Recent Advances in Aflatoxins Detection Based on Nanomaterials
Abstract
1. Introduction
2. Metal Nanomaterials for Aflatoxins Detection
3. Metal Oxides and Hydroxides Nanomaterials for Aflatoxins Detection
4. Carbon Nanomaterials for Aflatoxins Detection
4.1. Graphene Nanomaterials
4.2. Carbon Nanotube Nanomaterials
4.3. Other Carbon Nanomaterials
5. Other Nanomaterials for Aflatoxins Detection
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 94, v-412. [Google Scholar]
- Carlson, M.A.; Bargeron, C.B.; Benson, R.C.; Fraser, A.B.; Phillips, T.E.; Velky, J.T.; Groopman, J.D.; Strickland, P.T.; Ko, H.W. An automated, handheldbiosensor for aflatoxin. Biosens. Bioelectron. 2000, 14, 841–848. [Google Scholar] [CrossRef]
- Jaimez, J.; Fente, C.A.; Vazquez, B.I.; Franco, C.M.; Cepeda, A.; Mahuzier, G.; Prognon, P. Application of the assay of aflatoxins by liquid chromatography with fluorescence detection in food analysis. J. Chromatogr. A 2000, 882, 1–10. [Google Scholar] [CrossRef]
- Shyu, R.H.; Shyu, H.F.; Liu, H.W.; Tang, S.S. Colloidal gold-based immunochromatographic assay for detection of ricin. Toxicon 2002, 40, 255–258. [Google Scholar] [CrossRef]
- Egner, P.A.; Wang, J.B.; Zhu, Y.R.; Zhang, B.C.; Wu, Y.; Zhang, Q.N.; Qian, G.S.; Kuang, S.Y.; Gange, S.J.; Jacobson, L.P. Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 14601–14606. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, Y.; Shao, S.; Cai, Z.; Feng, L.; Pan, H.; Wang, Z. Simultaneous determination of multi-component mycotoxin contaminants in foods and feeds by ultra-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2007, 1143, 48–64. [Google Scholar] [CrossRef]
- Papp, E.; Klara, H.; Záray, G.; Mincsovics, E. Liquid chromatographic determination of aflatoxins. Microchem. J. 2002, 73, 39–46. [Google Scholar] [CrossRef]
- Caceres, I.; Khoury, A.E.; Khoury, R.E.; Lorber, S.; Bailly, J.D. Aflatoxin Biosynthesis and Genetic Regulation: A Review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, 364. [Google Scholar]
- Cunniff, P. Official Methods of Analysis of AOAC International, Method 923.03. Trends Food Sci. Technol. 1995, 6, 382. [Google Scholar]
- Huguette, C.; Michel, L.; Jean, M.F. Determination of Aflatoxin M1 in Milk by Liquid Chromatography with Fluorescence Detection. J. Assoc. Off. Anal. Chem. 2020, 67, 49–51. [Google Scholar]
- Wu, W.; Yu, C.D.; Wang, Q.; Zhao, F.Y.; He, H.; Liu, C.Z.; Yang, Q.L. Research advances of DNA aptasensors for foodborne pathogen detection. Crit. Rev. Food Sci. Nutr. 2019, 60, 2353–2368. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, N.; Wang, H.; Zhao, Q. An immunoassay for ochratoxin A using tetramethylrhodamine-labeled ochratoxin A as a probe based on a binding-induced change in fluorescence intensity. Analyst 2020, 145, 651–655. [Google Scholar] [CrossRef]
- Wu, W.; Yu, C.D.; Chen, J.H.; Yang, Q.L. Fluorometric detection of copper ions using click chemistry and the target-induced conjunction of split DNAzyme fragments. Int. J. Environ. Anal. Chem. 2019, 100, 324–332. [Google Scholar] [CrossRef]
- Alfaro, C.V.; Broto-Puig, F.; Agut, M.; Cornelias, L. Study of the production of aflatoxins B1, G1, B2 and G2 on cashew nuts by Aspergillus parasiticus CECT2681 by means of ultra-performance liquid chromatography. Afinidad Barc. 2013, 70, 170–174. [Google Scholar]
- Lv, J.; Yang, Y. Determination of aflatoxin b1 and b2 in peanut and peanut oil using cloud point extraction followed by ultra-high-performance liquid chromatography. J. Liquid Chromatogr. Relat. Technol. 2013, 36, 1421–1436. [Google Scholar] [CrossRef]
- Yibadatihan, S.; Jinap, S.; Mahyudin, N.A. Simultaneous determination of multi-mycotoxins in palm kernel cake (PKC) using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Food Addit. Contam. Part A 2014, 31, 2071–2079. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, B.H.; Hsu, Y.T.; Yu, F.Y. Sensitive competitive direct enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip for detecting aflatoxin M1 in milk. Food Control 2011, 22, 964–969. [Google Scholar] [CrossRef]
- Nardo, F.D.; Cavalera, S.; Baggiani, C.; Chiarello, M.; Pazzi, M.; Anfossi, L. Enzyme Immunoassay for Measuring Aflatoxin B1 in Legal Cannabis. Toxins 2020, 12, 265. [Google Scholar] [CrossRef]
- Di Nardo, F.; Alladio, E.; Baggiani, C.; Cavalera, S.; Giovannoli, C.; Spano, G.; Anfossi, L. Colour-encoded lateral flow immunoassay for the simultaneous detection of aflatoxin B1 and type-B fumonisins in a single Test line. Talanta 2019, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.F.; Xu, H.R. Application of multiplexing fiber optic laser induced fluorescence spectroscopy for detection of aflatoxin B1 contaminated pistachio kernels. Food Chem. 2019, 290, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Tang, X.; Feng, L.; Yao, G.-L.; Chen, J. Development of quantitative magnetic beads-based flow cytometry fluorescence immunoassay for aflatoxin B1. Microchem. J. 2020, 155, 104715. [Google Scholar] [CrossRef]
- Vdovenko, M.M.; Lu, C.C.; Yu, F.Y.; Sakharov, I.Y. Development of ultrasensitive direct chemiluminescent enzyme immunoassay for determination of aflatoxin M1 in milk. Food Chem. 2014, 158, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, X.; Chen, L.J.; Qian, H.L.; Yan, X.P. p-Bromophenol Enhanced Bienzymatic Chemiluminescence Competitive Immunoassay for Ultrasensitive Determination of Aflatoxin B1. Anal. Chem. 2019, 91, 13191–13197. [Google Scholar] [CrossRef] [PubMed]
- Adányi, N.; Levkovets, I.A.; Rodriguez-Gil, S.; Ronald, A.; Váradi, M.; Szendr, I. Development of immunosensor based on OWLS technique for determining Aflatoxin B1 and Ochratoxin A. Biosens. Bioelectron. 2007, 22, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, Z.B.; Grauby-Heywang, C.; Beven, L.; Cassagnere, S.; Bouhacina, T.C. Development of an ultrasensitive label-free immunosensor for fungal aflatoxin B1 detection. Biochem. Eng. J. 2019, 150, 107262. [Google Scholar] [CrossRef]
- Qian, J.S.; Lau, S.P.; Yuan, J.K. Aqueous Manganese Dioxide Ink for High Performance Capacitive Energy Storage Devices. Mrs Adv. 2016, 1, 3573–3578. [Google Scholar] [CrossRef]
- Shaabani, A.; Hezarkhani, Z.; Badali, E. Wool supported manganese dioxide nano-scale dispersion: A biopolymer based catalyst for the aerobic oxidation of organic compounds. RSC Adv. 2015, 5, 61759–61767. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, R.; Sinha, M.; Kumar, R.; Bhalla, V. MoS2based digital response platform for aptamer based fluorescent detection of pathogens. Microchim. Acta 2016, 183, 1501–1506. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Shen, Q.H.; Yu, H.S.; Han, D.D.; Zhong, F.L.; Yang, Y.J. Non-enzymatic sensing of hydrogen peroxide using a glassy carbon electrode modified with the layered MoS2-reduced graphene oxide and Prussian Blue. Microchim. Acta 2017, 184, 4587–4595. [Google Scholar] [CrossRef]
- Fei, Q.; Liu, Y.N.; Kong, R.M.; You, J.M. A versatile DNA detection scheme based on the quenching of fluorescent silver nanoclusters by MoS2 nanosheets: Application to aptamer-based determination of hepatitis B virus and of dopamine. Microchim. Acta 2017, 184, 1–8. [Google Scholar]
- Lu, Y.; Yu, J.; Ye, W.C.; Yao, X.; Zhou, P.P.; Zhang, H.X.; Zhao, S.Q.; Jia, L.P. Spectrophotometric determination of mercury(II) ions based on their stimulation effect on the peroxidase-like activity of molybdenum disulfide nanosheets. Microchim. Acta 2016, 183, 2481–2489. [Google Scholar] [CrossRef]
- Qing, Y.; Li, X.; Chen, S.; Zhou, X.P.; Luo, M.; Xu, X.; Li, C.R.; Qiu, J.F. Differential pulse voltammetric ochratoxin A assay based on the use of an aptamer and hybridization chain reaction. Microchim. Acta 2017, 184, 863–870. [Google Scholar] [CrossRef]
- Abnous, K.; Danesh, N.M.; Alibolandi, M.; Ramezani, M.; Taghdisi, S.M. Amperometric aptasensor for ochratoxin A based on the use of a gold electrode modified with aptamer, complementary DNA, SWCNTs and the redox marker Methylene Blue. Microchim. Acta 2017, 184, 1151–1159. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.L.; Wu, W. Graphene-based Steganographic Aptasensor for Information Computing and Monitoring Toxins of Biofilm in Food. Front. Microbiol. 2020, 10, 3139. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Li, Z.J.; Wang, Y.; Yang, Q.L. Nanomaterial-based Optical Biosensors for the Detection of Foodborne Bacteria. Food Rev. Int. 2020, 1–30. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; Khan, R. A highly sensitive amperometric immunosensor probe based on gold nanoparticle functionalized poly (3, 4-ethylenedioxythiophene) doped with graphene oxide for efficient detection of aflatoxin B 1. Synth. Met. 2018, 235, 136–144. [Google Scholar] [CrossRef]
- Srivastava, S.A.S.; Singh, C.; Ali, M.A.; Srivastava, A.; Sumana, G.; Malhotra, B. Protein conjugated carboxylated gold@reduced graphene oxide for aflatoxin B1 detection. RSC Adv. 2014, 5, 5406–5414. [Google Scholar] [CrossRef]
- Srivastava, S.; Kumar, V.; Arora, K.; Singh, C.; Ali, M.A.; Puri, N.K.; Malhotra, B.D. Antibody conjugated metal nanoparticle decorated graphene sheets for a mycotoxin sensor. RSC Adv. 2016, 6, 56518–56526. [Google Scholar] [CrossRef]
- Linting, Z.; Ruiyi, L.; Zaijun, L.; Qianfang, X.; Yinjun, F.; Junkang, L. An immunosensor for ultrasensitive detection of aflatoxin B_1 with an enhanced electrochemical performance based on graphene/conducting polymer/gold nanoparticles/the ionic liquid composite film on modified gold electrode with electrodeposition. Sens. Actuators B Chem. 2012, 174, 359–365. [Google Scholar] [CrossRef]
- Althagafi, I.I.; Ahmed, S.A.; El-Said, W.A.; Geng, J. Fabrication of gold/graphene nanostructures modified ITO electrode as highly sensitive electrochemical detection of Aflatoxin B1. PLoS ONE 2019, 14, e0210652. [Google Scholar] [CrossRef] [PubMed]
- Hamid, J.S.; Mohammad, R.; Mohammad, D.N.; Mona, A.; Khalil, A.; Mohammad, T.S. A novel electrochemical aptasensor for detection of aflatoxin M 1 based on target-induced immobilization of gold nanoparticles on the surface of electrode. Biosens. Bioelectron. 2018, 117, 487–492. [Google Scholar]
- Xu, X.; Liu, X.; Li, Y.; Ying, Y. A simple and rapid optical biosensor for detection of aflatoxin B1 based on competitive dispersion of gold nanorods. Biosens. Bioelectron. 2013, 47, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kumar, V.; Ali, M.A.; Solanki, P.R.; Srivastava, A.; Sumana, G.; Saxena, P.S.; Joshi, A.G.; Malhotra, B.D. Electrophoretically deposited reduced graphene oxide platform for food toxin detection. Nanoscale 2013, 5, 3043–3051. [Google Scholar] [CrossRef]
- Singh, J.; Roychoudhury, A.; Srivastava, M.; Solanki, P.; Lee, D.-W.; Lee, S.; Malhotra, B. A highly efficient rare earth metal oxide nanorods based platform for aflatoxin detection. J. Mater. Chem. B 2013, 1, 4493. [Google Scholar] [CrossRef]
- Ruchika, C.; Jay, S.; Pratima, R.S.; Basu, T.; Richard, O.K.; Malhotra, B.D. Electrochemical piezoelectric reusable immunosensor for aflatoxin B1 detection. Biochem. Eng. J. 2015, 103, 103–113. [Google Scholar]
- Gan, N.; Zhou, J.; Xiong, P.; Hu, F.; Cao, Y.; Li, T.; Jiang, Q. An Ultrasensitive Electrochemiluminescent Immunoassay for Aflatoxin M1 in Milk, Based on Extraction by Magnetic Graphene and Detection by Antibody-Labeled CdTe Quantumn Dots-Carbon Nanotubes Nanocomposite. Toxins 2013, 5, 865–883. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, Y.; Shen, G.; Wang, S.; Yu, R. Electrochemical immunosensor based on Pd-Au nanoparticles supported on functionalized PDDA-MWCNT nanocomposites for aflatoxin B1 detection. Anal. Biochem. 2015, 494, 10–15. [Google Scholar] [CrossRef]
- Singh, C.; Srivastava, S.; Ali, M.A.; Gupta, T.K.; Sumana, G.; Srivastava, A.; Mathur, R.B.; Malhotra, B.D. Carboxylated multiwalled carbon nanotubes based biosensor for aflatoxin detection. Sens. Actuators B Chem. 2013, 185, 258–264. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.-R.; Wang, W.-C.; Xue, J.; Huang, Y.-L.; Yang, X.-X.; Tan, B.; Zhou, X.-P.; Shao, C.; Ding, S.-J.; et al. A novel electrochemical immunosensor for highly sensitive detection of aflatoxin B1 in corn using single-walled carbon nanotubes/chitosan. Food Chem. 2016, 192, 197–202. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Hu, C.; Wu, H.; Yang, Y.; Huang, C.; Jia, N. Highly sensitive electrochemical impedance spectroscopy immunosensor for the detection of AFB(1) in olive oil. Food Chem. 2015, 176, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Chen, J.H.; Cao, H.; Yao, D.S.; Liu, D.L. Amperometric biosensor for aflatoxin B1 based on aflatoxin-oxidase immobilized on multiwalled carbon nanotubes. Food Control 2011, 22, 43–49. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Xu, L.; Feng, Y.; Lu, X. Electrochemical sensor for determination of aflatoxin B1based on multiwalled carbon nanotubes-supported Au/Pt bimetallic nanoparticles. J. Solid State Electrochem. 2014, 18, 2487–2496. [Google Scholar] [CrossRef]
- Srivastava, S.; Ali, M.A.; Umrao, S.; Parashar, U.K.; Srivastava, A.; Sumana, G.; Malhotra, B.D.; Pandey, S.S.; Hayase, S. Graphene Oxide-Based Biosensor for Food Toxin Detection. Appl. Biochem. Biotechnol. 2014, 174, 960–970. [Google Scholar] [CrossRef]
- Khoshfetrat, S.M.; Bagheri, H.; Mehrgardi, M.A. Visual electrochemiluminescence biosensing of aflatoxin M1 based on luminol-functionalized, silver nanoparticle-decorated graphene oxide. Biosens. Bioelectron. 2018, 100, 382–388. [Google Scholar] [CrossRef]
- Wang, D.; Hu, W.; Xiong, Y.; Xu, Y.; Li, C. Multifunctionalized reduced graphene oxide-doped polypyrrole/pyrrolepropylic acid nanocomposite impedimetric immunosensor to ultra-sensitively detect small molecular aflatoxin B1. Biosens. Bioelectron. 2015, 63, 185–189. [Google Scholar] [CrossRef]
- Geleta, G.S.; Zhao, Z.; Wang, Z. A novel reduced graphene oxide/molybdenum disulfide/polyaniline nanocomposite-based electrochemical aptasensor for detection of aflatoxin B1. Analyst 2018, 143, 1644–1649. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Z.; Yang, G.; Chen, X.; Gou, G.; Liu, W. Electrochemical immunosensor for Aflatoxin B1 based on polyaniline/graphene nanohybrids decorated with Au nanoparticle. Electrochemistry 2017, 85, 384–390. [Google Scholar] [CrossRef][Green Version]
- Shadjou, R.; Hasanzadeh, M.; Heidar Oor, M.; Shadjou, N. Electrochemical monitoring of aflatoxin M1 in milk samples using silver nanoparticles dispersed on α-cyclodextrin-GQDs nanocomposite. J. Mol. Recognit. 2018, 31, e2699. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, S.; Zhang, Q.; Gong, L.; Dai, H.; Lin, Y. Magnetic functionalized electrospun nanofibers for magnetically controlled ultrasensitive label-free electrochemiluminescent immune detection of aflatoxin B1. Sens. Actuators B Chem. 2016, 222, 707–713. [Google Scholar] [CrossRef]
- Wang, X.; Niessner, R.; Knopp, D. Magnetic Bead-Based Colorimetric Immunoassay for Aflatoxin B1 Using Gold Nanoparticles. Sensors 2014, 14, 21535–21548. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Reinhard, N.; Dietmar, K. Controlled growth of immunogold for amplified optical detection of aflatoxin B1. Analyst 2015, 140, 1453–1458. [Google Scholar]
- Zhang, J.; Xia, Y.K.; Chen, M.; Wu, D.Z.; Cai, S.X.; Liu, M.M.; He, W.H.; Chen, J.H. A fluorescent aptasensor based on DNA-scaffolded silver nanoclusters coupling with Zn(II)-ion signal-enhancement for simultaneous detection of OTA and AFB_1. Sens. Actuators B Chem. 2016, 235, 79–85. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Wang, X.; Wang, X.; Li, F. Development of a chemiluminescent aptasensor for ultrasensitive and selective detection of aflatoxin B1 in peanut and milk. Talanta 2019, 201, 52–57. [Google Scholar] [CrossRef]
- Wang, B.; Zheng, J.; Ding, A.; Xu, L.; Chen, J.; Li, C.M. Highly sensitive aflatoxin B1 sensor based on DNA-guided assembly of fluorescent probe and TdT-assisted DNA polymerization. Food Chem. 2019, 294, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Sabet, F.S.; Hosseini, M.; Khabbaz, H.; Dadmehr, M.; Ganjali, M.R. FRET-based aptamer biosensor for selective and sensitive detection of aflatoxin B1 in peanut and rice. Food Chem. 2017, 220, 527–532. [Google Scholar] [CrossRef]
- Jans, H.; Huo, Q. Gold nanoparticle-enabled biological and chemical detection and analysis. Chem. Soc. Rev. 2012, 41, 2849–2866. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Sun, J.; Xianyu, Y.; Jiang, X. Point-of-care biochemical assays using gold nanoparticle-implemented microfluidics. Chem. Soc. Rev. 2014, 43, 6239–6253. [Google Scholar] [CrossRef]
- Zhang, D.; Li, P.; Zhang, Q.; Li, R.; Zhang, W.; Ding, X.; Li, C.M. A naked-eye based strategy for semiquantitative immunochromatographic assay. Anal. Chim. Acta 2012, 740, 74–79. [Google Scholar] [CrossRef]
- Song, S.; Liu, N.; Zhao, Z.; Ediage, E.N.; Wu, S.; Sun, C.; Saeger, S.D.; Wu, A. Multiplex Lateral Flow Immunoassay for Mycotoxin Determination. Anal. Chem. 2014, 86, 4995–50001. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; D’Arco, G.; Calderara, M.; Baggiani, C.; Giovannoli, C.; Giraudi, G. Development of a quantitative lateral flow immunoassay for the detection of aflatoxins in maize. Food Addit. Contam. 2011, 28, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Akbarian, F.; Heydari, E.; Rezaei, B. A novel aptasensor based on 3D-reduced graphene oxide modified gold nanoparticles for determination of arsenite. Biosens. Bioelectron. 2018, 122, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhou, D.; Gong, J.; Liu, C.; Chen, J. Highly Sensitive Aptasensor for Trace Arsenic(III) Detection Using DNAzyme as the Biocatalytic Amplifier. Anal. Chem. 2019, 91, 1724–1727. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, L.; Li, J.; Chen, X.; Lan, J.; Yan, A.; Lei, Y.; Yang, S.; Yang, H.; Chen, J. A Ratiometric Fluorescent Bioprobe Based on Carbon Dots and Acridone Derivate for Signal Amplification Detection Exosomal microRNA. Anal. Chem. 2018, 90, 8969–8976. [Google Scholar] [CrossRef]
- Shu, J.; Qiu, Z.; Wei, Q.; Zhuang, J.; Tang, D. Cobalt-Porphyrin-Platinum-Functionalized Reduced Graphene Oxide Hybrid Nanostructures: A Novel Peroxidase Mimetic System For Improved Electrochemical Immunoassay. Sci. Rep. 2015, 5, 15113. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar]
- Wang, X.; Niessner, R.; Tang, D.; Knopp, D. Review: Nanoparticle-Based Immunosensors and Immunoassays for Aflatoxins. Anal. Chim. Acta 2016, 912, 10–23. [Google Scholar] [CrossRef]
- Reverté, L.; Prieto-Simón, B.; Campàs, M. New advances in electrochemical biosensors for the detection of toxins: Nanomaterials, magnetic beads and microfluidics systems. A review. Anal. Chim. Acta 2015, 908, 8–21. [Google Scholar] [CrossRef]
- Rijian, M.; Lei, H.; Xiemin, Y.; Tiantian, S.; Chunxia, Z.; Zhe, W.; Pengzhi, H.; Shengli, S.; Chengyong, L. A novel aflatoxin B1 biosensor based on a porous anodized alumina membrane modified with graphene oxide and an aflatoxin B1 aptamer. Electrochem. Commun. 2018, 95, 9–13. [Google Scholar]
- Goud, K.Y.; Hayat, A.; Catanante, G.L.; Satyanarayana, M.; Gobi, K.V.; Marty, J.L. An electrochemical aptasensor based on functionalized graphene oxide assisted electrocatalytic signal amplification of methylene blue for aflatoxin B1 detection. Electrochim. Acta 2017, 244, 96–103. [Google Scholar] [CrossRef]
- Mondal, K.; Ali, A.; Srivastava, S.; Malhotra, B.D.; Sharma, A. Electrospun functional micro/nanochannels embedded in porous carbon electrodes for microfluidic biosensing. Sens. Actuators B Chem. 2016, b229, 82–91. [Google Scholar] [CrossRef]
- Tudorache, M.; Bala, C. Sensitive Aflatoxin B1 Determination Using a Magnetic Particles-Based Enzyme-Linked Immunosorbent Assay. Sensors 2008, 8, 7571–7580. [Google Scholar] [CrossRef]
- Urusov, A.; Petrakova, A.; Vozniak, M.; Zherdev, A.; Dzantiev, B. Rapid Immunoenzyme Assay of Aflatoxin B1 Using Magnetic Nanoparticles. Sensors 2014, 14, 21843–21857. [Google Scholar] [CrossRef] [PubMed]
- Radoi, A.; Targa, M.; Prieto-Simon, B.; Marty, J.L. Enzyme-Linked Immunosorbent Assay (ELISA) based on superparamagnetic nanoparticles for aflatoxin M-1 detection. Talanta 2008, 77, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, L.; Pal, S.; Bhand, S. Miniaturised hybrid immunoassay for high sensitivity analysis of aflatoxin M1 in milk. Biosens. Bioelectron. 2011, 26, 2601–2606. [Google Scholar] [CrossRef] [PubMed]
- Rhouati, A.; Catanante, G.; Nunes, G.; Hayat, A.; Marty, J.-L. Label-Free Aptasensors for the Detection of Mycotoxins. Sensors 2016, 16, 2178. [Google Scholar] [CrossRef] [PubMed]
- Beloglazova, N.V.; Speranskaya, E.S.; Wu, A.; Wang, Z.; Sanders, M.; Goftman, V.V.; Zhang, D.; Goryacheva, I.Y.; De Saeger, S. Novel multiplex fluorescent immunoassays based on quantum dot nanolabels for mycotoxins determination. Biosens. Bioelectron. 2014, 62, 59–65. [Google Scholar] [CrossRef]
- Fernández-Argüelles, M.T.; Costa-Fernández, J.M.; Pereiro, R.; Sanz-Medel, A. Simple bio-conjugation of polymer-coated quantum dots with antibodies for fluorescence-based immunoassays. Analyst 2008, 133, 444. [Google Scholar] [CrossRef]
- Speranskaya, E.S.; Beloglazova, N.V.; Abe, S.; Aubert, T.; Hens, Z. Hydrophilic, Bright CuInS2 Quantum Dots as Cd-Free Fluorescent Labels in Quantitative Immunoassay. Langmuir Acs J. Surf. Colloids 2014, 30, 7567–7575. [Google Scholar] [CrossRef]
- Xu, W.; Xiong, Y.; Lai, W.; Xu, Y.; Li, C.; Xie, M. A homogeneous immunosensor for AFB1 detection based on FRET between different-sized quantum dots. Biosens. Bioelectron. 2014, 56, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Seok, Y.; Byun, J.-Y.; Shim, W.-B.; Kim, M.-G. A structure-switchable aptasensor for aflatoxin B1 detection based on assembly of an aptamer/split DNAzyme. Anal. Chim. Acta 2015, 886, 182–187. [Google Scholar] [CrossRef] [PubMed]
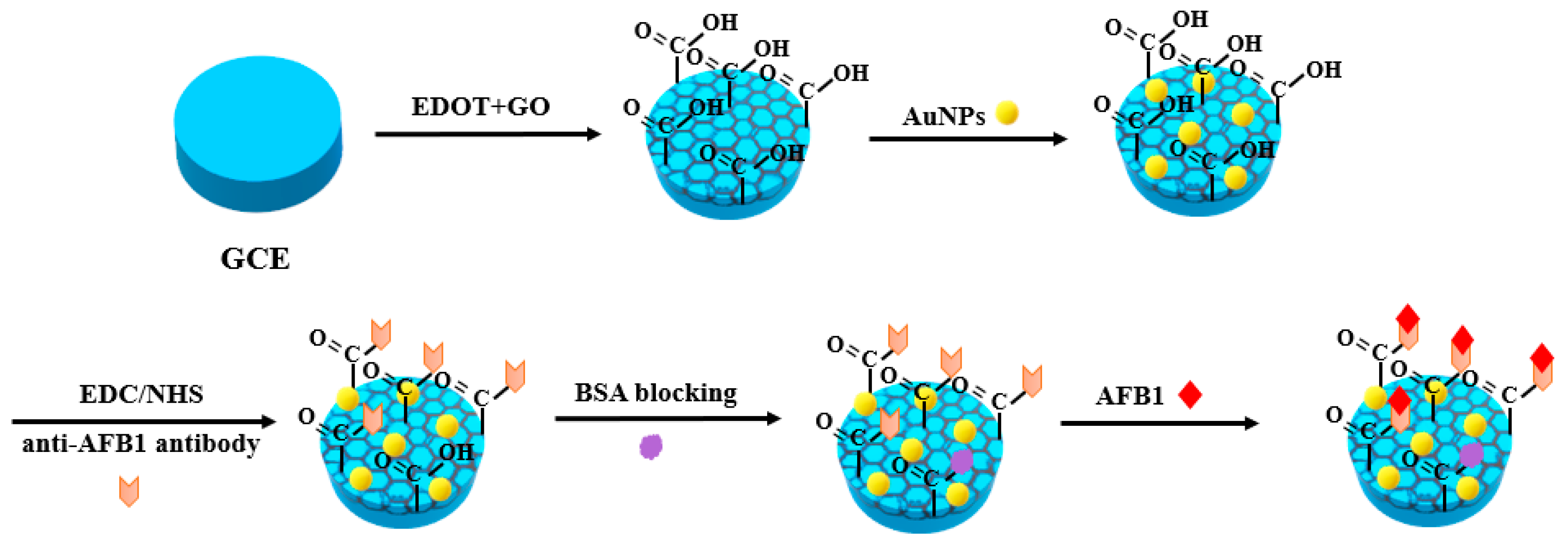
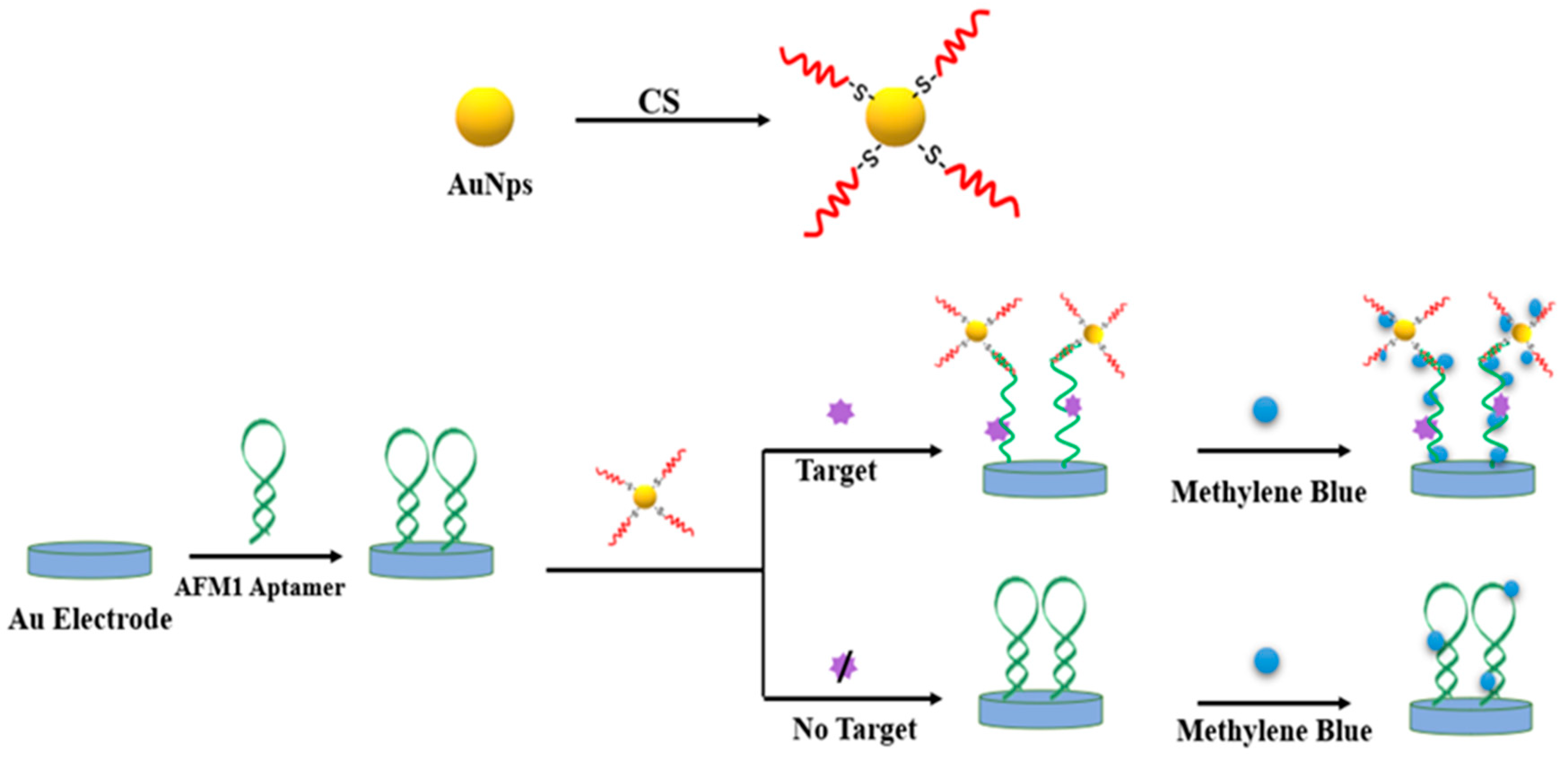
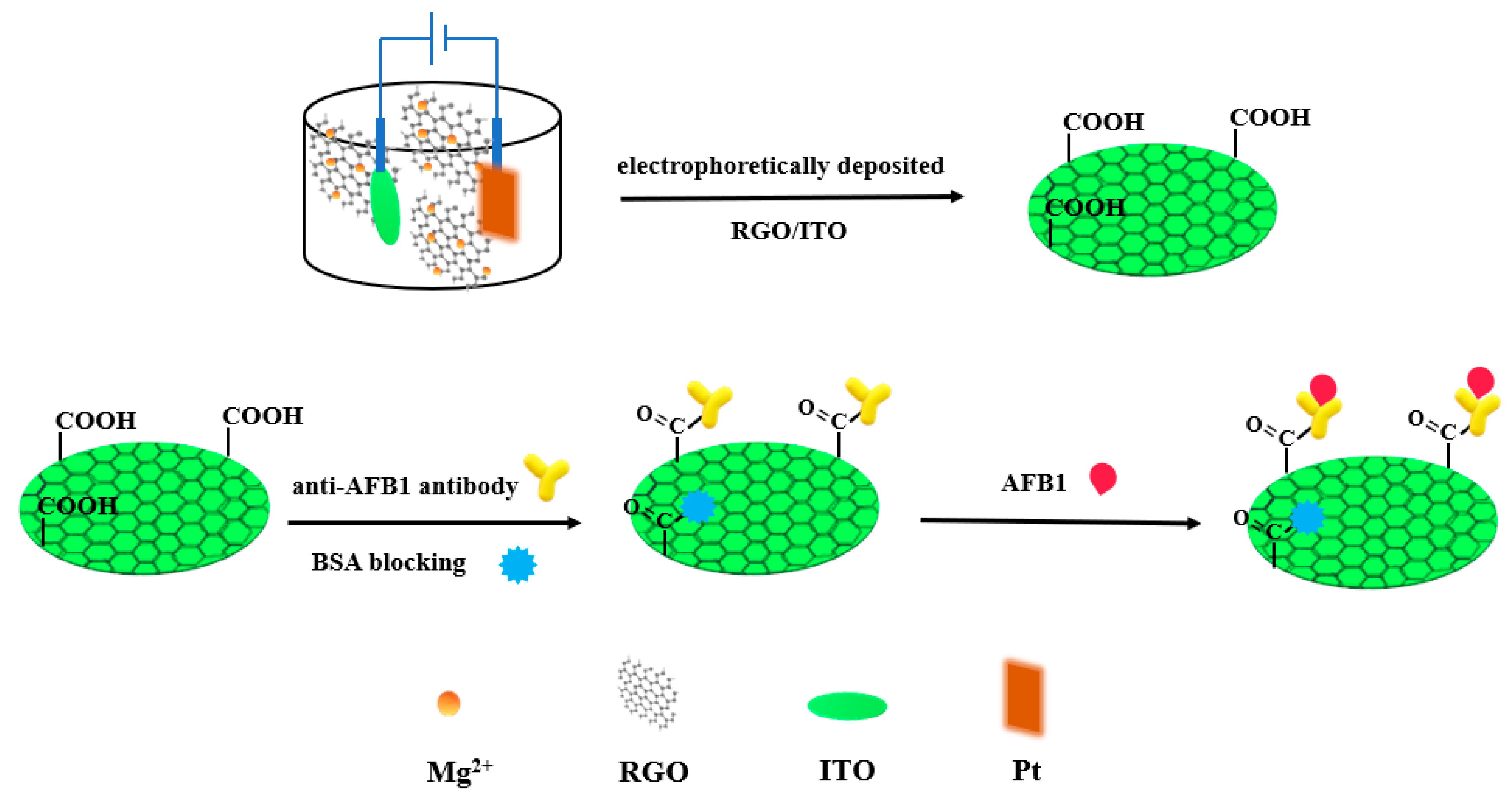
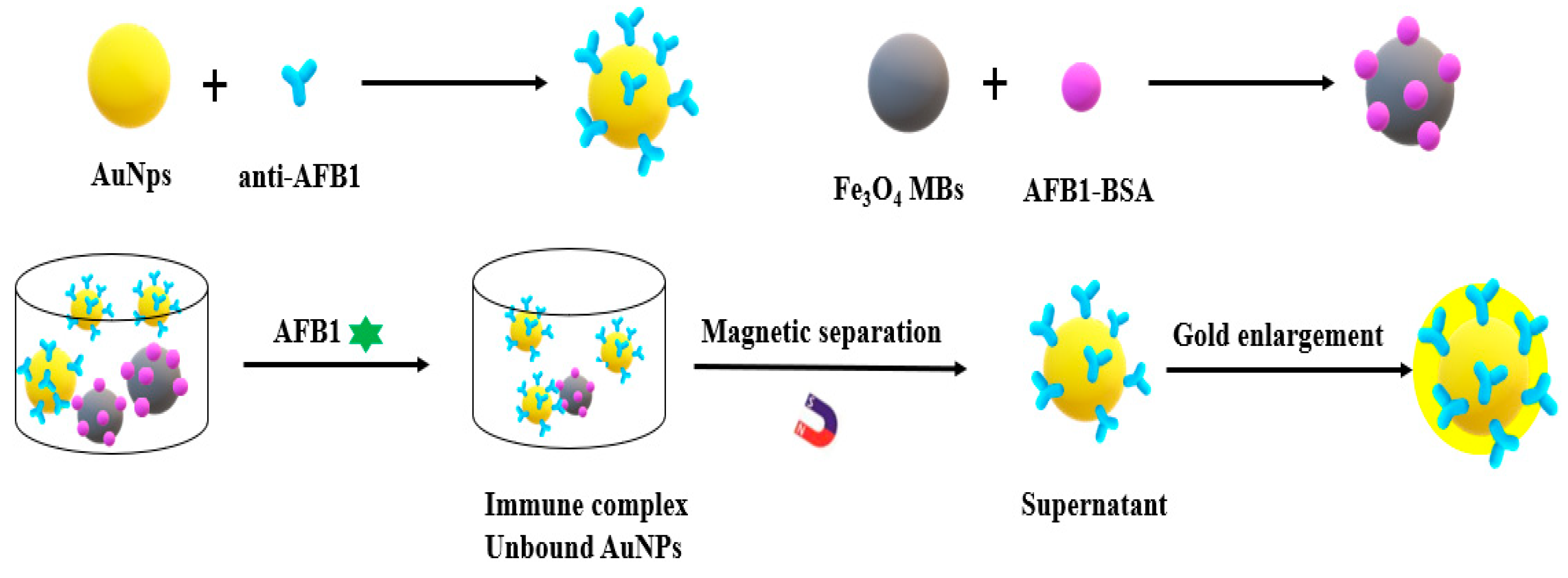
| Detection Method | Advantages | Disadvantages |
|---|---|---|
| TLC | Simple equipment, low cost and easy operation. | Cumbersome steps, poor sensitivity, high detection limit and reagent are harmful to operators. |
| HPLC | Good repeatability, low detection limit and high sensitivity. | Needs derivation, complex operation and high instrument cost. |
| UPLC | Fast detection speed, short experimental period, no derivative and high sensitivity. | High instrument cost. |
| LC-MS | Simple pretreatment, high selectivity and multi-component analysis. | Complex equipment operation and high instrument cost. |
| ELISA | Large number of samples can be analysed simultaneously, high sensitivity and accuracy, does not require extensive sample cleanup. | Short reagent life, higher false positive probability. |
| LFIA | Fast detection speed, low cost, easy operation, simple equipment, short experimental period. | Poor repeatability, difficult to quantify, poor sensitivity. |
| Type of Nanomaterials/NPs | Properties | Receptor Molecules | Target | Detection Signal | Linear Range | LOD | Real Sample | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Metal nanomaterials | AuNPs | High extinction coefficients, Good stability and conductivity, Good photoelectric performance, Good biocompatibility, High specific surface area, Easy to modify. | Antibody/PEDOT | AFB1 | Electrochemical signal | 0.5–20 ng/mL 20–60 ng/mL | 0.09 ng/mL | Corn | [37] |
| AuNPs | Antibody | AFB1 | Electrochemical signal | 0.1–12 ng/mL | 0.1 ng/mL | - | [38] | ||
| Ni NPs | Antibody | AFB1 | Electrochemical signal | 1–8 ng/mL | 0.16 ng/mL | - | [39] | ||
| AuNPs | Antibody/DPB | AFB1 | Electrochemical signal | - | 1.0 × l0−15 mol/L | Peanut, rice, milk, flour, soybean | [40] | ||
| AuNPs | Antibody | AFB1 | Electrochemical signal | 100 ng/mL–1 pg/mL | 6.9 pg/mL | Peanut | [41] | ||
| AuNPs | Aptamer/CS | AFM1 | Electrochemical signal | 2–600 ng/L | 0.9 ng/L | Milk, serum | [42] | ||
| GNRs | Antibody | AFB1 | Colorimetric signal | 0.5–20 ng/ml | 0.16 ng/mL | Peanut | [43] | ||
| Metal oxides and hydroxides | ITO | One-dimensional morphology, High electronic conductivity, Physicochemical stability, High specific surface area. | Antibody | AFB1 | Electrochemical signal | 0.125–1.5 ng/mL | 0.15 ng/mL | - | [44] |
| Sm2O3 nanorods | Antibody | AFB1 | Electrochemical signal | 10–700 pg/mL | 57.82 pg mL−1 cm−2 | - | [45] | ||
| Fe3O4 nanoparticles | Antibody | AFB1 | Electrochemical signal | 0.05–5 ng/mL | 0.07 ng/mL | Food | [46] | ||
| Carbon nanomaterials | CNTs | One-dimensional atomic sheet structure, Large surface area, Stable chemical properties, High electrical conductivity, Mechanical strength. | Antibody/ CdTe QDs | AFM1 | Electrochemiluminescence signal | 1.0–1.0 × 105 pg/mL | 0.3 pg/mL | Milk | [47] |
| CNTs | Antibody/ PDDA | AFB1 | Electrochemical signal | 0.05–25 ng/mL | 0.03 ng/mL | Rice | [48] | ||
| c-MWCNTs | Antibody | AFB1 | Electrochemical signal | 0.25–1.375 ng/mL | 0.08 ng/mL | - | [49] | ||
| SWCNTs | Antibody/α-NP | AFB1 | Electrochemical signal | 0.01–100 ng/mL | 3.5 pg/mL | Corn meal | [50] | ||
| MWCNTs | Antibody/[BMIM]PF6 | AFB1 | Electrochemical signal | 0.1–10 ng/mL | 0.03 ng/mL | Olive oil | [51] | ||
| MWCNT | Enzyme | AFB1 | Electrochemical signal | 3.2 × 10−9–721 × 10−9 moL/L | 1.6 × 10−9 moL/L | - | [52] | ||
| MWCNTs | MIP | AFB1 | Electrochemical signal | 1 × 10−10–l × 10−5 mol/L | 0.03 nmol/L | Rapeseed oil, hogwash oil | [53] | ||
| GO | Two-dimensional carbon nanomaterials, Excellent optical and electrical properties, High surface area, High strength and toughness, Good heat conduction performance, Facile functionalization and biocompatibility. | Antibody | AFB1 | Electrochemical signal | 0.5–5 ng/mL | 0.23 ng/mL | - | [54] | |
| GO | Aptamer | AFM1 | Electrochemiluminescence signal | 5–150 ng/mL | 0.01 ng/mL | Milk | [55] | ||
| rGO | Antibody/PPy-PPa | AFB1 | Electrochemical signal | 10 fg/mL–10 pg/mL | 10 fg/mL | - | [56] | ||
| rGO | Aptamer/ polyaniline | AFB1 | Electrochemical signal | 1.0 × 10−17–1.0 × l0−15 g/mL | 2 × 10−18 g/mL | Wine | [57] | ||
| Graphene | Antibody/ PANI | AFB1 | Electrochemical signal | 0.05–25 ng/mL | 0.034 ng/mL | Rice | [58] | ||
| GQDs | Zero-dimensional atomic, Good dispersion, More abundant active sites, Good biocompatibility and photostability, Better chemical and physical properties, High water solubility. | Antibody/α-cyclodextrin | AFM1 | Electrochemical signal | 0.015–25 mmol/L | 2 μmol/L | Milk | [59] | |
| CNHs | Antibody/L-F3O4-NFs | AFB1 | Electrochemiluminescence signal | 0.05–200 ng/mL | 0.02 ng/mL | Corn | [60] | ||
| Other nanomaterials | MBs | High resistivity and permeability, Excellent sensitivity, Good dispersion and suspension, High binding rate, Controllable size, Easy functionalization. | Antibody | AFB1 | Colorimetric signal | 20–800 ng/L | 12 ng/L | Corn | [61] |
| MBs | Antibody | AFB1 | Colorimetric signal | 0.01–1 ng/mL | 7 pg/mL | Maize | [62] | ||
| MBs | Aptamer/DNA-scaffolded AgNCs | AFB1 | Fluorescent signal | 0.001–0.050 ng/mL | 0.3 pg/mL | Wheat, rice, corn | [63] | ||
| MBs | Aptamer/HCR | AFB1 | Fluorescent signal | 0.5–40 ng/mL | 0.2 ng/mL | Peanut, milk | [64] | ||
| MNPs | Aptamer/ PAPDI | AFB1 | Fluorescent signal | - | 0.01 nM | Maize | [65] | ||
| QDs | Good light stability, Good biocompatibility, Long fluorescence life, Excellent sensitivity. | Aptamer/QDs | AFB1 | Fluorescent signal | 10–400 nmol/L | 3.4 nmol/L | Rice, peanut | [66] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.; Wang, Q.; Yang, Q.; Wu, W. Recent Advances in Aflatoxins Detection Based on Nanomaterials. Nanomaterials 2020, 10, 1626. https://doi.org/10.3390/nano10091626
Yan C, Wang Q, Yang Q, Wu W. Recent Advances in Aflatoxins Detection Based on Nanomaterials. Nanomaterials. 2020; 10(9):1626. https://doi.org/10.3390/nano10091626
Chicago/Turabian StyleYan, Chunlei, Qi Wang, Qingli Yang, and Wei Wu. 2020. "Recent Advances in Aflatoxins Detection Based on Nanomaterials" Nanomaterials 10, no. 9: 1626. https://doi.org/10.3390/nano10091626
APA StyleYan, C., Wang, Q., Yang, Q., & Wu, W. (2020). Recent Advances in Aflatoxins Detection Based on Nanomaterials. Nanomaterials, 10(9), 1626. https://doi.org/10.3390/nano10091626




