Magnetic Mg-Fe/LDH Intercalated Activated Carbon Composites for Nitrate and Phosphate Removal from Wastewater: Insight into Behavior and Mechanisms
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Chemicals
2.2. Synthesis of Magnetic Mg-Fe/LDH Composites
2.3. Characterization Methods for Magnetic SBAC-MgFe Composites
2.4. Sorption Methodology for the Removal of Nitrate and Phosphate Ions
2.5. The Effects of Coexisting Ions
2.6. Equilibrium, Isotherm, and Kinetic Studies
2.7. Sequenced Adsorption/Regeneration Experiments
3. Results and Discussion
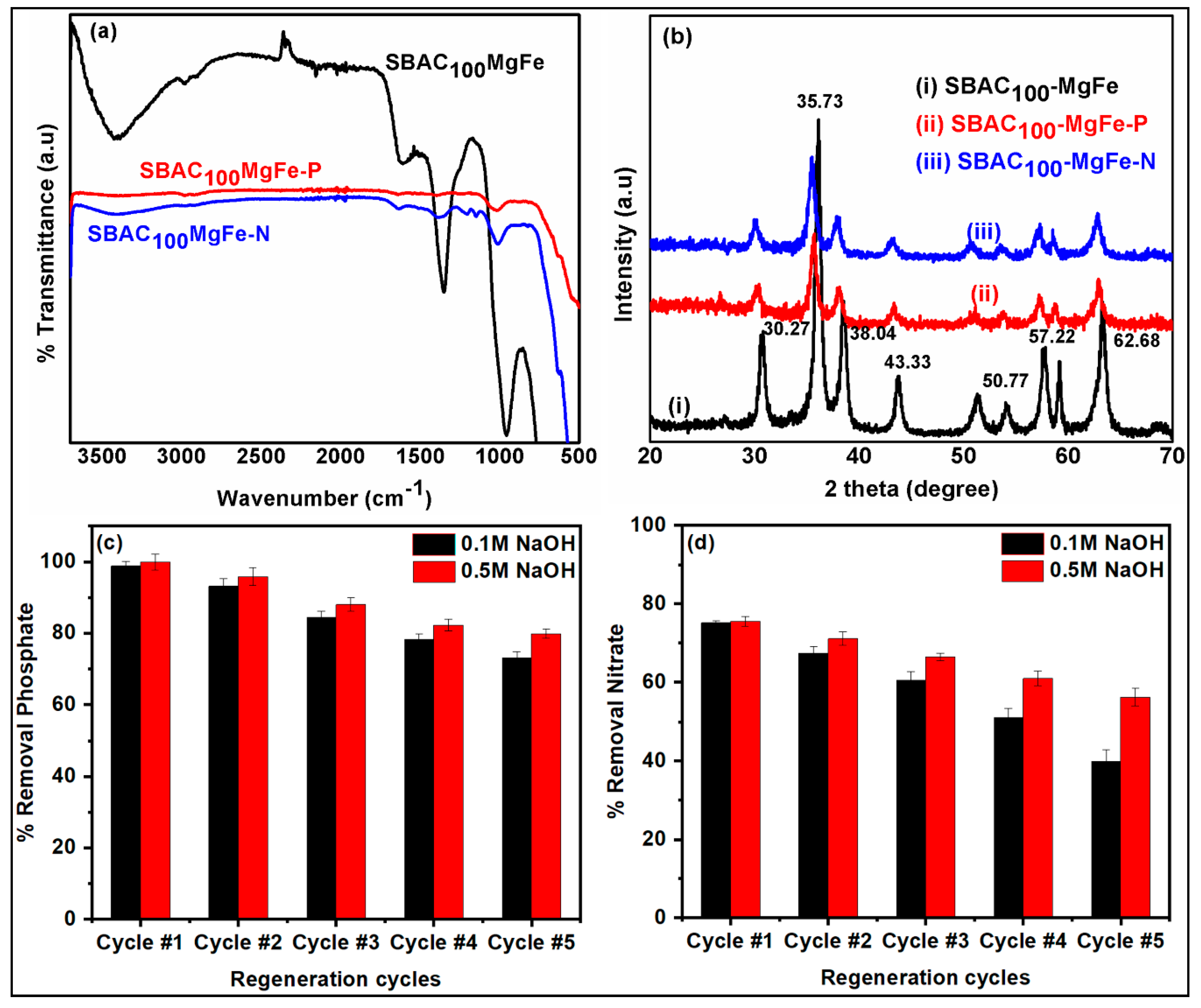
3.1. Characterization of Magnetic SBAC-MgFe Composites
3.2. Effects of pH, Adsorbent Dose, Contact Time, and Coexisting Ions on Adsorption
3.2.1. Initial Solution pH
3.2.2. Adsorbent Dosage
3.2.3. Contact Time and Kinetic Modeling
3.2.4. Coexisting Ions
3.3. Adsorption Isotherm Modeling Studies
3.4. Thermodynamic Modeling Studies
3.5. Mechanism Insight
3.6. Regeneration Studies and Reusability Performance
3.7. Removal Performance of SBAC-MgFe Composite in Real Wastewater
3.8. Comparison with Other Carbon-Based LDH Composites and Cost Estimation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhateria, R.; Jain, D. Water quality assessment of lake water: A review. Sustain. Water Resour. Manag. 2016, 2, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Keeney, D.; Olson, R.A. Sources of nitrate to ground water. Crit. Rev. Environ. Contr. 1986, 16, 257–304. [Google Scholar] [CrossRef]
- Mainstone, C.P.; Parr, W. Phosphorus in rivers—Ecology and management. Sci. Total Environ. 2002, 282–283, 25–47. [Google Scholar] [CrossRef]
- Imhoff, P.T.; Nakhli, S.A.A. Reducing Stormwater Runoff and Pollutant Loading with Biochar Addition to Highway Greenways; University of Delaware: Washington, DC, USA, 2017; p. 51. [Google Scholar]
- Chen, L.F.; Liang, H.W.; Lu, Y.; Cui, C.H.; Yu, S.H. Synthesis of an attapulgite clay@carbon nanocomposite adsorbent by a hydrothermal carbonization process and their application in the removal of toxic metal ions from water. Langmuir 2011, 27, 8998–9004. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Chao, C.; Waqas, M.; Arp, H.P.; Zhu, Y.G. Sewage sludge biochar influence upon rice (Oryza sativa L) yield, metal bioaccumulation and greenhouse gas emissions from acidic paddy soil. Environ. Sci. Technol. 2013, 47, 8624–8632. [Google Scholar] [CrossRef]
- Puga, A.; Abreu, C.; Melo, L.; Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef]
- Zubair, M.; Mu’azu, N.D.; Jarrah, N.; Blaisi, N.I.; Aziz, H.A.; Al-Harthi, M.A. Adsorption Behavior and Mechanism of Methylene Blue, Crystal Violet, Eriochrome Black T, and Methyl Orange Dyes onto Biochar-Derived Date Palm Fronds Waste Produced at Different Pyrolysis Conditions. Water Air Soil Pollut. 2020, 231. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Niazi, N.K.; Hassan, N.E.E.; Bibi, I.; Wang, H.; Tsang, D.C.W.; Ok, Y.S.; Bolan, N.; Rinklebe, J. Wood-based biochar for the removal of potentially toxic elements in water and wastewater: A critical review. Int. Mater. Rev. 2018, 64, 216–247. [Google Scholar] [CrossRef]
- Hibino, T.; Tsunashima, A. Characterization of Repeatedly Reconstructed Mg−Al Hydrotalcite-like Compounds: Gradual Segregation of Aluminum from the Structure. Chem. Mater. 1998, 10, 4055–4061. [Google Scholar] [CrossRef]
- Gupta, N.K.; Saifuddin, M.; Kim, S.; Kim, K.S. Microscopic, spectroscopic, and experimental approach towards understanding the phosphate adsorption onto Zn–Fe layered double hydroxide. J. Mol. Liq. 2020, 297, 111935. [Google Scholar] [CrossRef]
- Santos, L.C.; da Silva, A.F.; dos Santos Lins, P.V.; da Silva Duarte, J.L.; Ide, A.H.; Meili, L. Mg-Fe layered double hydroxide with chloride intercalated: Synthesis, characterization and application for efficient nitrate removal. Environ. Sci. Pollut. Res. 2020, 27, 5890–5900. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Pan, G.; Lundehøj, L.; Nielsen, U.G.; Shi, Y.; Hansen, H.C.B. Phosphate capture by ultrathin MgAl layered double hydroxide nanoparticles. Appl. Clay Sci. 2019, 177, 82–90. [Google Scholar] [CrossRef]
- Tran, H.N.; Nguyen, H.C.; Woo, S.H.; Nguyen, T.V.; Vigneswaran, S.; Hosseini-Bandegharaei, A.; Rinklebe, J.; Kumar Sarmah, A.; Ivanets, A.; Dotto, G.L.; et al. Removal of various contaminants from water by renewable lignocellulose-derived biosorbents: A comprehensive and critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2155–2219. [Google Scholar] [CrossRef]
- Ivanets, A.; Kitikova, N.; Shashkova, I.; Matrunchik, Y.; Kul’bitskaya, L.; Sillanpää, M. Non-acidic synthesis of phosphatized dolomite and its sorption behaviour towards Pb2+, Zn2+, Cu2+, Cd2+, Ni2+, Sr2+ and Co2+ ions in multicomponent aqueous solution. Environ. Technol. Innov. 2016, 6, 152–164. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Liu, T.; Yuan, Z.; Guo, J. Efficient nitrate removal from synthetic groundwater via in situ utilization of short-chain fatty acids from methane bioconversion. Chem. Eng. J. 2020, 393, 124594. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Shashkova, I.L.; Kitikova, N.V.; Kul’bitskaya, L.V.; Matrunchik, Y.V. Study of the interaction of mono-, di-, and trisubstituted sodium orthophosphates with thermally activated dolomite. Russ. J. Appl. Chem. 2015, 88, 1757–1762. [Google Scholar] [CrossRef]
- Zubair, M.; Manzar, M.S.; Mu’azu, N.D.; Anil, I.; Blaisi, N.I.; Al-Harthi, M.A. Functionalized MgAl-layered hydroxide intercalated date-palm biochar for Enhanced Uptake of Cationic dye: Kinetics, isotherm and thermodynamic studies. Appl. Clay Sci. 2020, 190, 105587. [Google Scholar] [CrossRef]
- Xue, L.; Gao, B.; Wan, Y.; Fang, J.; Wang, S.; Li, Y.; Muñoz-Carpena, R.; Yang, L. High efficiency and selectivity of MgFe-LDH modified wheat-straw biochar in the removal of nitrate from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2016, 63, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Yin, Q.; Wang, R.; Zhao, Z. Application of Mg–Al-modified biochar for simultaneous removal of ammonium, nitrate, and phosphate from eutrophic water. J. Clean. Prod. 2018, 176, 230–240. [Google Scholar] [CrossRef]
- You, H.; Li, W.; Zhang, Y.; Meng, Z.; Shang, Z.; Feng, X.; Ma, Y.; Lu, J.; Li, M.; Niu, X. Enhanced removal of NO3-N from water using Fe-Al modified biochar: Behavior and mechanism. Water Sci. Technol. 2019, 80, 2003–2012. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Zubair, M.; Jarrah, N.; Alagha, O.; Al-Harthi, M.A.; Essa, M.H. Sewage Sludge ZnCl2-Activated Carbon Intercalated MgFe-LDH Nanocomposites: Insight of the Sorption Mechanism of Improved Removal of Phenol from Water. Int. J. Mol. Sci. 2020, 21, 1563. [Google Scholar] [CrossRef] [Green Version]
- Liadi, M.A.; Mu’azu, N.D.; Jarrah, N.; Zubair, M.; Alagha, O.; Al-Harthi, M.A.; Essa, M.H. Comparative performance study of ZnCl2 and NaOH sludge based activated carbon for simultaneous aqueous uptake of phenolic compounds. Int. J. Environ. Anal. Chem. 2020, 1–25. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. 1998, 76, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The constitution and fundamental properties of solids and liquids Part I Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Peng, Y.; Sun, Y.; Sun, R.; Zhou, Y.; Tsang, D.C.W.; Chen, Q. Optimizing the synthesis of Fe/Al (Hydr)oxides-Biochars to maximize phosphate removal via response surface model. J. Clean. Prod. 2019, 237, 117770. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.; Han, X.; Wu, Z.; Lai, C.; Wang, J.; Deng, Q.; Zeng, Z.; Deng, S. Simultaneous and efficient removal of Cr(VI) and methyl orange on LDHs decorated porous carbons. Chem. Eng. J. 2018, 352, 306–315. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Jarrah, N.; Zubair, M.; Alagha, O. Removal of phenolic compounds from water using sewage sludge-based activated carbon adsorption: A review. Int. J. Environ. Res. Public Health 2017, 14, 1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Kazeem, T.S.; Zubair, M.; Daud, M.; Mu’azu, N.D.; Al-Harthi, M.A. Graphene/ternary layered double hydroxide composites: Efficient removal of anionic dye from aqueous phase. Korean J. Chem. Eng. 2019, 36, 1057–1068. [Google Scholar] [CrossRef]
- Durrani, S.K.; Naz, S.; Mehmood, M.; Nadeem, M.; Siddique, M. Structural, impedance and Mössbauer studies of magnesium ferrite synthesized via sol–gel auto-combustion process. J. Saudi Chem. Soc. 2017, 21, 899–910. [Google Scholar] [CrossRef] [Green Version]
- Ivanets, A.I.; Srivastava, V.; Roshchina, M.Y.; Sillanpää, M.; Prozorovich, V.G.; Pankov, V.V. Magnesium ferrite nanoparticles as a magnetic sorbent for the removal of Mn2+, Co2+, Ni2+ and Cu2+ from aqueous solution. Ceram. Int. 2018, 44, 9097–9104. [Google Scholar] [CrossRef]
- Valeikiene, L.; Roshchina, M.; Grigoraviciute-Puroniene, I.; Prozorovich, V.; Zarkov, A.; Ivanets, A.; Kareiva, A. On the Reconstruction Peculiarities of Sol–Gel Derived Mg2−xMx/Al1 (M = Ca, Sr, Ba) Layered Double Hydroxides. Crystals 2020, 10, 470. [Google Scholar] [CrossRef]
- Kang, D.; Yu, X.; Tong, S.; Ge, M.; Zuo, J.; Cao, C.; Song, W. Performance and mechanism of Mg/Fe layered double hydroxides for fluoride and arsenate removal from aqueous solution. Chem. Eng. J. 2013, 228, 731–740. [Google Scholar] [CrossRef]
- Morrell, D.G. Catalysis of Organic Reactions, 1st ed.; CRC Press: New York, NY, USA, 2019; p. 712. [Google Scholar]
- Xu, Q.; Wei, Y.; Liu, Y.; Ji, X.; Yang, L.; Gu, M. Preparation of Mg/Fe spinel ferrite nanoparticles from Mg/Fe-LDH microcrystallites under mild conditions. Solid State Sci. 2009, 11, 472–478. [Google Scholar] [CrossRef]
- Hu, F.P.; Wang, M.; Peng, X.M.; Qiu, F.X.; Zhang, T.; Dai, H.L.; Liu, Z.M.; Cao, Z. High-efficient adsorption of phosphates from water by hierarchical CuAl/biomass carbon fiber layered double hydroxide. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 314–323. [Google Scholar] [CrossRef]
- Azam, H.M.; Alam, S.T.; Hasan, M.; Yameogo, D.D.S.; Kannan, A.D.; Rahman, A.; Kwon, M.J. Phosphorous in the environment: Characteristics with distribution and effects, removal mechanisms, treatment technologies, and factors affecting recovery as minerals in natural and engineered systems. Environ. Sci. Pollut. Res. Int. 2019, 26, 20183–20207. [Google Scholar] [CrossRef]
- Diaz, O.A.; Reddy, K.R.; Moore, P.A. Solubility of inorganic phosphorus in stream water as influenced by pH and calcium concentration. Water Res. 1994, 28, 1755–1763. [Google Scholar] [CrossRef]
- Yuan, L.; Qiu, Z.; Yuan, L.; Tariq, M.; Lu, Y.; Yang, J.; Li, Z.; Lyu, S. Adsorption and mechanistic study for phosphate removal by magnetic Fe3O4-doped spent FCC catalysts adsorbent. Chemosphere 2019, 219, 183–190. [Google Scholar] [CrossRef]
- Wei, A.; Ma, J.; Chen, J.; Zhang, Y.; Song, J.; Yu, X. Enhanced nitrate removal and high selectivity towards dinitrogen for groundwater remediation using biochar-supported nano zero-valent iron. Chem. Eng. J. 2018, 353, 595–605. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Shashkova, I.L.; Kitikova, N.V.; Morozov, Y. The kinetic studies of the cobalt ion removal from aqueous solutions by dolomite-based sorbent. Int. J. Environ. Sci. Technol. 2016, 13, 2561–2568. [Google Scholar] [CrossRef]
- Wei, J.; Meng, X.; Wen, X.; Song, Y. Adsorption and recovery of phosphate from water by amine fiber, effects of co-existing ions and column filtration. J. Environ. Sci. 2020, 87, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, P.; Elanchezhiyan, S.S.D.; Preethi, J.; Meenakshi, S.; Park, C.M. Mechanistic performance of polyaniline-substituted hexagonal boron nitride composite as a highly efficient adsorbent for the removal of phosphate, nitrate, and hexavalent chromium ions from an aqueous environment. Appl. Surf. Sci. 2020, 511, 145543. [Google Scholar] [CrossRef]
- Berkessa, Y.W.; Mereta, S.T.; Feyisa, F.F. Simultaneous removal of nitrate and phosphate from wastewater using solid waste from factory. Appl. Water Sci. 2019, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Nzihou, A.; Sharrock, P. Role of Phosphate in the Remediation and Reuse of Heavy Metal Polluted Wastes and Sites. Waste Biomass Valoriz. 2010, 1, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Anil, I.; Gunday, S.T.; Bozkurt, A.; Alagha, O. Design of Crosslinked Hydrogels Comprising Poly(Vinylphosphonic Acid) and Bis[2-(Methacryloyloxy)Ethyl] Phosphate as an Efficient Adsorbent for Wastewater Dye Removal. Nanomaterials 2020, 10, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halajnia, A.; Oustan, S.; Najafi, N.; Khataee, A.R.; Lakzian, A. The adsorption characteristics of nitrate on Mg–Fe and Mg–Al layered double hydroxides in a simulated soil solution. Appl. Clay Sci. 2012, 70, 28–36. [Google Scholar] [CrossRef]
- Yan, L.G.; Yang, K.; Shan, R.R.; Yan, T.; Wei, J.; Yu, S.J.; Yu, H.Q.; Du, B. Kinetic, isotherm and thermodynamic investigations of phosphate adsorption onto core-shell Fe(3)O(4)@LDHs composites with easy magnetic separation assistance. J. Colloid Interface Sci. 2015, 448, 508–516. [Google Scholar] [CrossRef]
- Ivanets, A.I.; Srivastava, V.; Kitikova, N.V.; Shashkova, I.L.; Sillanpää, M. Kinetic and thermodynamic studies of the Co(II) and Ni(II) ions removal from aqueous solutions by Ca-Mg phosphates. Chemosphere 2017, 171, 348–354. [Google Scholar] [CrossRef]
- Halajnia, A.; Oustan, S.; Najafi, N.; Khataee, A.R.; Lakzian, A. Adsorption–desorption characteristics of nitrate, phosphate and sulfate on Mg–Al layered double hydroxide. Appl. Clay Sci. 2013, 80–81, 305–312. [Google Scholar] [CrossRef]
- Tong, X.; Yang, Z.; Xu, P.; Li, Y.; Niu, X. Nitrate adsorption from aqueous solutions by calcined ternary Mg-Al-Fe hydrotalcite. Water Sci. Technol. 2017, 75, 2194–2203. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, L.; Xu, P.; Zhang, X.; Niu, X.; Zhou, S. The adsorption of nitrate from aqueous solution onto calcined Mg/Fe hydrotalcite. Desalin. Water Treat. 2014, 54, 3400–3411. [Google Scholar] [CrossRef]
- Bolbol, H.; Fekri, M.; Hejazi-Mehrizi, M. Layered double hydroxide–loaded biochar as a sorbent for the removal of aquatic phosphorus: Behavior and mechanism insights. Arab. J. Geosci. 2019, 12, 503. [Google Scholar] [CrossRef]
- Chitrakar, R.; Tezuka, S.; Hosokawa, J.; Makita, Y.; Sonoda, A.; Ooi, K.; Hirotsu, T. Uptake properties of phosphate on a novel Zr-modified MgFe-LDH(CO(3)). J. Colloid Interface Sci. 2010, 349, 314–320. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lundehøj, L.; Nielsen, U.G. An investigation of the phosphate removal mechanism by MgFe layered double hydroxides. Appl. Clay Sci. 2020, 189. [Google Scholar] [CrossRef]
- Sun, X.; Imai, T.; Sekine, M.; Higuchi, T.; Yamamoto, K.; Kanno, A.; Nakazono, S. Adsorption of phosphate using calcined Mg3–Fe layered double hydroxides in a fixed-bed column study. J. Ind. Eng. Chem. 2014, 20, 3623–3630. [Google Scholar] [CrossRef]
- Wan, S.; Wang, S.; Li, Y.; Gao, B. Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions. J. Ind. Eng. Chem. 2017, 47, 246–253. [Google Scholar] [CrossRef]
- Sasai, R.; Norimatsu, W.; Matsumoto, Y. Nitrate-ion-selective exchange ability of layered double hydroxide consisting of MgII and FeIII. J. Hazard. Mater. 2012, 215–216, 311–314. [Google Scholar] [CrossRef]
- Baldermann, A.; Fleischhacker, Y.; Schmidthaler, S.; Wester, K.; Nachtnebel, M.; Eichinger, S. Removal of Barium from Solution by Natural and Iron(III) Oxide-Modified Allophane, Beidellite and Zeolite Adsorbents. Materials 2020, 13, 2582. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Zhang, N.; Chen, N.; Lei, Z.; Shimizu, K.; Zhang, Z. Efficient phosphate removal from wastewater by MgAl-LDHs modified hydrochar derived from tobacco stalk. Bioresour. Technol. Rep. 2019, 8, 100348. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, J.-W.; Song, K.G.; Choi, K.; Lee, Y.J.; Jung, K.-W. Adsorption and mechanistic study for phosphate removal by rice husk-derived biochar functionalized with Mg/Al-calcined layered double hydroxides via co-pyrolysis. Compos. Part B Eng. 2019, 176, 107209. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, L.; Yu, H.; Yan, T.; Li, X. Adsorption of phosphate from aqueous solution by vegetable biochar/layered double oxides: Fast removal and mechanistic studies. Bioresour. Technol. 2019, 284, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Alagha, O.; Manzar, M.S.; Zubair, M.; Anil, I.; Mu’azu, N.D.; Qureshi, A. Comparative Adsorptive Removal of Phosphate and Nitrate from Wastewater Using Biochar-MgAl LDH Nanocomposites: Coexisting Anions Effect and Mechanistic Studies. Nanomaterials 2020, 10, 336. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Gupta, V.K. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
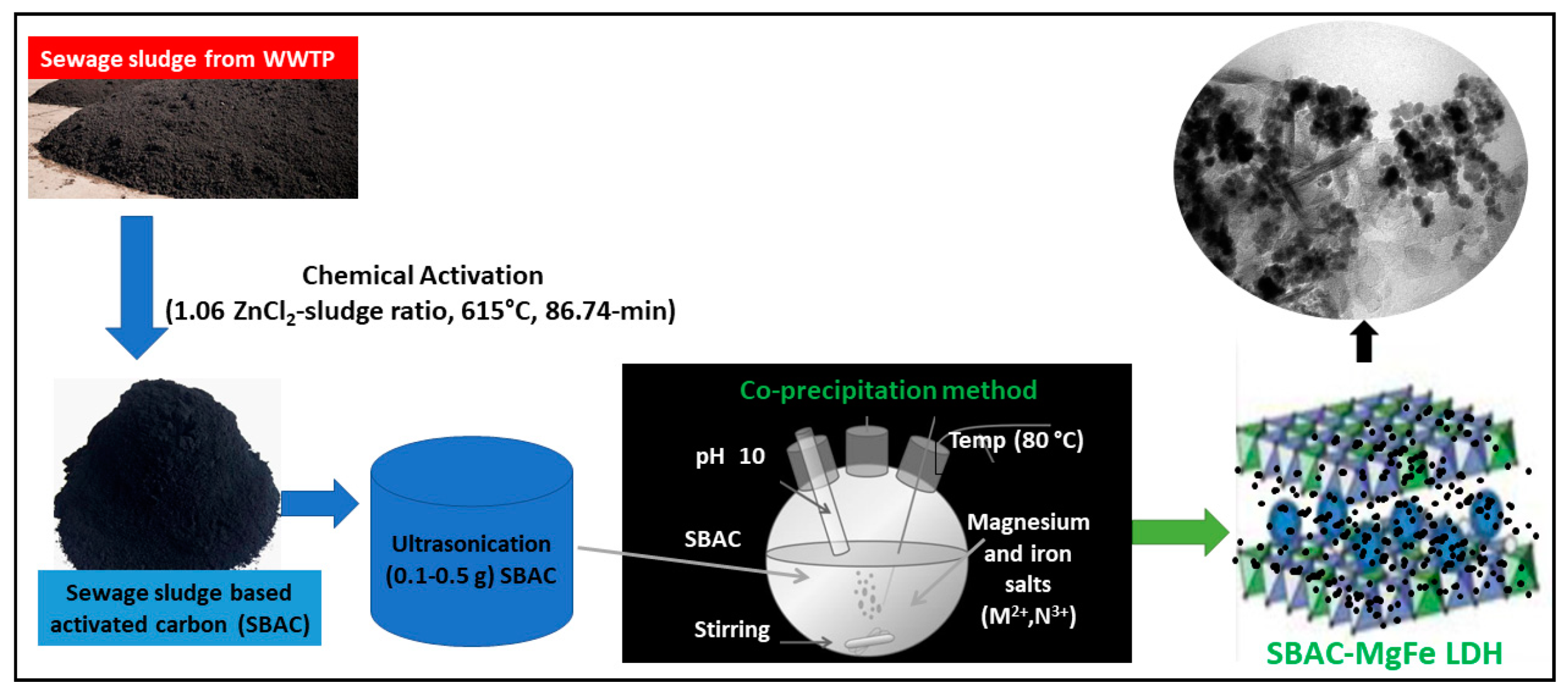

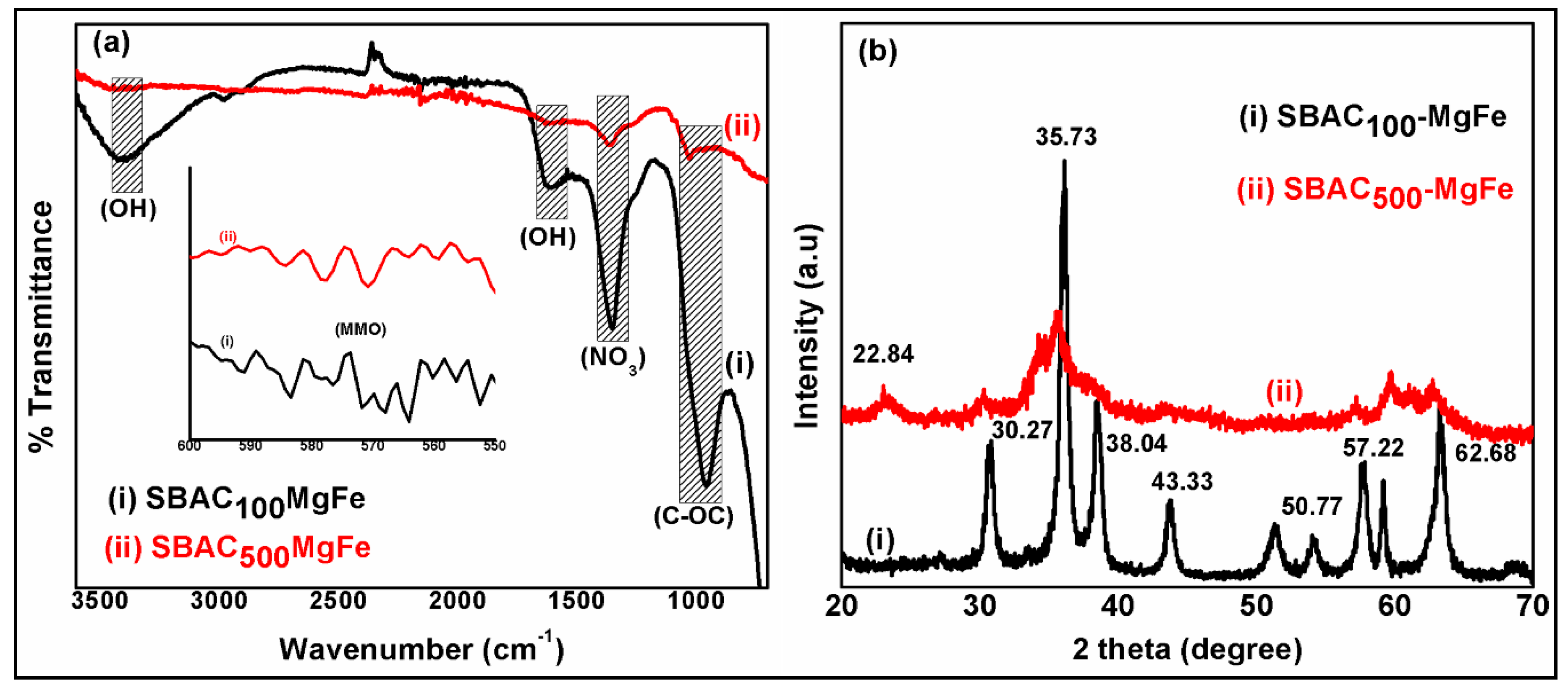
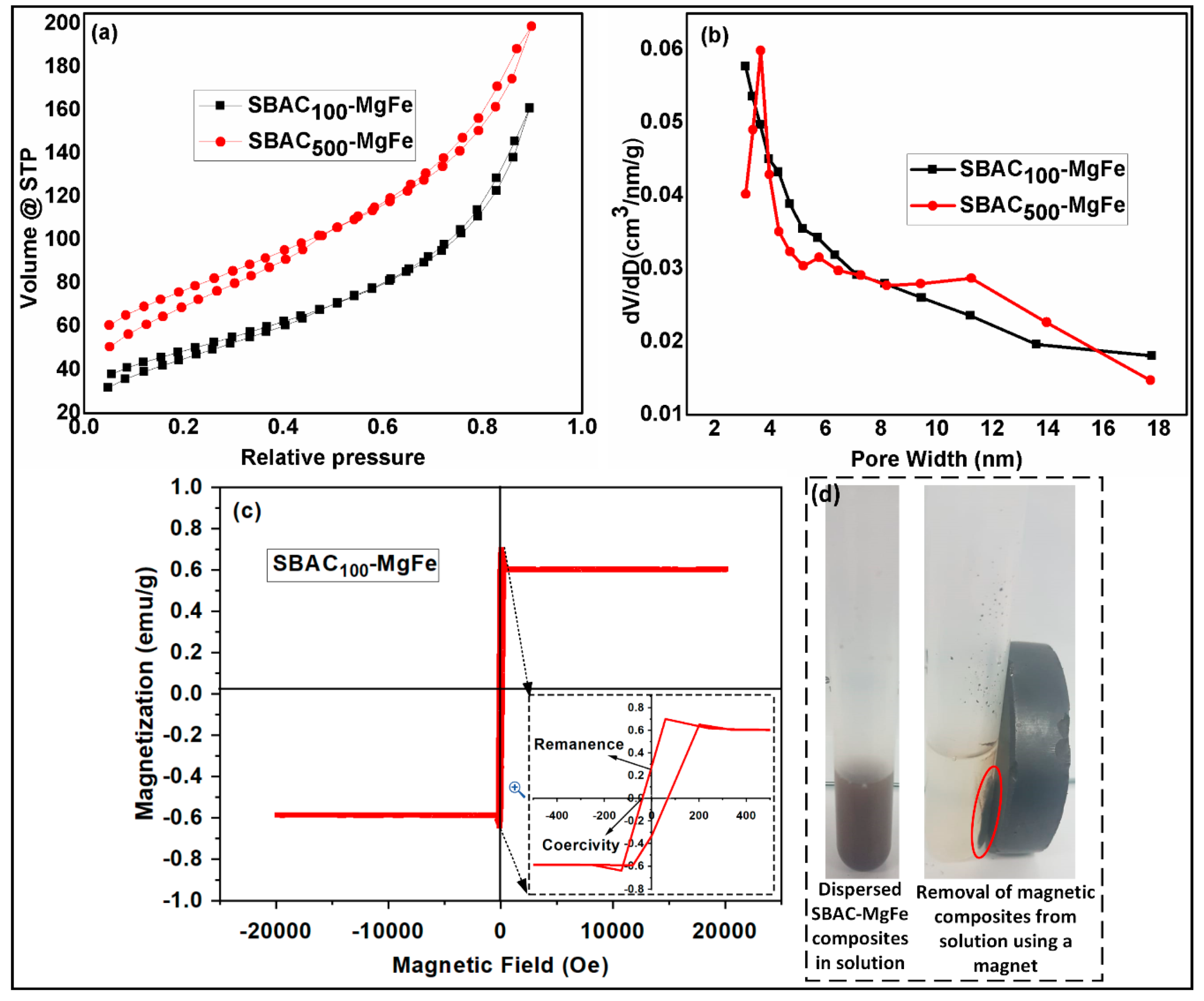
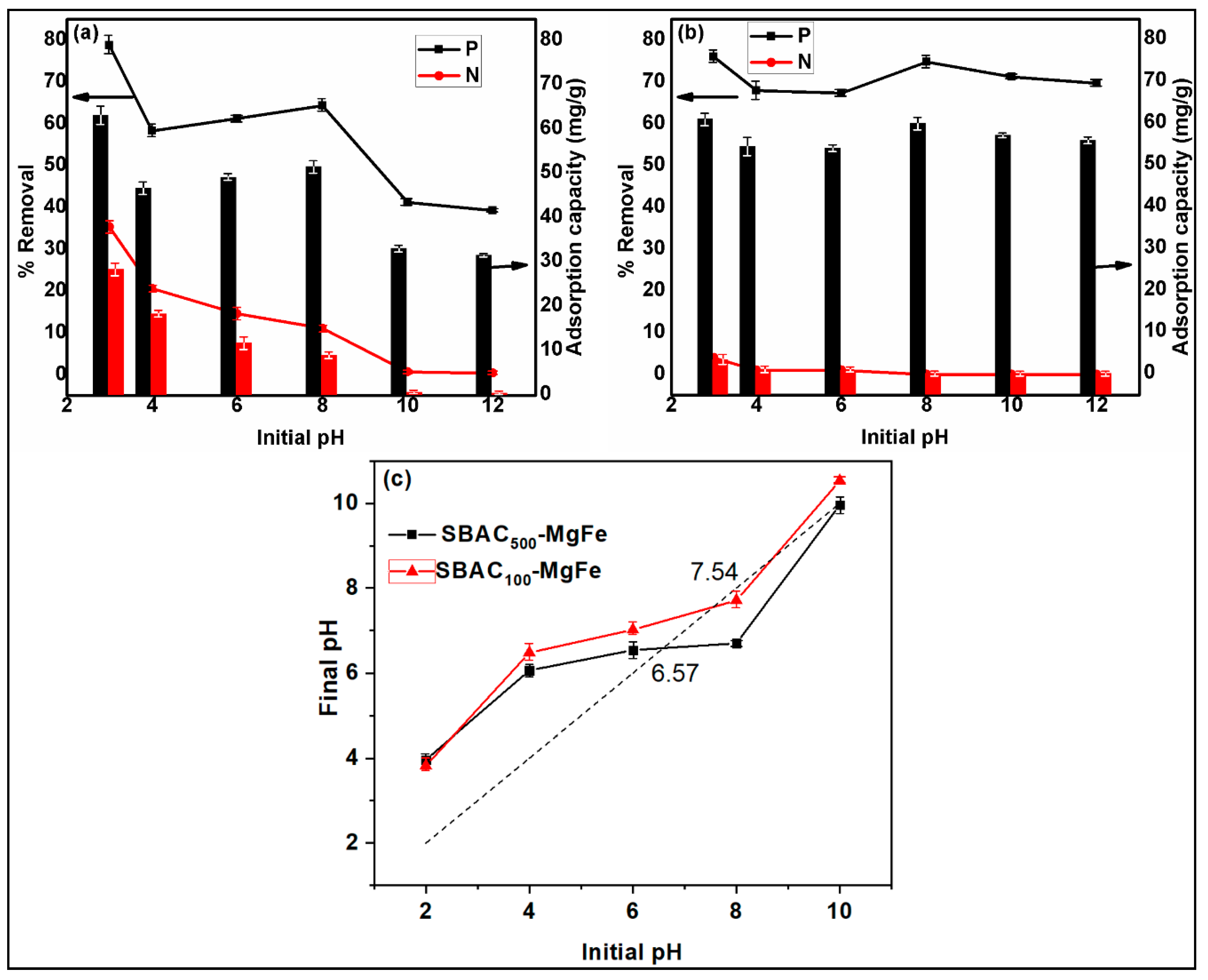



| Composite | SBAC | Mg(II) Salt | Fe (III) Salt | SBAC (Carbon) | Mg(II) | Fe(III) |
|---|---|---|---|---|---|---|
| Theoretical (g) | Actual (%) | |||||
| SBAC100MgFe | 0.1 | 2.54 | 4.04 | 12.6 | 13.3 | 55.3 |
| SBAC500MgFe | 0.5 | 2.54 | 4.04 | 20.8 | 8.2 | 39.6 |
| Textural Properties | SBAC100MgFe | SBAC500MgFe |
|---|---|---|
| BET surface area (m2/g) | 169 | 257 |
| Pore volume (cm3/g) | 0.21 | 0.23 |
| Pore radius (based on BJH) (nm) | 1.82 | 1.72 |
| Pollutant | C0 | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|---|
| qe (exp) | qe | k1 | R2 | qe | k2 × 10−5 | R2 | ||
| Phosphate | 10 | 63.1 | 67.7 | 0.0416 | 0.992 | 99.0 | 6.03 | 0.899 |
| 30 | 95.9 | 99.7 | 0.0451 | 0.990 | 135 | 6.07 | 0.901 | |
| 50 | 113 | 115 | 0.0161 | 0.994 | 170 | 3.20 | 0.703 | |
| Nitrate | 10 | 27.9 | 31.9 | 0.0184 | 0.972 | 15.0 | 17.5 | 0.219 |
| 30 | 39.3 | 42.5 | 0.0140 | 0.978 | 20.2 | 13.1 | 0.115 | |
| 50 | 32.2 | 43.1 | 0.0322 | 0.930 | 12.8 | 21.1 | 0.303 | |
| Pollutant | T (K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| qmax | KL | R2 | KF | 1/n | R2 | ||
| Phosphate (pH 3) | 298 | 104.1 | 0.89 | 0.989 | 46.1 | 0.25 | 0.927 |
| 308 | 108.5 | 0.91 | 0.985 | 47.7 | 0.26 | 0.935 | |
| 318 | 109.9 | 1.12 | 0.974 | 50.3 | 0.25 | 0.944 | |
| Phosphate (pH 6) | 298 | 65.5 | 0.51 | 0.981 | 28.1 | 0.23 | 0.932 |
| 308 | 68.8 | 0.57 | 0.977 | 30.5 | 0.23 | 0.915 | |
| 318 | 73.4 | 0.58 | 0.981 | 32.0 | 0.23 | 0.917 | |
| Nitrate (pH 3) | 298 | 46.3 | 0.25 | 0.988 | 15.1 | 0.30 | 0.993 |
| 308 | 51.7 | 0.24 | 0.987 | 15.8 | 0.31 | 0.988 | |
| 318 | 54.5 | 0.23 | 0.981 | 16.7 | 0.32 | 0.995 | |
| Nitrate (pH 6) | 298 | 20.8 | 0.21 | 0.925 | 6.66 | 0.29 | 0.981 |
| 308 | 25.4 | 0.23 | 0.999 | 8.94 | 0.26 | 0.977 | |
| 318 | 25.7 | 0.31 | 0.987 | 10.7 | 0.22 | 0.977 | |
| T (K) | Kd | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (J/mol K) | |
|---|---|---|---|---|---|
| Phosphate (pH 3) | 298 | 30.0 | −8.43 | ||
| 308 | 31.8 | −8.86 | 5.30 | 46.0 | |
| 318 | 34.3 | −9.35 | |||
| Phosphate (pH 6) | 298 | 12.6 | −6.27 | ||
| 308 | 15.5 | −7.01 | 11.8 | 60.7 | |
| 318 | 16.9 | −7.48 | |||
| Nitrate (pH 3) | 298 | 4.35 | −3.64 | ||
| 308 | 4.50 | −3.85 | 4.90 | 28.5 | |
| 318 | 4.92 | −4.21 | |||
| Nitrate (pH 6) | 298 | 1.36 | −0.76 | ||
| 308 | 1.95 | −1.71 | 18.8 | 65.8 | |
| 318 | 2.18 | −2.06 |
| Adsorbent | Adsorbate | pH | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|
| Sugar cane leaves biochar/(4:1) MgAl | Phosphorous | 3 | 81.8 | [63] |
| Pinecone flakes biochar/MgFe | Phosphorous | 2 | 17.5 | [56] |
| Tobacco stalk biochar/MgAl | Phosphate | 2 | 41.2 | [64] |
| Rice husk biochar/MgAl calcined | Phosphate | 4 | 121 | [65] |
| Cabbage biochar/MgAl calcined | Phosphate | 2 | 127 | [66] |
| Rape biochar/MgAl calcined | Phosphate | 2 | 133 | [66] |
| Date palm fronds biochar/MgAl | Phosphate/Nitrate | 3 | 146/31.9 | [67] |
| Sewage sludge-activated carbon/MgFe LDH | Phosphate/Nitrate | 3 | 104/46.3 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alagha, O.; Manzar, M.S.; Zubair, M.; Anil, I.; Mu’azu, N.D.; Qureshi, A. Magnetic Mg-Fe/LDH Intercalated Activated Carbon Composites for Nitrate and Phosphate Removal from Wastewater: Insight into Behavior and Mechanisms. Nanomaterials 2020, 10, 1361. https://doi.org/10.3390/nano10071361
Alagha O, Manzar MS, Zubair M, Anil I, Mu’azu ND, Qureshi A. Magnetic Mg-Fe/LDH Intercalated Activated Carbon Composites for Nitrate and Phosphate Removal from Wastewater: Insight into Behavior and Mechanisms. Nanomaterials. 2020; 10(7):1361. https://doi.org/10.3390/nano10071361
Chicago/Turabian StyleAlagha, Omar, Mohammad Saood Manzar, Mukarram Zubair, Ismail Anil, Nuhu Dalhat Mu’azu, and Aleem Qureshi. 2020. "Magnetic Mg-Fe/LDH Intercalated Activated Carbon Composites for Nitrate and Phosphate Removal from Wastewater: Insight into Behavior and Mechanisms" Nanomaterials 10, no. 7: 1361. https://doi.org/10.3390/nano10071361
APA StyleAlagha, O., Manzar, M. S., Zubair, M., Anil, I., Mu’azu, N. D., & Qureshi, A. (2020). Magnetic Mg-Fe/LDH Intercalated Activated Carbon Composites for Nitrate and Phosphate Removal from Wastewater: Insight into Behavior and Mechanisms. Nanomaterials, 10(7), 1361. https://doi.org/10.3390/nano10071361







