Switching Quantum Interference in Phenoxyquinone Single Molecule Junction with Light
Abstract
1. Introduction
2. Computational Methods
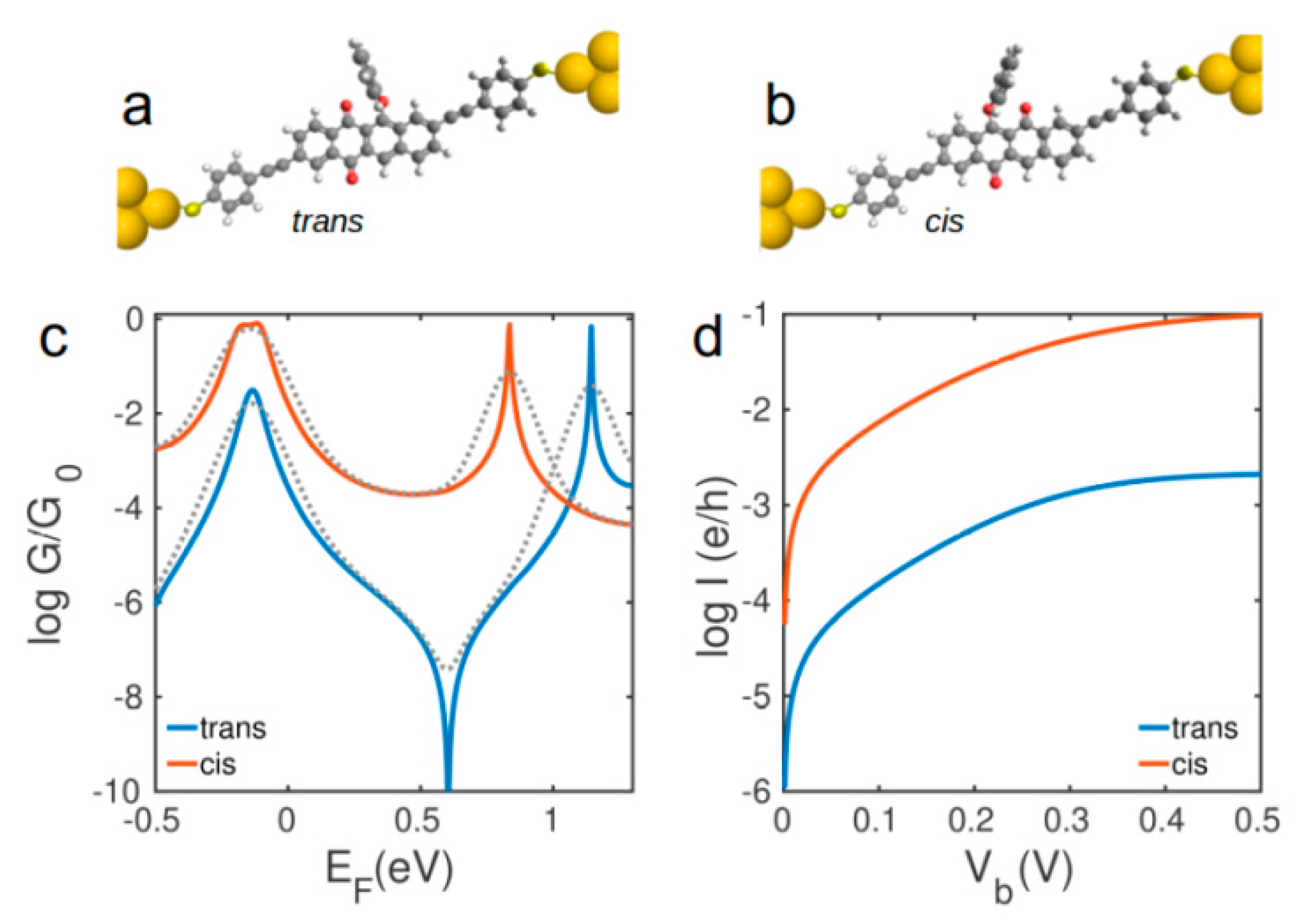
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Seong, H.C.; Kim, B.; Frisbie, C.D. Electrical resistance of long conjugated molecular wires. Science 2008, 320, 1482–1486. [Google Scholar] [CrossRef]
- Reed, M.A.; Zhou, C.; Muller, C.J.; Burgin, T.P.; Tour, J.M. Conductance of a molecular junction. Science 1997, 278, 252–254. [Google Scholar] [CrossRef]
- Tao, N.J. Electron transport in molecular junctions. Nat. Nanotechnol. 2006, 1, 173–181. [Google Scholar] [CrossRef]
- Russew, M.-M.; Hecht, S. Photoswitches: From Molecules to Materials. Adv. Mater. 2010, 22, 3348–3360. [Google Scholar] [CrossRef]
- Goulet-Hanssens, A.; Eisenreich, F.; Hecht, S. Enlightening Materials with Photoswitches. Adv. Mater. 2020, 1905966. [Google Scholar] [CrossRef]
- Chikkaraddy, R.; De Nijs, B.; Benz, F.; Barrow, S.J.; Scherman, O.A.; Rosta, E.; Demetriadou, A.; Fox, P.; Hess, O.; Baumberg, J.J. Single-molecule strong coupling at room temperature in plasmonic nanocavities. Nature 2016, 535, 127–130. [Google Scholar] [CrossRef]
- Harzmann, G.D.; Frisenda, R.; Van Der Zant, H.S.J.; Mayor, M. Single-Molecule Spin Switch Based on Voltage-Triggered Distortion of the Coordination Sphere. Angew. Chem. Int. Ed. 2015, 54, 13425–13430. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhong, J.Q.; Lin, J.D.; Hu, W.P.; Wu, K.; Xu, G.Q.; Wee, A.T.S.; Chen, W. Towards single molecule switches. Chem. Soc. Rev. 2015, 44, 2998–3022. [Google Scholar] [CrossRef]
- Canary, J.W. Redox-triggered chiroptical molecular switches. Chem. Soc. Rev. 2009, 38, 747. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, L.; Samorì, P. Coupling carbon nanomaterials with photochromic molecules for the generation of optically responsive materials. Nat. Commun. 2016, 7, 11118. [Google Scholar] [CrossRef]
- Huang, X.; Li, T. Recent progress in the development of molecular-scale electronics based on photoswitchable molecules. J. Mater. Chem. C 2020, 8, 821–848. [Google Scholar] [CrossRef]
- Bouas-Laurent, H.; Dürr, H. Organic photochromism (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 639–665. [Google Scholar] [CrossRef]
- Jia, C.; Wang, J.; Yao, C.; Cao, Y.; Zhong, Y.; Liu, Z.; Liu, Z.; Guo, X. Conductance switching and mechanisms in single-molecule junctions. Angew. Chem. Int. Ed. 2013, 52, 8666–8670. [Google Scholar] [CrossRef]
- Kim, Y.; Hellmuth, T.J.; Sysoiev, D.; Pauly, F.; Pietsch, T.; Wolf, J.; Erbe, A.; Huhn, T.; Groth, U.; Steiner, U.E.; et al. Charge transport characteristics of diarylethene photoswitching single-molecule junctions. Nano Lett. 2012. [Google Scholar] [CrossRef]
- Dulić, D.; van der Molen, S.J.; Kudernac, T.; Jonkman, H.T.; de Jong, J.J.D.; Bowden, T.N.; van Esch, J.; Feringa, B.L.; van Wees, B.J. One-way optoelectronic switching of photochromic molecules on gold. Phys. Rev. Lett. 2003, 91, 207402. [Google Scholar] [CrossRef]
- Marquardt, C.W.; Grunder, S.; Błaszczyk, A.; Dehm, S.; Hennrich, F.; Löhneysen, H.V.; Mayor, M.; Krupke, R. Electroluminescence from a single nanotube-molecule-nanotube junction. Nat. Nanotechnol. 2010, 5, 863–867. [Google Scholar] [CrossRef]
- Roldan, D.; Kaliginedi, V.; Cobo, S.; Kolivoska, V.; Bucher, C.; Hong, W.; Royal, G.; Wandlowski, T. Charge transport in photoswitchable dimethyldihydropyrene-type single-molecule junctions. J. Am. Chem. Soc. 2013, 135, 5974–5977. [Google Scholar] [CrossRef]
- Purkait, M.K.; Sinha, M.K.; Mondal, P.; Singh, R. Photoresponsive Membranes. Interface Sci. Technol. 2018, 25, 115–144. [Google Scholar] [CrossRef]
- Rau, H. Photoisomerization of Azobenzenes. In Photoreactive Organic Thin Films; Elsevier: Amsterdam, The Netherlands, 2002; pp. 3–47. [Google Scholar]
- Martin, S.; Haiss, W.; Higgins, S.J.; Nichols, R.J. The Impact of E—Z Photo-Isomerization on Single Molecular Conductance. Nano Lett. 2010, 10, 2019–2023. [Google Scholar] [CrossRef]
- Qin, M.; Huang, Y.; Li, F.; Song, Y. Photochromic sensors: A versatile approach for recognition and discrimination. J. Mater. Chem. C 2015, 3, 9265–9275. [Google Scholar] [CrossRef]
- Osella, S.; Samorì, P.; Cornil, J. Photoswitching Azobenzene Derivatives in Single Molecule Junctions: A Theoretical Insight into the I/V Characteristics. J. Phys. Chem. C 2014, 118, 18721–18729. [Google Scholar] [CrossRef]
- Mativetsky, J.M.; Pace, G.; Elbing, M.; Rampi, M.A.; Mayor, M.; Samorì, P. Azobenzenes as Light-Controlled Molecular Electronic Switches in Nanoscale Metal—Molecule—Metal Junctions. J. Am. Chem. Soc. 2008, 130, 9192–9193. [Google Scholar]
- Kumar, A.S.; Ye, T.; Takami, T.; Yu, B.C.; Flatt, A.K.; Tour, J.M.; Weiss, P.S. Reversible photo-switching of single azobenzene molecules in controlled nanoscale environments. Nano Lett. 2008, 8, 1644–1648. [Google Scholar] [CrossRef]
- Xiang, D.; Wang, X.; Jia, C.; Lee, T.; Guo, X. Molecular-Scale Electronics: From Concept to Function. Chem. Rev. 2016, 116, 4318–4440. [Google Scholar] [CrossRef]
- Liu, X.; Sangtarash, S.; Reber, D.; Zhang, D.; Sadeghi, H.; Shi, J.; Xiao, Z.Y.; Hong, W.; Lambert, C.J.; Liu, S.X. Gating of Quantum Interference in Molecular Junctions by Heteroatom Substitution. Angew. Chem. Int. Ed. 2017, 56, 173–176. [Google Scholar] [CrossRef]
- Park, I.S.; Heo, E.J.; Kim, J.M. A photochromic phenoxyquinone based cyanide ion sensor. Tetrahedron Lett. 2011, 52, 2454–2457. [Google Scholar] [CrossRef]
- Pace, G.; Ferri, V.; Grave, C.; Elbing, M.; von Hanisch, C.; Zharnikov, M.; Mayor, M.; Rampi, M.A.; Samori, P. Cooperative light-induced molecular movements of highly ordered azobenzene self-assembled monolayers. Proc. Natl. Acad. Sci. USA 2007, 104, 9937–9942. [Google Scholar] [CrossRef]
- Moresco, F.; Meyer, G.; Rieder, K.-H.; Tang, H.; Gourdon, A.; Joachim, C. Conformational Changes of Single Molecules Induced by Scanning Tunneling Microscopy Manipulation: A Route to Molecular Switching. Phys. Rev. Lett. 2001, 86, 672–675. [Google Scholar] [CrossRef]
- Soler, J.M.; Artacho, E.; Gale, J.D.; García, A.; Junquera, J.; Ordejón, P.; Sánchez-Portal, D. The SIESTA method for ab initio order- N materials simulation. J. Phys. Condens. Matter. 2002, 14, 2745–2779. [Google Scholar] [CrossRef]
- Sadeghi, H. Theory of electron, phonon and spin transport in nanoscale quantum devices. Nanotechnology 2018, 29, 373001. [Google Scholar] [CrossRef]
- Ferrer, J.; Lambert, C.J.; García-Suárez, V.M.; Manrique, D.Z.; Visontai, D.; Oroszlany, L.; Rodríguez-Ferradás, R.; Grace, I.; Bailey, S.W.D.; Gillemot, K.; et al. GOLLUM: A next-generation simulation tool for electron, thermal and spin transport. New J. Phys. 2014, 16, 093029. [Google Scholar] [CrossRef]
- Jia, C.; Migliore, A.; Xin, N.; Huang, S.; Wang, J.; Yang, Q.; Wang, S.; Chen, H.; Wang, D.; Feng, B.; et al. Covalently bonded single-molecule junctions with stable and reversible photoswitched conductivity. Science 2016, 352, 1443–1445. [Google Scholar] [CrossRef]
- Kermack, W.O.; Robinson, R. LI.—An explanation of the property of induced polarity of atoms and an interpretation of the theory of partial valencies on an electronic basis. J. Chem. Soc. Trans. 1922, 121, 427–440. [Google Scholar] [CrossRef]



© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daaoub, A.; Sangtarash, S.; Sadeghi, H. Switching Quantum Interference in Phenoxyquinone Single Molecule Junction with Light. Nanomaterials 2020, 10, 1544. https://doi.org/10.3390/nano10081544
Daaoub A, Sangtarash S, Sadeghi H. Switching Quantum Interference in Phenoxyquinone Single Molecule Junction with Light. Nanomaterials. 2020; 10(8):1544. https://doi.org/10.3390/nano10081544
Chicago/Turabian StyleDaaoub, Abdalghani, Sara Sangtarash, and Hatef Sadeghi. 2020. "Switching Quantum Interference in Phenoxyquinone Single Molecule Junction with Light" Nanomaterials 10, no. 8: 1544. https://doi.org/10.3390/nano10081544
APA StyleDaaoub, A., Sangtarash, S., & Sadeghi, H. (2020). Switching Quantum Interference in Phenoxyquinone Single Molecule Junction with Light. Nanomaterials, 10(8), 1544. https://doi.org/10.3390/nano10081544




