Kinetic and Thermodynamic Studies on Synthesis of Mg-Doped LiMn2O4 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Mg-Doped LiMn2O4 Nanoparticles
2.2. Materials’ Characterization
2.3. Kinetic Principle of Solid-State Reactions
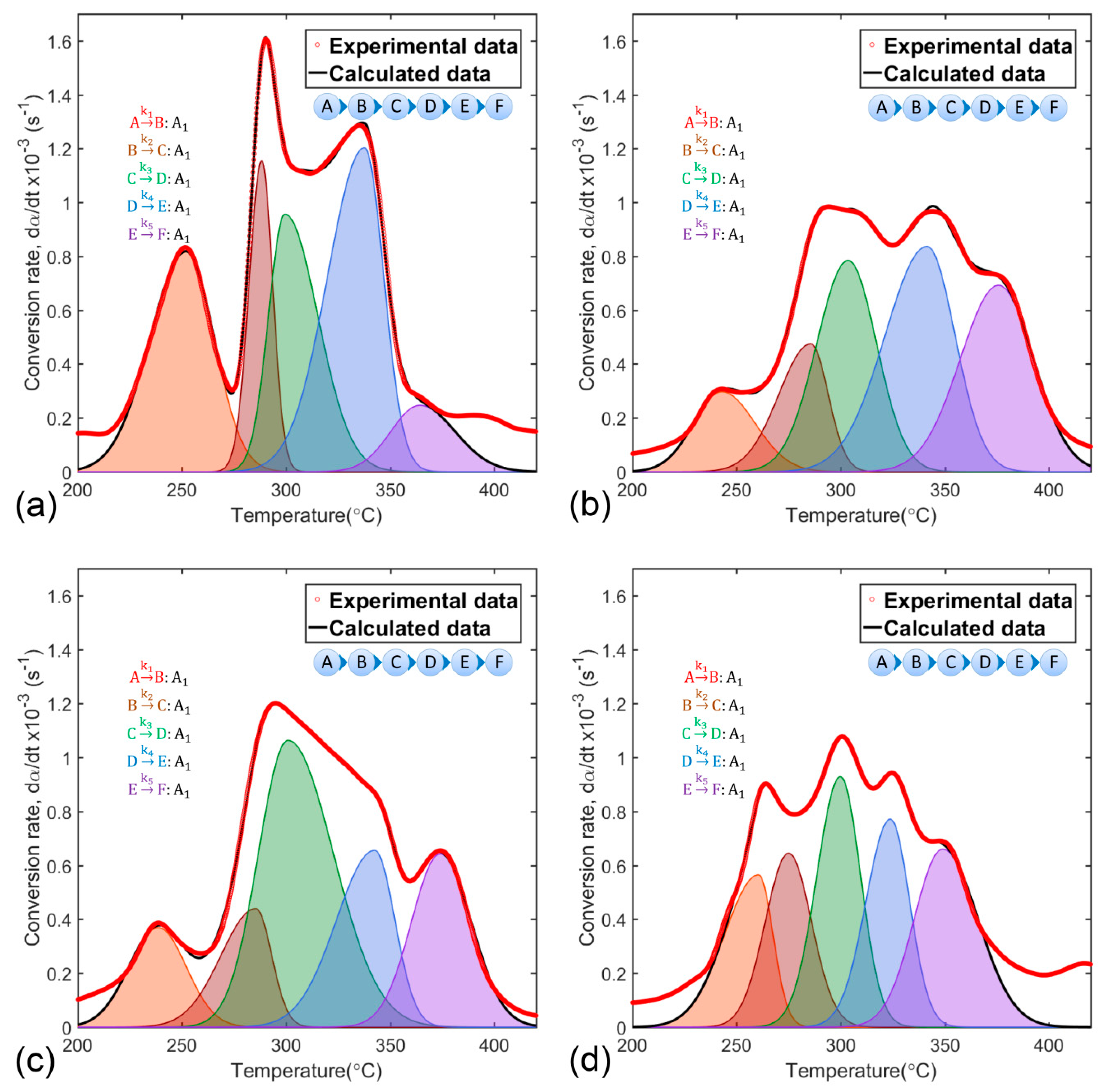
2.4. Deconvolution Function
3. Results and Discussion
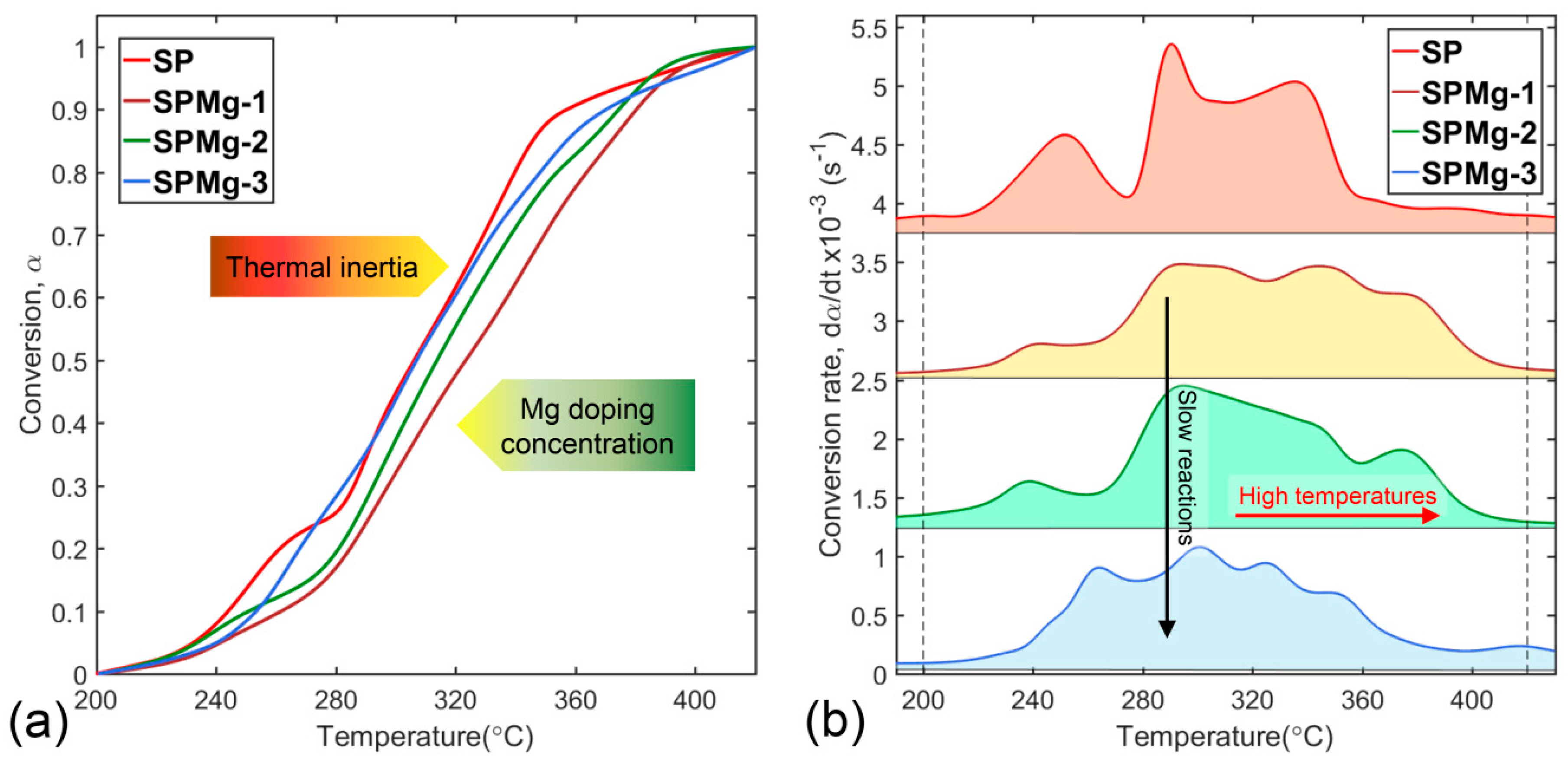
3.1. Thermogravimetric Data Analysis
3.2. Thermal Decomposition of Synthesis Precursors
- Ammonia evolution reaction:
- Dehydroxylation reaction:
- Decarboxylation reaction:
- Thermo-oxidative reaction of organic composition:
3.3. Determination of Thermal Decomposition Kinetic Parameters of Polymeric Matrix
- Dehydroxylation reaction:
- Decarboxylation reaction:
- Multistage combustion reaction:where is the dry synthesis precursor after dehydration; , , and are solid intermediate products; is the final solid product; and is reaction rate constant (subscripts from 1 to 5 correspond to reactions 23 to 27, respectively).
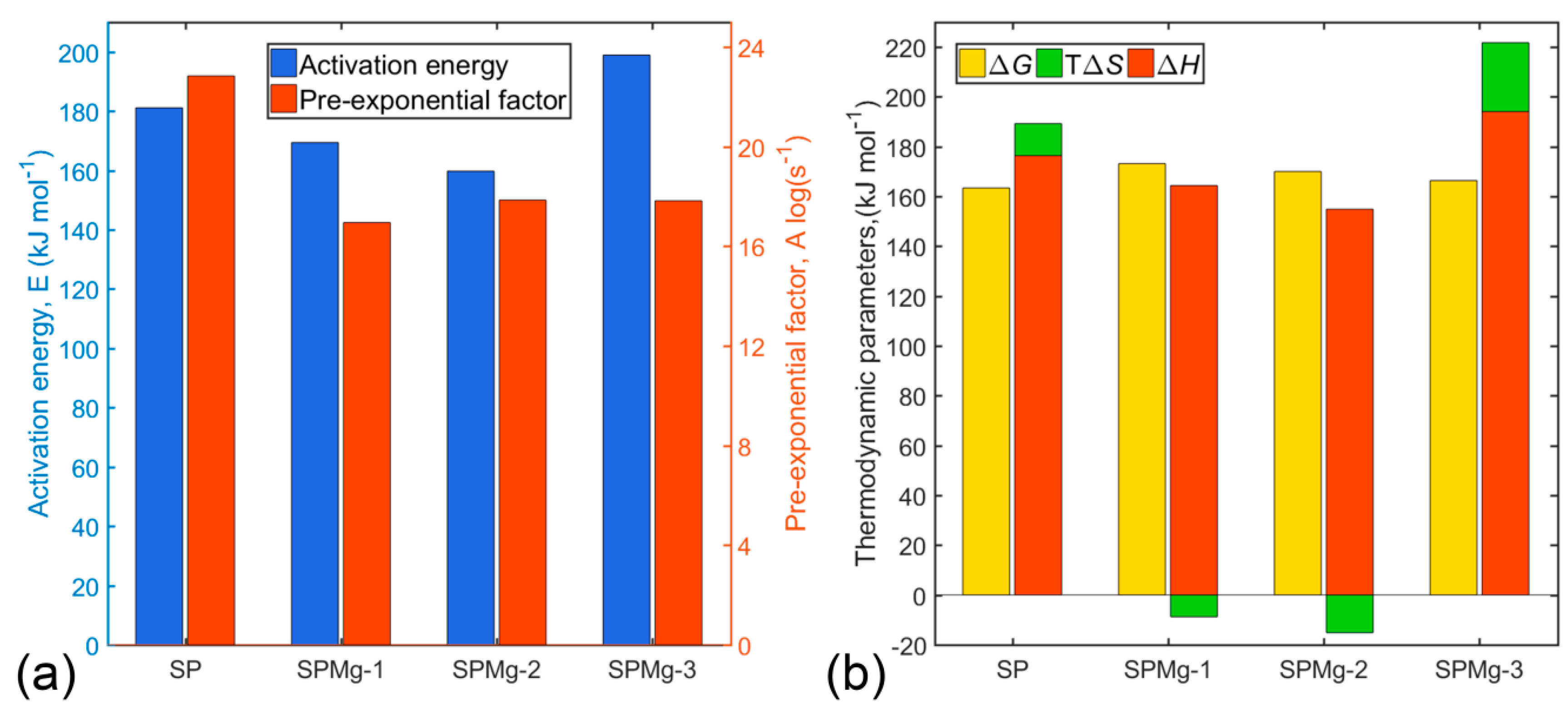
3.4. Transition State Thermodynamic Parameters of Polymeric Matrix Thermal Decomposition
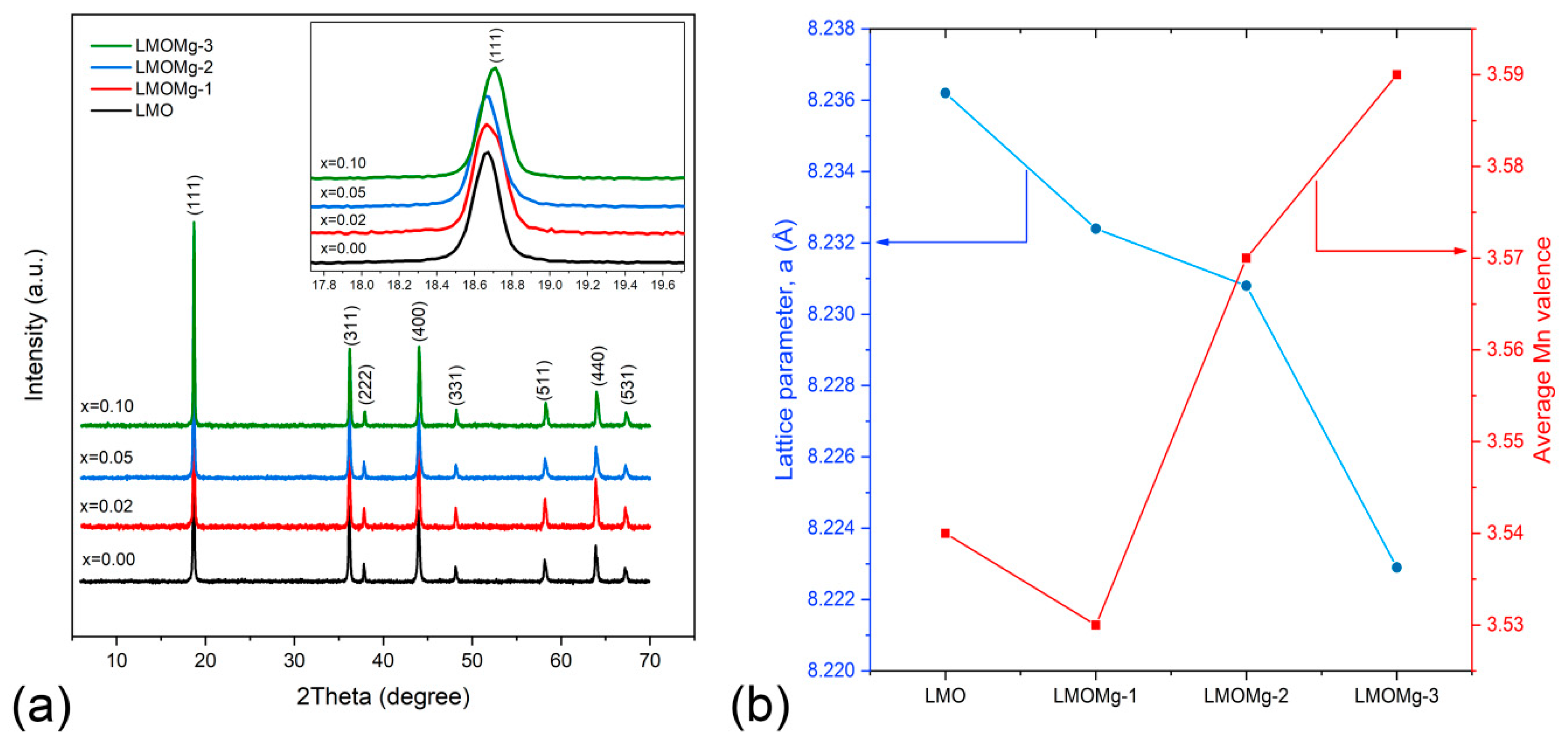
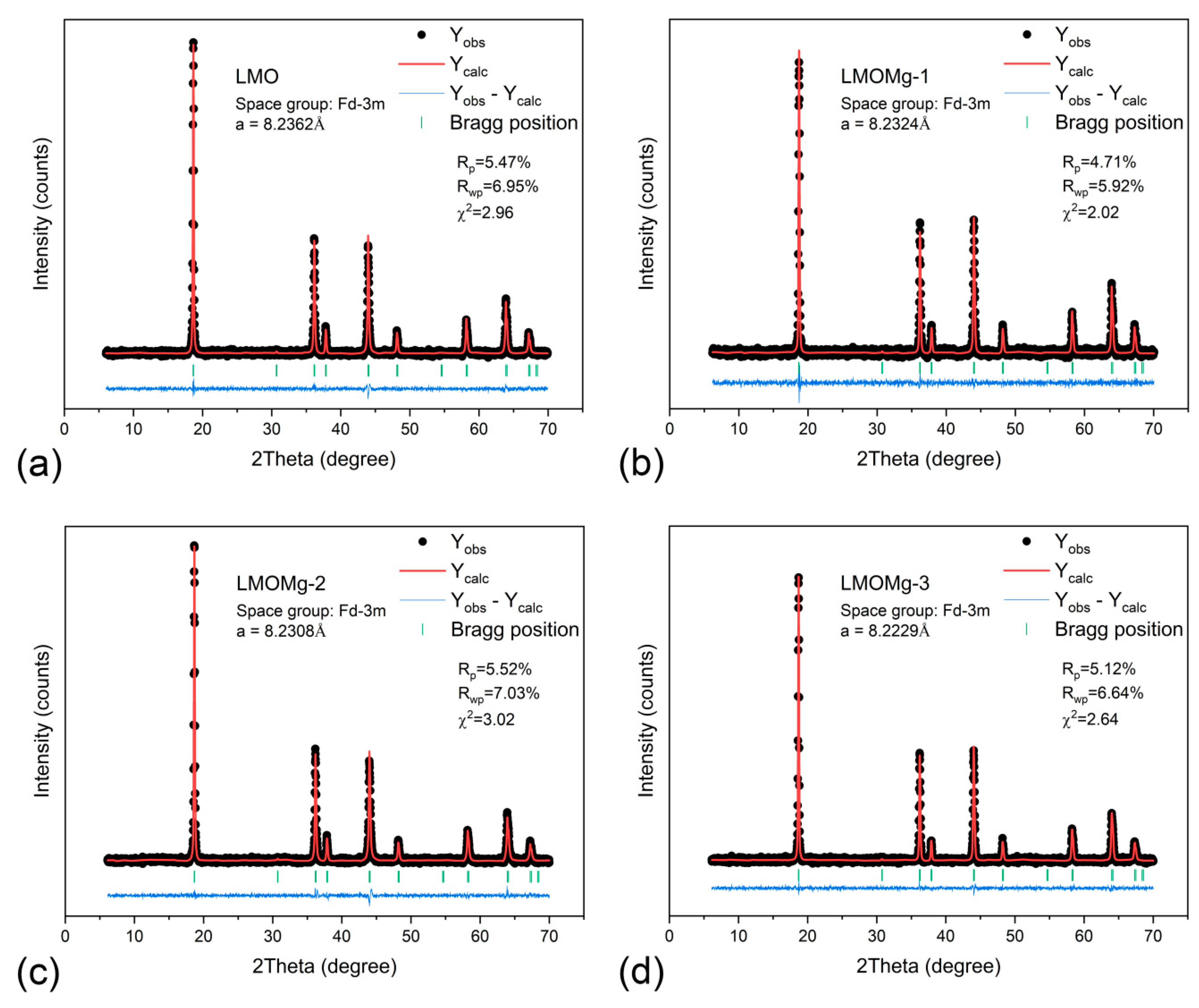
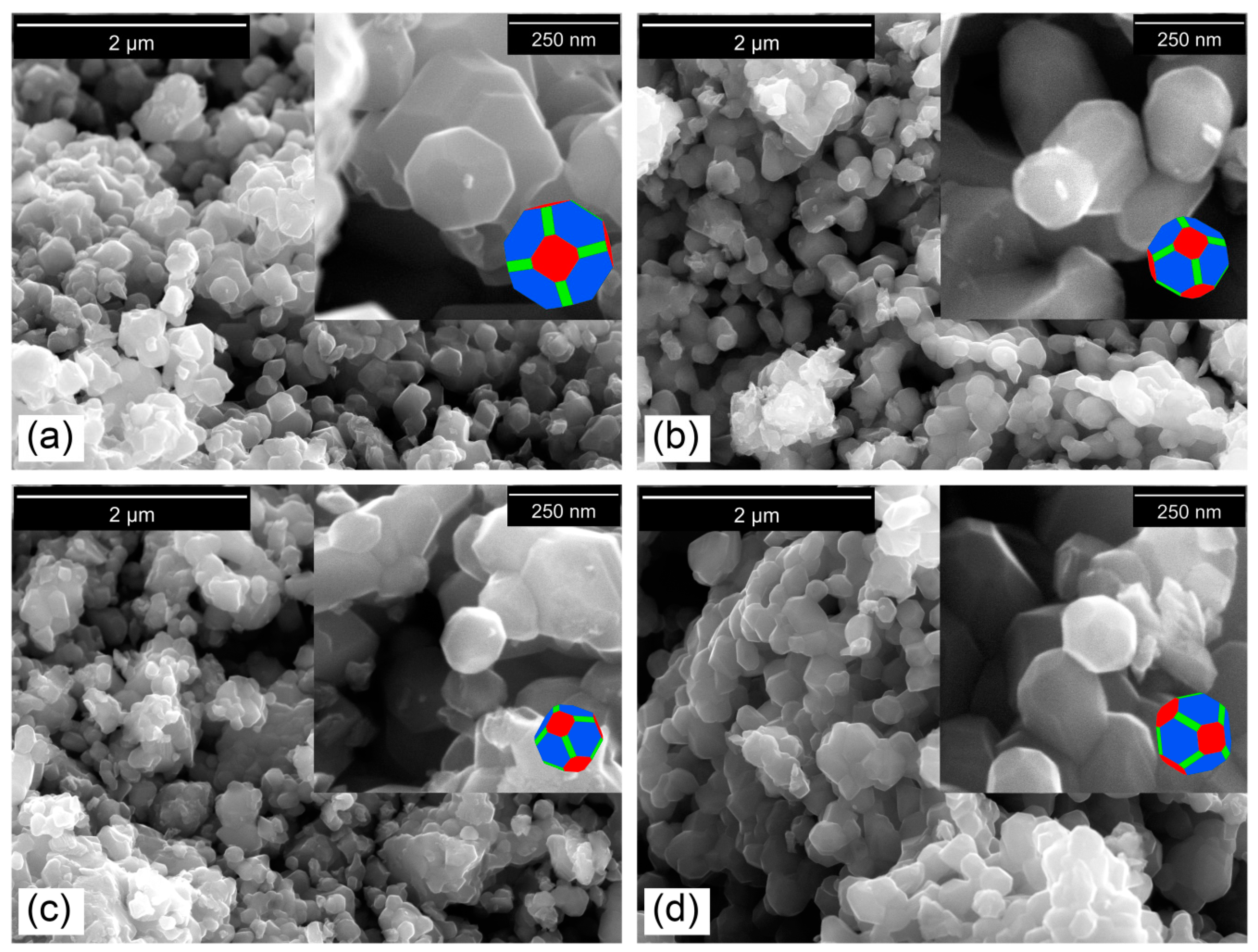
3.5. Stoichiometric, Structural, and Morphological Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stan, A.; Świerczyński, M.; Stroe, D.; Teodorescu, R.; Andreasen, S.J. Lithium ion battery chemistries from renewable energy storage to automotive and back-up power applications—An overview. In Proceedings of the 2014 International Conference on Optimization of Electrical and Electronic Equipment (OPTIM), Brain, Romania, 22–24 May 2014; pp. 713–720. [Google Scholar]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Khan, M.A.; Zou, W.; Xu, J.; Zhang, L.; Zhang, J. Recent progress in advanced electrode materials, separators, and electrolytes for lithium batteries. J. Mater. Chem. A 2018, 6, 20564–20620. [Google Scholar] [CrossRef]
- Bresser, D.; Hosoi, K.; Howell, D.; Li, H.; Zeisel, H.; Amine, K.; Passerini, S. Perspectives of automotive battery R&D in China, Germany, Japan, and the USA. J. Power Sources 2018, 382, 176–178. [Google Scholar] [CrossRef]
- Liu, X.; Li, K.; Li, X. The Electrochemical Performance and Applications of Several Popular Lithium-ion Batteries for Electric Vehicles—A Review. Biomed. Eng. Syst. Technol. 2018, 925, 201–213. [Google Scholar] [CrossRef]
- Miao, Y.; Hynan, P.; von Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2016, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Potapenko, A.V.; Kirillov, S.A. Lithium manganese spinel materials for high-rate electrochemical applications. J. Energy Chem. 2014, 23, 543–558. [Google Scholar] [CrossRef]
- Ahmad, M. Review on Synthesis, Characterizations, and Electrochemical Properties of Cathode Materials for Lithium Ion Batteries. J. Mater. Sci. Eng. 2016, 5. [Google Scholar] [CrossRef]
- Xiao, W.; Xin, C.; Li, S.; Jie, J.; Gu, Y.; Pan, F.; Zheng, J.-X. Insight into fast Li diffusion in Li-excess spinel lithium manganese oxide. J. Mater. Chem. A 2018, 6, 9893–9898. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Zaghib, K.; Groult, H. Comparative Issues of Cathode Materials for Li-Ion Batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Ben, L.; Sun, Y.; Chen, B.; Yang, Z.; Gu, L.; Huang, X. Electrochemical behavior, and surface structural change of LiMn2O4charged to 5.1 V. J. Mater. Chem. A 2014, 2, 14519–14527. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Zhan, C.; Wu, T.; Lu, J.; Amine, K. Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes—A critical review. Energy Environ. Sci. 2018, 11, 243–257. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Chen, X.; Wu, H.; Zhang, Y. Mg 2+ and Ti 4+ Co–Doped Spinel LiMn 2 O 4 as Lithium-Ion Battery Cathode. Chemistry 2019, 4, 9583–9589. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Streipert, B.; Röser, S.; Wagner, R.; Laskovic, I.C.; Winter, M. Determining oxidative stability of battery electrolytes: Validity of common electrochemical stability window (ESW) data and alternative strategies. Phys. Chem. Chem. Phys. 2017, 19, 16078–16086. [Google Scholar] [CrossRef]
- Dou, S. Review, and prospects of Mn-based spinel compounds as cathode materials for lithium-ion batteries. Ionics 2015, 21, 3001–3030. [Google Scholar] [CrossRef]
- Xu, G.; Liu, Z.; Zhang, C.; Cui, G.; Chen, L. Strategies for improving the cyclability and thermo-stability of LiMn2O4-based batteries at elevated temperatures. J. Mater. Chem. A 2015, 3, 4092–4123. [Google Scholar] [CrossRef]
- Susanto, D.; Kim, H.; Kim, J.-Y.; Lim, S.; Yang, J.; Choi, S.A.; Chung, K.Y. Effect of (Mg, Al) double doping on the thermal decomposition of LiMn2O4 cathodes investigated by time-resolved X-ray diffraction. Curr. Appl. Phys. 2015, 15, S27–S31. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Tan, H.; Yang, Z.; Zeng, J. Preparation and Doping Mode of Doped LiMn2O4 for Li-Ion Batteries. Energies 2013, 6, 1718–1730. [Google Scholar] [CrossRef]
- Zhao, H.; Li, F.; Liu, X.; Cheng, C.; Zhang, Z.; Wu, Y.; Xiong, W.; Chen, B. Effects of equimolar Mg (II) and Si (IV) co-doping on the electrochemical properties of spinel LiMn2−2xMgxSixO4 prepared by citric acid assisted sol–gel method. Electrochimica Acta 2015, 151, 263–269. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Anjaneya, K.C.; Periasamy, P.; Tripathi, V.S.; Manjannaa, J. Structural, electrical, and electrochemical studies of LiNi0.4 M 0.1Mn1.5O4 (M = Co, Mg) solid solutions for lithium ion battery. Bull. Mater. Sci. 2016, 39, 1279–1284. [Google Scholar] [CrossRef][Green Version]
- Zhao, H.; Liu, X.; Cheng, C.; Li, Q.; Zhang, Z.; Wu, Y.; Chen, B.; Xiong, W. Synthesis and electrochemical characterizations of spinel LiMn1.94MO4 (M = Mn0.06, Mg0.06, Si0.06, (Mg0.03Si0.03)) compounds as cathode materials for lithium-ion batteries. J. Power Sources 2015, 282, 118–128. [Google Scholar] [CrossRef]
- Xiao, Z.H.; Cui, Q.-Q.; Li, X.-L.; Wang, H.-L.; Zhou, Q. Ionothermal synthesis for Mg-doped LiMn1.5Ni0.5O4 spinel with structural stability and high-rate performance. Ionics 2014, 21, 1261–1267. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wu, J.; Zhuang, X.; Ma, M.; Jiang, Z. Surface modification of Mg-doped spinel with different Li-containing manganese oxides. Ionics 2015, 21, 1851–1856. [Google Scholar] [CrossRef]
- Wen, W.; Ju, B.; Wang, X.; Wu, C.; Shu, H.; Yang, X. Effects of magnesium and fluorine co-doping on the structural and electrochemical performance of the spinel LiMn2O4 cathode materials. Electrochimica Acta 2014, 147, 271–278. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, N.; Zhang, X.; Zhao, C.; Xu, Y. Microwave synthesis of LiMg0.05Mn1.95O4 and electrochemical performance at elevated temperature for lithium-ion batteries. J. Solid State Electrochem. 2013, 18, 569–575. [Google Scholar] [CrossRef]
- Liu, M.; Huang, H.-T.; Lin, C.-M.; Chen, J.-M.; Liao, S.-C. Mg gradient-doped LiNi0.5Mn1.5O4 as the cathode material for Li-ion batteries. Electrochimica Acta 2014, 120, 133–139. [Google Scholar] [CrossRef]
- Andre, D.; Kim, S.-J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: An automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Massarotti, V.; Capsoni, D.; Bini, M. Stability of LiMn2O4 and new high temperature phases in air, O2 and N2. Solid State Commun. 2002, 122, 317–322. [Google Scholar] [CrossRef]
- Molenda, M.; Dziembaj, R.; Podstawka, E.; Proniewicz, L. Changes in local structure of lithium manganese spinels (Li:Mn = 1:2) characterised by XRD, DSC, TGA, IR, and Raman spectroscopy. J. Phys. Chem. Solids 2005, 66, 1761–1768. [Google Scholar] [CrossRef]
- Amatucci, G.; Tarascon, J.-M. Optimization of Insertion Compounds Such as LiMn [sub 2] O [sub 4] for Li-Ion Batteries. J. Electrochem. Soc. 2002, 149, K31. [Google Scholar] [CrossRef]
- Paulsen, J.M.; Dahn, J.R. Phase Diagram of Li−Mn−O Spinel in Air. Chem. Mater. 1999, 11, 3065–3079. [Google Scholar] [CrossRef]
- Berbenni, V.; Marini, A. Thermoanalytical (TGA-DSC) and high temperature X-ray diffraction (HT-XRD) study of the thermal decomposition processes in Li2CO3–MnO mixtures. J. Anal. Appl. Pyrolysis 2002, 64, 43–58. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Oh, I.-H.; Kim, K.Y. Synthesis of Spinel LiMn2O4by the Sol−Gel Method for a Cathode-Active Material in Lithium Secondary Batteries. Ind. Eng. Chem. Res. 1997, 36, 4839–4846. [Google Scholar] [CrossRef]
- Hon, Y.-M.; Fung, K.-Z.; Hon, M.H. Effect of Temperature and Atmosphere on Phase Stability and Morphology of LiMn2O4 Powder Synthesized by Citric Acid Gel Process. J. Ceram. Soc. Jpn. 2000, 108, 462–468. [Google Scholar] [CrossRef][Green Version]
- Zhao, M.S.; Song, X.P. Synthesizing kinetics and characteristics for spinel LiMn2O4 with the precursor using as lithium-ion battery cathode material. J. Power Sources 2007, 164, 822–828. [Google Scholar] [CrossRef]
- Lin, J.; Yu, M.; Lin, C.; Liu, X. Multiform Oxide Optical Materials via the Versatile Pechini-Type Sol−Gel Process: Synthesis and Characteristics. J. Phys. Chem. C 2007, 111, 5835–5845. [Google Scholar] [CrossRef]
- Dimesso, L. Pechini Processes: An Alternate Approach of the Sol–Gel Method, Preparation, Properties, and Applications. In Handbook of Sol-Gel Science and Technology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; pp. 1–22. [Google Scholar]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horizons 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Liu, W.; Farrington, G.C.; Chaput, F.; Dunn, B. Synthesis and Electrochemical Studies of Spinel Phase LiMn [sub 2] O [sub 4] Cathode Materials Prepared by the Pechini Process. J. Electrochem. Soc. 1996, 143, 879–884. [Google Scholar] [CrossRef]
- Han, Y.-S.; Kim, H.-G. Synthesis of LiMn2O4 by modified Pechini method and characterization as a cathode for rechargeable Li/LiMn2O4 cells. J. Power Sources 2000, 88, 161–168. [Google Scholar] [CrossRef]
- Son, J.; Kim, H.; Park, Y. New preparation method and electrochemical property of LiMn2O4 electrode. Electrochimica Acta 2004, 50, 453–459. [Google Scholar] [CrossRef]
- Xiong, L.; Xu, Y.; Tao, T.; Goodenough, J.B. Synthesis, and electrochemical characterization of multi-cations doped spinel LiMn2O4 used for lithium ion batteries. J. Power Sources 2012, 199, 214–219. [Google Scholar] [CrossRef]
- Amaral, F.A.D.; Santana, L.K.; Campos, I.O.; Fagundes, W.S.; Xavier, F.F.S.; Canobre, S.C. Pechini Synthesis of Nanostructured Li1.05M0.02Mn1.98O4 (M = Al3+ or Ga3+). Mater. Res. 2015, 18, 250–259. [Google Scholar] [CrossRef]
- Ptáček, P.; Opravil, T.; Šoukal, F. A Brief Introduction to the History of Chemical Kinetics. In Introducing the Effective Mass of Activated Complex and the Discussion on the Wave Function of this Instanton; IntechOpen: London, UK, 2018. [Google Scholar]
- Vyazovkin, S. Some Basics En Route to Isoconversional Methodology. In Isoconversional Kinetics of Thermally Stimulated Processes; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2015; pp. 1–25. [Google Scholar]
- Šesták, J.; Berggren, G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim. Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
- Šesták, J. Šesták–Berggren equation: Now questioned but formerly celebrated—What is right. J. Therm. Anal. Calorim. 2015, 127, 1117–1123. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Solid-State Kinetic Models: Basics and Mathematical Fundamentals. J. Phys. Chem. B 2006, 110, 17315–17328. [Google Scholar] [CrossRef]
- Vlaev, L.T.; Georgieva, V.; Tavlieva, M.P. On the Kinetic Mechanism of Non-Isothermal Degradation of Solids. In Reactions and Mechanisms in Thermal Analysis of Advanced Materials; Wiley: Hoboken, NJ, USA, 2015; pp. 547–578. [Google Scholar]
- Hill, J. Principles and Practices of Thermal Analysis and Calorimetry. In Characterization of Materials; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Perejoόn, A.; Saánchez-Jimeénez, P.E.; Criado, J.M.; Peérez-Maqueda, L.A. Kinetic Analysis of Complex Solid-State Reactions. A New Deconvolution Procedure. J. Phys. Chem. B 2011, 115, 1780–1791. [Google Scholar] [CrossRef]
- Vyazovkin, S. Thermogravimetric Analysis. In Characterization of Materials; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Prime, R.B.; Bair, H.E.; Vyazovkin, S.; Gallagher, P.K.; Riga, A. Thermogravimetric Analysis (TGA). In Thermal Analysis of Polymers; Menczel, J.D., Prime, R.B., Eds.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- van Werde, K.; Mondelaers, D.; Vanhoyland, G.; Nelis, D.; van Bael, M.K.; Mullens, J.; van Poucke, L.C.; van der Veken, B.; Desseyn, H.O. Thermal decomposition of the ammonium zinc acetate citrate precursor for aqueous chemical solution deposition of ZnO. J. Mater. Sci. 2002, 37, 81–88. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Hebanowska, E.; Nowak-Wiczk, G.; Makowski, M.; Chmurzyński, L. Thermal behaviour of citric acid and isomeric aconitic acids. J. Therm. Anal. Calorim. 2010, 104, 731–735. [Google Scholar] [CrossRef]
- Apelblat, A. Citric Acid; Springer International Publishing AG: Cham, Switzerland, 2014. [Google Scholar]
- Predoana, L.; Jitianu, A.; Voicescu, M.; Apostol, N.G.; Zaharescu, M. Study of formation of LiCoO2 using a modified Pechini aqueous sol–gel process. J. Sol-Gel Sci. Technol. 2015, 74, 406–418. [Google Scholar] [CrossRef]
- Allan, J.; Bonner, J.; Werninck, A.; Bowley, H.; Gerrard, D. Thermal studies on itaconic acid compounds of some transition metal ions. Thermochim. Acta 1987, 122, 295–303. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Thermogravimetric analysis. A review. Anal. 1963, 88, 906. [Google Scholar] [CrossRef]
- Sunde, T.O.L.; Grande, T.; Einarsrud, M.-A. Modified Pechini Synthesis of Oxide Powders and Thin Films. In Handbook of Sol-Gel Science and Technology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; pp. 1–30. [Google Scholar]
- Petrykin, V.; Kakihana, M. Chemistry and Applications of Polymeric Gel Precursors. In Handbook of Sol-Gel Science and Technology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; pp. 81–112. [Google Scholar]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Geological Institute of Hungary (Magyar Állami Földtani Intézet): Budapest, Hungary, 2011. [Google Scholar]
- Liu, X.W.; Feng, Y.; Li, H.R.; Zhang, P.; Wang, P. Thermal decomposition kinetics of magnesite from thermogravimetric data. J. Therm. Anal. Calorim. 2011, 107, 407–412. [Google Scholar] [CrossRef]
- Stern, K.H. High Temperature Properties and Thermal Decomposition of Inorganic Salts with Oxyanions. In High Temperature Properties and Thermal Decomposition of Inorganic Salts with Oxyanions; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Beyer, H.; Meini, S.; Tsiouvaras, N.; Piana, M.; Gasteiger, H.A. Thermal and electrochemical decomposition of lithium peroxide in non-catalyzed carbon cathodes for Li–air batteries. Phys. Chem. Chem. Phys. 2013, 15, 11025. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.; Mansuetto, M.; Dees, D.; Vissers, D. The thermal stability of lithium-manganese-oxide spinel phases. Mater. Res. Bull. 1996, 31, 133–140. [Google Scholar] [CrossRef]
- Thackeray, M.; Mansuetto, M.; Bates, J. Structural stability of LiMn2O4 electrodes for lithium batteries. J. Power Sources 1997, 68, 153–158. [Google Scholar] [CrossRef]
- Luo, C.; Martin, M. Stability, and defect structure of spinels Li1 + x Mn2 − x O4 − δ: I. In situ investigations on the stability field of the spinel phase. J. Mater. Sci. 2007, 42, 1955–1964. [Google Scholar] [CrossRef]
- Fedunik-Hofman, L.; Bayon, A.; Donne, S.W. Kinetics of Solid-Gas Reactions and Their Application to Carbonate Looping Systems. Energies 2019, 12, 2981. [Google Scholar] [CrossRef]
- Cai, J.; Wu, W.; Liu, R. Isoconversional Kinetic Analysis of Complex Solid-State Processes: Parallel and Successive Reactions. Ind. Eng. Chem. Res. 2012, 51, 16157–16161. [Google Scholar] [CrossRef]
- Pomerantsev, A.L.; Kutsenova, A.V.; Rodionova, O.Y. Kinetic analysis of non-isothermal solid-state reactions: Multi-stage modeling without assumptions in the reaction mechanism. Phys. Chem. Chem. Phys. 2017, 19, 3606–3615. [Google Scholar] [CrossRef] [PubMed]
- Opfermann, J. Kinetic Analysis Using Multivariate Non-linear Regression. I. Basic concepts. I. Basic concepts. J. Therm. Anal. Calorim. 2000, 60, 641–658. [Google Scholar] [CrossRef]
- Nakano, M.; Wada, T.; Koga, N. Exothermic Behavior of Thermal Decomposition of Sodium Percarbonate: Kinetic Deconvolution of Successive Endothermic and Exothermic Processes. J. Phys. Chem. A 2015, 119, 9761–9769. [Google Scholar] [CrossRef] [PubMed]
- Vlaev, L.; Nedelchev, N.; Gyurova, K.; Zagorcheva, M. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J. Anal. Appl. Pyrolysis 2008, 81, 253–262. [Google Scholar] [CrossRef]
- Georgieva, V.; Vlaev, L.T.; Gyurova, K. Non-Isothermal Degradation Kinetics of CaCO3 from Different Origin. J. Chem. 2013, 1–12. [Google Scholar] [CrossRef]
- Eyring, H. The Activated Complex and the Absolute Rate of Chemical Reactions. Chem. Rev. 1935, 17, 65–77. [Google Scholar] [CrossRef]
- Ptáček, P.; Šoukal, F.; Opravil, T. Introduction to the Transition State Theory. In Introducing the Effective Mass of Activated Complex and the Discussion on the Wave Function of this Instanton; IntechOpen: London, UK, 2018. [Google Scholar]
- Perez-Benito, J.F. Some Considerations on the Fundamentals of Chemical Kinetics: Steady State, Quasi-Equilibrium, and Transition State Theory. J. Chem. Educ. 2017, 94, 1238–1246. [Google Scholar] [CrossRef]
- Hettema, H. Unity of Chemistry and Physics: The Theory of Absolute Reaction Rates; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2017; Volume 7, pp. 69–86. [Google Scholar]
- Hashem, A.; Abdel-Ghany, A.; Abuzeid, H.M.; El-Tawil, R.; Indris, S.; Ehrenberg, H.; Mauger, A.; Julien, C.M. EDTA as chelating agent for sol-gel synthesis of spinel LiMn 2 O 4 cathode material for lithium batteries. J. Alloy. Compd. 2018, 737, 758–766. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Varadaraju, U.; Gopalan, R.; Prakash, R. Structural stability, and superior electrochemical performance of Sc-doped LiMn2O4 spinel as cathode for lithium ion batteries. Electrochimica Acta 2019, 301, 342–351. [Google Scholar] [CrossRef]
- Subramania, A.; Angayarkanni, N.; Priya, A.R.S.; Gangadharan, R.; Vasudevan, T. Synthesis and characterization of LiMgyMn2-yO4 cathode materials by a modified Pechini process for lithium batteries. Bull. Mater. Sci. 2005, 28, 663–667. [Google Scholar] [CrossRef]
- Hashem, A.; Abbas, S.M.; Hou, X.; Eid, A.; Abdel-Ghany, A.E. Facile one step synthesis method of spinel LiMn2O4 cathode material for lithium batteries. Heliyon 2019, 5, e02027. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, D.; Wang, Y.; Li, F.; Wang, G.; Wu, T.; Wang, Z.; Li, Y.; Su, J. Sol-Gel Synthesis of Silicon-Doped Lithium Manganese Oxide with Enhanced Reversible Capacity and Cycling Stability. Materials 2018, 11, 1455. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-M.; Zhao, L.-C. Structure, and electrochemical properties of LiMn2O4. Trans. Nonferrous Met. Soc. China 2007, 17, 659–664. [Google Scholar] [CrossRef]
- Douafer, S.; Lahmar, H.; Benamira, M.; Rekhila, G.; Trari, M. Physical and photoelectrochemical properties of the spinel LiMn 2 O 4 and its application in photocatalysis. J. Phys. Chem. Solids 2018, 118, 62–67. [Google Scholar] [CrossRef]
- Chand, P.; Bansal, V.; Sukriti; Singh, V. Effect of pH values on structural, optical, electrical, and electrochemical properties of spinel LiMn2O4 cathode materials. J. Sci. Adv. Mater. Devices 2019, 4, 245–251. [Google Scholar] [CrossRef]
- Jiang, C.; Tang, Z.; Wang, S.; Zhang, Z. A truncated octahedral spinel LiMn 2 O 4 as high-performance cathode material for ultrafast and long-life lithium-ion batteries. J. Power Sour. 2017, 357, 144–148. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, K.; Cho, W.; Shin, W.H.; Kanno, R.; Choi, J.W. A Truncated Manganese Spinel Cathode for Excellent Power and Lifetime in Lithium-Ion Batteries. Nano Lett. 2012, 12, 6358–6365. [Google Scholar] [CrossRef]
- Zhou, S.; Mei, T.; Wang, X.; Qian, Y. Crystal structural design of exposed planes: Express channels, high-rate capability cathodes for lithium-ion batteries. Nanoscale 2018, 10, 17435–17455. [Google Scholar] [CrossRef]
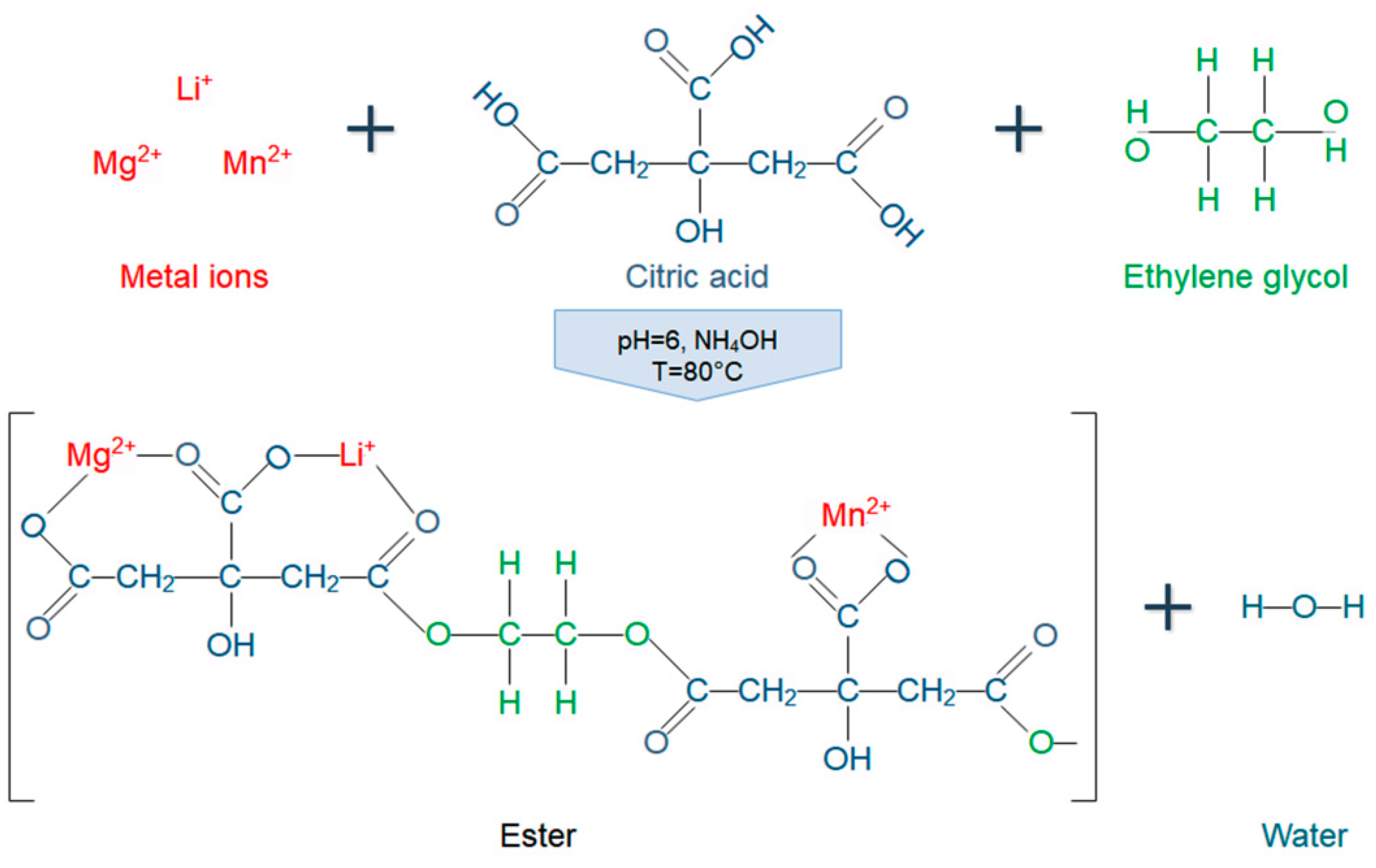
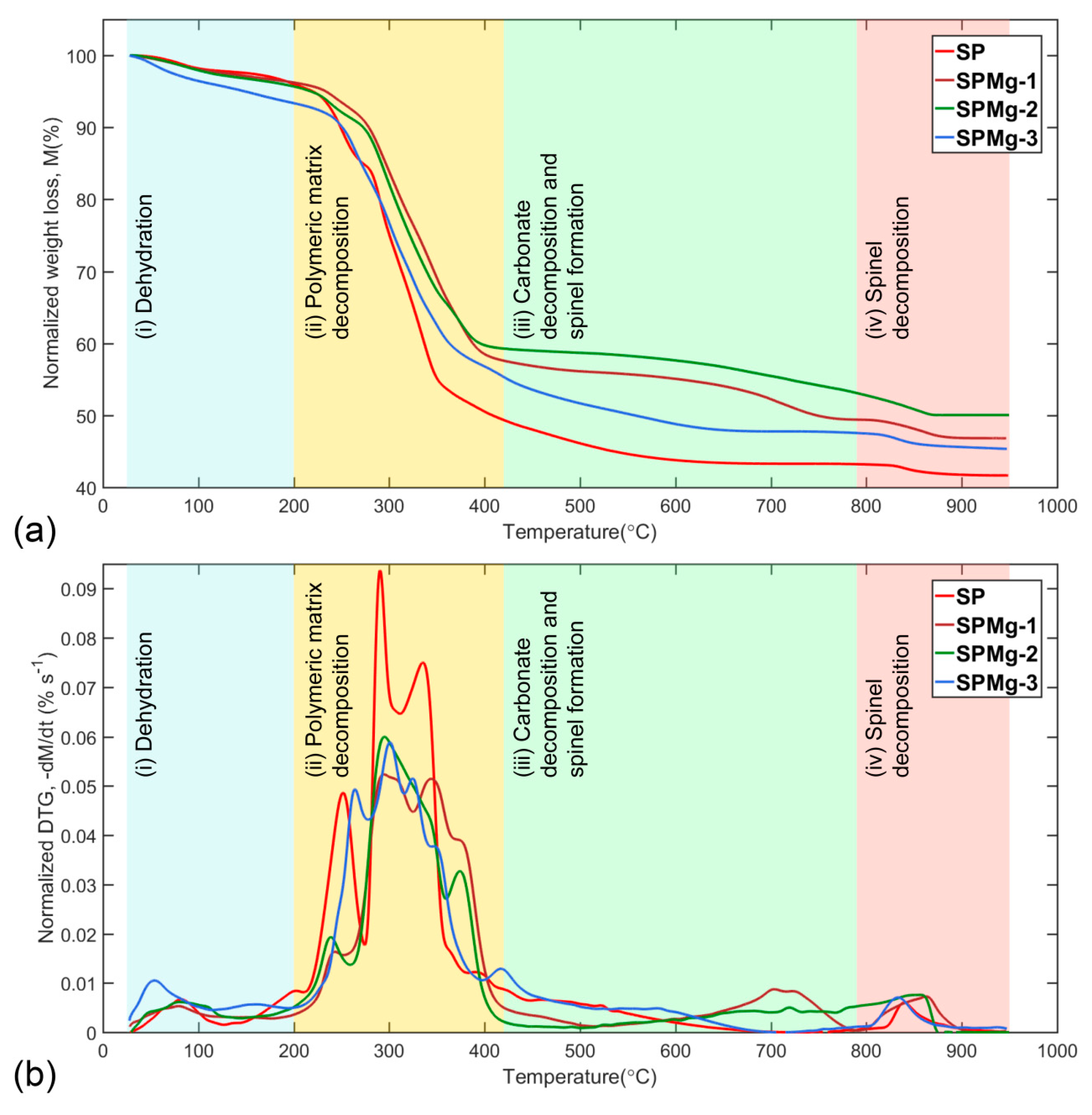
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | ||
|---|---|---|---|---|---|
| Sample | Temperature range | 25 °C–200 °C | 200 °C–420 °C | 420 °C–790 °C | 790 °C–950 °C |
| SP x = 0.00 | Mass loss (%) | 2.19 | 46.02 | 8.51 | 1.61 |
| Max. decomposition rate (% s−1) | 0.67 × 10−2 | 9.37 × 10−2 | 1.22 × 10−2 | 0.6 × 10−2 | |
| SPMg-1 x = 0.02 | Mass loss (%) | 3.35 | 40.65 | 6.6 | 2.58 |
| Max. decomposition rate (% s−1) | 0.53 × 10−2 | 5.24 × 10−2 | 0.87 × 10−2 | 0.73 × 10−2 | |
| SPMg-2 x = 0.05 | Mass loss (%) | 3.13 | 38.2 | 4.94 | 3.69 |
| Max. decomposition rate (% s−1) | 0.61 × 10−2 | 6 × 10−2 | 0.5 × 10−2 | 0.76 × 10−2 | |
| SPMg-3 x = 0.10 | Mass loss (%) | 6.45 | 36.61 | 9.16 | 2.31 |
| Max. decomposition rate (% s−1) | 1.05 × 10−2 | 5.89 × 10−2 | 1.29 × 10−2 | 0.71 × 10−2 |
| Synthesis Precursor | Peak Number | Reaction Model | ||
|---|---|---|---|---|
| SP x = 0.00 | 1 | Avrami–Erofeev equation, n = 1 | 157.68 | 13.77 |
| 2 | 249.19 | 21.72 | ||
| 3 | 133.79 | 12.80 | ||
| 4 | 189.41 | 14.56 | ||
| 5 | 257.35 | 18.78 | ||
| SPMg-1 x = 0.02 | 1 | Avrami–Erofeev equation, n = 1 | 135.66 | 11.95 |
| 2 | 187.88 | 15.43 | ||
| 3 | 170.99 | 14.67 | ||
| 4 | 157.76 | 12.44 | ||
| 5 | 180.74 | 13.65 | ||
| SPMg-2 x = 0.05 | 1 | Avrami–Erofeev equation, n = 1 | 123.54 | 11.56 |
| 2 | 206.82 | 17.86 | ||
| 3 | 101.50 | 6.95 | ||
| 4 | 261.91 | 20.42 | ||
| 5 | 206.18 | 14.63 | ||
| SPMg-3 x = 0.10 | 1 | Avrami–Erofeev equation, n = 1 | 209.10 | 18.09 |
| 2 | 148.30 | 11.91 | ||
| 3 | 198.30 | 16.33 | ||
| 4 | 194.48 | 15.21 | ||
| 5 | 181.01 | 12.44 |
| Synthesis Precursor | Peak Number | |||||
|---|---|---|---|---|---|---|
| SP x = 0.00 | 1 | 0.23 | 251.94 | 160.57 | 14.07 | 0.98 |
| 2 | 0.12 | 288.36 | 265.02 | 23.75 | ||
| 3 | 0.23 | 299.17 | 129.57 | 9.90 | ||
| 4 | 0.30 | 330.75 | 181.37 | 13.78 | ||
| 5 | 0.12 | 343.27 | 233.96 | 18.07 | ||
| Total | 1.00 | Average | 181.13 | 22.84 | ||
| SPMg-1 x = 0.02 | 1 | 0.07 | 242.99 | 142.64 | 12.44 | 0.99 |
| 2 | 0.15 | 285.67 | 209.40 | 17.78 | ||
| 3 | 0.21 | 303.59 | 165.53 | 13.04 | ||
| 4 | 0.30 | 341.46 | 159.56 | 11.59 | ||
| 5 | 0.27 | 375.87 | 168.39 | 11.48 | ||
| Total | 1.00 | Average | 169.41 | 16.96 | ||
| SPMg-2 x = 0.05 | 1 | 0.10 | 238.71 | 149.12 | 13.24 | 0.99 |
| 2 | 0.13 | 285.52 | 213.13 | 18.13 | ||
| 3 | 0.43 | 300.99 | 94.02 | 6.30 | ||
| 4 | 0.14 | 342.55 | 240.03 | 18.61 | ||
| 5 | 0.21 | 374.04 | 215.91 | 15.44 | ||
| Total | 1.00 | Average | 159.77 | 17.86 | ||
| SPMg-3 x = 0.10 | 1 | 0.14 | 260.59 | 207.85 | 18.70 | 0.92 |
| 2 | 0.14 | 275.00 | 154.45 | 12.86 | ||
| 3 | 0.24 | 299.86 | 211.79 | 17.53 | ||
| 4 | 0.23 | 323.86 | 218.74 | 17.33 | ||
| 5 | 0.26 | 348.98 | 189.34 | 13.87 | ||
| Total | 1.00 | Average | 198.92 | 17.91 |
| Synthesis Precursor | Peak Number | (J mol−1 K−1) | (kJ mol−1) | (kJ mol−1) | ||
|---|---|---|---|---|---|---|
| SP x = 0.00 | 1 | 0.23 | 525.09 | 11.40 | 156.21 | 150.22 |
| 2 | 0.12 | 561.51 | 196.19 | 260.35 | 150.18 | |
| 3 | 0.23 | 572.32 | −69.07 | 124.81 | 164.34 | |
| 4 | 0.30 | 603.90 | 4.69 | 176.35 | 173.51 | |
| 5 | 0.12 | 616.42 | 86.63 | 228.83 | 175.44 | |
| Total | 1.00 | Average | 22.40 | 176.35 | 163.42 | |
| SPMg-1 x = 0.02 | 1 | 0.07 | 516.14 | −19.74 | 138.35 | 148.53 |
| 2 | 0.15 | 558.82 | 81.91 | 204.75 | 158.98 | |
| 3 | 0.21 | 576.74 | −9.02 | 160.74 | 165.94 | |
| 4 | 0.30 | 614.61 | −37.46 | 154.45 | 177.48 | |
| 5 | 0.27 | 649.02 | −39.85 | 163.00 | 188.86 | |
| Total | 1.00 | Average | −12.91 | 164.42 | 173.19 | |
| SPMg-2 x = 0.05 | 1 | 0.10 | 511.86 | −4.31 | 144.86 | 147.07 |
| 2 | 0.13 | 558.67 | 88.63 | 208.49 | 158.97 | |
| 3 | 0.43 | 574.14 | −138.01 | 89.25 | 168.48 | |
| 4 | 0.14 | 615.70 | 96.92 | 234.91 | 175.24 | |
| 5 | 0.21 | 647.19 | 35.91 | 210.53 | 187.29 | |
| Total | 1.00 | Average | −28.04 | 154.88 | 170.04 | |
| SPMg-3 x = 0.10 | 1 | 0.14 | 533.74 | 99.92 | 203.41 | 150.08 |
| 2 | 0.14 | 548.15 | −12.04 | 149.89 | 156.49 | |
| 3 | 0.24 | 573.01 | 76.97 | 207.03 | 162.92 | |
| 4 | 0.23 | 597.01 | 72.84 | 213.78 | 170.29 | |
| 5 | 0.26 | 622.13 | 6.18 | 184.17 | 180.32 | |
| Total | 1.00 | Average | 48.39 | 194.08 | 166.37 |
| Sample Name | Nominal | Experimental | ||
|---|---|---|---|---|
| Stoichiometry | Average Mn Valence | Stoichiometry | Average Mn Valence | |
| LMO | LiMn2O4 | 3.50 | Li1.03Mn1.97O4 | 3.54 |
| LMOMg-1 | LiMg0.02Mn1.98O4 | 3.52 | Li1.01Mg0.02Mn1.97O4 | 3.53 |
| LMOMg-2 | LiMg0.05Mn1.95O4 | 3.54 | Li1.03Mg0.05Mn1.92O4 | 3.57 |
| LMOMg-3 | LiMg0.10Mn1.90O4 | 3.58 | Li1.01Mg0.10Mn1.89O4 | 3.59 |
| Sample Name | LMO | LMOMg-1 | LMOMg-2 | LMOMg-3 |
|---|---|---|---|---|
| Symmetry | cubic | |||
| Space group | ||||
| Lattice parameter (Å) | 8.2362 | 8.2324 | 8.2308 | 8.2229 |
| Unit cell volume (Å3) | 558.6968 | 557.9358 | 557.6075 | 556.0020 |
| Rp (%) | 5.47 | 4.71 | 5.52 | 5.12 |
| Rwp (%) | 6.95 | 5.92 | 7.03 | 6.64 |
| χ2 | 2.96 | 2.02 | 3.02 | 2.64 |
| I(311)/I(400) | 0.87 | 0.82 | 0.87 | 0.83 |
| FWHM111 (°) | 0.148 | 0.170 | 0.152 | 0.146 |
| FWHM311 (°) | 0.155 | 0.173 | 0.171 | 0.167 |
| FWHM400 (°) | 0.159 | 0.175 | 0.183 | 0.178 |
| Dc Average (nm) | 56.51 | 50.47 | 51.91 | 53.45 |
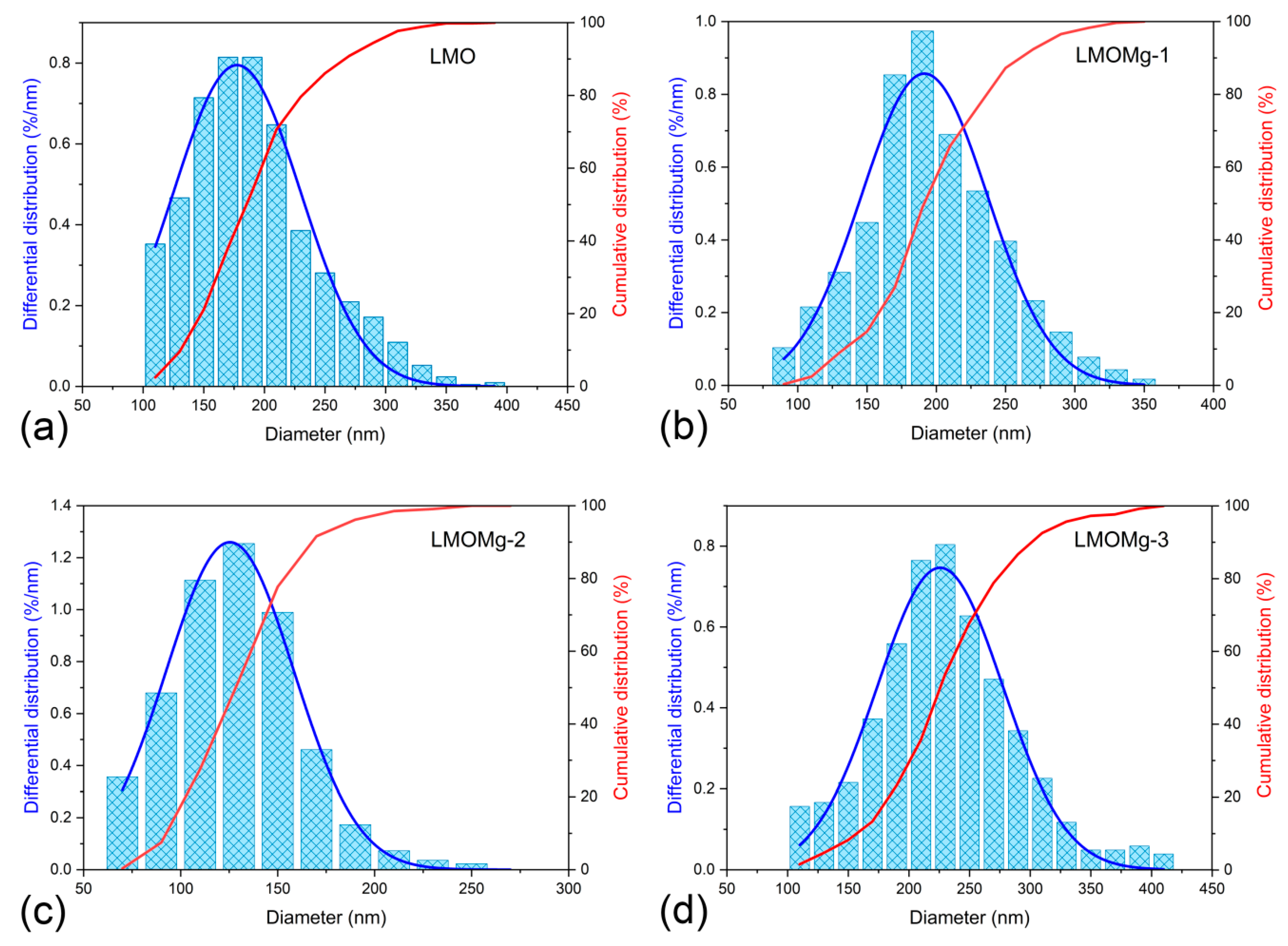
| Sample | LMO | LMOMg-1 | LMOMg-2 | LMOMg-3 |
|---|---|---|---|---|
| Mean diameter (nm) | 177.5 | 191.4 | 125.4 | 225.7 |
| Standard Deviation (nm) | 52.3 | 45.7 | 32.9 | 51.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llusco, A.; Grageda, M.; Ushak, S. Kinetic and Thermodynamic Studies on Synthesis of Mg-Doped LiMn2O4 Nanoparticles. Nanomaterials 2020, 10, 1409. https://doi.org/10.3390/nano10071409
Llusco A, Grageda M, Ushak S. Kinetic and Thermodynamic Studies on Synthesis of Mg-Doped LiMn2O4 Nanoparticles. Nanomaterials. 2020; 10(7):1409. https://doi.org/10.3390/nano10071409
Chicago/Turabian StyleLlusco, Aleksei, Mario Grageda, and Svetlana Ushak. 2020. "Kinetic and Thermodynamic Studies on Synthesis of Mg-Doped LiMn2O4 Nanoparticles" Nanomaterials 10, no. 7: 1409. https://doi.org/10.3390/nano10071409
APA StyleLlusco, A., Grageda, M., & Ushak, S. (2020). Kinetic and Thermodynamic Studies on Synthesis of Mg-Doped LiMn2O4 Nanoparticles. Nanomaterials, 10(7), 1409. https://doi.org/10.3390/nano10071409





