Enhancement of Chemical Stability and Dermal Delivery of Cordyceps militaris Extracts by Nanoemulsion
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungi Materials
2.2. Chemical Materials
2.3. Extraction of C. militaris
2.3.1. Maceration
2.3.2. Sequential Maceration
2.3.3. Infusion
2.4. Determination of Chemical Compositions of C. militaris Extracts
2.4.1. Cordycepin Content Determination Using High Performance Liquid Chromatography (HPLC)
2.4.2. Total Phenolic Content Determination by Folin-Ciocalteu (FC) Method
2.4.3. Total Flavonoid Content Determination by Aluminum Chloride Colorimetric Method
2.5. Antioxidant Activities Determination of C. militaris Extracts
2.5.1. 2,2′-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.5.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.3. 2,2′-Azino-bis3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) Assay
2.5.4. Determination of Lipid Peroxidation Inhibition by Ferric Thiocyanate (FTC) Assay
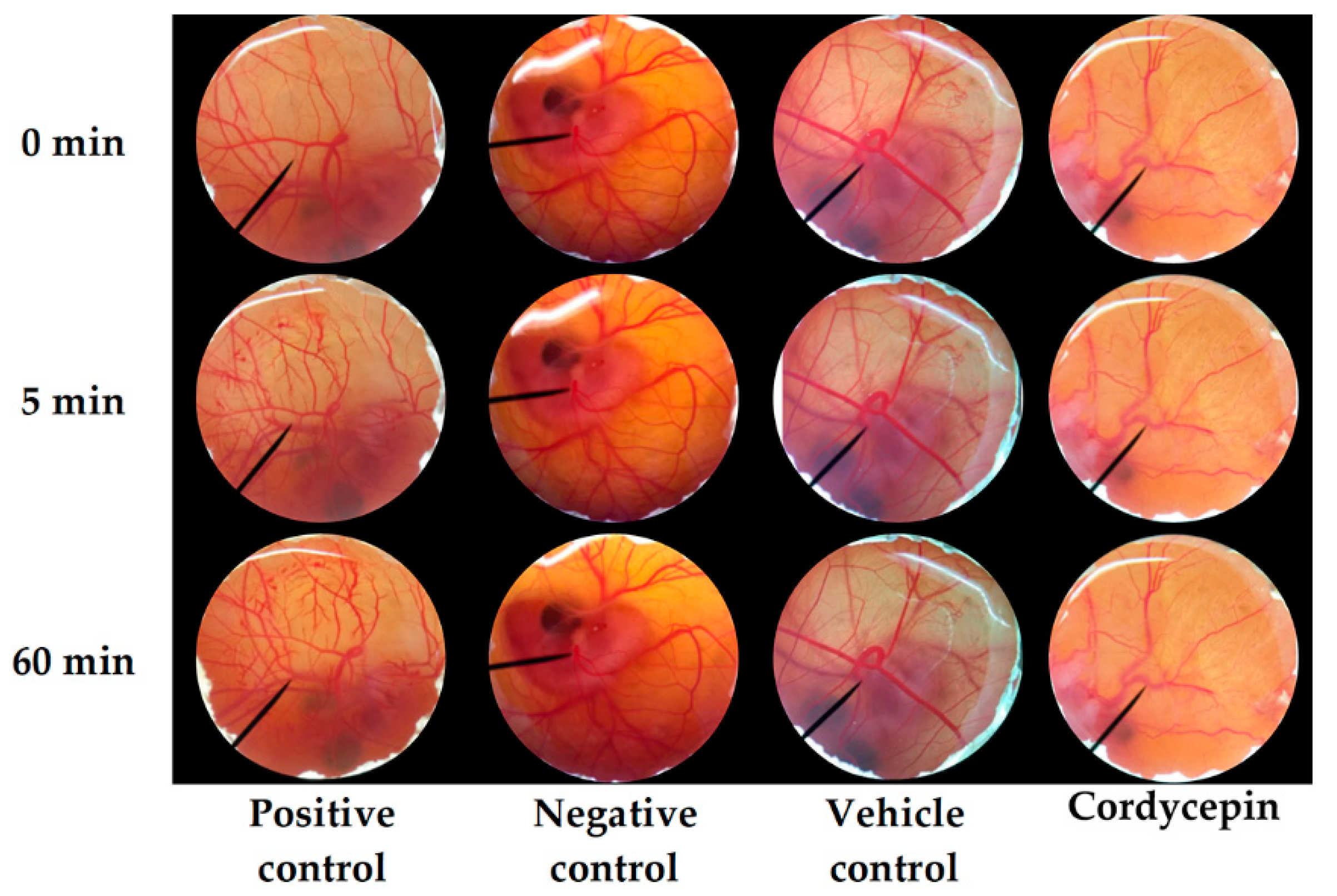
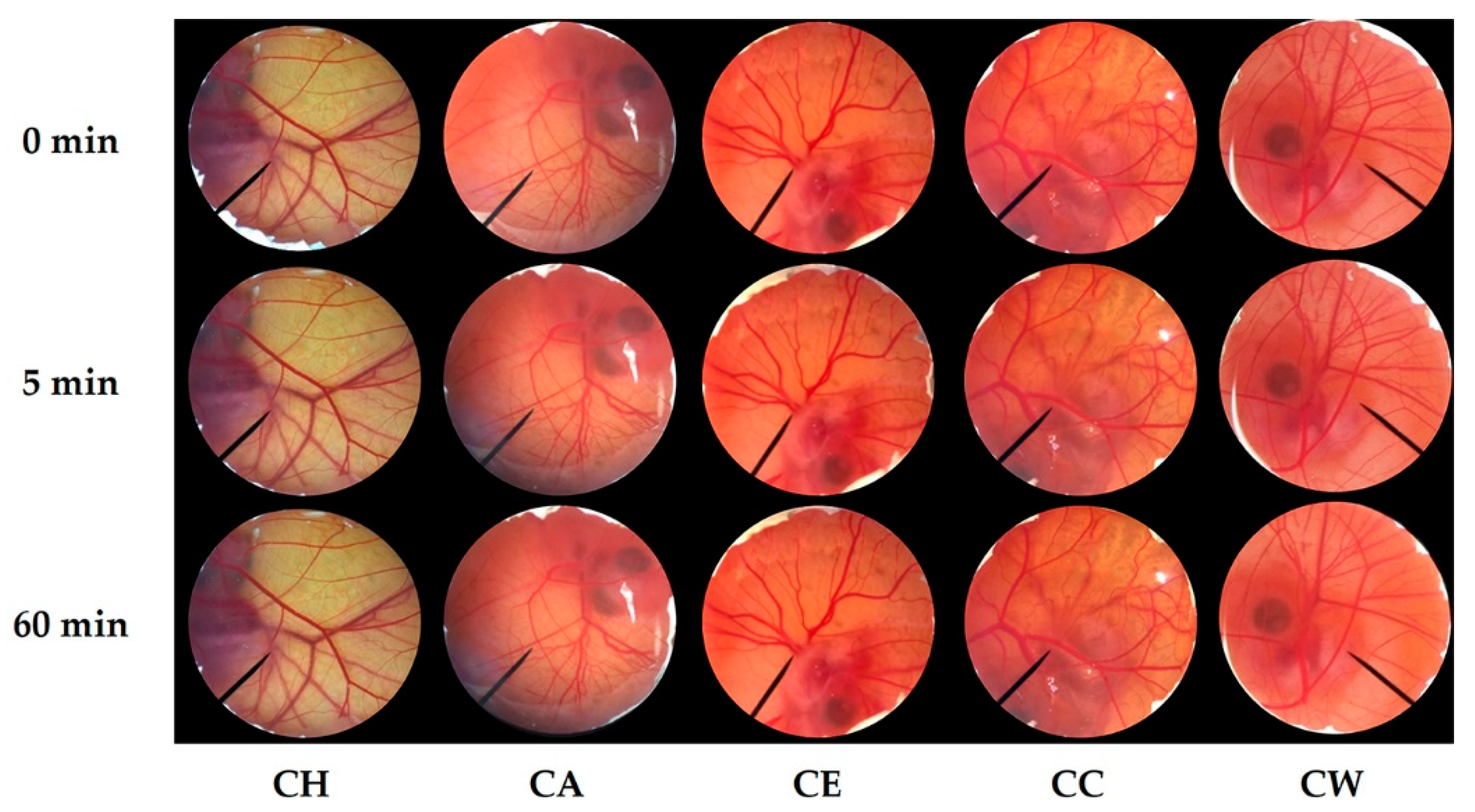
2.6. Irritation Test of C. militaris Extracts by Hen’s Egg Test Chorioallantoic Membrane (HET-CAM) Assay
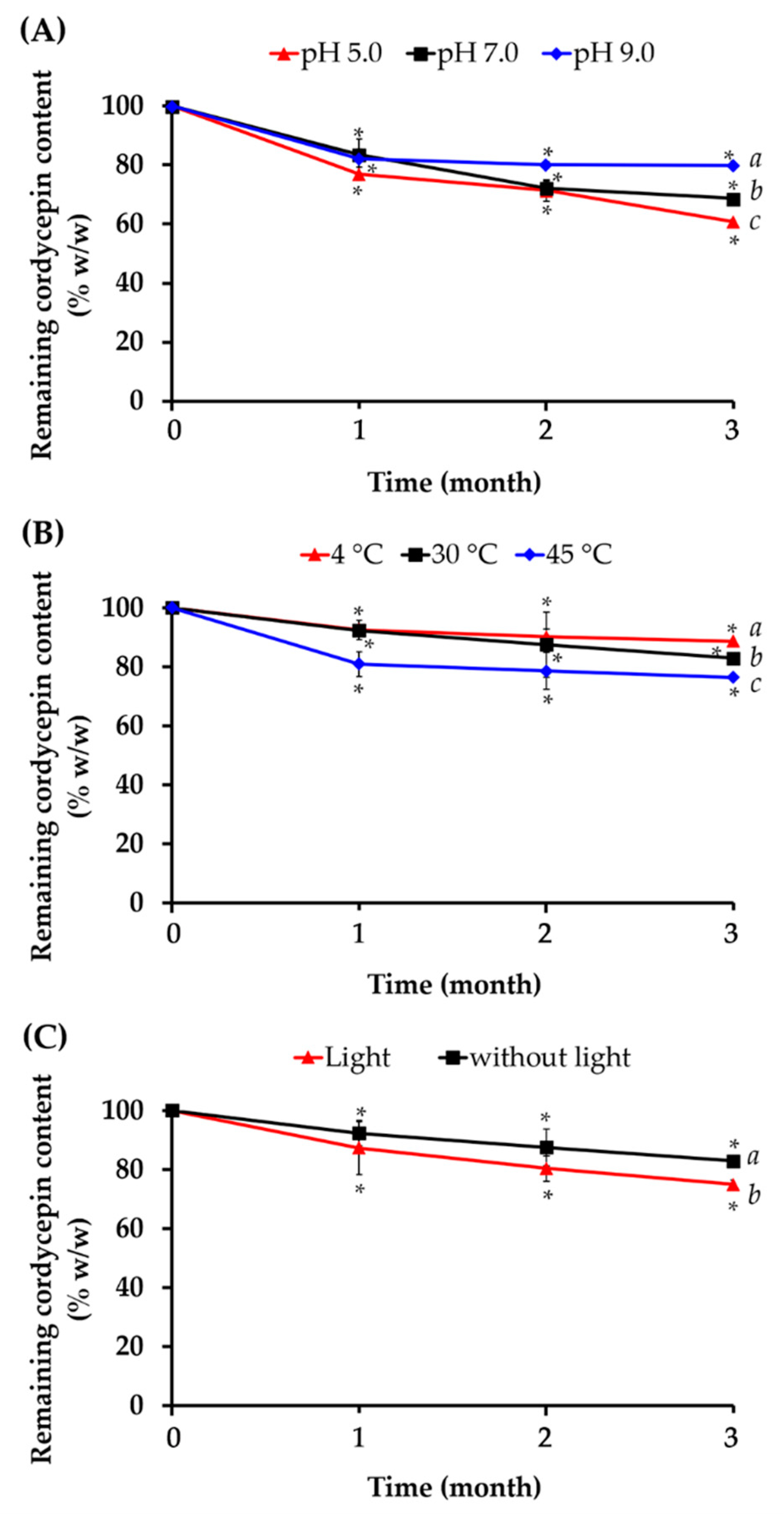
2.7. Stability of Cordycepin in C. militaris Extracts
2.7.1. Effect of pH
2.7.2. Effect of Temperature
2.7.3. Effect of Light
2.8. Development of Nanoemulsion
2.8.1. Nanoemulsion Preparation
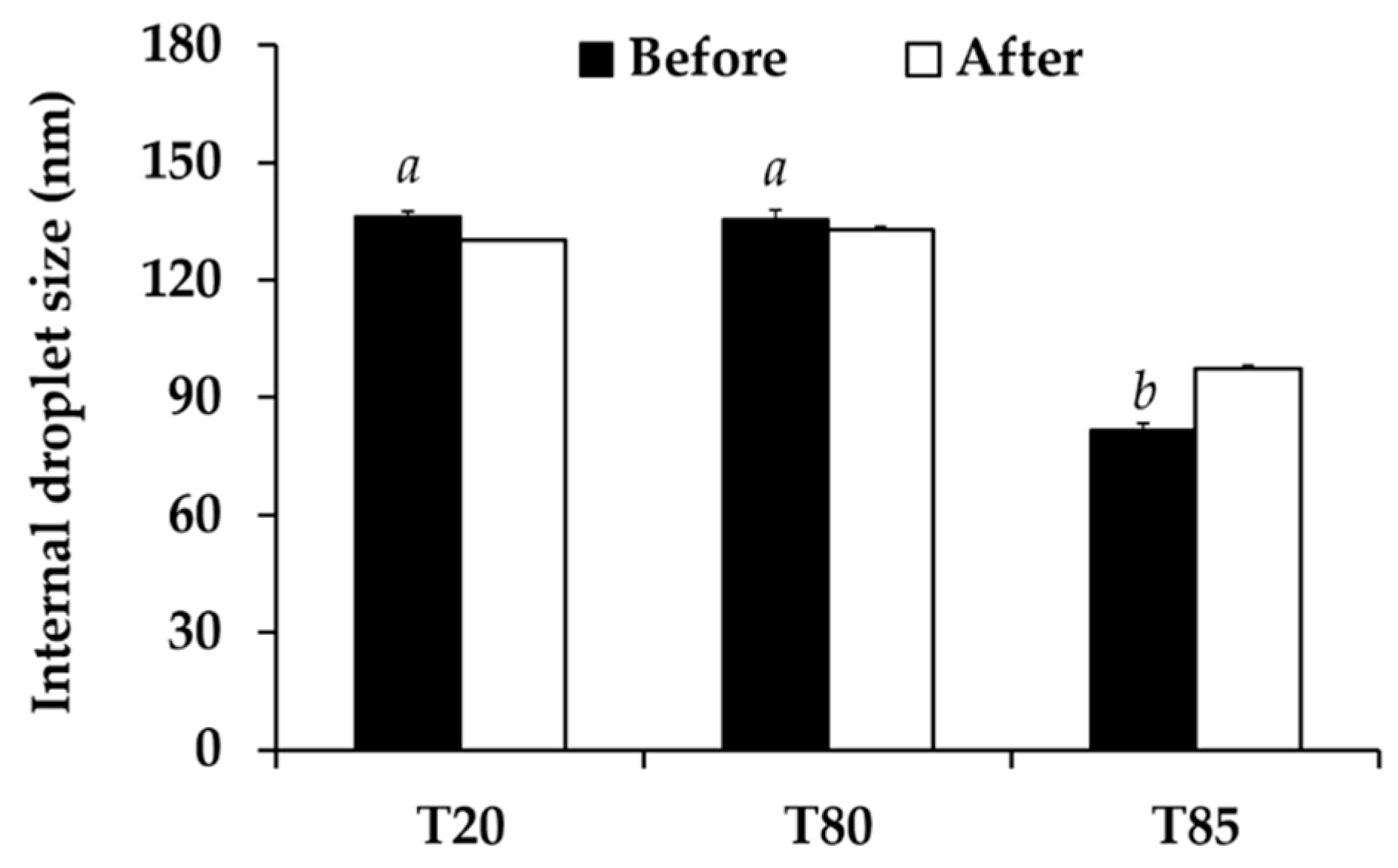
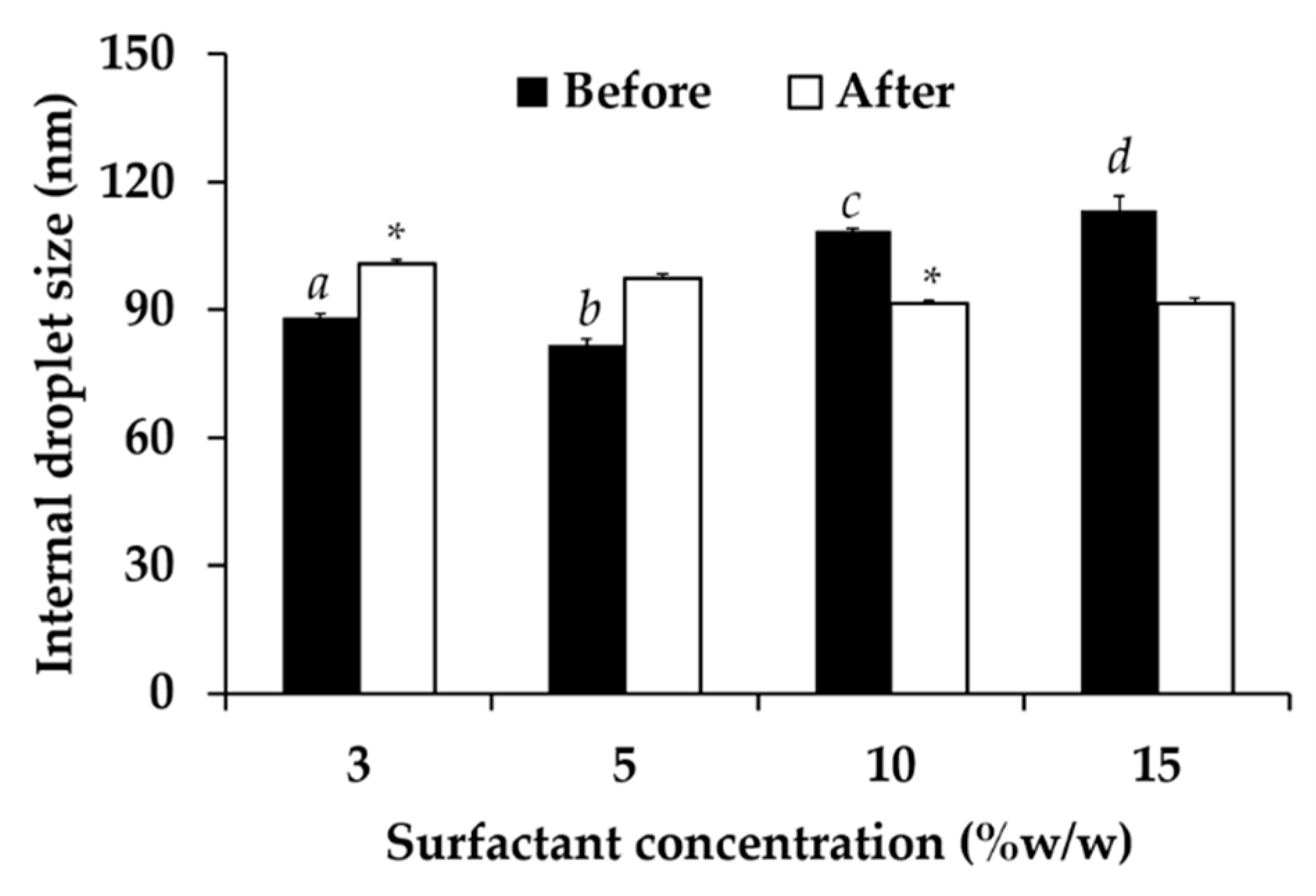
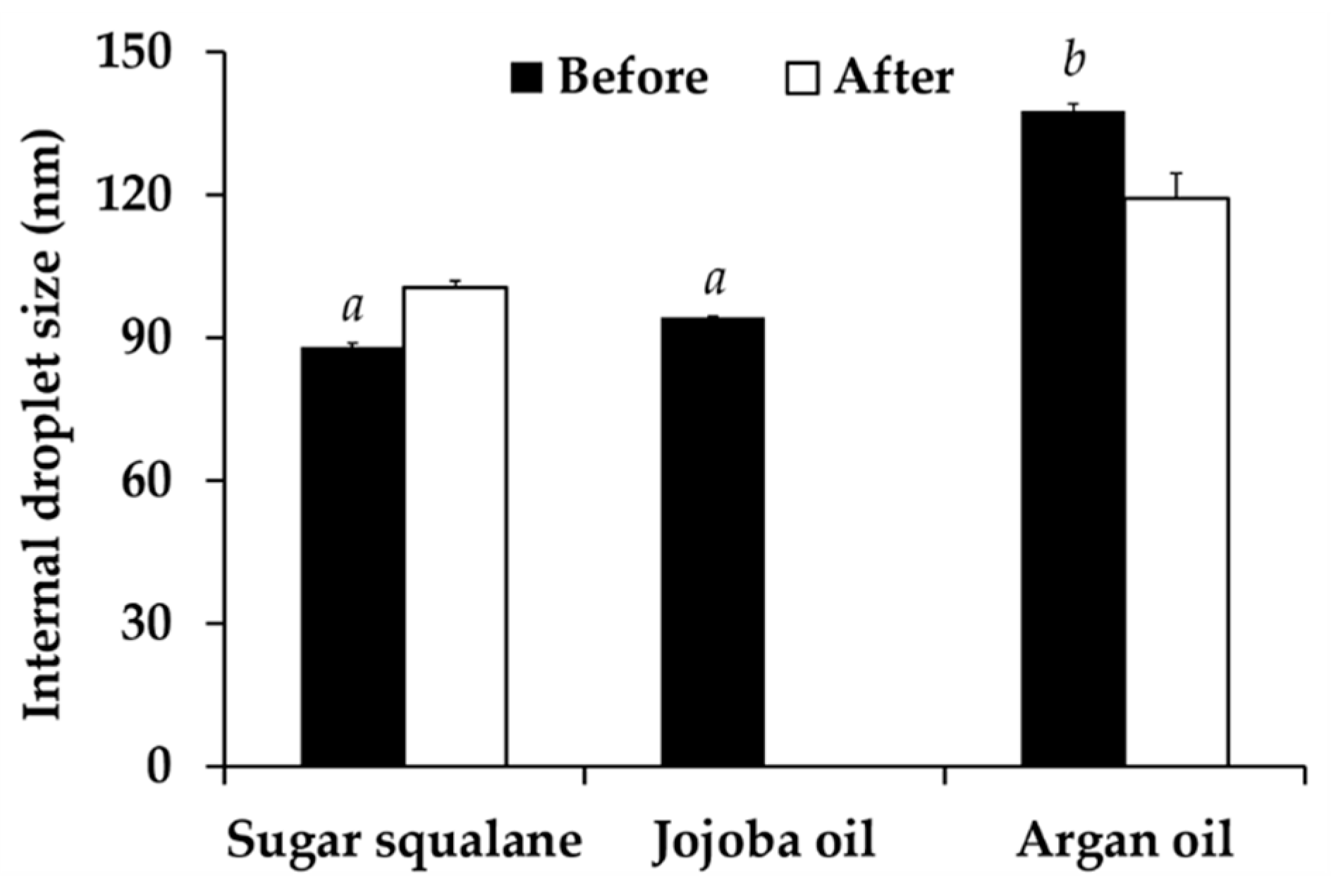
2.8.2. Characterizations of Nanoemulsions
2.8.3. Stability of Nanoemulsions
2.9. Development of Nanoemulsion Containing C. militaris Extracts
2.9.1. Preparation of Nanoemulsion Containing C. militaris Extract
2.9.2. Characterizations of Nanoemulsions Containing C. militaris Extract
2.9.3. Stability of Nanoemulsions Containing C. militaris Extract
2.10. Cytotoxicity on Human Keratinocyte (HaCaT) Cells of Nanoemulsions Containing C. militaris Extract by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl Tetrazolium Bromide (MTT) Assay
2.10.1. Human Keratinocyte (HaCaT) Cell Culture and Condition
2.10.2. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl Tetrazolium Bromide (MTT) Assay
2.11. In Vivo Irritation Test in Human Using Human Patch Test
2.12. Release Profile of C. militaris Extract from Nanoemulsions
2.13. Skin Permeation and Skin Retention of C. militaris Extract from Nanoemulsions
2.13.1. Skin Preparation
2.13.2. Skin Permeation Determination
2.13.3. Skin Retention Determination
2.14. Statistical Analysis
3. Results and Discussion
3.1. C. militaris Extracts
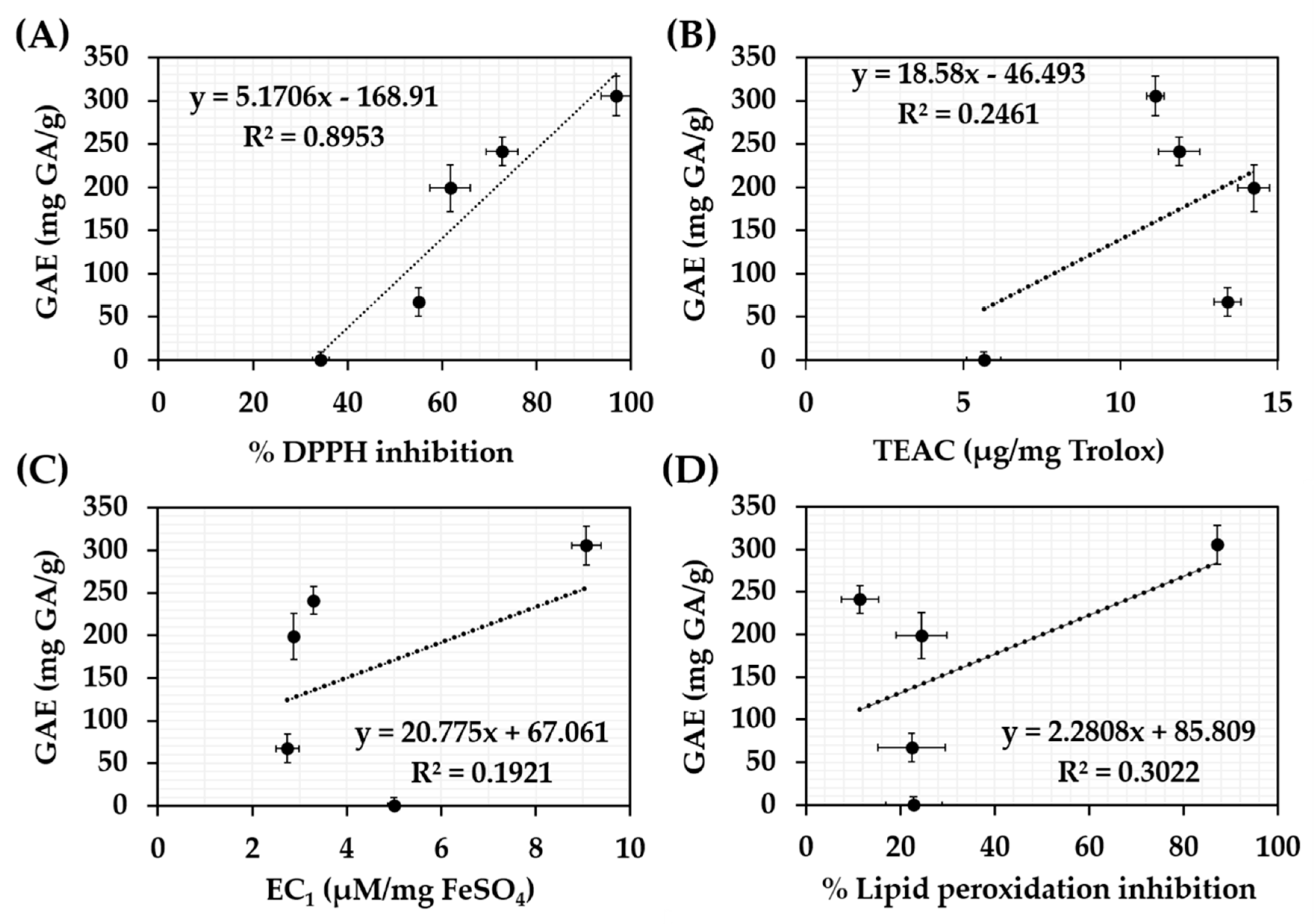
3.2. Chemical Composition of C. militaris Extracts
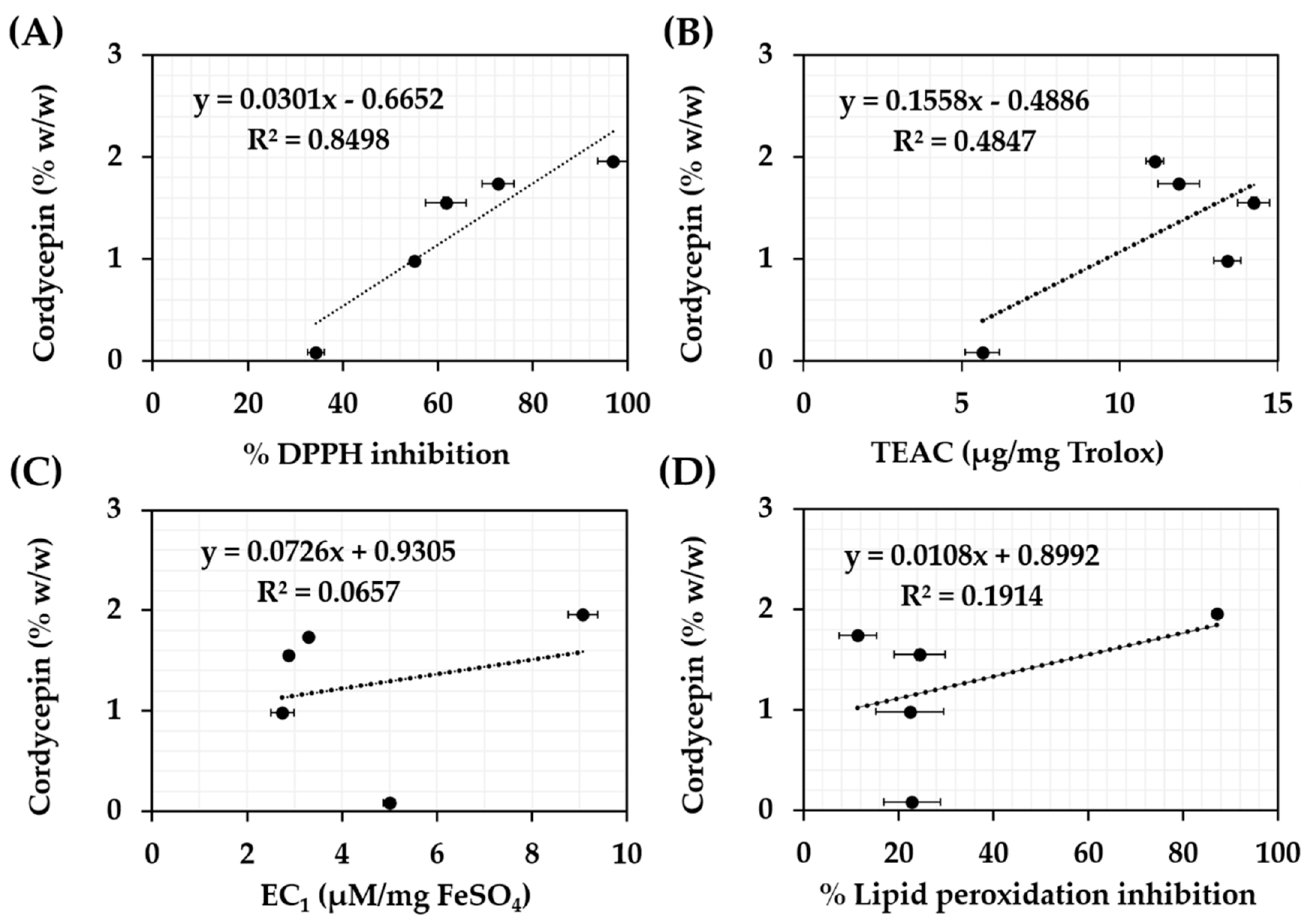
3.3. Antioxidant Activities of C. militaris Extracts
3.4. Irritation Profiles of C. militaris Extracts
3.5. Stability Profile of Cordycepin in C. militaris Extracts
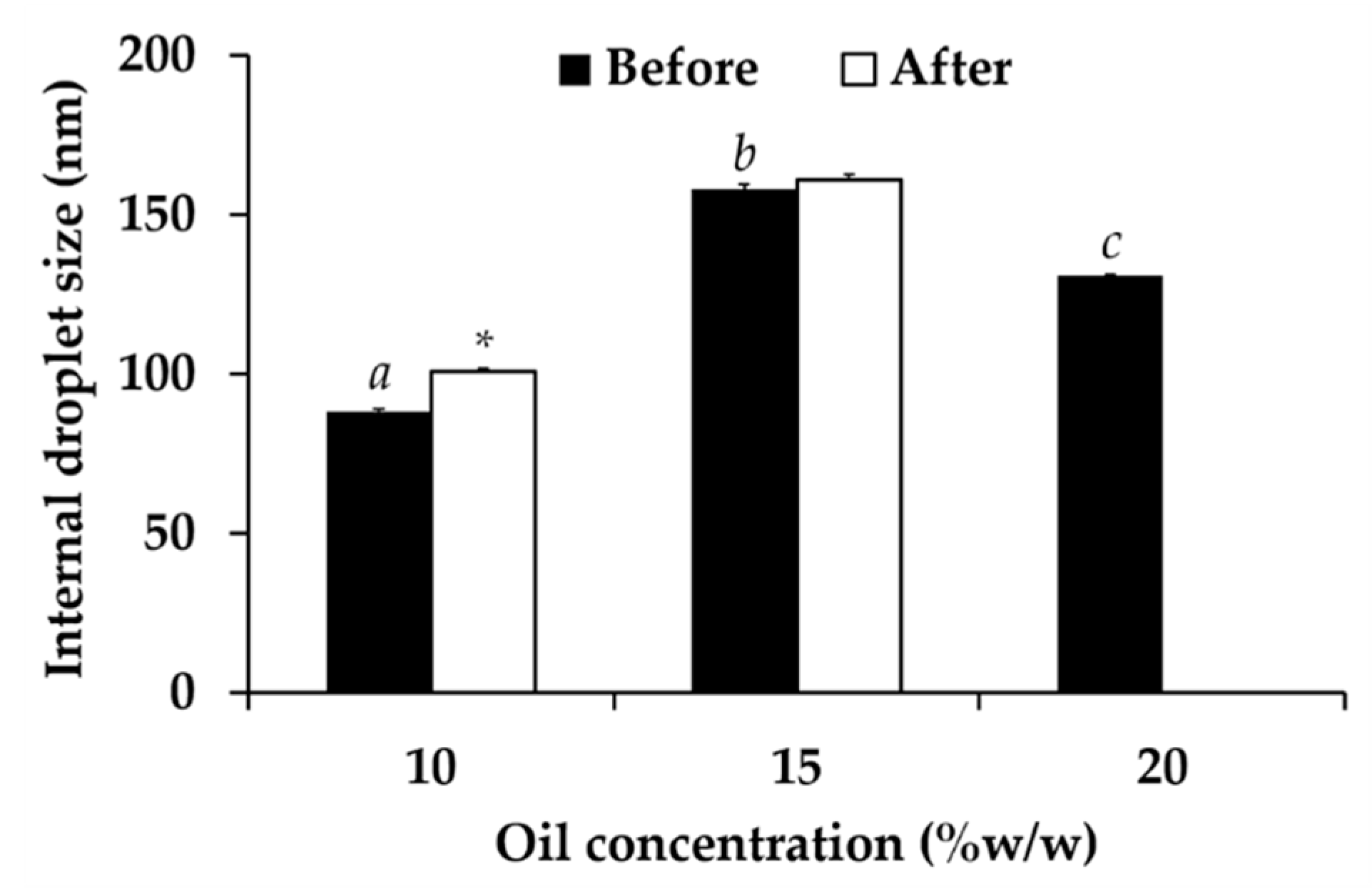
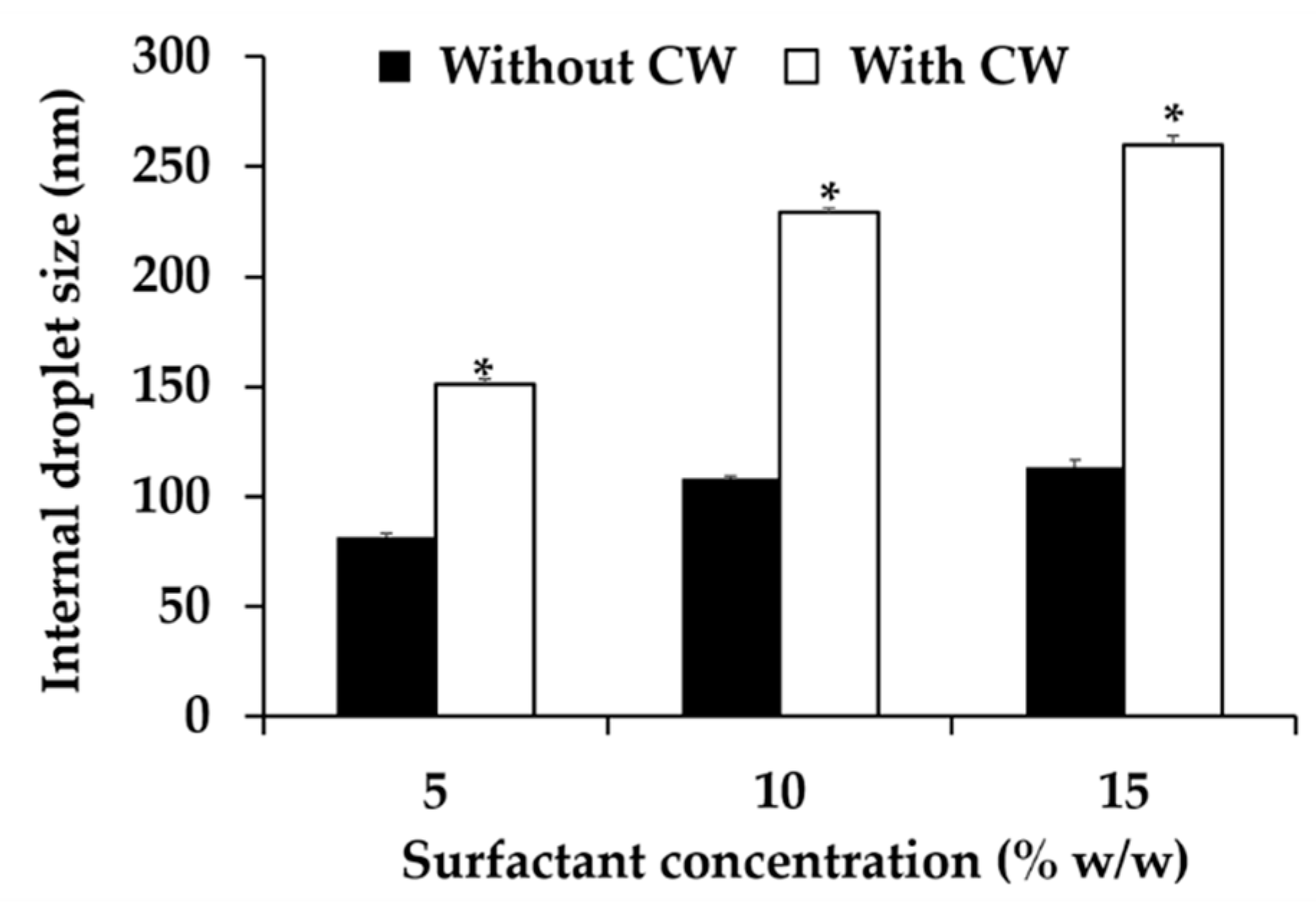
3.6. Nanoemulsion Development
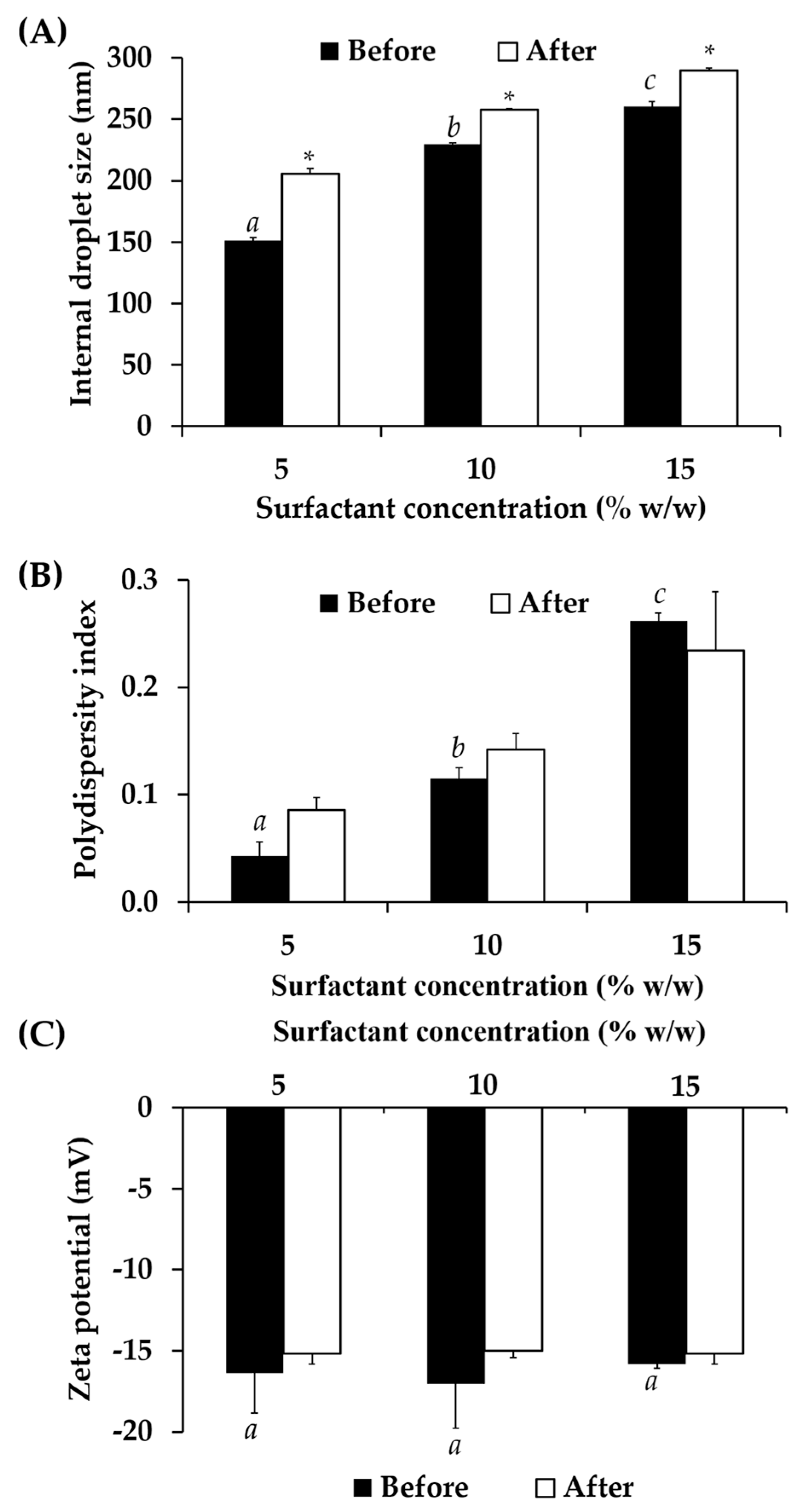
3.7. Nanoemulsion Containing C. militaris Extracts
3.7.1. Physical Stability of Nanoemulsion Containing C. militaris Extracts
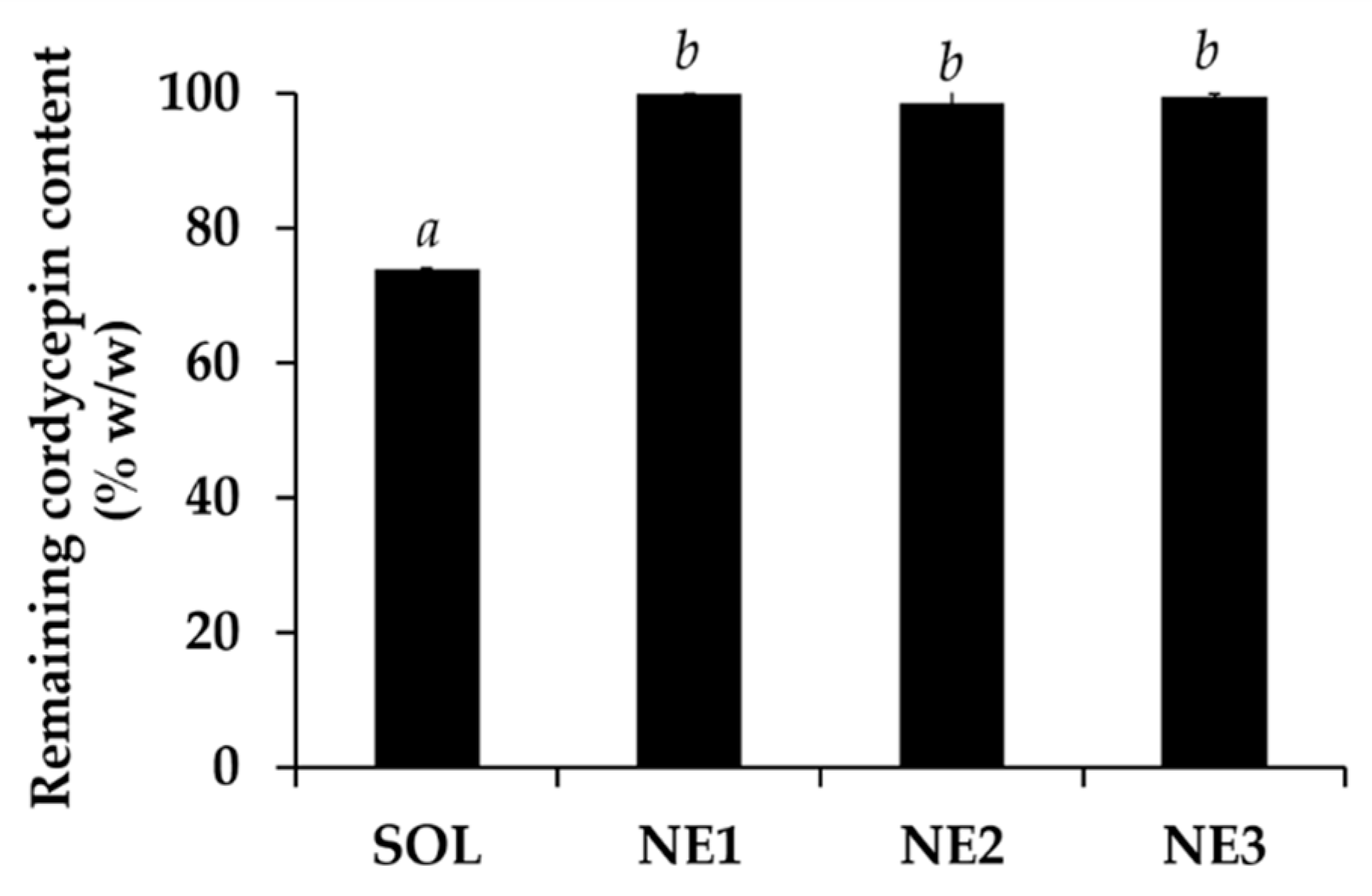
3.7.2. Chemical Stability of Nanoemulsion Containing C. militaris Extracts
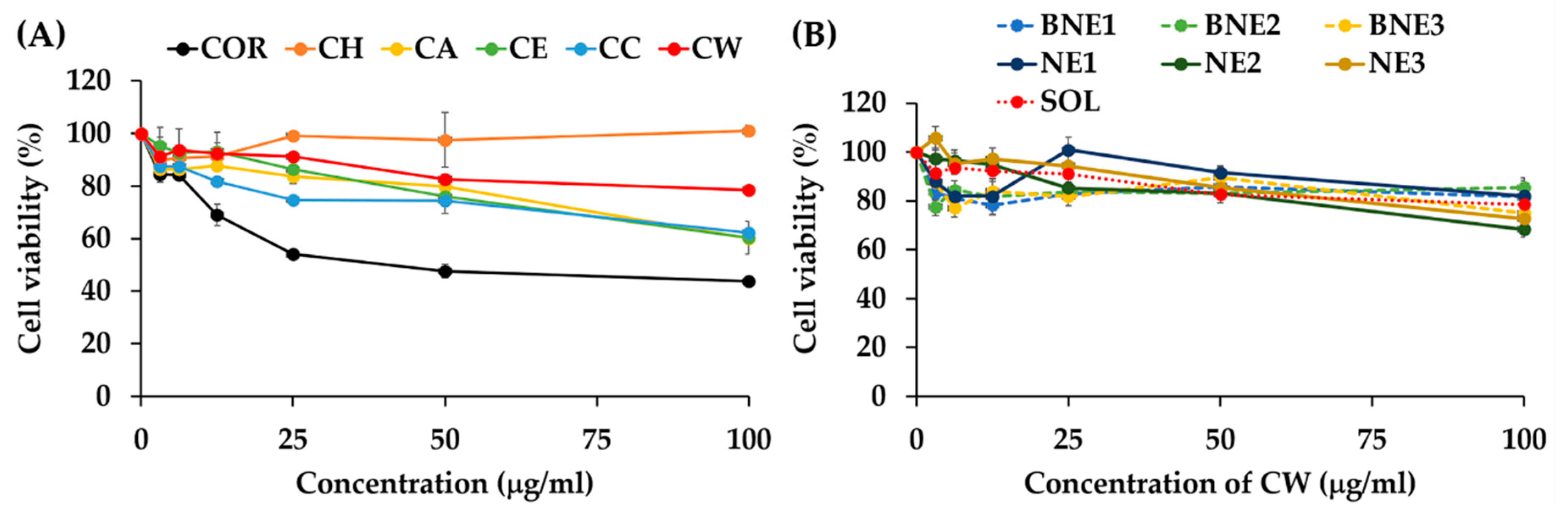
3.8. Cytotoxicity on HaCaT Cells of Nanoemulsions Containing C. militaris Extract
3.9. Irritation Effect of Nanoemulsions Containing C. militaris Extract on Human Volunteers
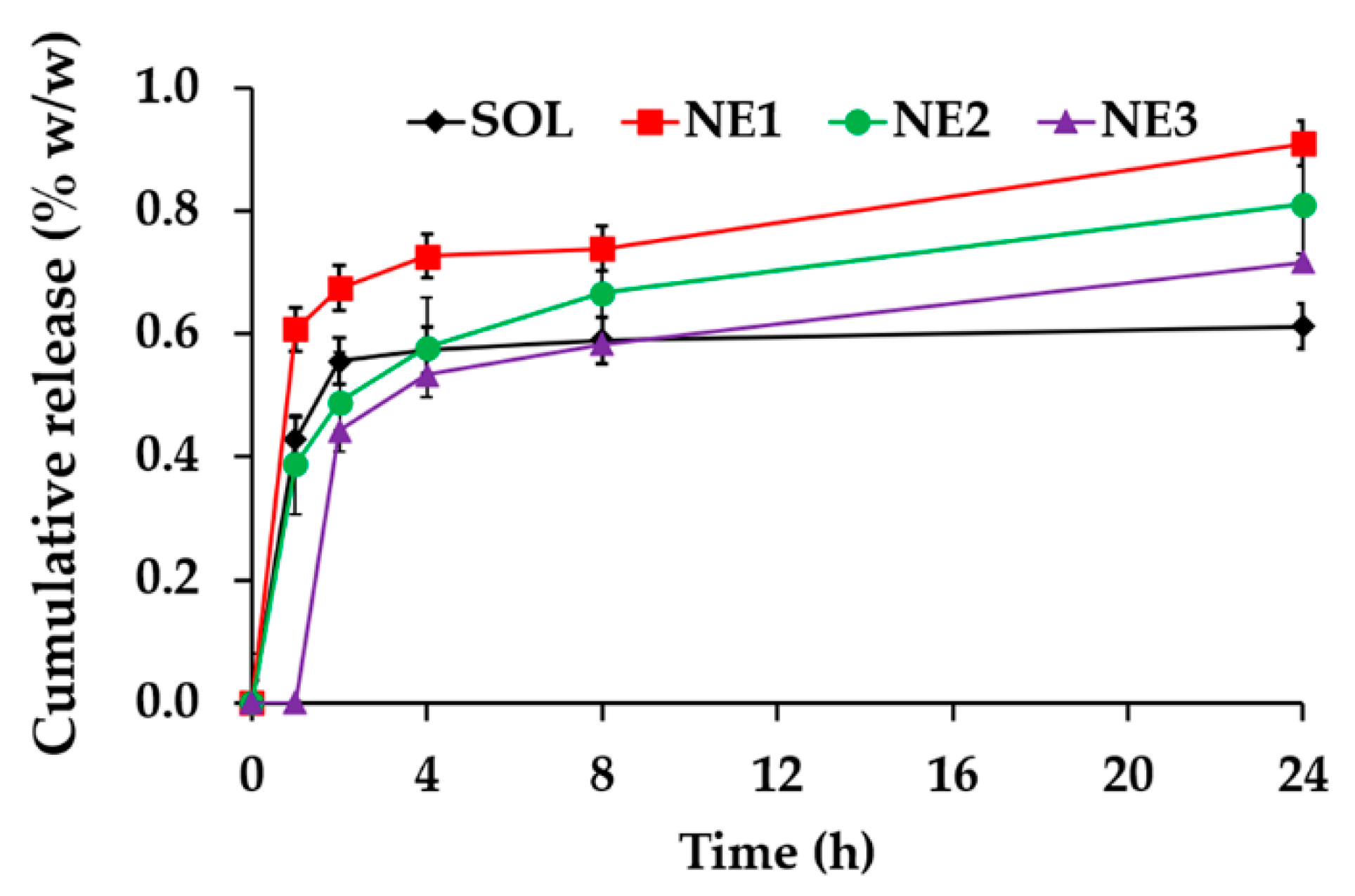
3.10. Release Profile of C. militaris Extract from Nanoemulsions
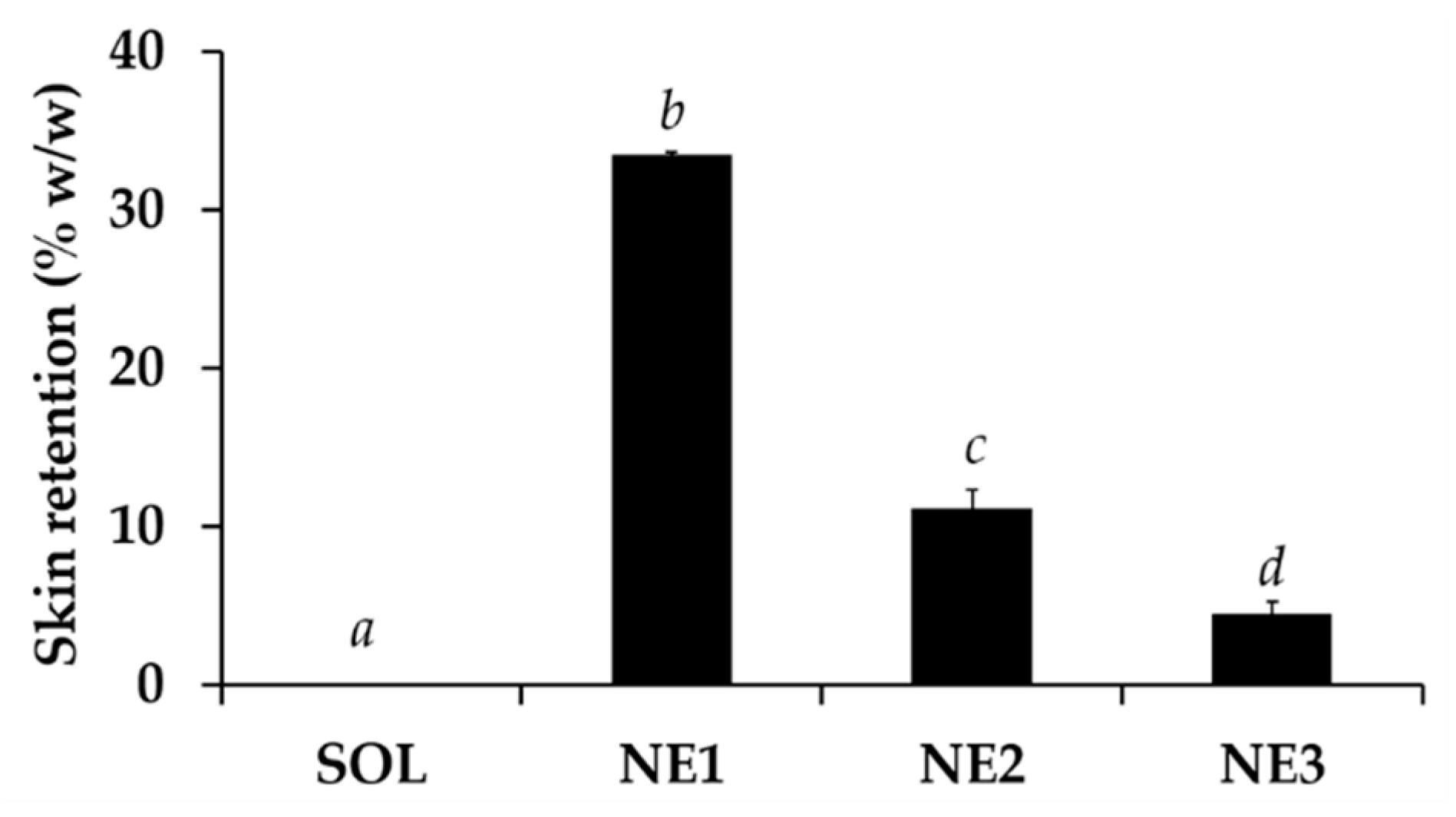
3.11. Skin Permeation and Skin Retention of C. militaris Extract from Nanoemulsions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bawadekji, A.; Al Ali, K.; Al Ali, M. A review of the bioactive compound and medicinal value of Cordyceps militaris. J. North. Basic Applied Sci. 2016, 347, 1–13. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Duan, Y.; Zhang, H.; Ma, H. Advance in Cordyceps militaris (Linn) Link polysaccharides: Isolation, structure, and bioactivities: A review. Int. J. Biol. Macromol. 2019, 132, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Mehra, A.; Zaidi, K.U.; Mani, A.; Thawani, V. The health benefits of Cordyceps militaris—A review. Kavaka 2017, 48, 27–32. [Google Scholar]
- Lee, J.B.; Radhi, M.; Cipolla, E.; Gandhi, R.D.; Sarmad, S.; Zgair, A.; Kim, T.H.; Feng, W.; Qin, C.; Adrower, C.; et al. A novel nucleoside rescue metabolic pathway may be responsible for therapeutic effect of orally administered cordycepin. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Jiang, Z.; Luo, P.; Liu, L.; Huang, Y.; Wang, H.; Wang, Y.; Long, L.; Tan, X.; et al. Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Garg, S.; Garg, A.; Shukla, A.; Dev, S.K.; Bishnoi, R.S.; Kumar, M. Review on antioxidant evaluation methods: And models In-vitro in-vivo. Asian J. Pharm. Pharmacol. 2018, 4, 147–154. [Google Scholar] [CrossRef]
- Pirakathiswaran, G.; Selvan, A.; Dhasarathan, P. Anticancer and antioxidant activity of potential of 1-Benzyl Indole-3-Carboxy Aldehydine Thio Semi Carbazone Schiff base ligand. Res. J. Chem. Environ. 2020, 24, 6. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef]
- Wu, P.K.; Tao, Z.; Ouyang, Z.; Cao, J.Y.; Geng, D.; Liu, J.; Wang, C.M. The anti-tumor effects of cordycepin-loaded liposomes on the growth of hepatoma 22 tumors in mice and human hepatoma BEL-7402 cells in culture. Drug Dev. Ind. Pharm. 2016, 42, 1424–1433. [Google Scholar] [CrossRef]
- Tang, H.; Chen, C.; Zou, Y.; Lou, H.; Zheng, Q.; Guo, L.; Lin, J.; Ye, Z.; Yun, F. Purification and structural characterization of a novel natural pigment: Cordycepene from edible and medicinal mushroom Cordyceps militaris. Appl. Microbiol. Biotechnol. 2019, 103, 7943–7952. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, Q.; Chen, Y.; Wang, X.; Zhou, X. Determination and analysis of cordycepin and adenosine in the products of Cordyceps spp. Afr. J. Microbiol. Res. 2009, 3, 957–961. [Google Scholar]
- Chaiyana, W.; Anuchapreeda, S.; Punyoyai, C.; Neimkhum, W.; Lee, K.H.; Lin, W.C.; Lue, S.C.; Viernstein, H.; Mueller, M. Ocimum sanctum Linn. as a natural source of skin anti-ageing compounds. Ind. Crops Prod. 2019, 127, 217–224. [Google Scholar] [CrossRef]
- Tachakittirungrod, S.; Okonogi, S.; Chowwanapoonpohn, S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem. 2007, 103, 381–388. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: New York, NY, USA, 1999; pp. 152–178. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Marrkham, K.P. Techniques of Flavonoids Identification; Academic Press: London, UK, 1982; pp. 36–51. [Google Scholar] [CrossRef]
- Brem, B.; Seger, C.; Pacher, T.; Hartl, M.; Hadacek, F.; Hofer, O.; Vajrodaya, S.; Greger, H. Antioxidant dehydrotocopherols as a new chemical character of Stemona species. Phytochem. 2004, 65, 2719–2729. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Okonogi, S.; Duangrat, C.; Anuchpreeda, S.; Tachakittirungrod, S.; Chowwanapoonpohn, S. Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chem. 2007, 103, 839–846. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Poomanee, W.; Chaiyana, W.; Intasai, N.; Leelapornpisid, P. Biological activities and characterization of the pod extracts from sompoi (Acacia concinna linn) grown in northern Thailand. Int. J. Pharm. Pharm. Sci. 2015, 7, 237–241. [Google Scholar]
- Niehaus Jr, W.G.; Samuelsson, B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968, 6, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Punyoyai, C.; Somwongin, S.; Leelapornpisid, P.; Ingkaninan, K.; Waranuch, N.; Srivilai, J.; Thitipramote, N.; Wisuitiprot, W.; Schuster, R.; et al. Inhibition of 5α-reductase, IL-6 secretion, and oxidation process of Equisetum debile Roxb. ex vaucher extract as functional food and nutraceuticals ingredients. Nutrients 2017, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Luepke, N.P.; Kemper, F.H. The HET-CAM test: An alternative to the Draize eye test. Food Chem. Toxicol. 1986, 24, 495–496. [Google Scholar] [CrossRef]
- Freire, P.L.; Stamford, T.C.; Albuquerque, A.J.; Sampaio, F.C.; Cavalcante, H.M.; Macedo, R.O.; Galembeck, A.; Flores, M.A.; Rosenblatt, A. Action of silver nanoparticles towards biological systems: Cytotoxicity evaluation using hen’s egg test and inhibition of Streptococcus mutans biofilm formation. Int. J. Antimicrob. Agents. 2015, 45, 183–187. [Google Scholar] [CrossRef]
- Sharma, S.; Sahni, J.K.; Ali, J.; Baboota, S. Effect of high-pressure homogenization on formulation of TPGS loaded nanoemulsion of rutin-pharmacodynamic and antioxidant studies. Drug Dev. 2015, 22, 541–551. [Google Scholar] [CrossRef]
- El Kinawy, O.S.; Petersen, S.; Ulrich, J. Technological aspects of nanoemulsion formation of low-fat foods enriched with vitamin E by high-pressure homogenization. Chem. Eng. Technol. 2012, 35, 937–940. [Google Scholar] [CrossRef]
- Laothaweerungsawat, N.; Neimkhum, W.; Anuchapreeda, S.; Sirithunyalug, J.; Chaiyana, W. Transdermal delivery enhancement of carvacrol from Origanum vulgare L. essential oil by microemulsion. Int. J. Pharm. 2020, 579, 119052. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 1997, 21, A.3B.1–A.3B.2. [Google Scholar] [CrossRef]
- Basketter, D.A.; Chamberlain, M.; Griffiths, H.A.; Rowson, M.; Whittle, E.; York, M. The classification of skin irritants by human patch test. Food Chem. Toxicol. 1997, 35, 845–852. [Google Scholar] [CrossRef]
- Kitano, M. Updating of OECD guidelines for the testing of chemicals. Wat. Sci Tech. 1992, 25, 465. [Google Scholar] [CrossRef]
- Chaiyana, W.; Rades, T.; Okonogi, S. Characterization and in vitro permeation study of microemulsions and liquid crystalline systems containing the anticholinesterase alkaloidal extract from Tabernaemontana divaricata. Int. J. Pharm. 2013, 452, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Singh, S.B.; Pandey, S. Phytochemical screening and physicochemical parameters of crude drugs: A brief review. Int. J. Pharm. Sci. Rev. Res. 2013, 2, 53–60. [Google Scholar] [CrossRef]
- Quy, T.N.; Xuan, T.D.; Andriana, Y.; Tran, H.D.; Khanh, T.D.; Teschke, R. Cordycepin Isolated from Cordyceps militaris: Its Newly Discovered Herbicidal Property and Potential Plant-Based Novel Alternative to Glyphosate. Molecules 2019, 24, 2901. [Google Scholar] [CrossRef]
- Abarca-Vargas, R.; Pena Malacara, C.F.; Petricevich, V.L. Characterization of chemical compounds with antioxidant and cytotoxic activities in bougainvillea x buttiana holttum and standl, (Var. rose) extracts. Antioxidants 2016, 5, 45. [Google Scholar] [CrossRef]
- Widyawati, P.S.; Budianta, T.D.W.; Kusuma, F.A.; Wijaya, E.L. Difference of solvent polarity to phytochemical content and antioxidant activity of Pluchea indicia less leaves extracts. Int. J. Pharmacogn. Phytochem. 2014, 6, 850–855. [Google Scholar]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Abramovič, H.; Grobin, B.; Poklar Ulrih, N.; Cigić, B. Relevance and standardization of in vitro antioxidant assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018, 2018, 4608405. [Google Scholar] [CrossRef]
- Khan, H.; Saeed, M.; Khan, M.A.; Khan, I.; Ahmad, M.; Muhammad, N.; Khan, A. Antimalarial and free radical scavenging activities of rhizomes of Polygonatum verticillatum supported by isolated metabolites. Med. Chem. Res. 2012, 21, 1278–1282. [Google Scholar] [CrossRef]
- Somwongin, S.; Chantawannakul, P.; Chaiyana, W. Antioxidant activity and irritation property of venoms from Apis species. Toxicon 2018, 145, 32–39. [Google Scholar] [CrossRef]
- Bursal, E.; Köksal, E. Evaluation of reducing power and radical scavenging activities of water and ethanol extracts from sumac (Rhus coriaria L.). Food Res. Int. 2011, 44, 2217–2221. [Google Scholar] [CrossRef]
- Ionita, P. Is DPPH stable free radical a good scavenger for oxygen active species. Chem. Pap. 2005, 59, 11–16. [Google Scholar]
- Mao, L.C.; Pan, X.; Que, F.; Fang, X.H. Antioxidant properties of water and ethanol extracts from hot air-dried and freeze-dried daylily flowers. Eur. Food Res. Technol. 2006, 222, 236–241. [Google Scholar] [CrossRef]
- Yassa, N.; Beni, H.R.; Hadjiakhoondi, A. Free radical scavenging and lipid peroxidation activity of the Shahani black grape. Pak. J. Biol. Sci. 2008, 11. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Shanab, S.M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Palanisamy, U.D.; Ling, L.T. Potential antioxidants from tropical plants. In Food Industrial Processes-Methods and Equipment; IntechOpen: London, UK, 2012; pp. 63–74. [Google Scholar] [CrossRef]
- Yu, H.M.; Wang, B.S.; Huang, S.C.; Duh, P.D. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. J. Agric. Food Chem. 2006, 54, 3132–3138. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Chen, R.; Jin, C.; Li, H.; Liu, Z.; Lu, J.; Li, S.; Yang, S. Ultrahigh pressure extraction of polysaccharides from Cordyceps militaris and evaluation of antioxidant activity. Sep. Purif. Technol. 2014, 134, 90–99. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, D.; Xu, D.; Wu, J.; Bian, X. A study on Cordyceps militaris polysaccharide purification, composition and activity analysis. Afr. J. Biotechnol. 2008, 7, 4004–4009. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, Y.; Zhang, L.; Cui, S.; Sun, Y. Structural characterization and antioxidant and immunomodulation activities of polysaccharides from the spent rice substrate of Cordyceps militaris. Food Sci. Biotechnol. 2015, 24, 24–25. [Google Scholar] [CrossRef]
- Khanum, R.; Thevanayagam, H. Lipid peroxidation: Its effects on the formulation and use of pharmaceutical emulsions. Asian J. Pharm. Sci. 2017, 12, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Calhelha, R.C.; Ćirić, A.; Van Griensven, L.J.; Soković, M.; Ferreira, I.C. The methanolic extract of Cordyceps militaris (L.) Link fruiting body shows antioxidant, antibacterial, antifungal and antihuman tumor cell lines properties. Food Chem. Toxicol. 2013, 62, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Chutvirasakul, B. Stability-Indicating Method to Determine Bioactive Nucleosides in Crude Drugs, Extracts, and products from Cordyceps sinensis and Cordyceps militaris. Thai J. Pharm. Sci. 2017, 41, 52–60. [Google Scholar] [CrossRef]
- Prasad, R.; Tewari, S.P.; Singh, J.K. Microstructural and wear characterization of friction stir processed ZrB2/AA7075 in-situ composites. Materials Research Express 2019, 6, 086514. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, S.M.; Mo, F.K.; Zhong, D.F. Investigation of microemulsion system for transdermal delivery of meloxicam. Int. J. Pharm. 2006, 321, 117–123. [Google Scholar] [CrossRef]
- Yaghmur, A.; Aserin, A.; Mizrahi, Y.; Nerd, A.; Garti, N. Argan oil-in-water emulsions: Preparation and stabilization. J. Am. Oil Chem. Soc. 1999, 76, 15–18. [Google Scholar] [CrossRef]
- Kubitschek-KM, A.R.J.; Zero, J.M. Development of jojoba oil (Simmondsia chinensis (Link) CK Schneid.) based nanoemulsions. Lat. Am. J. Pharm. 2014, 33, 459–463. [Google Scholar]
- Sessa, M.; Tsao, R.; Liu, R.; Ferrari, G.; Donsì, F. Evaluation of the stability and antioxidant activity of nanoencapsulated resveratrol during in vitro digestion. J. Agric. Food Chem. 2011, 59, 12352–12360. [Google Scholar] [CrossRef]
- Brosin, A.; Wolf, V.; Mattheus, A.; Heise, H. Use of XTT-assay to assess the cytotoxicity of different surfactants and metal salts in human keratinocytes (HaCaT). A feasible method for in vitro testing of skin irritants. Acta derm-venereol 1997, 77, 26–28. [Google Scholar] [CrossRef]
- Mackay, D.R.; Saggers, G.C.; Kotwal, N.; Manders, E.K. Stretching skin: Undermining is more important than intraoperative expansion. Plast. Reconstr. Surg. 1990, 86, 722–730. [Google Scholar] [CrossRef]
- Ferderber, K.; Hook, S.; Rades, T. Phosphatidyl choline-based colloidal systems for dermal and transdermal drug delivery. J. Liposome Res. 2009, 19, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.M.; Yardley, H.J. Lipid compositions of cells isolated from pig, human, and rat epidermis. J. Lipid Res. 1975, 16, 434–440. [Google Scholar] [PubMed]
- Cilurzo, F.; Minghetti, P.; Sinico, C. Newborn pig skin as model membrane in in vitro drug permeation studies: A technical note. Aaps Pharmscitech. 2007, 8, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Songkro, S.; Purwo, Y.; Becket, G.; Rades, T. Investigation of newborn pig skin as an in vitro animal model for transdermal drug delivery. STP Pharma Sci. 2003, 13, 133–139. [Google Scholar]

| Extracts | External Appearance | Yield (% w/w) |
|---|---|---|
| CC | Brownish semisolid mass | 7.6 ± 2.2 a |
| CH | Brownish semisolid mass | 1.8 ± 0.6 c |
| CA | Brownish semisolid mass | 0.7 ± 0.3 d |
| CE | Brownish semisolid mass | 3.6 ± 1.4 b |
| CW | Brownish powder | 0.5 ± 0.1 e |
| Extracts | Cordycepin Content (% w/w) | Total Phenolics Content (mg GA/g extract) | Total Flavonoids Content (mg QE/g extract) |
|---|---|---|---|
| CC | 1.74 ± 0.01 b | 241.1 ± 16.6 b | 5.06 ± 0.04 a |
| CH | 0.08 ± 0.00 e | 0.0 ± 9.8 e | 0.00 ± 0.03 c |
| CA | 0.98 ± 0.01 d | 67.2 ± 16.7 d | 1.52 ± 1.39 b |
| CE | 1.55 ± 0.05 c | 199.0 ± 27.1 c | 1.99 ± 0.20 b |
| CW | 1.96 ± 0.01 a | 305.6 ± 22.8 a | 5.06 ± 0.04 a |
| Samples | EC1 (µM FeSO4/mg) | TEAC (µg Trolox/mg) | Inhibition (%) | |
|---|---|---|---|---|
| DPPH | Lipid Peroxidation | |||
| l-Ascorbic acid | 10.4 ± 0.5 a | 12.4 ± 0.0 b | 93.8 ± 0.5 a | - |
| Trolox | - | - | - | 65.6 ± 1.7 b |
| Cordycepin | 1.3 ± 0.0 e | 0.0 ± 0.5 e | 12.6 ± 0.5 e | 31.9 ± 3.6 c |
| CH | 5.0 ± 0.1 c | 5.7 ± 0.5 d | 34.3 ± 1.8 d | 22.8 ± 6.0 d,e |
| CA | 2.8 ± 0.3 d | 13.4 ± 0.4 a,b | 55.0 ± 0.2 d | 22.4 ± 7.1 d,e |
| CE | 2.9 ± 0.1 d | 14.2 ± 0.5 a | 61.7 ± 4.3 c | 24.5 ± 5.4 d |
| CC | 3.3 ± 0.0 d | 11.9 ± 0.7 b,c | 72.7 ± 3.4 b | 11.4 ± 3.9 e |
| CW | 9.1 ± 0.3 b | 11.1 ± 0.3 c | 96.9 ± 3.1 a | 87.2 ± 1.0 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsup, P.; Yeerong, K.; Neimkhum, W.; Sirithunyalug, J.; Anuchapreeda, S.; To-anun, C.; Chaiyana, W. Enhancement of Chemical Stability and Dermal Delivery of Cordyceps militaris Extracts by Nanoemulsion. Nanomaterials 2020, 10, 1565. https://doi.org/10.3390/nano10081565
Marsup P, Yeerong K, Neimkhum W, Sirithunyalug J, Anuchapreeda S, To-anun C, Chaiyana W. Enhancement of Chemical Stability and Dermal Delivery of Cordyceps militaris Extracts by Nanoemulsion. Nanomaterials. 2020; 10(8):1565. https://doi.org/10.3390/nano10081565
Chicago/Turabian StyleMarsup, Pachabadee, Kankanit Yeerong, Waranya Neimkhum, Jakkapan Sirithunyalug, Songyot Anuchapreeda, Chaiwat To-anun, and Wantida Chaiyana. 2020. "Enhancement of Chemical Stability and Dermal Delivery of Cordyceps militaris Extracts by Nanoemulsion" Nanomaterials 10, no. 8: 1565. https://doi.org/10.3390/nano10081565
APA StyleMarsup, P., Yeerong, K., Neimkhum, W., Sirithunyalug, J., Anuchapreeda, S., To-anun, C., & Chaiyana, W. (2020). Enhancement of Chemical Stability and Dermal Delivery of Cordyceps militaris Extracts by Nanoemulsion. Nanomaterials, 10(8), 1565. https://doi.org/10.3390/nano10081565








