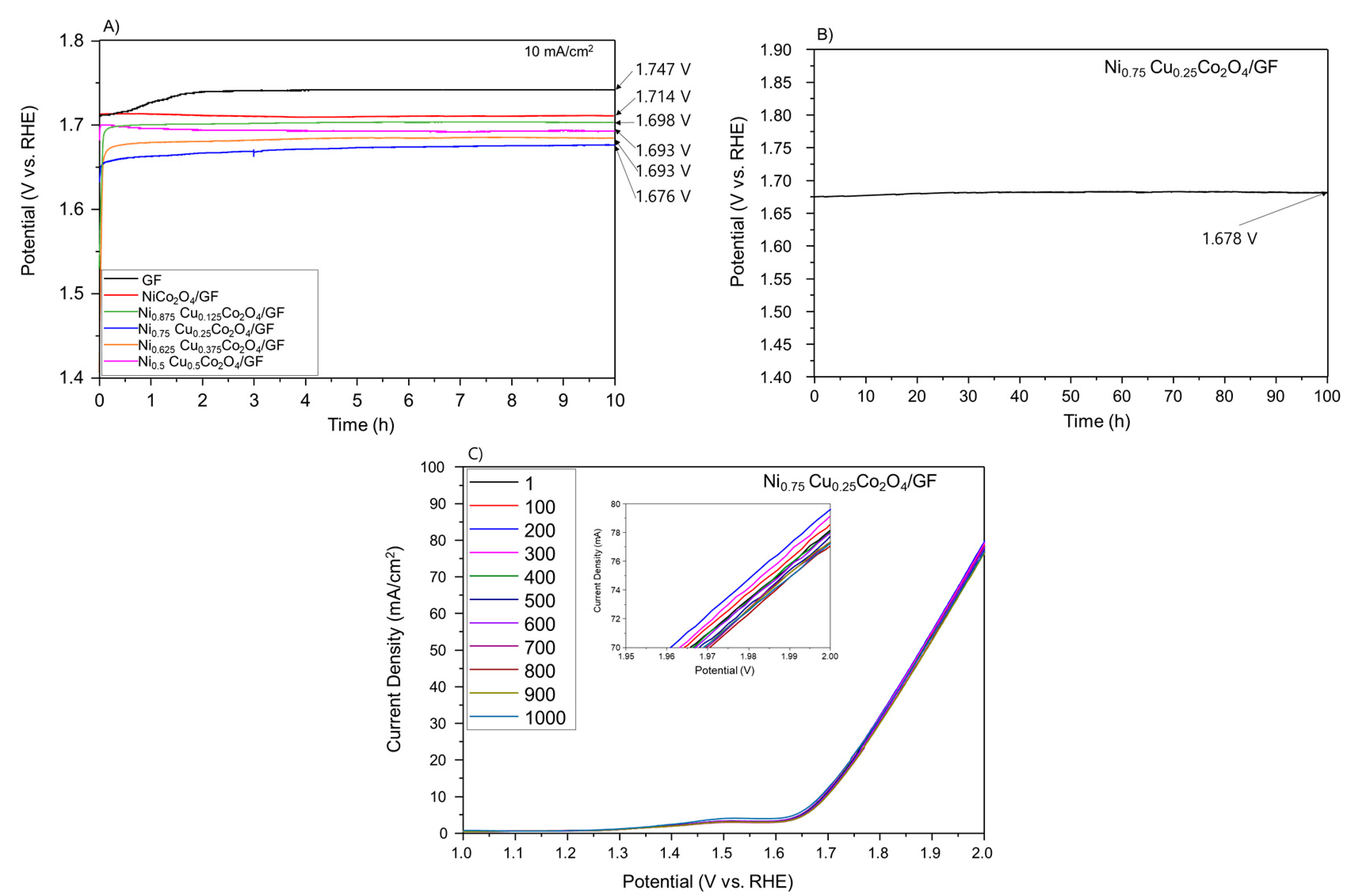
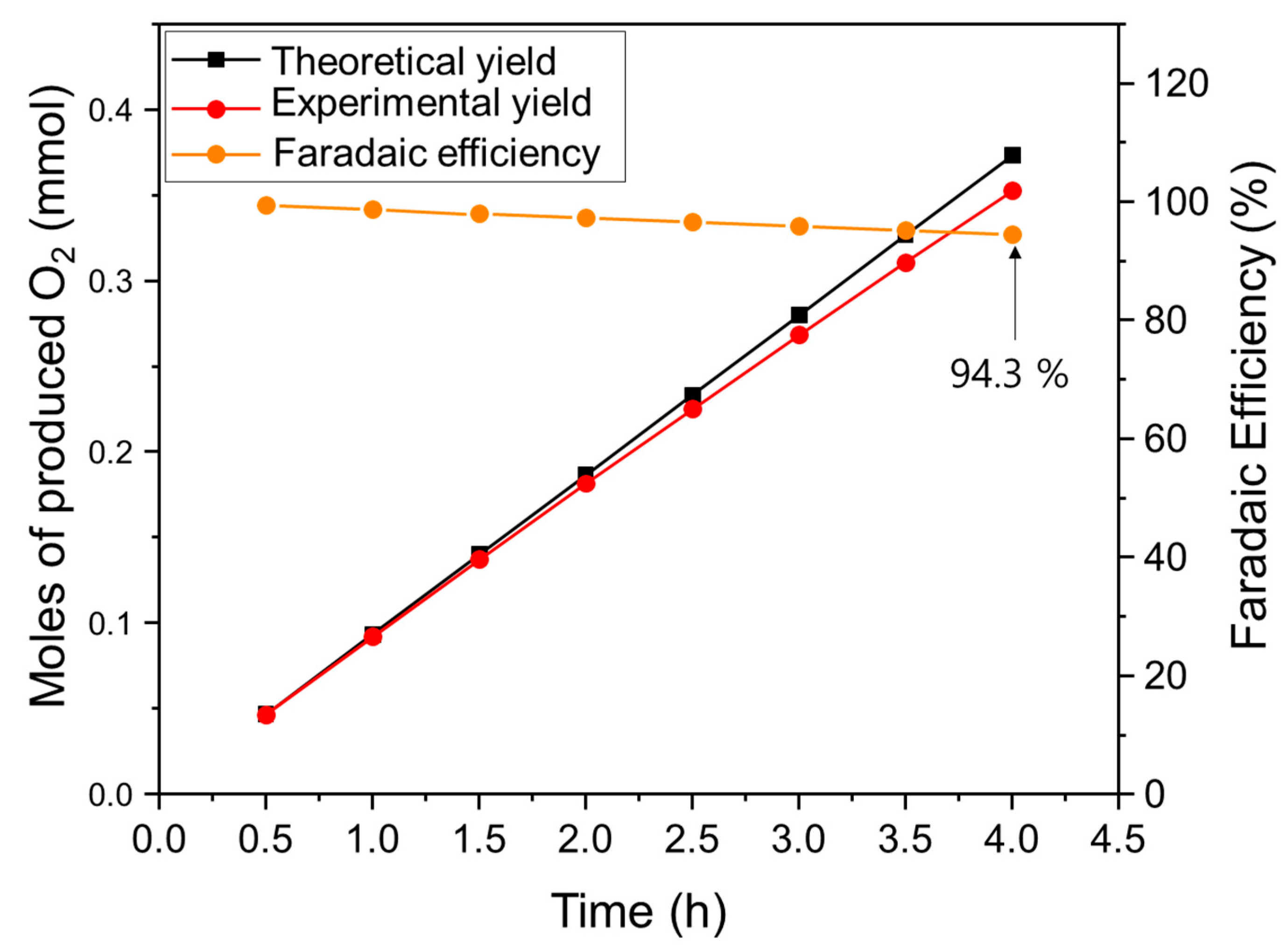
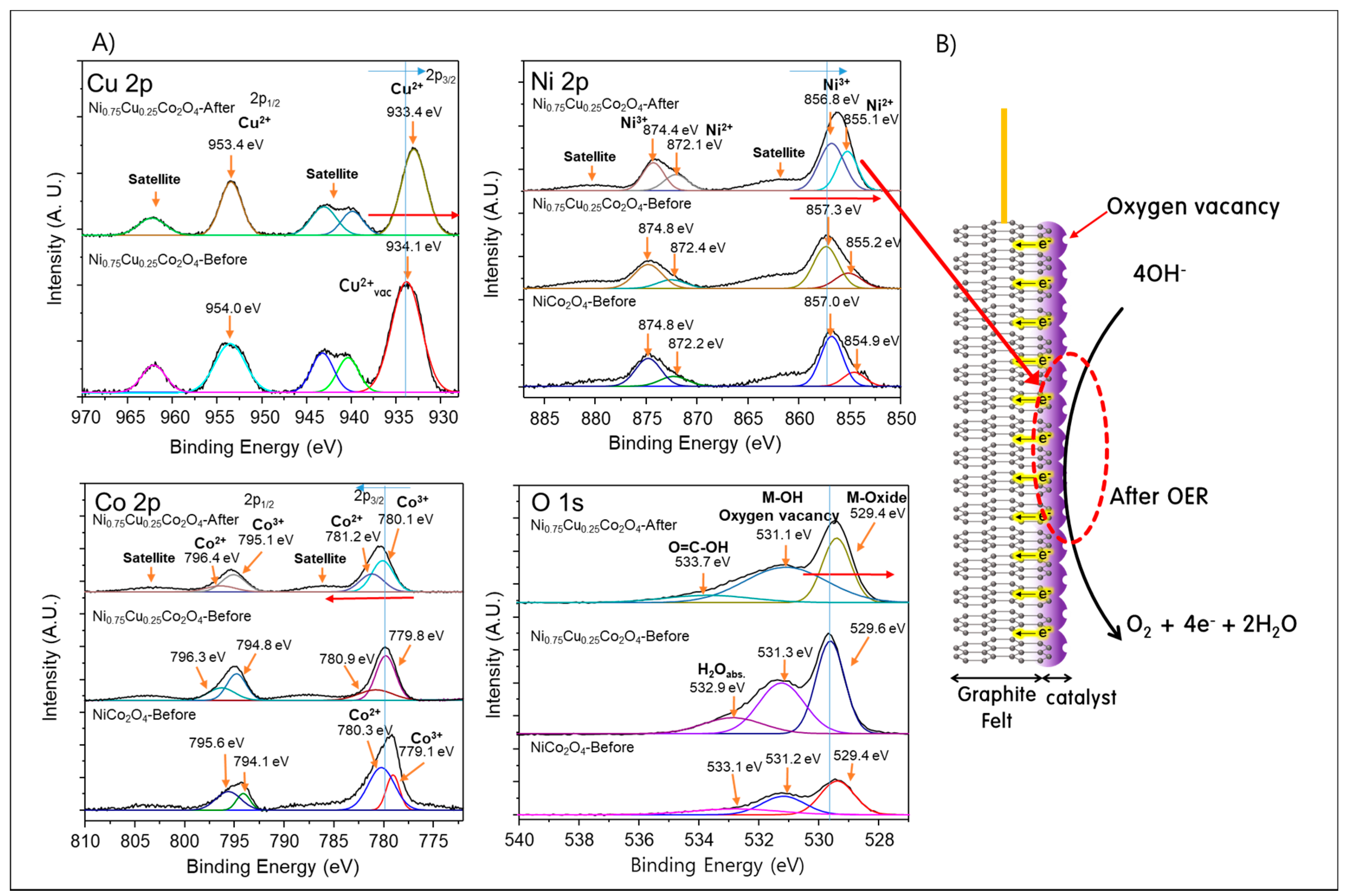
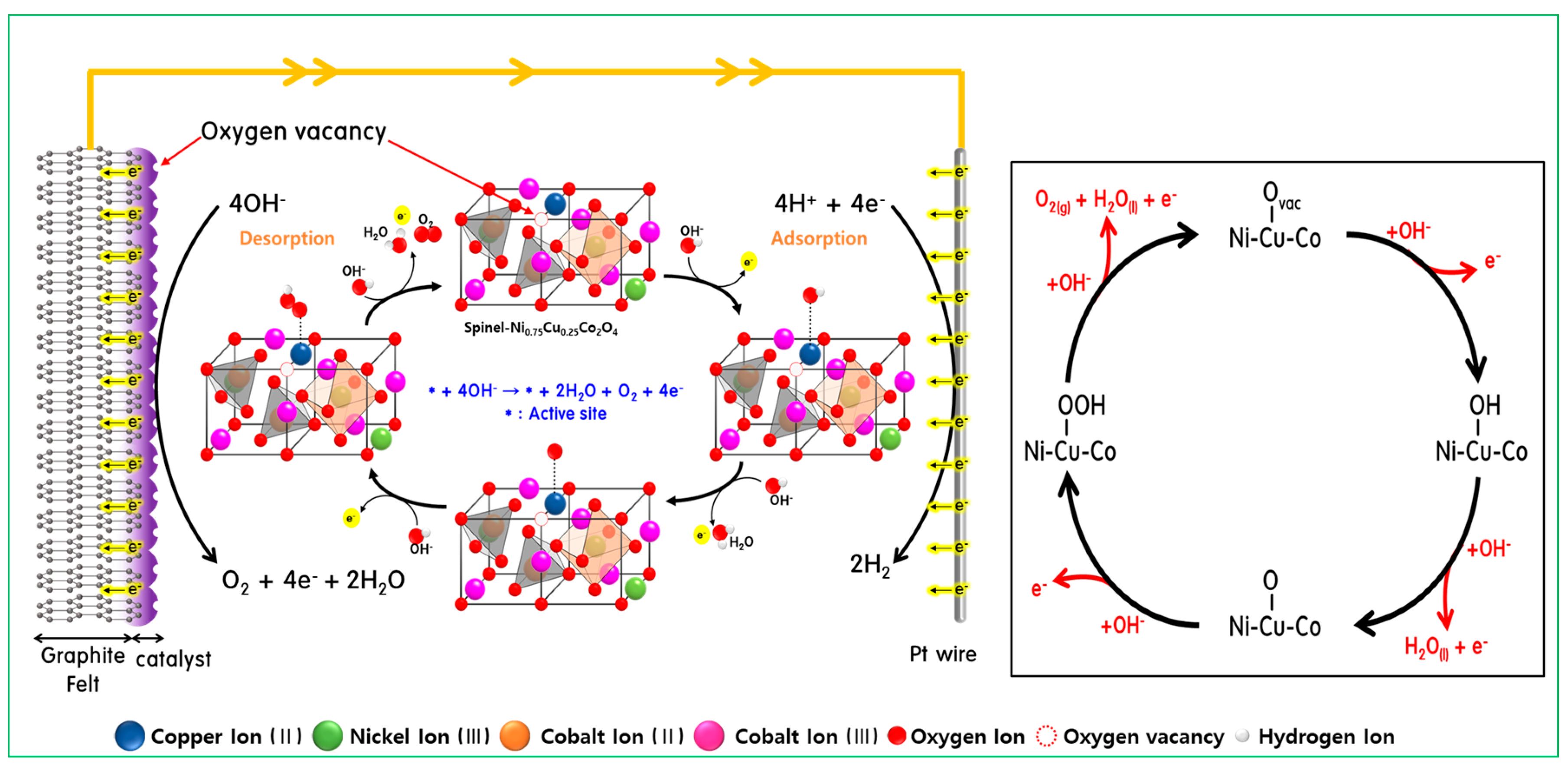
| Atomic (%) | Ni | Cu | Co | O | C | |
|---|---|---|---|---|---|---|
| Sample | ||||||
| Graphite Felt | - | - | - | 10.49 | 89.51 | |
| NiCo2O4/GF | 7.46 | - | 14.88 | 29.82 | 47.84 | |
| Ni0.875Cu0.125Co2O4/GF | 6.70 | 0.98 | 15.40 | 31.00 | 45.92 | |
| Ni0.75Cu0.25Co2O4/GF | 5.51 | 1.70 | 14.98 | 32.11 | 45.70 | |
| Ni0.625Cu0.375Co2O4/GF | 4.59 | 2.74 | 14.62 | 29.27 | 48.78 | |
| NiCuCo2O4/GF | 3.93 | 3.52 | 14.83 | 29.64 | 48.08 | |
| Sample | Support | Overpotential (j = 10 mA cm−2) | Tafel Slope (mV dec−1) | Ref. |
|---|---|---|---|---|
| Ni0.75Cu0.25Co2O4 | Graphite Felt | 509 mV | 119 | This work |
| Co-P | Graphite Felt | 530 mV (j = 5 mA cm−2) | 133 | [41] |
| CuS/CuFeS2 | Carbon Felt | 400 mV | 171 | [42] |
| NiCu(I) | Ni foam | 252 mV | 54 | [43] |
| NiFe2O4 | Ni plate | 520 mV | 223 | [44] |
| CuFe2O4 | Ni plate | 540 mV | 238 | [44] |
| Fe-Co(OH)2 | Glassy Carbon | 290 mV | 69 | [45] |
| NiCo2S4/RGO | Glassy Carbon | 366 mV | 65 | [46] |
| NiCo2O4/CoNx-NMC NMC (Nitrogen doped Mesoporous Carbon) | Glassy Carbon | 370 mV | 99 | [47] |
| (Co0.21Ni0.25Cu0.54)3Se2 | Glassy Carbon | 241 mV | 53 | [48] |
| FeCoNi alloy | N-doped graphene | 288 mV | 57 | [49] |
| RuO2 | Glassy Carbon | 300 mV | 54 | [49] |
| IrO2 | Glassy Carbon | 314 mV | - | [50] |
| Sample | Graphite Felt | NiCo2O4 | Ni0.875Cu0.125Co2O4 | Ni0.75Cu0.25Co2O4 | Ni0.625Cu0.375Co2O4 | Ni0.5Cu0.5Co2O4 | |
|---|---|---|---|---|---|---|---|
| Factor | |||||||
| RS (Ω) (Solution Resistance) | 2.960 | 2.768 | 2.627 | 2.654 | 2.613 | 3.012 | |
| Rct (Ω) (Charge Transfer Resistance) | 10.39 | 1.814 | 1.250 | 0.728 | 1.613 | 1.631 | |
| Total resistance (Ω) | 13.35 | 4.582 | 3.877 | 3.382 | 4.226 | 4.643 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Park, B.H.; Choi, J.; Kim, S.; Kim, T.; Youn, Y.-S.; Son, N.; Kim, J.H.; Kang, M. Enhanced Electrochemical Properties and OER Performances by Cu Substitution in NiCo2O4 Spinel Structure. Nanomaterials 2020, 10, 1727. https://doi.org/10.3390/nano10091727
Park H, Park BH, Choi J, Kim S, Kim T, Youn Y-S, Son N, Kim JH, Kang M. Enhanced Electrochemical Properties and OER Performances by Cu Substitution in NiCo2O4 Spinel Structure. Nanomaterials. 2020; 10(9):1727. https://doi.org/10.3390/nano10091727
Chicago/Turabian StylePark, Hyerim, Byung Hyun Park, Jaeyoung Choi, Seyeon Kim, Taesung Kim, Young-Sang Youn, Namgyu Son, Jae Hong Kim, and Misook Kang. 2020. "Enhanced Electrochemical Properties and OER Performances by Cu Substitution in NiCo2O4 Spinel Structure" Nanomaterials 10, no. 9: 1727. https://doi.org/10.3390/nano10091727
APA StylePark, H., Park, B. H., Choi, J., Kim, S., Kim, T., Youn, Y.-S., Son, N., Kim, J. H., & Kang, M. (2020). Enhanced Electrochemical Properties and OER Performances by Cu Substitution in NiCo2O4 Spinel Structure. Nanomaterials, 10(9), 1727. https://doi.org/10.3390/nano10091727