Oriented Carbon Nanostructures from Plasma Reformed Resorcinol-Formaldehyde Polymer Gels for Gas Sensor Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of RF Xerogel
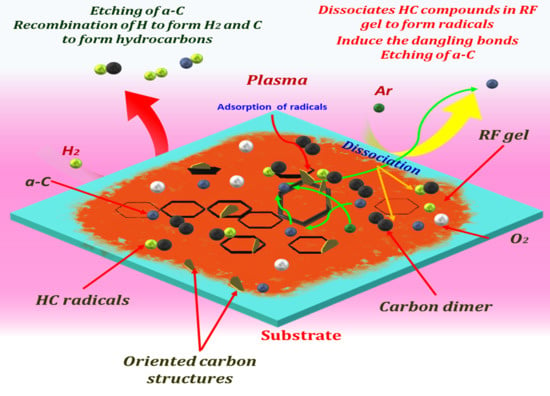
2.2. Plasma-Assisted Synthesis of OCNs
2.3. Gas Sensing Measurement
3. Characterization Techniques
4. Results and Discussions
5. Gas Sensor Properties
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santhosh, N.; Filipič, G.; Tatarova, E.; Baranov, O.; Kondo, H.; Sekine, M.; Hori, M.; Ostrikov, K.; Cvelbar, U. Oriented Carbon Nanostructures by Plasma Processing: Recent Advances and Future Challenges. Micromachines 2018, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Meyyappan, M. A review of plasma enhanced chemical vapor deposition of carbon nanotubes. J. Phys. D Appl. Phys. 2009, 42, 213001. [Google Scholar] [CrossRef]
- Wang, H.; Moore, J.J. Low temperature growth mechanisms of vertically aligned carbon nanofibers and nanotubes by radio frequency-plasma enhanced chemical vapor deposition. Carbon 2012. [Google Scholar] [CrossRef]
- Bo, Z.; Cui, S.; Yu, K.; Lu, G.; Mao, S.; Chen, J. Note: Continuous synthesis of uniform vertical graphene on cylindrical surfaces. Rev. Sci. Instrum. 2011, 82. [Google Scholar] [CrossRef]
- Maruyama, J.; Maruyama, S.; Fukuhara, T.; Chashiro, K.; Uyama, H. Ordered mesoporous structure by graphitized carbon nanowall assembly. Carbon 2018, 126, 452–455. [Google Scholar] [CrossRef]
- Wu, Y.; Qiao, P.; Chong, T.; Shen, Z. Carbon nanowalls grown by microwave plasma enhanced chemical vapor deposition. Adv. Mater. 2002, 14, 64–67. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, B.; Zong, B.; Sun, H.; Shen, Z.; Feng, Y. Carbon nanowalls and related materials. J. Mater. Chem. 2004, 14, 469. [Google Scholar] [CrossRef]
- Shiji, K.; Hiramatsu, M.; Enomoto, A.; Nakamura, M.; Amano, H.; Hori, M. Vertical growth of carbon nanowalls using rf plasma-enhanced chemical vapor deposition. Diam. Relat. Mater. 2005, 14, 831–834. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science (80-) 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005. [Google Scholar] [CrossRef]
- Melechko, A.V.; Merkulov, V.I.; McKnight, T.E.; Guillorn, M.A.; Klein, K.L.; Lowndes, D.H.; Simpson, M.L. Vertically aligned carbon nanofibers and related structures: Controlled synthesis and directed assembly. J. Appl. Phys. 2005. [Google Scholar] [CrossRef]
- Yap, H.Y.; Ramaker, B.; Sumant, A.V.; Carpick, R.W. Growth of mechanically fixed and isolated vertically aligned carbon nanotubes and nanofibers by DC plasma-enhanced hot filament chemical vapor deposition. Diam. Relat. Mater. 2006. [Google Scholar] [CrossRef]
- Wang, J.; Ito, T. CVD growth and field emission characteristics of nano-structured films composed of vertically standing and mutually intersecting nano-carbon sheets. Diam. Relat. Mater. 2007, 16, 589–593. [Google Scholar] [CrossRef]
- Hojati-Talemi, P.; Simon, G.P. Field emission study of graphene nanowalls prepared by microwave-plasma method. Carbon 2011, 49, 2875–2877. [Google Scholar] [CrossRef]
- Yu, K.; Bo, Z.; Lu, G.; Mao, S.; Cui, S.; Zhu, Y.; Chen, X.; Ruoff, R.S.; Chen, J. Growth of carbon nanowalls at atmospheric pressure for one-step gas sensor fabrication. Nanoscale Res. Lett. 2011, 6. [Google Scholar] [CrossRef]
- Krivchenko, V.A.; Itkis, D.M.; Evlashin, S.A.; Semenenko, D.A.; Goodilin, E.A.; Rakhimov, A.T.; Stepanov, A.S.; Suetin, N.V.; Pilevsky, A.A.; Voronin, P.V. Carbon nanowalls decorated with silicon for lithium-ion batteries. Carbon 2012, 50, 1438–1442. [Google Scholar] [CrossRef]
- Tanaike, O.; Kitada, N.; Yoshimura, H.; Hatori, H.; Kojima, K.; Tachibana, M. Lithium insertion behavior of carbon nanowalls by dc plasma CVD and its heat-treatment effect. Solid State Ionics 2009, 180, 381–385. [Google Scholar] [CrossRef]
- Wu, J.; Shao, Y.; Wang, B.; Ostrikov, K.K.; Feng, J.; Cheng, Q. Plasma-Produced Vertical Carbonous Nanoflakes for Li-Ion Batteries. Plasma Process. Polym. 2016. [Google Scholar] [CrossRef]
- Machino, T.; Takeuchi, W.; Kano, H.; Hiramatsu, M.; Hori, M. Synthesis of platinum nanoparticles on two-dimensional carbon nanostructures with an ultrahigh aspect ratio employing supercritical fluid chemical vapor deposition process. Appl. Phys. Express 2009, 2. [Google Scholar] [CrossRef]
- Giorgi, L.; Makris, T.D.; Giorgi, R.; Lisi, N.; Salernitano, E. Electrochemical properties of carbon nanowalls synthesized by HF-CVD. Sens. Actuators B Chem. 2007, 126, 144–152. [Google Scholar] [CrossRef]
- Segundo, E.H.; Fontana, L.C.; Recco, A.A.C.; Scholtz, J.S.; Nespolo Vomstein, M.A.; Becker, D. Graphene nanosheets obtained through graphite powder exfoliation in pulsed underwater electrical discharge. Mater. Chem. Phys. 2018, 217, 1–4. [Google Scholar] [CrossRef]
- Bo, Z.; Yang, Y.; Chen, J.; Yu, K.; Yan, J.; Cen, K. Plasma-enhanced chemical vapor deposition synthesis of vertically oriented graphene nanosheets. Nanoscale 2013, 5, 5180. [Google Scholar] [CrossRef]
- Levchenko, I.; Ostrikov, K.K.; Zheng, J.; Li, X.; Keidar, M.; Teo, K.B.K. Scalable graphene production: Perspectives and challenges of plasma applications. Nanoscale 2016, 8, 10511–10527. [Google Scholar] [CrossRef] [PubMed]
- Chuang, A.T.H.; Robertson, J.; Boskovic, B.O.; Koziol, K.K.K. Three-dimensional carbon nanowall structures. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef]
- Van Nang, L.; Kim, E.-T. Controllable Synthesis of High-Quality Graphene Using Inductively-Coupled Plasma Chemical Vapor Deposition. J. Electrochem. Soc. 2012, 159, K93. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhu, M.Y.; Outlaw, R.A.; Zhao, X.; Manos, D.M.; Holloway, B.C.; Mammana, V.P. Free-standing subnanometer graphite sheets. Appl. Phys. Lett. 2004, 85, 1265–1267. [Google Scholar] [CrossRef]
- Hussain, S.; Kovacevic, E.; Berndt, J.; Marath Santhosh, N.; Pattyn, C.; Dias, A.I.V.A.; Strunskus, T.; Ammar, M.-R.; Jagodar, A.; Gaillard, M.; et al. Low-temperature low power PECVD synthesis of vertically aligned graphene. Nanotechnology 2020, in press. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Shiji, K.; Amano, H.; Hori, M. Fabrication of vertically aligned carbon nanowalls using capacitively coupled plasma-enhanced chemical vapor deposition assisted by hydrogen radical injection. Appl. Phys. Lett. 2004, 84, 4708–4710. [Google Scholar] [CrossRef]
- Kondo, S.; Hori, M.; Yamakawa, K.; Den, S.; Kano, H.; Hiramatsu, M.; Yamakawa, M.H.; Hiramatsu, S.D.K.; Hori, M.; Yamakawa, K.; et al. Highly reliable growth process of carbon nanowalls using radical injection plasma-enhanced chemical vapor deposition. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2008, 26, 1294–1300. [Google Scholar] [CrossRef]
- Kumar, S.; Martin, P.; Bendavid, A.; Bell, J.; Ostrikov, K. Oriented graphenes from plasma-reformed coconut oil for supercapacitor electrodes. Nanomaterials 2019, 9, 1679. [Google Scholar] [CrossRef]
- Seo, D.H.; Rider, A.E.; Kumar, S.; Randeniya, L.K.; Ostrikov, K. Vertical graphene gas- and bio-sensors via catalyst-free, reactive plasma reforming of natural honey. Carbon 2013, 60, 221–228. [Google Scholar] [CrossRef]
- Seo, D.H.; Han, Z.J.; Kumar, S.; Ostrikov, K. Structure-controlled, vertical graphene-based, binder-free electrodes from plasma-reformed butter enhance supercapacitor performance. Adv. Energy Mater. 2013. [Google Scholar] [CrossRef]
- Seo, D.H.; Pineda, S.; Yick, S.; Bell, J.; Han, Z.J.; Ostrikov, K. Plasma-enabled sustainable elemental lifecycles: Honeycomb-derived graphenes for next-generation biosensors and supercapacitors. Green Chem. 2015, 17, 2164–2171. [Google Scholar] [CrossRef]
- Arif, A.F.; Balgis, R.; Ogi, T.; Mori, T.; Okuyama, K. Experimental and theoretical approach to evaluation of nanostructured carbon particles derived from phenolic resin via spray pyrolysis. Chem. Eng. J. 2015. [Google Scholar] [CrossRef]
- Yamazaki, M.; Kayama, M.; Ikeda, K.; Alii, T.; Ichihara, S. Nanostructured carbonaceous material with continuous pores obtained from reaction-induced phase separation of miscible polymer blends. Carbon 2004. [Google Scholar] [CrossRef]
- Pekala, R.W. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mat. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Liang, C.; Sha, G.; Guo, S. Resorcinol-formaldehyde aerogels prepared by supercritical acetone drying. J. Non-Cryst. Solids 2000. [Google Scholar] [CrossRef]
- Maldonado-Hódar, F.J.; Moreno-Castilla, C.; Rivera-Utrilla, J.; Hanzawa, Y.; Yamada, Y. Catalytic graphitization of carbon aerogels by transition metals. Langmuir 2000. [Google Scholar] [CrossRef]
- Oya, A.; Otani, S. Catalytic graphitization of carbons by various metals. Carbon 1979. [Google Scholar] [CrossRef]
- Kakunuri, M.; Kali, S.; Sharma, C.S. Catalytic graphitization of resorcinol-formaldehyde xerogel and its effect on lithium ion intercalation. J. Anal. Appl. Pyrolysis 2016. [Google Scholar] [CrossRef]
- Gaikwad, M.M.; Kakunuri, M.; Sharma, C.S. Enhanced catalytic graphitization of resorcinol formaldehyde derived carbon xerogel to improve its anodic performance for lithium ion battery. Mater. Today Commun. 2019. [Google Scholar] [CrossRef]
- Schulz, M.J.; Shanov, V.; Yin, Z.; Cahay, M. Nanotube Superfiber Materials: Science, Manufacturing, Commercialization; William Andrew: Norwich, NY, USA, 2019; ISBN 9780128126677. [Google Scholar]
- Santhosh, N.M.; Vasudevan, A.; Jurov, A.; Korent, A.; Slobodian, P.; Zavašnik, J.; Cvelbar, U. Improving sensing properties of entangled carbon nanotube-based gas sensors by atmospheric plasma surface treatment. Microelectron. Eng. 2020, 111403. [Google Scholar] [CrossRef]
- Lee, C.; Choi, R.; Kwak, E.; Lee, H.; Lee, I.; Seo, J.; Kim, H.; Kim, Y. Resorcinol-functionalized carbon nanoparticles with a stick-out nanostructure for stable hydrogen bonding with polyester microfibers. RSC Adv. 2013. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Maslova, O.A.; Ammar, M.R.; Guimbretière, G.; Rouzaud, J.N.; Simon, P. Determination of crystallite size in polished graphitized carbon by Raman spectroscopy. Phys. Rev. B Condens. Matter Mater. Phys. 2012. [Google Scholar] [CrossRef]
- Ammar, M.R.; Charon, E.; Rouzaud, J.N.; Aleon, J.; Guimbretière, G.; Simon, P. On a reliable structural characterization of polished carbons in meteorites by Raman microspectroscopy. Spectrosc. Lett. 2011, 44, 535–538. [Google Scholar] [CrossRef]
- Pol, V.G.; Shrestha, L.K.; Ariga, K. Tunable, functional carbon spheres derived from rapid synthesis of resorcinol-formaldehyde resins. ACS Appl. Mater. Interfaces 2014. [Google Scholar] [CrossRef]
- Li, M.; Chang, X.; Han, X.; Yin, W.; Ren, M. Resorcinol-formaldehyde resin based porous carbon materials with yolk-shell structure for high-performance supercapacitors. Synth. Met. 2016. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Yang, L.; Liu, Y.; Yao, J. Hollow melamine resin-based carbon spheres/graphene composite with excellent performance for supercapacitors. Electrochim. Acta 2015, 166, 310–319. [Google Scholar] [CrossRef]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A. Reduced graphene oxide-TiO2 nanocomposite as a promising visible-light-active photocatalyst for the conversion of carbon dioxide. Nanoscale Res. Lett. 2013, 8, 465. [Google Scholar] [CrossRef]
- Santhosh, N.M.; Filipič, G.; Kovacevic, E.; Jagodar, A.; Berndt, J.; Strunskus, T.; Kondo, H.; Hori, M.; Tatarova, E.; Cvelbar, U. N-Graphene Nanowalls via Plasma Nitrogen Incorporation and Substitution: The Experimental Evidence. Nano-Micro Lett. 2020, 12, 53. [Google Scholar] [CrossRef]
- Ostrikov, K. Reactive plasmas as a versatile nanofabrication tool. Rev. Mod. Phys. 2005. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Hori, M. Carbon Nanowalls: Synthesis and Emerging Applications; Springer: Vienna, Austria, 2010; ISBN 978-3-211-99717-8. [Google Scholar]
- Nageswaran, G.; Jothi, L.; Jagannathan, S. Plasma Assisted Polymer Modifications. In Non-Thermal Plasma Technology for Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ostrikov, K.; Neyts, E.C.; Meyyappan, M. Plasma nanoscience: From nano-solids in plasmas to nano-plasmas in solids. Adv. Phys. 2013, 62, 113–224. [Google Scholar] [CrossRef]
- Luo, Z.; Lim, S.; You, Y.; Miao, J.; Gong, H.; Zhang, J.; Wang, S.; Lin, J.; Shen, Z. Effect of ion bombardment on the synthesis of vertically aligned single-walled carbon nanotubes by plasma-enhanced chemical vapor deposition. Nanotechnology 2008. [Google Scholar] [CrossRef]
- Anoshkin, I.V.; Nasibulin, A.G.; Mudimela, P.R.; He, M.; Ermolov, V.; Kauppinen, E.I. Single-walled carbon nanotube networks for ethanol vapor sensing applications. Nano Res. 2013. [Google Scholar] [CrossRef]
- Zettl, A. Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science (80-) 2000. [Google Scholar] [CrossRef]
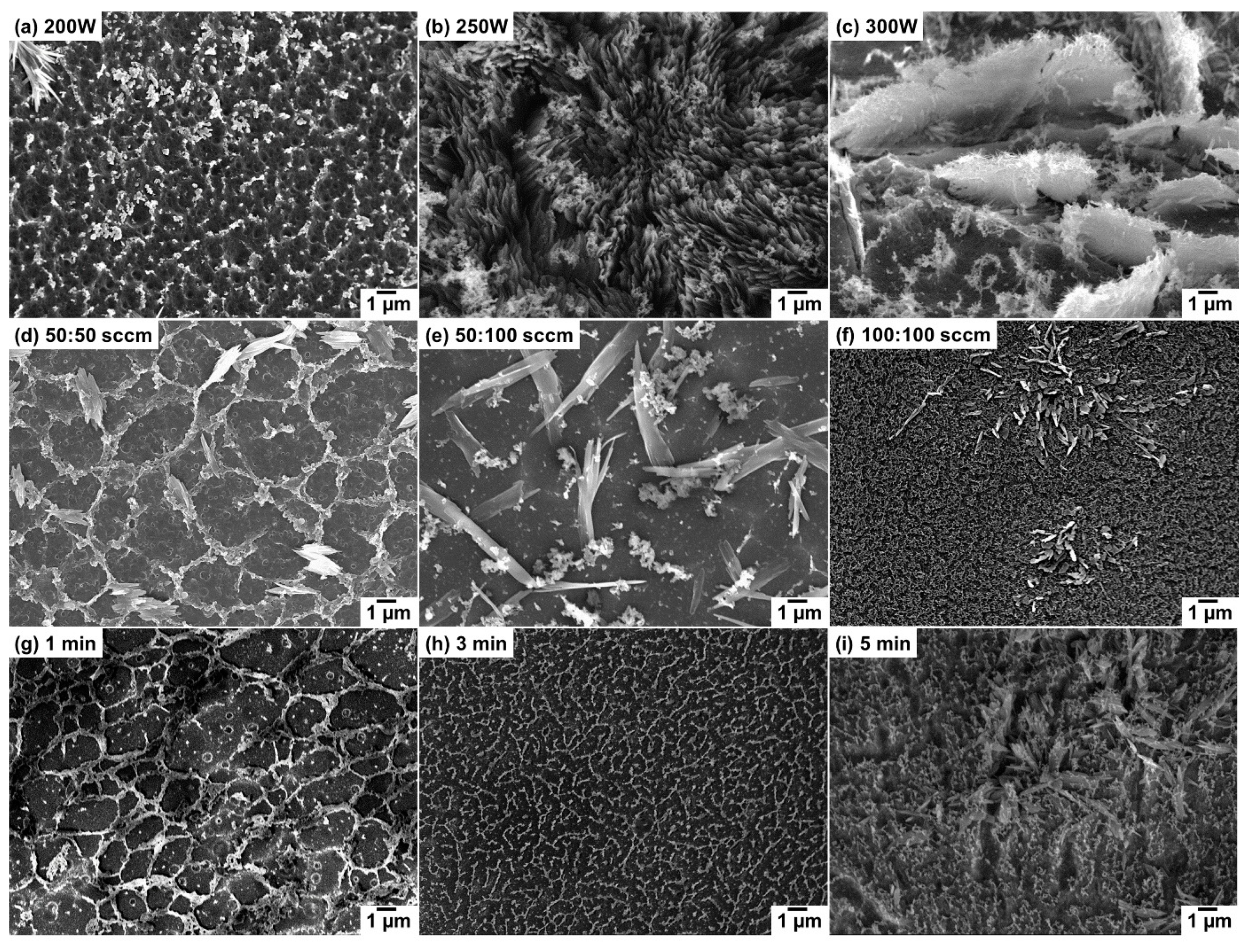

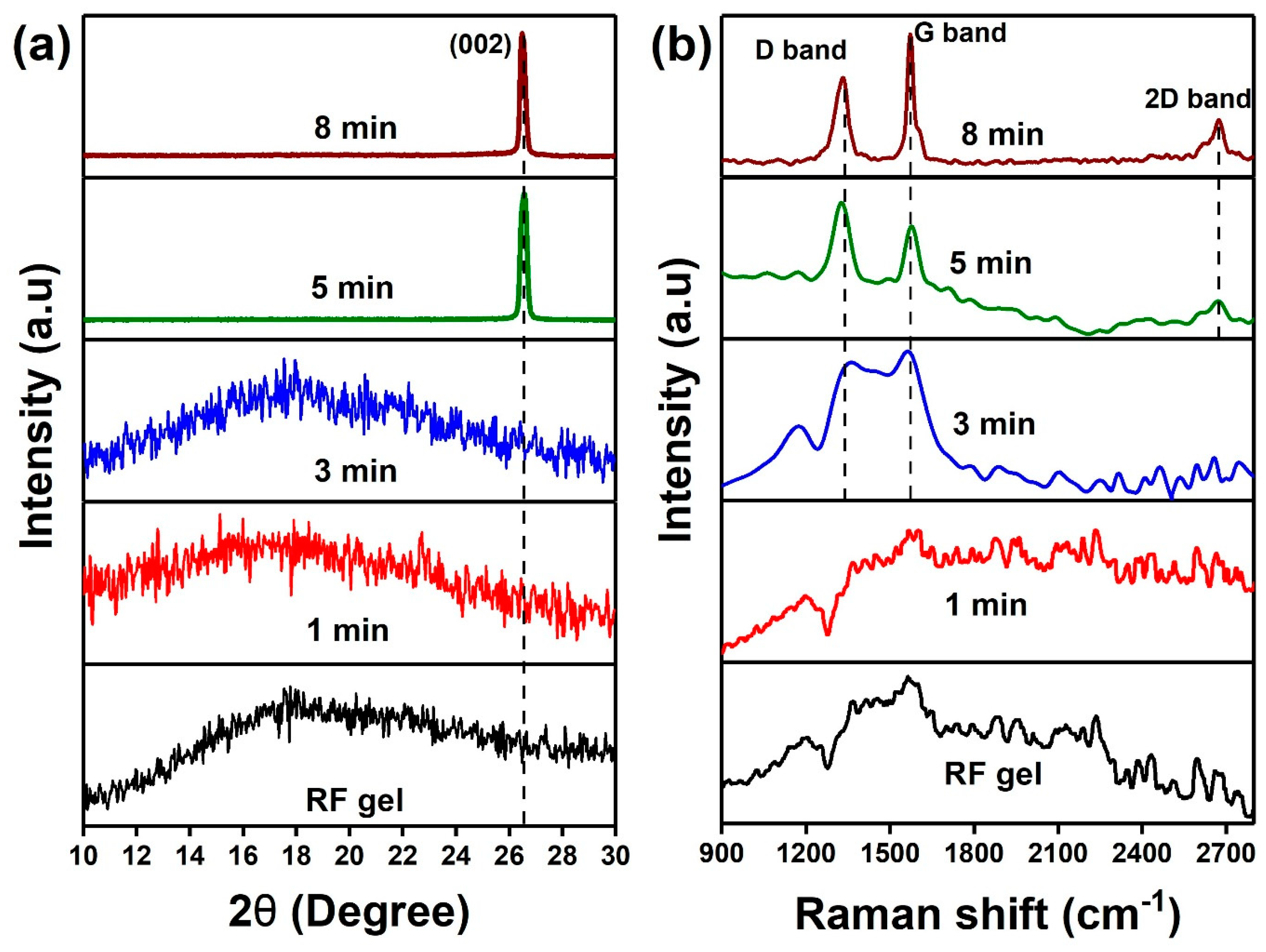
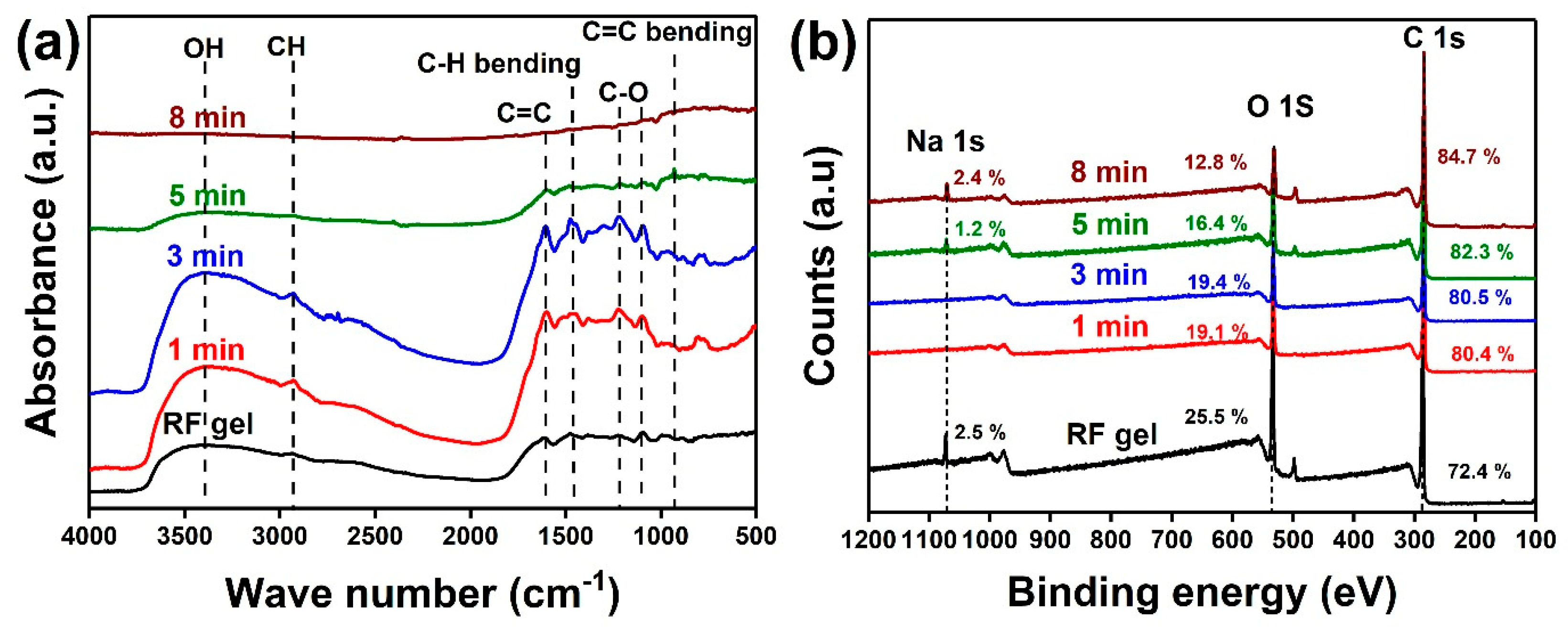
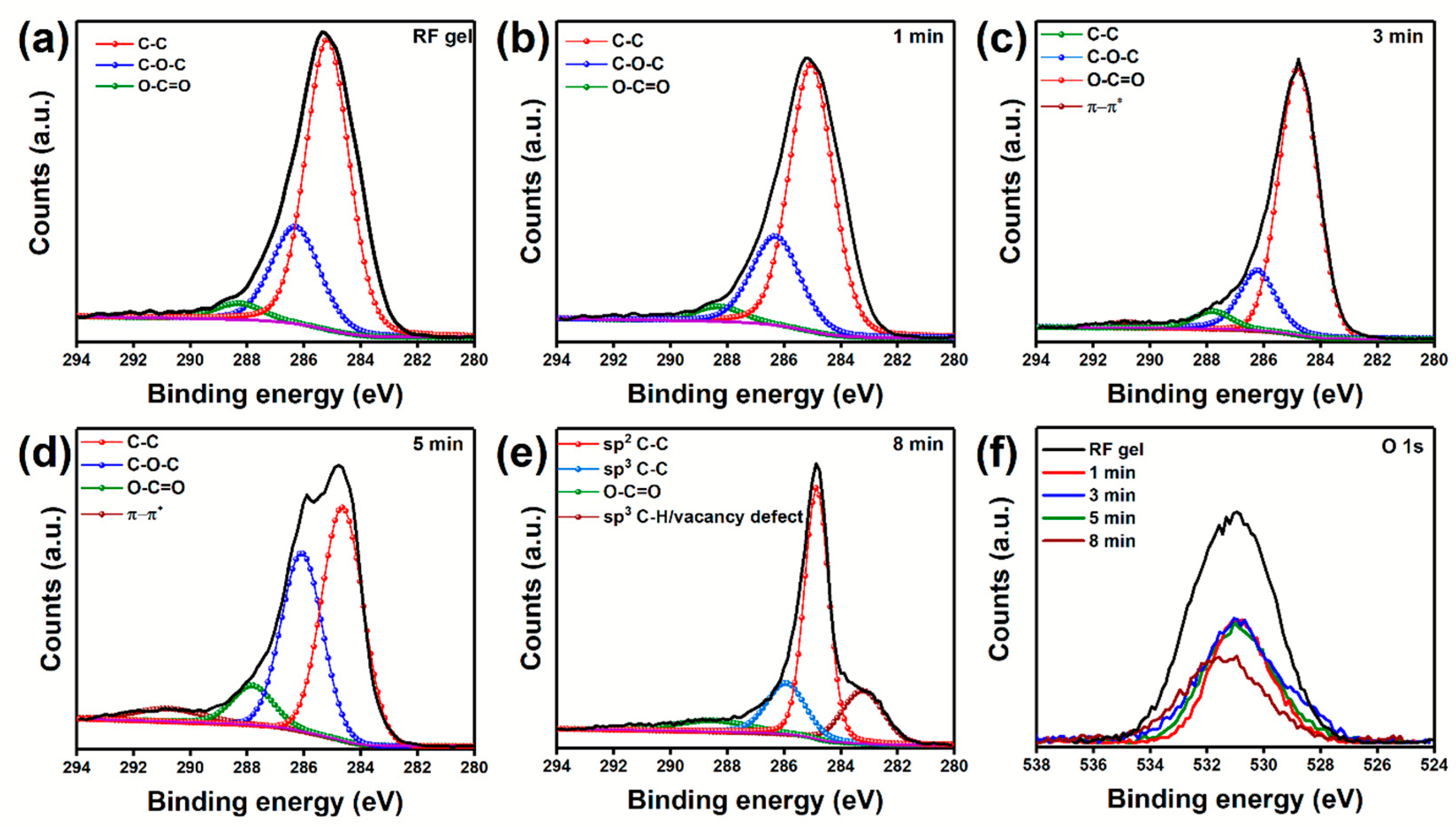
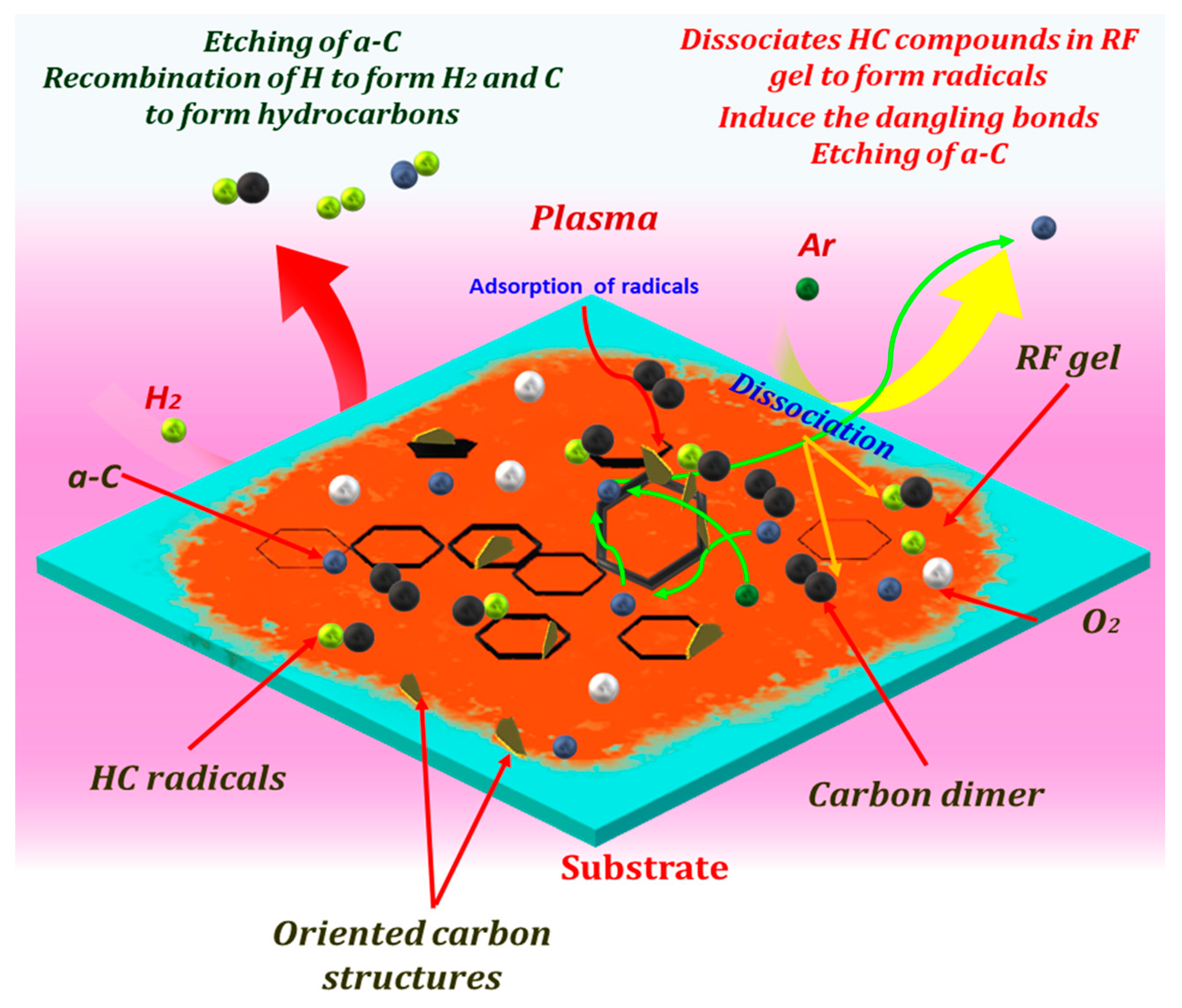

| Sample | Plasma Power (W) | Flow Rate Ar:H2 (sccm) | Growth Time (min) |
|---|---|---|---|
| 1 | 200 | 100:50 | 8 |
| 2 | 250 | 100:50 | 8 |
| 3 | 300 | 100:50 | 8 |
| 4 | 250 | 50:50 | 8 |
| 5 | 250 | 50:100 | 8 |
| 6 | 250 | 100:100 | 8 |
| 7 | 250 | 100:50 | 1 |
| 8 | 250 | 100:50 | 3 |
| 9 | 250 | 100:50 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santhosh, N.M.; Vasudevan, A.; Jurov, A.; Filipič, G.; Zavašnik, J.; Cvelbar, U. Oriented Carbon Nanostructures from Plasma Reformed Resorcinol-Formaldehyde Polymer Gels for Gas Sensor Applications. Nanomaterials 2020, 10, 1704. https://doi.org/10.3390/nano10091704
Santhosh NM, Vasudevan A, Jurov A, Filipič G, Zavašnik J, Cvelbar U. Oriented Carbon Nanostructures from Plasma Reformed Resorcinol-Formaldehyde Polymer Gels for Gas Sensor Applications. Nanomaterials. 2020; 10(9):1704. https://doi.org/10.3390/nano10091704
Chicago/Turabian StyleSanthosh, Neelakandan M., Aswathy Vasudevan, Andrea Jurov, Gregor Filipič, Janez Zavašnik, and Uroš Cvelbar. 2020. "Oriented Carbon Nanostructures from Plasma Reformed Resorcinol-Formaldehyde Polymer Gels for Gas Sensor Applications" Nanomaterials 10, no. 9: 1704. https://doi.org/10.3390/nano10091704
APA StyleSanthosh, N. M., Vasudevan, A., Jurov, A., Filipič, G., Zavašnik, J., & Cvelbar, U. (2020). Oriented Carbon Nanostructures from Plasma Reformed Resorcinol-Formaldehyde Polymer Gels for Gas Sensor Applications. Nanomaterials, 10(9), 1704. https://doi.org/10.3390/nano10091704