Investigation of WO3 Electrodeposition Leading to Nanostructured Thin Films
Abstract
1. Introduction
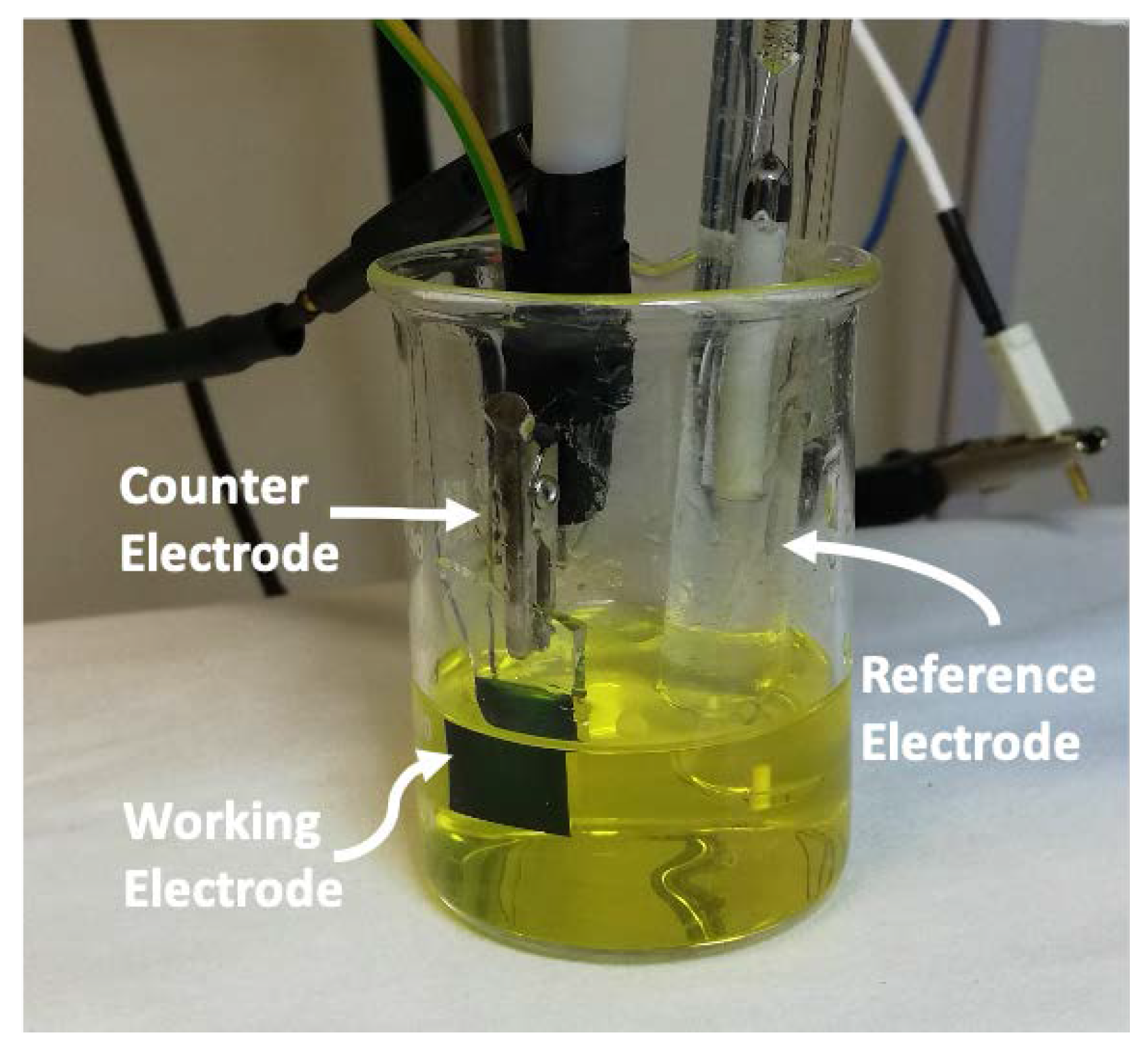
2. Materials and Methods
2.1. Chemicals
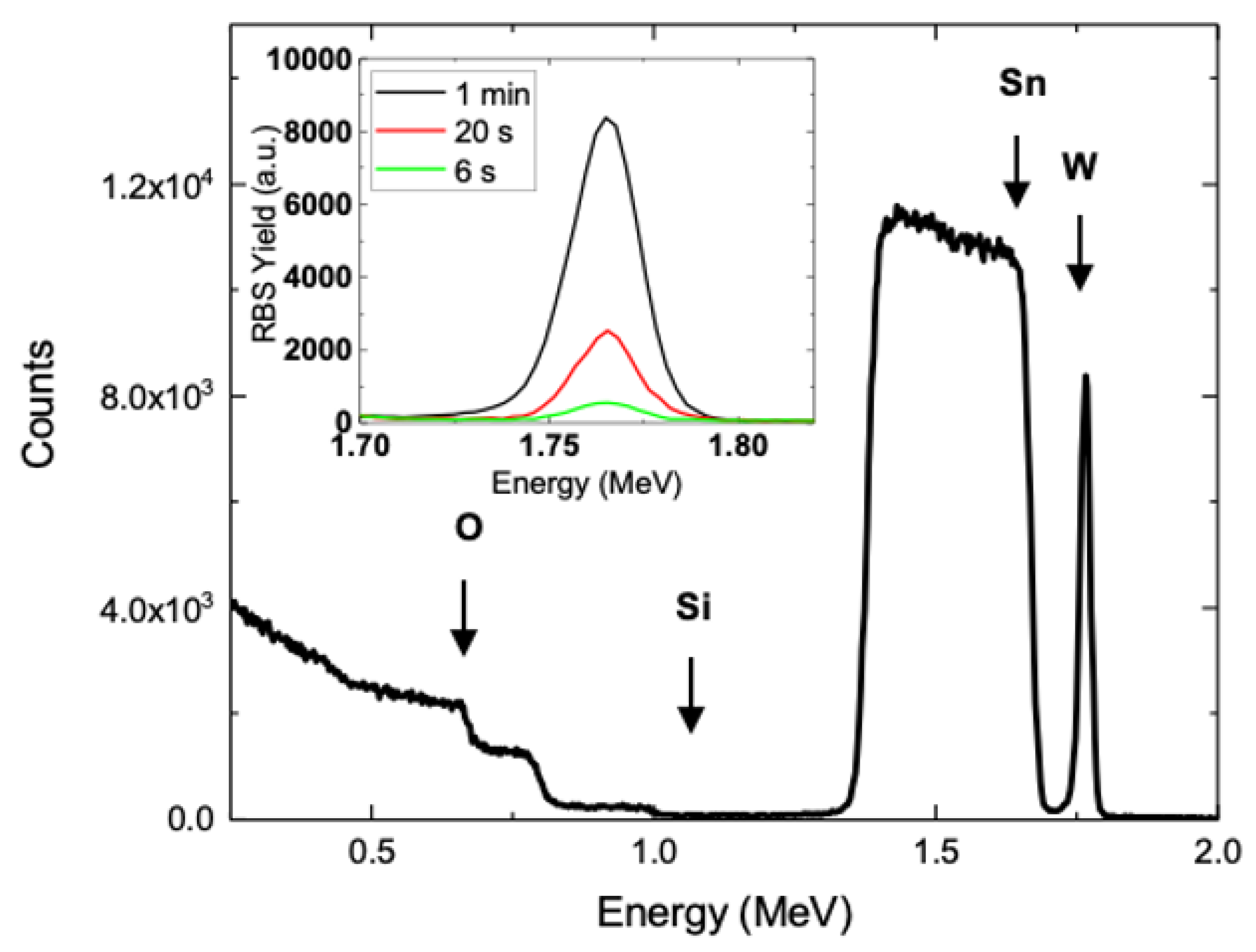
2.2. Characterization
3. Results and Discussion
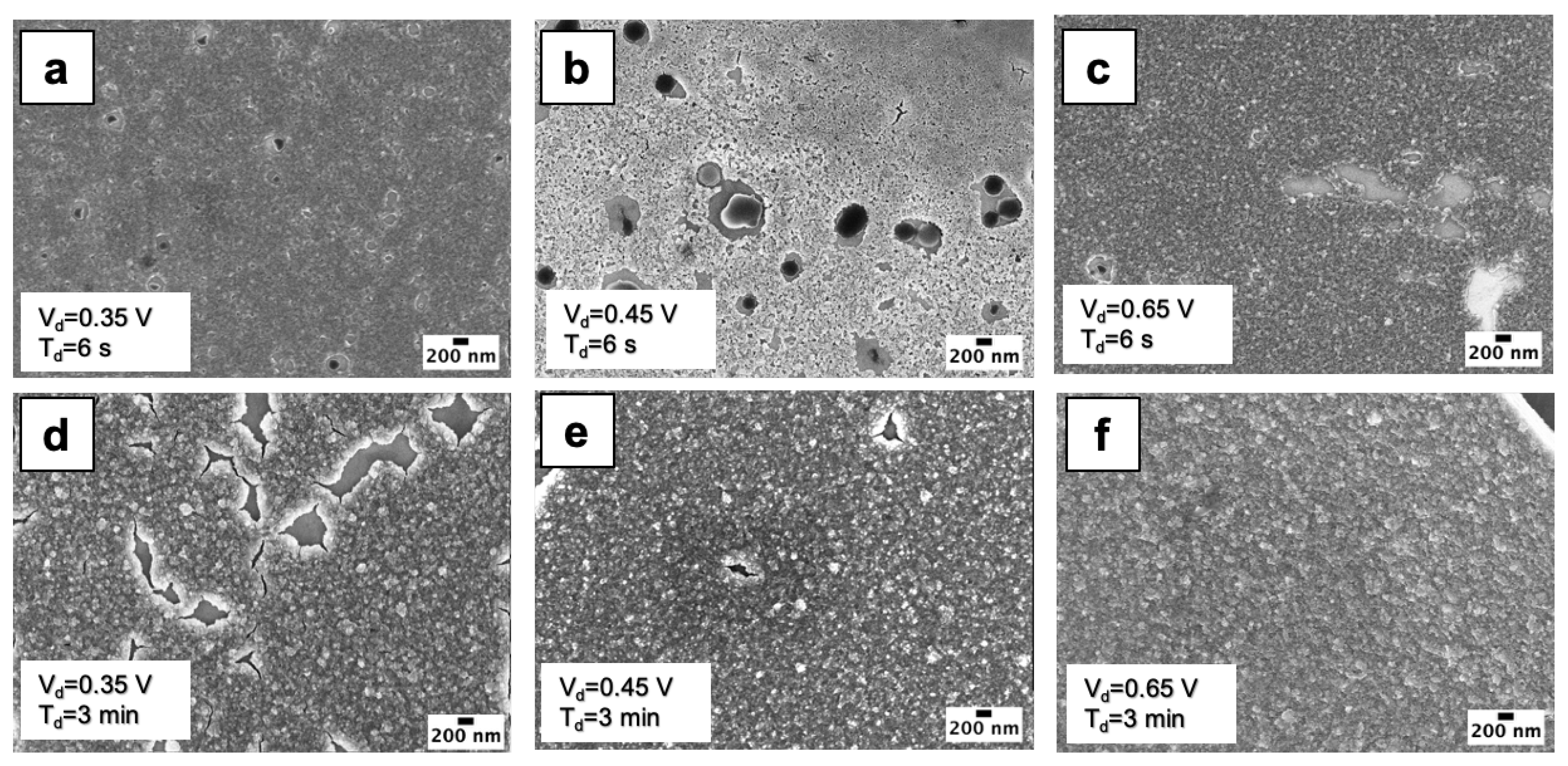
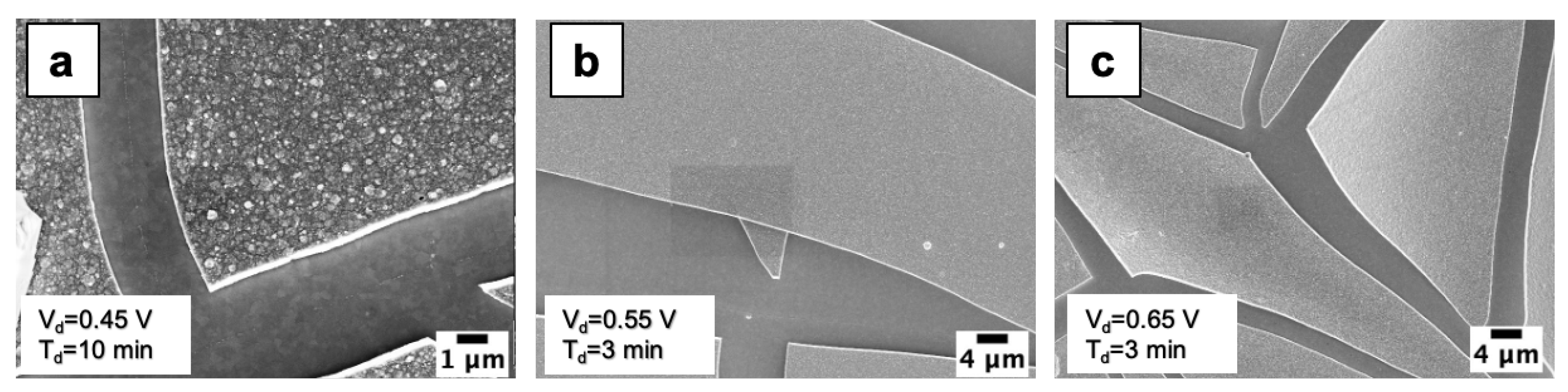
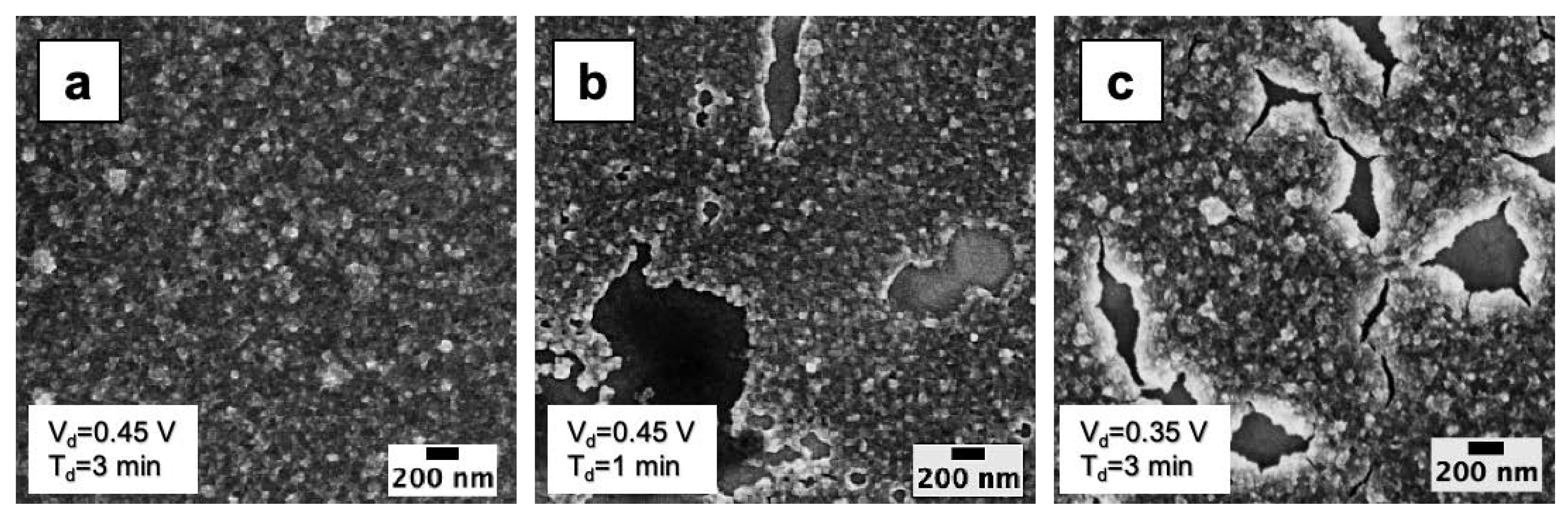
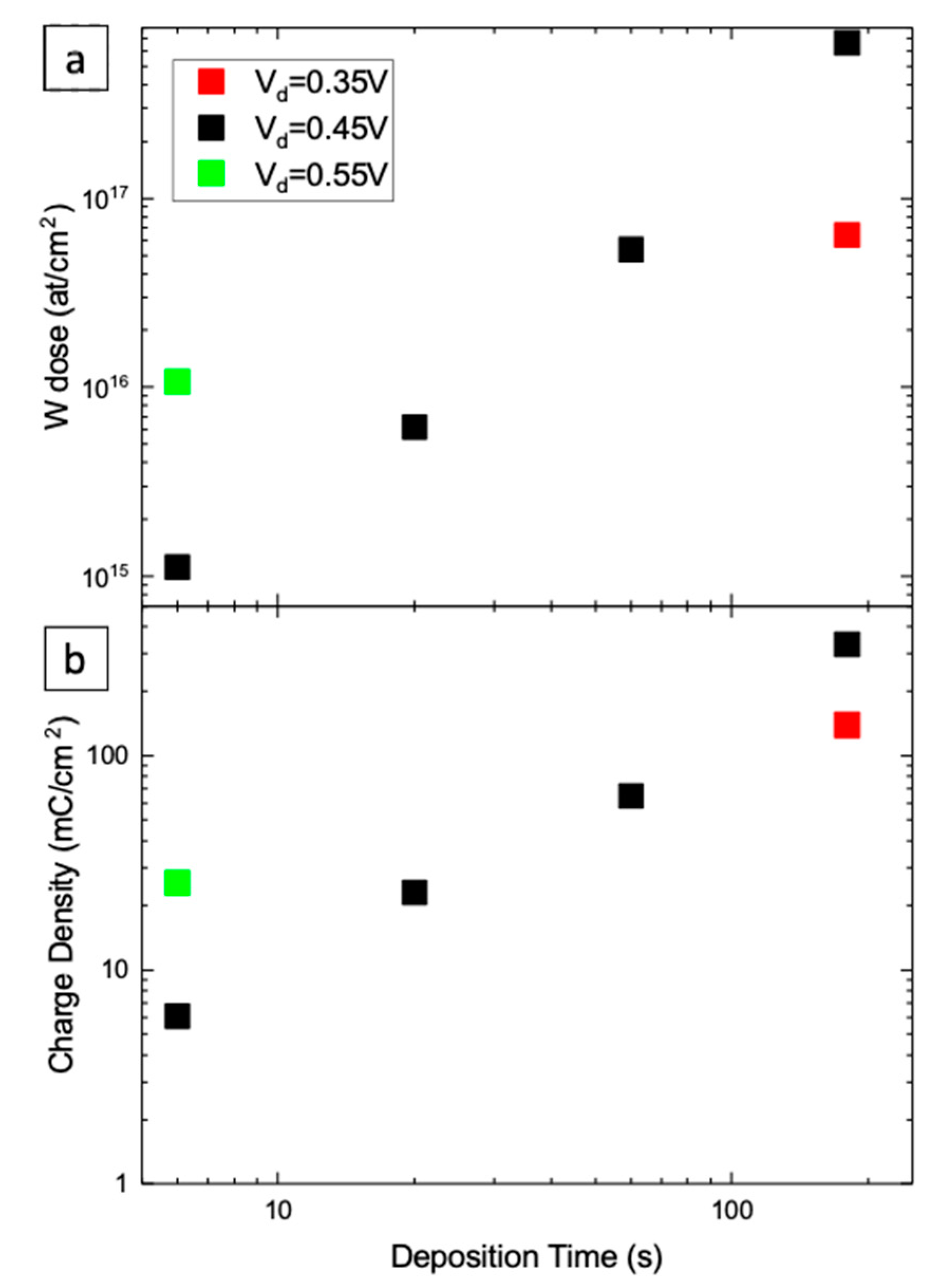
3.1. Morphological Analyses
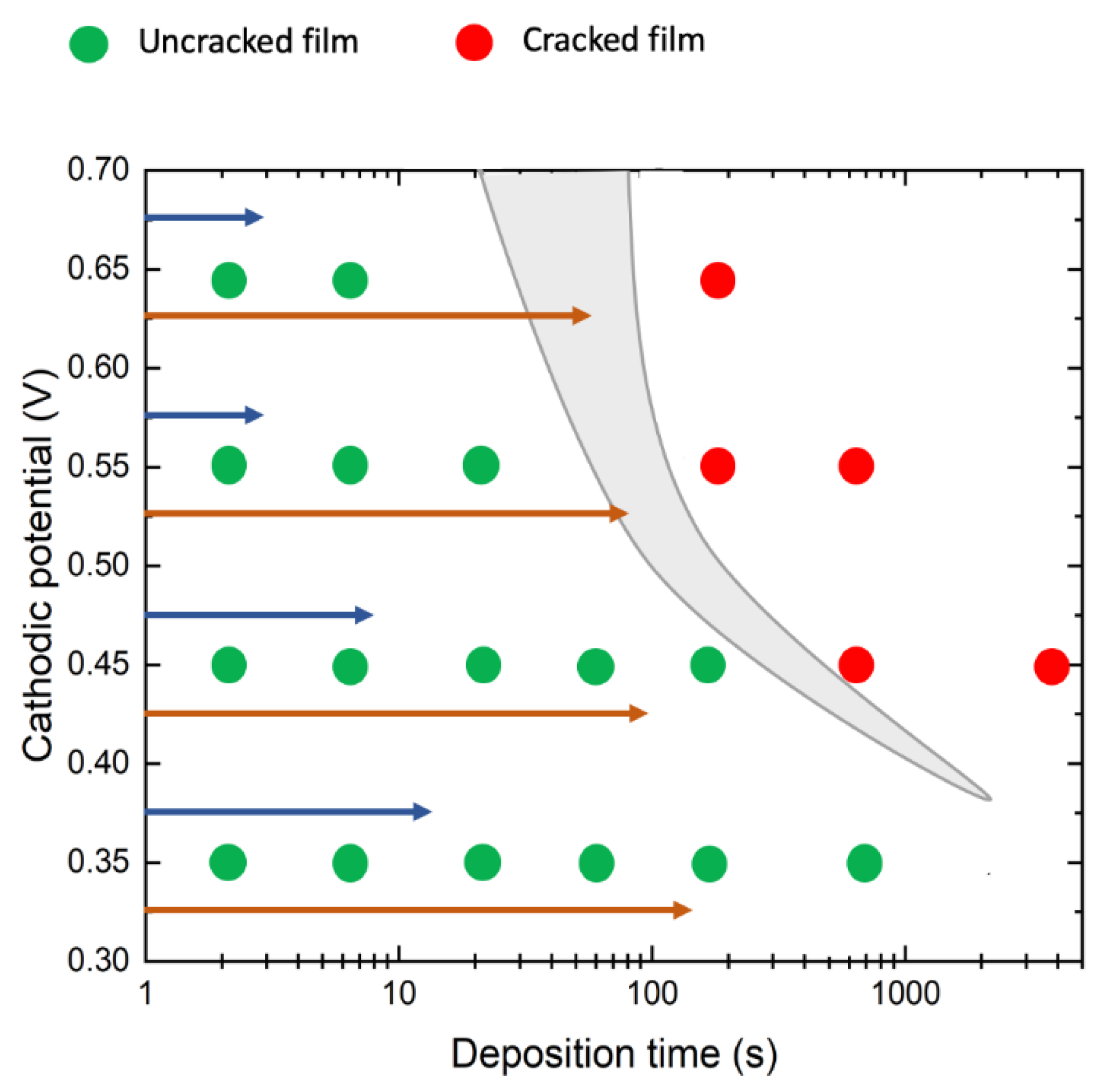
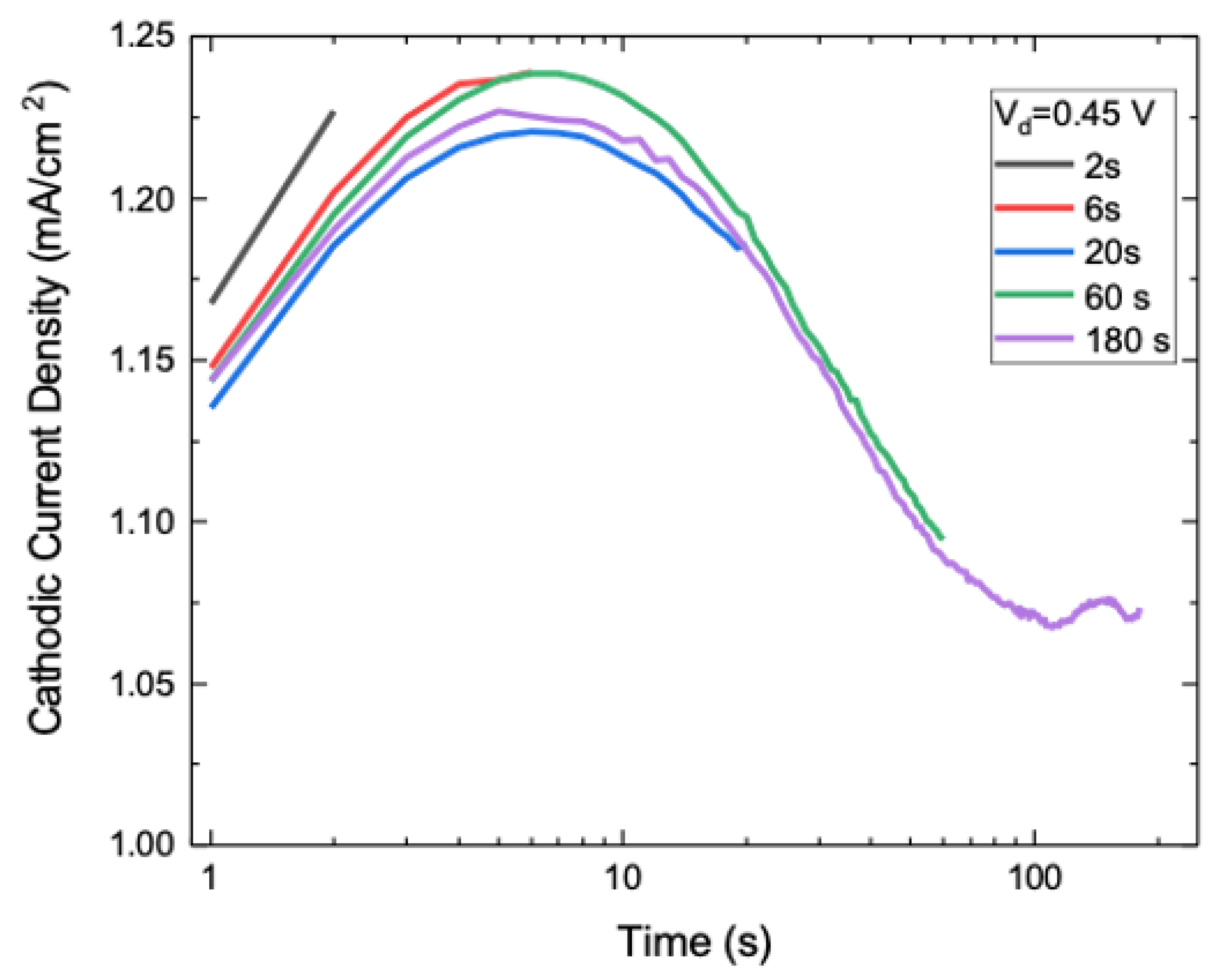
3.2. Nucleation and Growth of WO3 Films
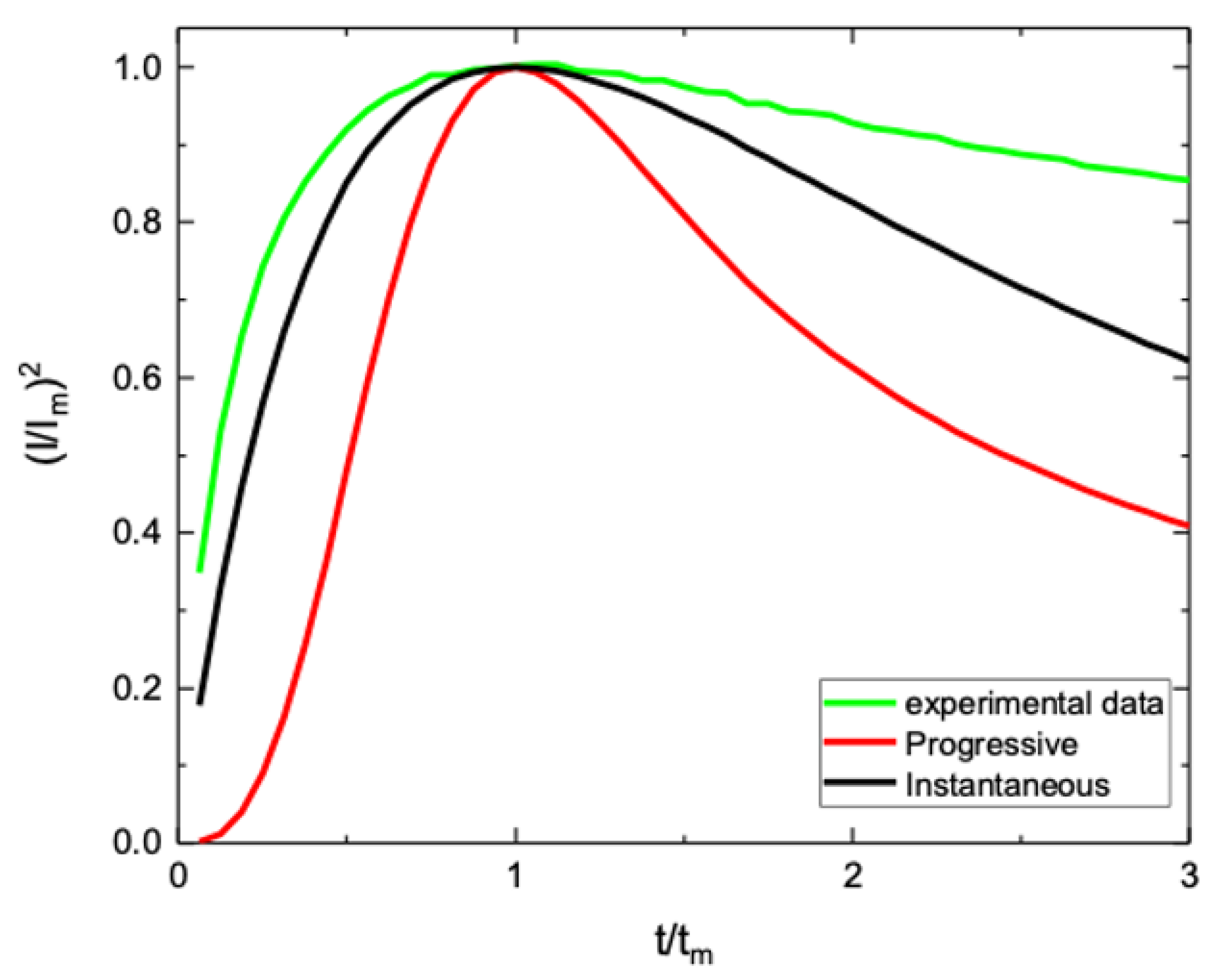
3.3. Model for WO3 Electrodeposition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deb, S.K. Opportunities and challenges in science and technology of WO3 for electrochromic and related applications. Sol. Energy Mater. Sol. Cells 2008, 92, 245–258. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromic tungsten oxide films: review of progress 1993–1998. Sol. Energy Mater. Sol. Cells 2000, 60, 201–262. [Google Scholar] [CrossRef]
- Thummavichai, K.; Xia, Y.; Zhu, Y. Recent progress in chromogenic research of tungsten oxides towards energy-related applications. Prog. Mater. Sci. 2017, 88, 281–324. [Google Scholar] [CrossRef]
- Ahsan, M.; Tesfamichael, T.; Ionescu, M.; Bell, J.; Motta, N. Low temperature CO sensitive nanostructured WO3 thin films doped with Fe. Sens. Actuators B Chem. 2012, 162, 14–21. [Google Scholar] [CrossRef]
- Kida, T.; Nishiyama, A.; Hua, Z.; Suematsu, K.; Yuasa, M.; Shimanoe, K. WO3 nanolamella gas sensor: porosity control using SnO2 nanoparticles for enhanced NO2 sensing. Langmuir 2014, 30, 2571–2579. [Google Scholar] [CrossRef]
- Ponzoni, A.; Comini, E.; Ferroni, M.; Sberveglieri, G. Nanostructured WO3 deposited by modified thermal evaporation for gas-sensing applications. Thin Solid Films 2005, 490, 81–85. [Google Scholar] [CrossRef]
- Grace, P.S.; Devadasan, J.J.; Jeyadheepan, K.; Thangam, G.J. Room temperature NH3-sensing properties of WO3 thin films synthesized by microprocessor controlled spray pyrolysis. IOSR J. Appl. Phys. 2017, 3, 52–56. [Google Scholar] [CrossRef]
- Shi, J.; Cheng, Z.; Gao, L.; Zhang, Y.; Xu, J.; Zhao, H. Facile synthesis of reduced graphene oxide/hexagonal WO3 nanosheets composites with enhanced H2S sensing properties. Sens. Actuators B Chem. 2016, 230, 736–745. [Google Scholar] [CrossRef]
- Kalantar-zadeh, K.; Fry, B. Nanotechnology-Enabled Sensors; Springer Science & Business Media: Medford, MA, USA, 2007. [Google Scholar]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-zadeh, K. Nanostructured tungsten oxide-properties, synthesis, and applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Lee, S.H.; Deshpande, R.; Parilla, P.A.; Jones, K.M.; To, B.; Mahan, A.H.; Dillon, A.C. Crystalline WO3 nanoparticles for highly improved electrochromic applications. Adv. Mater. 2006, 18, 763–766. [Google Scholar] [CrossRef]
- Karakurt, İ.; Boneberg, J.; Leiderer, P. Electrochromic switching of WO3 nanostructures and thin films. Appl. Phys. A 2006, 83, 1–3. [Google Scholar] [CrossRef]
- Nah, Y.C.; Ghicov, A.; Kim, D.; Schmuki, P. Enhanced electrochromic properties of self-organized nanoporous WO3. Electrochem. commun. 2008, 10, 1777–1780. [Google Scholar] [CrossRef]
- Kim, Y.O.; Yu, S.H.; Ahn, K.S.; Lee, S.K.; Kang, S.H. Enhancing the photoresponse of electrodeposited WO3 film: Structure and thickness effect. J. Electroanal. Chem. 2005, 752, 25–32. [Google Scholar] [CrossRef]
- Lemire, C.; Lollman, D.B.; Al Mohammad, A.; Gillet, E.; Aguir, K. Reactive RF magnetron sputtering deposition of WO3 thin films. Sens. Actuators B Chem. 2005, 84, 43–48. [Google Scholar] [CrossRef]
- Hong, K.; Xie, M.; Wu, H. Tungsten oxide nanowires synthesized by a catalyst-free method at low temperature. Nanotechnology 2006, 17, 4830. [Google Scholar] [CrossRef]
- Djaoued, Y.; Priya, S.; Balaji, S. Low temperature synthesis of nanocrystalline WO3 films by sol-gel process. J. Non Cryst. Solids 2008, 354, 673–679. [Google Scholar] [CrossRef]
- Deepa, M.; Kar, M.; Agnihotry, S.A. Electrodeposited tungsten oxide films: annealing effects on structure and electrochromic performance. Thin Solid Films 2004, 468, 32–42. [Google Scholar] [CrossRef]
- Kalhori, H.; Ranjbar, M.; Salamati, H.; Coey, J.M.D. Flower-like nanostructures of WO3: Fabrication and characterization of their in-liquid gasochromic effect. Sens. Actuators B Chem. 2016, 225, 535–543. [Google Scholar] [CrossRef]
- Poongodi, S.; Kumar, P.S.; Mangalaraj, D.; Ponpandian, N.; Meena, P.; Masuda, Y.; Lee, C. Electrodeposition of WO3 nanostructured thin films for electrochromic and H2S gas sensor applications. J. Alloys Compd. 2017, 719, 71–81. [Google Scholar] [CrossRef]
- Deepa, M.; Srivastava, A.K.; Sood, K.N.; Agnihotry, S.A. Nanostructured mesoporous tungsten oxide films with fast kinetics for electrochromic smart windows. Nanotechnology 2006, 17, 2625. [Google Scholar] [CrossRef]
- More, A.J.; Patil, R.S.; Dalavi, D.S.; Mali, S.S.; Hong, C.K.; Gang, M.G.; Patil, P.S. Electrodeposition of nano-granular tungsten oxide thin films for smart window application. Mater. Lett. 2014, 134, 298–301. [Google Scholar] [CrossRef]
- Baeck, S.H.; Jaramillo, T.; Stucky, G.D.; McFarland, E.W. Controlled electrodeposition of nanoparticulate tungsten oxide. Nano Lett. 2002, 2, 831–834. [Google Scholar] [CrossRef]
- Vijayakumar, E.; Yun, Y.H.; Quy, V.H.V.; Lee, Y.H.; Kang, S.H.; Ahn, K.S.; Lee, S.W. Development of tungsten trioxide using pulse and continuous electrodeposition and its properties in electrochromic devices. J. Electrochem. Soc. 2019, 166, D86. [Google Scholar] [CrossRef]
- Kwong, W.L.; Savvides, N.; Sorrel, C.C. Electrodeposited nanostructured WO3 thin films for photoelectrochemical applications. Electrochim. Acta 2012, 75, 371–380. [Google Scholar] [CrossRef]
- Leftheriotis, G.; Yianoulis, P. Development of electrodeposited WO3 films with modified surface morphology and improved electrochromic properties. Solid State Ionics 2008, 179, 2192–2197. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Deepa, M.; Singh, S.; Kishore, R.; Agnihotry, S.A. Microstructural and electrochromic characteristics of electrodeposited and annealed WO3 films. Solid State Ionics 2005, 176, 1161–1168. [Google Scholar] [CrossRef]
- Raciti, R.; Bahariqushchi, R.; Summonte, C.; Aydinli, A.; Terrasi, A.; Mirabella, S. Optical bandgap of semiconductor nanostructures: methods for experimental data analysis. J. Appl. Phys. 2017, 121, 234304. [Google Scholar] [CrossRef]
- Scharifker, B.; Hills, G. Theoretical and experimental studies of multiple nucleation. Electrochim. Acta 1983, 28, 879–889. [Google Scholar] [CrossRef]
- Meulenkamp, E.A. Mechanism of WO3 Electrodeposition from Peroxy-Tungstate Solution. J. Electrochem. Soc. 1997, 144, 1664. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mineo, G.; Ruffino, F.; Mirabella, S.; Bruno, E. Investigation of WO3 Electrodeposition Leading to Nanostructured Thin Films. Nanomaterials 2020, 10, 1493. https://doi.org/10.3390/nano10081493
Mineo G, Ruffino F, Mirabella S, Bruno E. Investigation of WO3 Electrodeposition Leading to Nanostructured Thin Films. Nanomaterials. 2020; 10(8):1493. https://doi.org/10.3390/nano10081493
Chicago/Turabian StyleMineo, G., F. Ruffino, S. Mirabella, and E. Bruno. 2020. "Investigation of WO3 Electrodeposition Leading to Nanostructured Thin Films" Nanomaterials 10, no. 8: 1493. https://doi.org/10.3390/nano10081493
APA StyleMineo, G., Ruffino, F., Mirabella, S., & Bruno, E. (2020). Investigation of WO3 Electrodeposition Leading to Nanostructured Thin Films. Nanomaterials, 10(8), 1493. https://doi.org/10.3390/nano10081493







