Towards Understanding the Role of Surface Gas Nanostructures: Effect of Temperature Difference Pretreatment on Wetting and Flotation of Sulfide Minerals and Pb-Zn Ore
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AFM and XPS Characterization
2.3. Contact Angle Measurement
2.4. Flotation Tests
3. Results
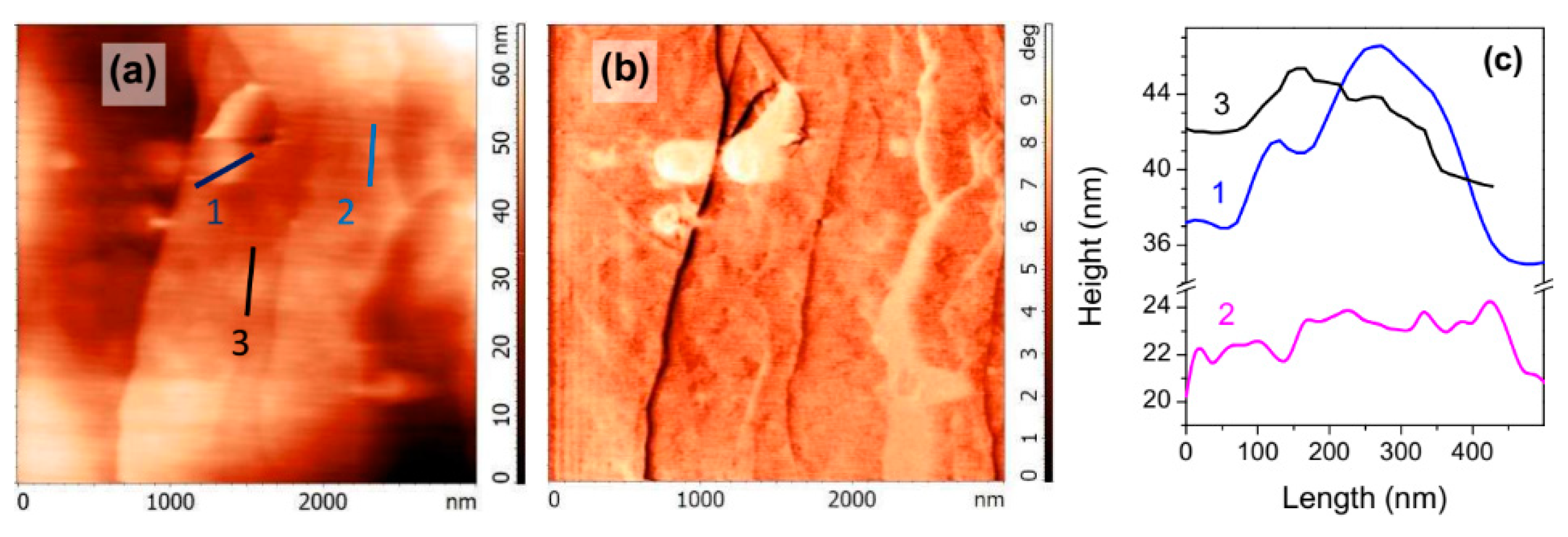
3.1. Atomic Force Microscopy
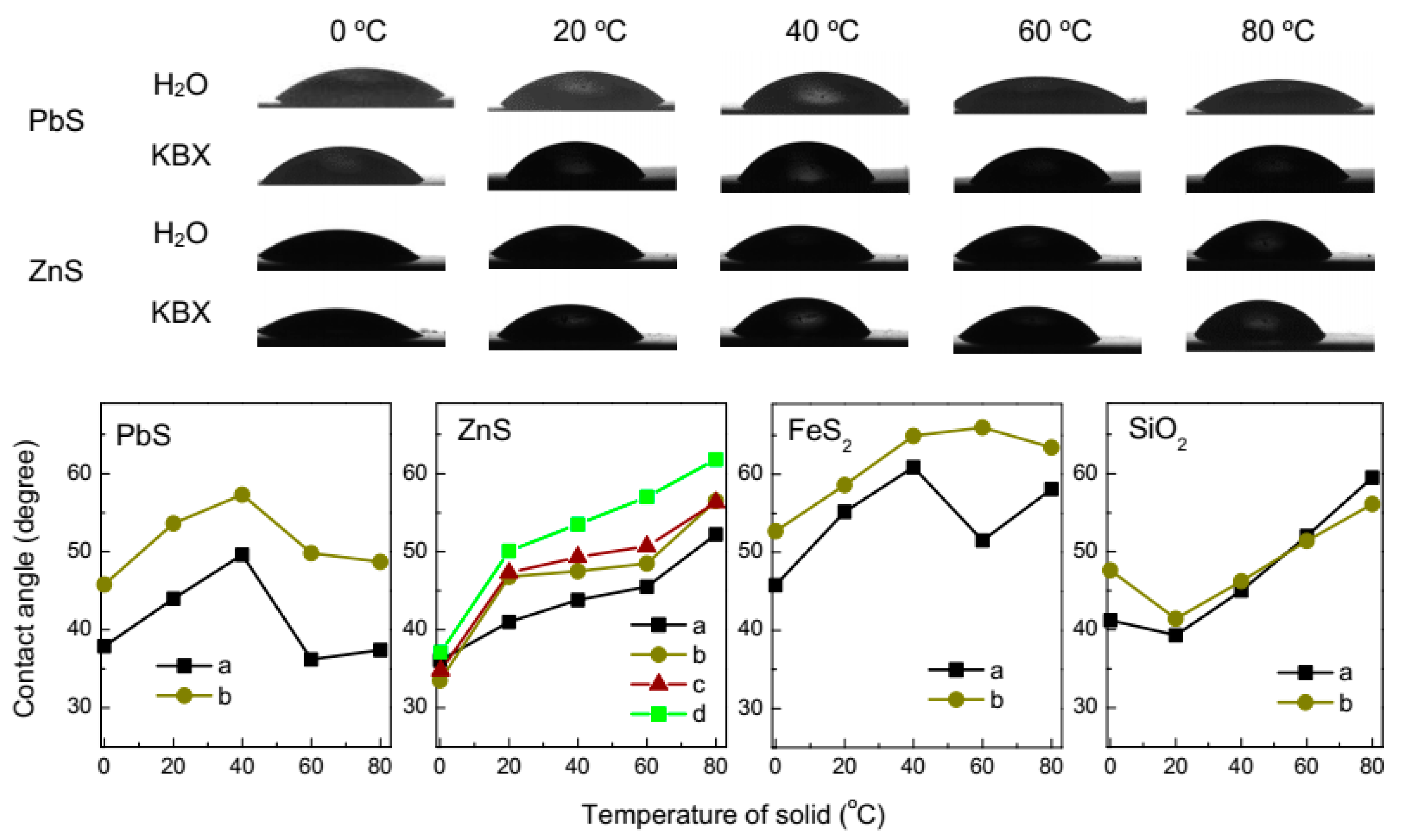
3.2. Contact Angle Measurement
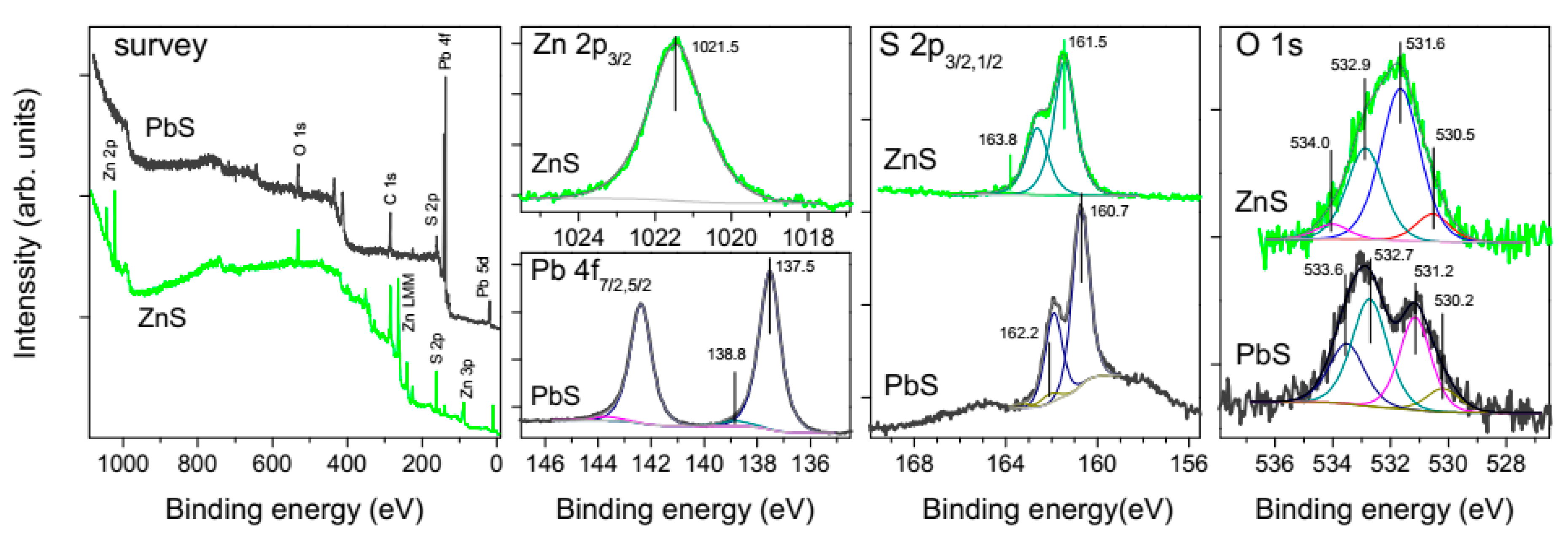
3.3. X-ray Photoelectron Spectroscopy Characterization
3.4. Single Mineral Flotation
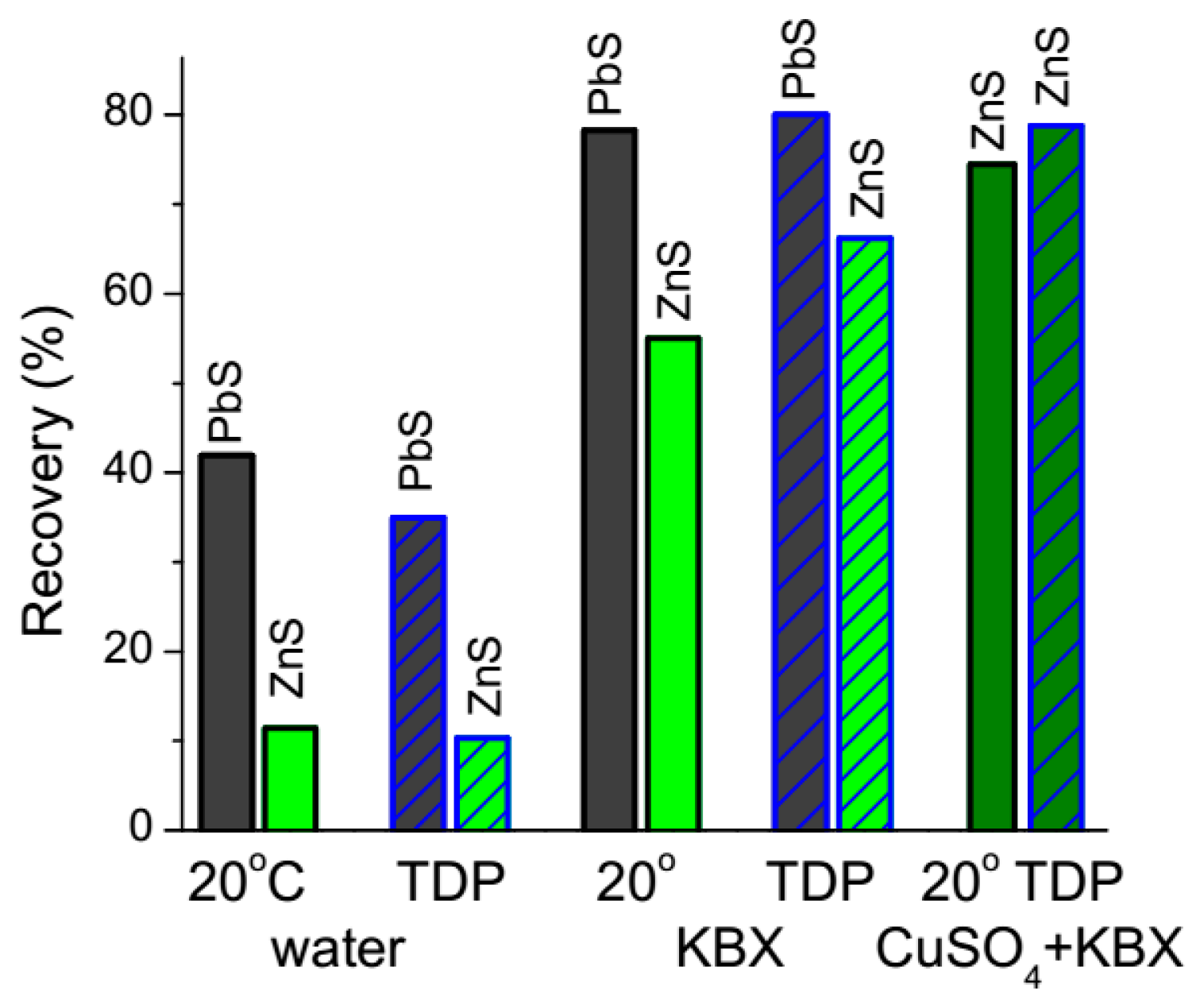
3.5. Flotation of Pb-Zn Sulfide Ore
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ishida, N.; Inoue, T.; Miyahara, M.; Higashitani, K. Nano bubbles on a hydrophobic surface in water observed by tapping-mode atomic force microscopy. Langmuir 2000, 16, 6377–6380. [Google Scholar] [CrossRef]
- An, H.; Liu, G.; Craig, V.S.J. Wetting of nanophases: Nanobubbles, nanodroplets and micropancakes on hydrophobic surfaces. Adv. Colloid Interface Sci. 2015, 222, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Alheshibri, M.; Qian, J.; Jehannin, M.; Craig, V.S.J. A history of nanobubbles. Langmuir 2016, 32, 11086–11100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lohse, D. Perspectives on surface nanobubbles. Biomicrofluidics 2014, 8, 041301. [Google Scholar] [CrossRef]
- Lohse, D.; Zhang, X. Surface nanobubbles and nanodroplets. Rev. Mod. Phys. 2015, 87, 981–1035. [Google Scholar] [CrossRef]
- Peng, H.; Hampton, M.A.; Nguyen, A.V. Nanobubbles do not sit alone at the solid–liquid interface. Langmuir 2013, 29, 6123–6130. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Pashley, R.M. The hydrophobic interaction is long range, decaying exponentially with distance. Nature 1982, 300, 341–342. [Google Scholar] [CrossRef]
- Meyer, E.E.; Rosenberg, K.J.; Israelachvili, J. Recent progress in understanding hydrophobic interactions. Proc. Natl. Acad. Sci. USA 2006, 103, 15739–15746. [Google Scholar] [CrossRef]
- Cao, P.; Xu, K.; Varghese, J.O.; Heath, J.R. The microscopic structure of adsorbed water on hydrophobic surfaces under ambient conditions. Nano Lett. 2011, 11, 5581–5586. [Google Scholar] [CrossRef]
- Tyrrell, J.W.G.; Attard, P. Atomic force microscope images of nanobubbles on a hydrophobic surface and corresponding force-separation data. Langmuir 2002, 18, 160–167. [Google Scholar] [CrossRef]
- Attard, P. Nanobubbles and the hydrophobic attraction. Adv. Colloid Interface Sci. 2003, 104, 75–91. [Google Scholar] [CrossRef]
- Hampton, M.A.; Nguyen, A.V. Nanobubbles and the nanobubble bridging capillary force. Adv. Colloid Interface Sci. 2010, 154, 30–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, J.; Fornasiero, D.; Ralston, J. Very small bubble formation at the solid−water interface. J. Phys. Chem. B 2003, 107, 6139–6147. [Google Scholar] [CrossRef]
- Schubert, H. Nanobubbles, hydrophobic effect, heterocoagulation and hydrodynamics in flotation. Int. J. Miner. Process. 2005, 78, 11–21. [Google Scholar] [CrossRef]
- Calgaroto, S.; Wilberg, K.Q.; Rubio, J. On the nanobubbles interfacial properties and future applications in flotation. Miner. Eng. 2014, 60, 33–40. [Google Scholar] [CrossRef]
- Hampton, M.A.; Nguyen, A.V. Accumulation of dissolved gases at hydrophobic surfaces in water and sodium chloride solutions: Implications for coal flotation. Miner. Eng. 2009, 22, 786–792. [Google Scholar] [CrossRef]
- Krasowska, M.; Malysa, K.; Beattie, D.A. Recent advances in studies of bubble-solid interactions and wetting film stability. Curr. Opin. Colloid Interface Sci. 2019, 44, 48–58. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Karacharov, A.A.; Likhatski, M.N. Effect of adsorption of butyl xanthate on galena, PbS, and HOPG surfaces as studied by atomic force microscopy and spectroscopy and XPS. Int. J. Miner. Process. 2015, 144, 81–89. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Karacharov, A.; Tomashevich, Y.; Shchukarev, A. Cryogenic XPS study of fast-frozen sulfide minerals: Flotation-related adsorption of n-butyl xanthate and beyond. J. Electron Spectrosc. Rel. Phenom. 2016, 206, 65–73. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Karacharov, A.; Tomashevich, Y.; Shchukarev, A. Interaction of sphalerite with potassium n-butyl xanthate and copper sulfate solutions studied by XPS of fast-frozen samples and zeta-potential measurement. Vacuum 2016, 125, 98–105. [Google Scholar] [CrossRef]
- Owens, C.L.; Schach, E.; Rudolph, M.; Nash, G.R. Surface nanobubbles on the carbonate mineral dolomite. RSC Adv. 2018, 8, 35448–35452. [Google Scholar] [CrossRef]
- Owens, C.L.; Schach, E.; Heinig, T.; Rudolph, M.; Nash, G.R. Surface nanobubbles on the rare earth fluorcarbonate mineral synchysite. J. Colloid Interface Sci. 2019, 552, 66–71. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Cao, Y. The hydrophobic force for bubble–particle attachment in flotation—A brief review. Phys. Chem. Chem. Phys. 2017, 19, 24421–24435. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Guo, W.; Gao, L.; Tang, Y.; Hu, J.; Dong, Y. Investigation on the temperature difference method for producing nanobubbles and their physical properties. ChemPhysChem 2012, 13, 2115–2118. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Peng, S.; Qiao, G.G.; Gutowski, V.; Lohse, D.; Zhang, X. Nanobubble formation on a warmer substrate. Soft Matter 2014, 10, 7857–7864. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Tan, B.H.; Zeng, Q.; Ohl, C.-D. Stability of nanobubbles formed at the interface between cold water and hot highly oriented pyrolytic graphite. Langmuir 2016, 32, 11212–11220. [Google Scholar] [CrossRef] [PubMed]
- Walczyk, W.; Schön, P.M.; Schönherr, H. The effect of PeakForce tapping mode AFM imaging on the apparent shape of surface nanobubbles. J. Phys. Condens. Matter 2013, 25, 184005. [Google Scholar] [CrossRef]
- Lu, Y.-H.; Yang, C.-W.; Fang, C.-K.; Ko, H.-C.; Hwang, I.-S. Interface-induced ordering of gas molecules confined in a small space. Sci. Rep. 2014, 4, 7189. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Romanchenko, A.S.; Shagaev, A.A. Scanning probe microscopy studies of PbS surfaces oxidized in air and etched in aqueous acid solutions. Appl. Surf. Sci. 2006, 252, 5645–5658. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Vorobyev, S.; Romanchenko, A.; Karasev, S.; Karacharov, A.; Zharkov, S. Ultrafine particles derived from mineral processing: A case study of the Pb–Zn sulfide ore with emphasis on lead-bearing colloids. Chemosphere 2016, 147, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Hayes, R.A.; Prestidge, C.A.; Ralston, J.; Smart, R.St.C. In-situ scanning tunnelling microscopy studies of galena surfaces under flotation-related conditions. Colloids Surf. Physicochem. Eng. Asp. 1996, 117, 117–129. [Google Scholar] [CrossRef]
- Higgins, S.R.; Hamers, R.J. Spatially-resolved electrochemistry of the lead sulfide (galena) (001) surface by electrochemical scanning tunneling microscopy. Surf. Sci. 1995, 324, 263–281. [Google Scholar] [CrossRef]
- De Giudici, G.; Rossi, A.; Fanfani, L.; Lattanzi, P. Mechanisms of galena dissolution in oxygen-saturated solutions: Evaluation of pH effect on apparent activation energies and mineral-water interface. Geochim. Cosmochim. Acta 2005, 69, 2321–2331. [Google Scholar] [CrossRef]
- Vorobyev, S.; Saikova, S.; Novikova, S.; Fetisova, O.; Zharkov, S.; Krylov, A.; Likhatski, M.; Mikhlin, Y. Colloidal and immobilized nanoparticles of lead xanthates. ACS Omega 2019, 4, 11472–11480. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.Y. Wetting of Real Surfaces; Walter de Gruyter GmbH: Berlin, Germany; Boston, FL, USA, 2013; pp. 5–6. [Google Scholar]
- Chau, T.T.; Bruckard, W.J.; Koh, P.T.L.; Nguyen, A.V. A review of factors that affect contact angle and implications for flotation practice. Adv. Colloid Interface Sci. 2009, 150, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.N.; Goh, S.W.; Lamb, R.N.; Woods, R. Interaction of thiol collectors with pre-oxidised sulfide minerals. Int. J. Miner. Process. 2003, 72, 163–174. [Google Scholar] [CrossRef]
- Piantadosi, C.; Smart, R.St.C. Statistical comparison of hydrophobic and hydrophilic species on galena and pyrite particles in flotation concentrates and tails from ToF-SIMS evidence. Int. J. Miner. Process. 2002, 64, 43–54. [Google Scholar] [CrossRef]
- Deng, M.; Karpuzov, D.; Liu, Q.; Xu, Z. Cryo-XPS study of xanthate adsorption on pyrite: Cryo-XPS study of xanthate adsorption on pyrite. Surf. Interface Anal. 2013, 45, 805–810. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.; Hu, J.; Wang, S.; Tai, R.; Gao, X.; Zhang, L. Interfacial gas nanobubbles or oil nanodroplets? Phys. Chem. Chem. Phys. 2017, 19, 1108–1114. [Google Scholar] [CrossRef]
- Kartio, I.; Laajalehto, K.; Suoninen, E. Characterization of the ethyl xanthate adsorption layer on galena (PbS) by synchrotron radiation excited photoelectron spectroscopy. Colloids Surf. Physicochem. Eng. Asp. 1999, 154, 97–101. [Google Scholar] [CrossRef]
- Nasluzov, V.; Shor, A.; Romanchenko, A.; Tomashevich, Y.; Mikhlin, Y. DFT+U and low-temperature XPS studies of Fe-depleted chalcopyrite (CuFeS2) surfaces: A focus on polysulfide species. J. Phys. Chem. C 2019, 123, 21031–21041. [Google Scholar] [CrossRef]
- Peng, Y.; Grano, S. Dissolution of fine and intermediate sized galena particles and their interactions with iron hydroxide colloids. J. Colloid Interface Sci. 2010, 347, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Krasowska, M.; Zawala, J.; Bradshaw-Hajek, B.H.; Ferri, J.K.; Beattie, D.A. Interfacial characterisation for flotation: 1. Solid-liquid interface. Curr. Opin. Colloid Interface Sci. 2018, 37, 61–73. [Google Scholar] [CrossRef]
- Pawliszak, P.; Zawala, J.; Ulaganathan, V.; Ferri, J.K.; Beattie, D.A.; Krasowska, M. Interfacial characterisation for flotation: 2. Air-water interface. Curr. Opin. Colloid Interface Sci. 2018, 37, 115–127. [Google Scholar] [CrossRef]
- Chanturiya, V.A.; Kondratiev, S.A. Contemporary understanding and developments in the flotation theory of non-ferrous ores. Miner. Process. Extr. Met. Rev. 2019, 40, 390–401. [Google Scholar] [CrossRef]
- Tarábková, H.; Janda, P. Nanobubble-assisted nanopatterning reveals the existence of liquid quasi-two-dimensional foams pinned to a water-immersed surface. Langmuir 2020, 36, 7200–7209. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhlin, Y.; Karacharov, A.; Vorobyev, S.; Romanchenko, A.; Likhatski, M.; Antsiferova, S.; Markosyan, S. Towards Understanding the Role of Surface Gas Nanostructures: Effect of Temperature Difference Pretreatment on Wetting and Flotation of Sulfide Minerals and Pb-Zn Ore. Nanomaterials 2020, 10, 1362. https://doi.org/10.3390/nano10071362
Mikhlin Y, Karacharov A, Vorobyev S, Romanchenko A, Likhatski M, Antsiferova S, Markosyan S. Towards Understanding the Role of Surface Gas Nanostructures: Effect of Temperature Difference Pretreatment on Wetting and Flotation of Sulfide Minerals and Pb-Zn Ore. Nanomaterials. 2020; 10(7):1362. https://doi.org/10.3390/nano10071362
Chicago/Turabian StyleMikhlin, Yuri, Anton Karacharov, Sergey Vorobyev, Alexander Romanchenko, Maxim Likhatski, Svetlana Antsiferova, and Svetlana Markosyan. 2020. "Towards Understanding the Role of Surface Gas Nanostructures: Effect of Temperature Difference Pretreatment on Wetting and Flotation of Sulfide Minerals and Pb-Zn Ore" Nanomaterials 10, no. 7: 1362. https://doi.org/10.3390/nano10071362
APA StyleMikhlin, Y., Karacharov, A., Vorobyev, S., Romanchenko, A., Likhatski, M., Antsiferova, S., & Markosyan, S. (2020). Towards Understanding the Role of Surface Gas Nanostructures: Effect of Temperature Difference Pretreatment on Wetting and Flotation of Sulfide Minerals and Pb-Zn Ore. Nanomaterials, 10(7), 1362. https://doi.org/10.3390/nano10071362





