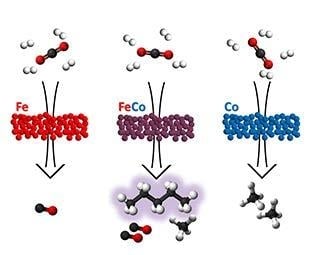
CO2 Hydrogenation over Unsupported Fe-Co Nanoalloy Catalysts
Abstract
1. Introduction
2. Materials and Methods
3. Results
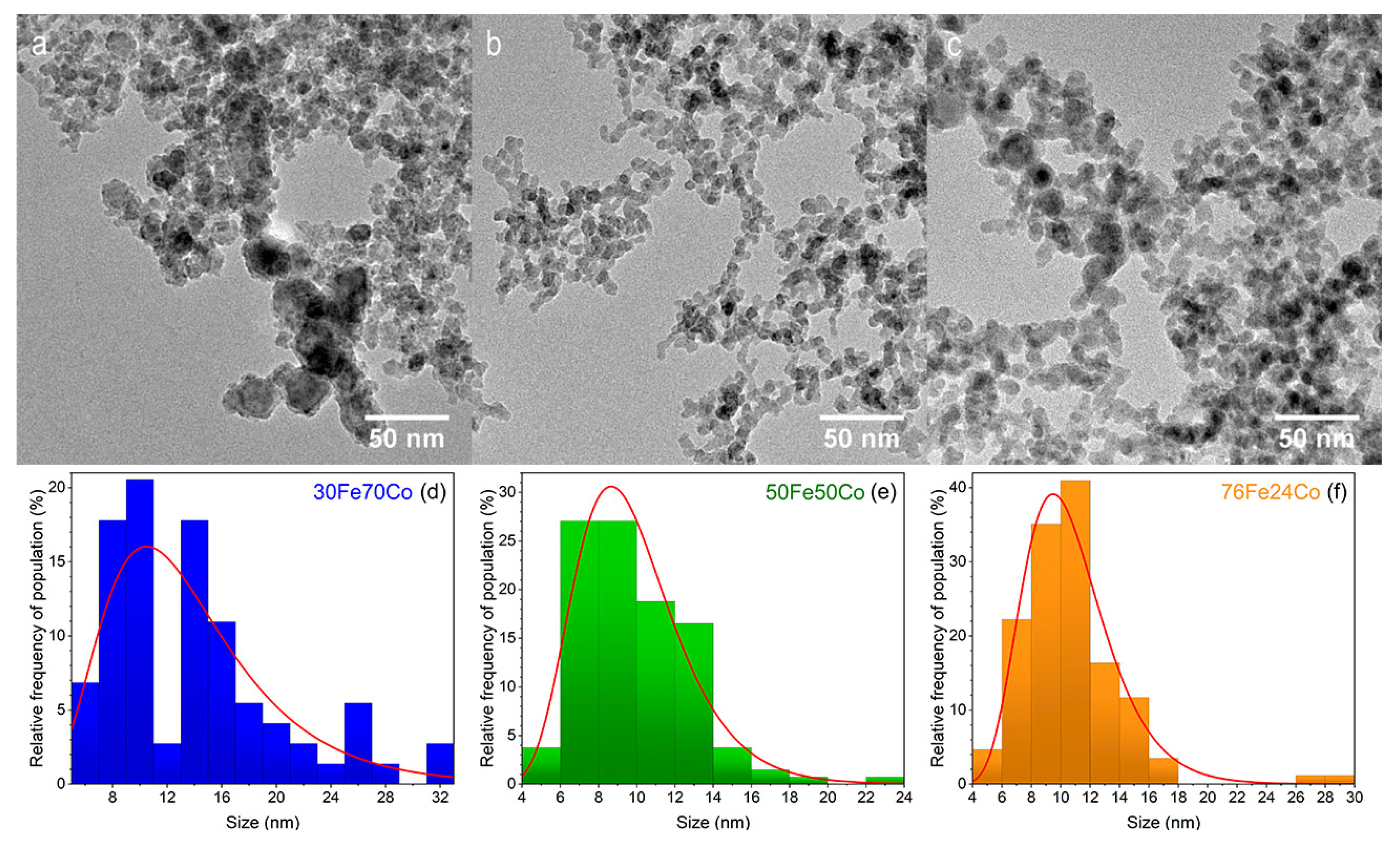
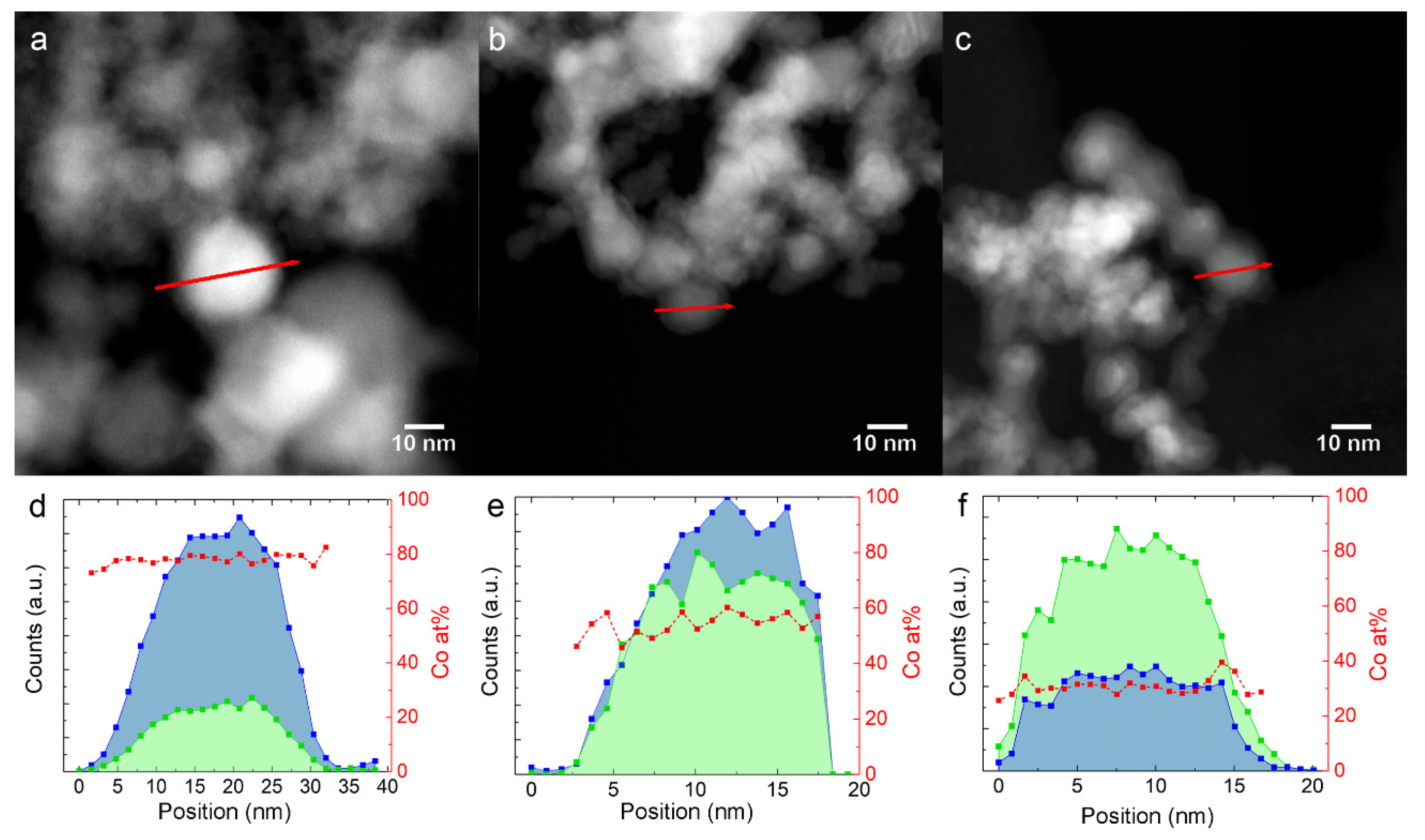
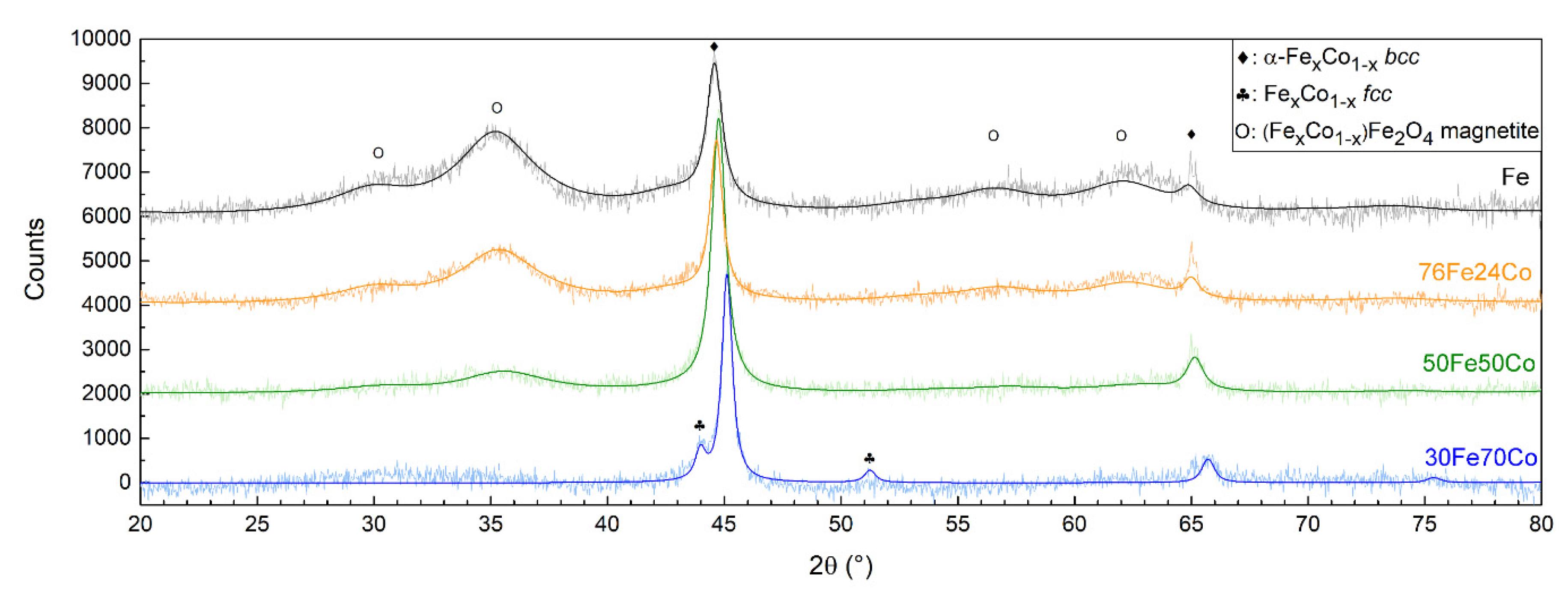
3.1. Structure, Morphology and Composition of Fe-Co Nanoalloys
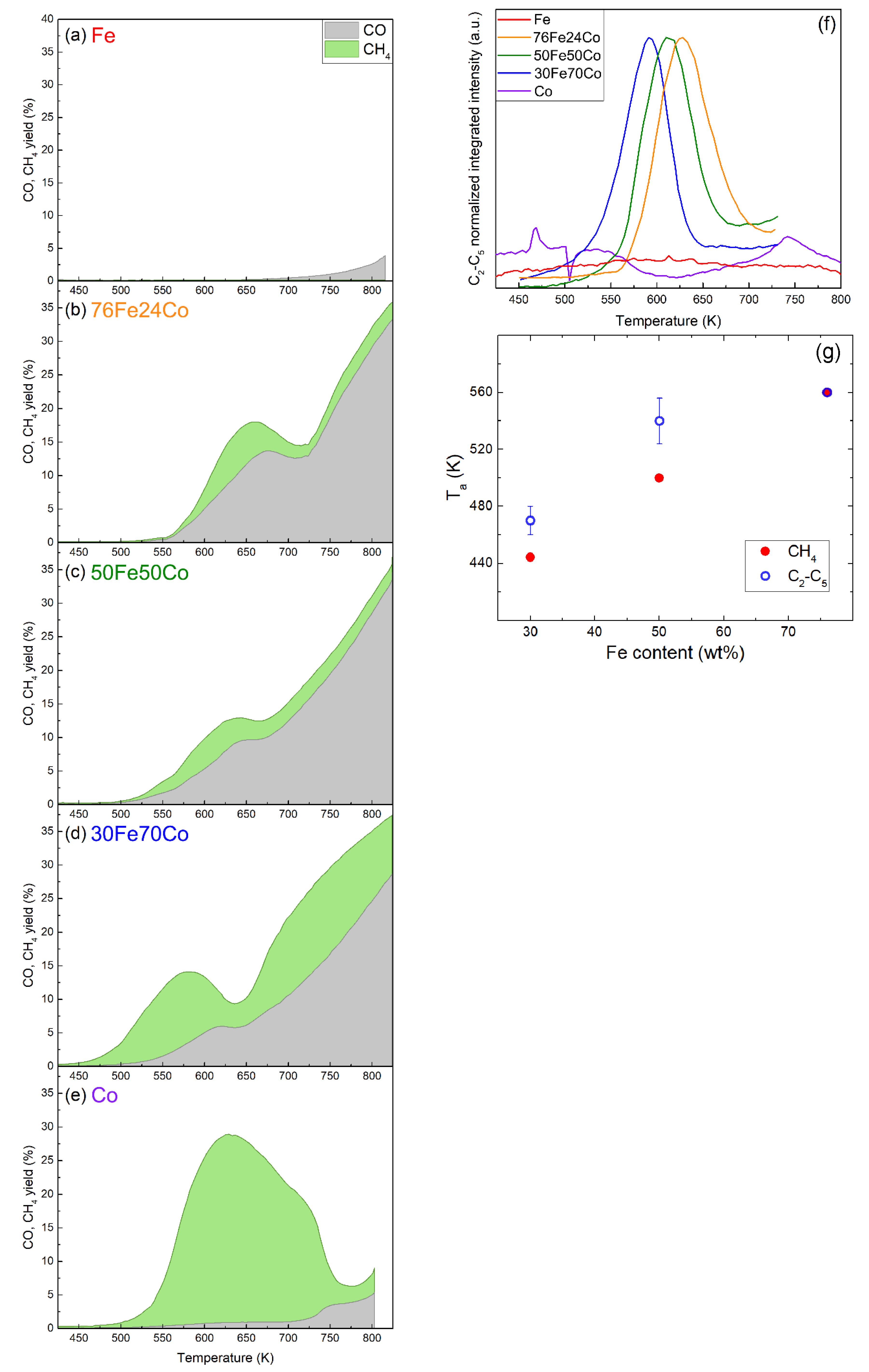
3.2. Catalytic Properties of Fe-Co Nanoalloys
4. Discussion
4.1. Structure, Morphology, and Composition of Fe-Co Nanoalloys
4.2. Catalytic Properties of Fe-Co Nanoalloys: Compositional Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Graves, C.; Ebbesen, S.D.; Mogensen, M.; Lackner, K.S. Sustainable hydrocarbon fuels by recycling CO2 and H2O with renewable or nuclear energy. Renew. Sustain. Energy Rev. 2011, 15, 1–23. [Google Scholar] [CrossRef]
- Züttel, A.; Mauron, P.; Kato, S.; Callini, E.; Holzer, M.; Huang, J. Storage of Renewable Energy by Reduction of CO2 with Hydrogen. Chim. Int. J. Chem. 2015, 69, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Li, J.; Pan, X.; Xiao, J.; Li, H.; Ma, H.; Wei, M.; Pan, Y.; Zhou, Z.; Li, M.; et al. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Li, S.; Bu, X.; Dang, S.; Liu, Z.; Wang, H.; Zhong, L.; Qiu, M.; Yang, C.; Cai, J.; et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 2017, 9, 1019–1024. [Google Scholar] [CrossRef]
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 15174. [Google Scholar] [CrossRef]
- Luo, M.; Davis, B.H. Fischer-Tropsch synthesis: Group II alkali-earth metal promoted catalysts. Appl. Catal. Gen. 2003, 246, 171–181. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef]
- Satthawong, R.; Koizumi, N.; Song, C.; Prasassarakich, P. Comparative Study on CO2 Hydrogenation to Higher Hydrocarbons over Fe-Based Bimetallic Catalysts. Top. Catal. 2014, 57, 588–594. [Google Scholar] [CrossRef]
- Boreriboon, N.; Jiang, X.; Song, C.; Prasassarakich, P. Fe-based bimetallic catalysts supported on TiO2 for selective CO2 hydrogenation to hydrocarbons. J. CO2 Util. 2018, 25, 330–337. [Google Scholar] [CrossRef]
- Satthawong, R.; Koizumi, N.; Song, C.; Prasassarakich, P. Bimetallic Fe–Co catalysts for CO2 hydrogenation to higher hydrocarbons. J. CO2 Util. 2013, 3–4, 102–106. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703. [Google Scholar] [CrossRef] [PubMed]
- Venturi, F.; Calizzi, M.; Bals, S.; Perkisas, T.; Pasquini, L. Self-assembly of gas-phase synthesized magnesium nanoparticles on room temperature substrates. Mater. Res. Express 2015, 2, 15007. [Google Scholar] [CrossRef]
- Calizzi, M.; Chericoni, D.; Jepsen, L.H.; Jensen, T.R.; Pasquini, L. Mg-Ti nanoparticles with superior kinetics for hydrogen storage. Int. J. Hydrogen Energy 2016, 41, 14447–14454. [Google Scholar] [CrossRef]
- Callini, E.; Pasquini, L.; Piscopiello, E.; Montone, A.; Antisari, M.V.; Bonetti, E. Hydrogen sorption in Pd-decorated Mg-MgO core-shell nanoparticles. Appl. Phys. Lett. 2009, 94, 221905. [Google Scholar] [CrossRef]
- Rossi, G.; Calizzi, M.; Di Cintio, V.; Magkos, S.; Amidani, L.; Pasquini, L.; Boscherini, F. Local Structure of V Dopants in TiO2 Nanoparticles: X-ray Absorption Spectroscopy, Including Ab-Initio and Full Potential Simulations. J. Phys. Chem. C 2016, 120, 7457–7466. [Google Scholar] [CrossRef]
- Patelli, N.; Calizzi, M.; Migliori, A.; Morandi, V.; Pasquini, L. Hydrogen Desorption Below 150 °C in MgH2-TiH2 Composite Nanoparticles: Equilibrium and Kinetic Properties. J. Phys. Chem. C 2017, 121, 11166–11177. [Google Scholar] [CrossRef]
- Patelli, N.; Migliori, A.; Morandi, V.; Pasquini, L. One-Step Synthesis of Metal/Oxide Nanocomposites by Gas Phase Condensation. Nanomaterials 2019, 9, 219. [Google Scholar] [CrossRef]
- Lutterotti, L.; Bortolotti, M.; Ischia, G.; Londarelli, I.; Wenk, H.-R. Rietveld texture analysis from diffraction images. Z. Krist. Suppl. 2007, 26, 125–130. [Google Scholar] [CrossRef]
- Mutschler, R.; Luo, W.; Moioli, E.; Züttel, A. Fast real time and quantitative gas analysis method for the investigation of the CO2 reduction reaction mechanism. Rev. Sci. Instrum. 2018, 89, 114102. [Google Scholar] [CrossRef]
- Mutschler, R.; Moioli, E.; Luo, W.; Gallandat, N.; Züttel, A. CO2 hydrogenation reaction over pristine Fe, Co, Ni, Cu and Al2O3 supported Ru: Comparison and determination of the activation energies. J. Catal. 2018, 366, 139–149. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook-SRD 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 15 February 2020).
- Hahn, H. Gas phase synthesis of nanocrystalline materials. Nanostruct. Mater. 1997, 9, 3–12. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2003; p. 3485. ISBN 978-1466571143. [Google Scholar]
- Patelli, N.; Migliori, A.; Morandi, V.; Pasquini, L. Interfaces within biphasic nanoparticles give a boost to magnesium-based hydrogen storage. Nano Energy 2020, 72, 104654. [Google Scholar] [CrossRef]
- Signorini, L.; Pasquini, L.; Savini, L.; Carboni, R.; Boscherini, F.; Bonetti, E.; Giglia, A.; Pedio, M.; Mahne, N.; Nannarone, S. Size-dependent oxidation in iron/iron oxide core-shell nanoparticles. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 68, 195423. [Google Scholar] [CrossRef]
- Nlebedim, I.C.; Moses, A.J.; Jiles, D.C. Non-stoichiometric cobalt ferrite, CoxFe3-xO4 (x = 1.0 to 2.0): Structural, magnetic and magnetoelastic properties. J. Magn. Magn. Mater. 2013, 343, 49–54. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Diamandescu, L.; Bibicu, I.; Leostean, C.; Borodi, G.; Barbu Tudoran, L. Structural and magnetic properties of CoxFe3-xO4 versus Co/Fe molar ratio. J. Magn. Magn. Mater. 2015, 394, 111–116. [Google Scholar] [CrossRef]
- Klencsár, Z.; Németh, P.; Sándor, Z.; Horváth, T.; Sajó, I.E.; Mészáros, S.; Mantilla, J.; Coaquira, J.A.H.; Garg, V.K.; Kuzmann, E.; et al. Structure and magnetism of Fe–Co alloy nanoparticles. J. Alloys Compd. 2016, 674, 153–161. [Google Scholar] [CrossRef]
- Liu, C.; Cundari, T.R.; Wilson, A.K. CO2 Reduction on Transition Metal (Fe, Co, Ni, and Cu) Surfaces: In Comparison with Homogeneous Catalysis. J. Phys. Chem. C 2012, 116, 5681–5688. [Google Scholar] [CrossRef]
- Willauer, H.D.; Ananth, R.; Olsen, M.T.; Drab, D.M.; Hardy, D.R.; Williams, F.W. Modeling and kinetic analysis of CO2 hydrogenation using a Mn and K-promoted Fe catalyst in a fixed-bed reactor. J. CO2 Util 2013, 3–4, 56–64. [Google Scholar] [CrossRef]

| Sample | (Å) | wt% Fe | wt% Co | ||||
|---|---|---|---|---|---|---|---|
| Fe | 15 (1) | 2.8729 (4) | 100 | 0 | -- | -- | -- |
| 76Fe24Co | 19 (1) | 2.8686 (2) | 76 (2) | 24 (2) | 47 (9) | 56 | 15 (7) |
| 50Fe50Co | 18 (1) | 2.8629 (2) | 50 (2) | 50 (2) | 56 (5) | 63 | 13 (5) |
| 30Fe70Co | 25 (1) | 2.8410 (2) | 30 (2) | 70 (2) | 56 (5) | 37 | 22 (7) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calizzi, M.; Mutschler, R.; Patelli, N.; Migliori, A.; Zhao, K.; Pasquini, L.; Züttel, A. CO2 Hydrogenation over Unsupported Fe-Co Nanoalloy Catalysts. Nanomaterials 2020, 10, 1360. https://doi.org/10.3390/nano10071360
Calizzi M, Mutschler R, Patelli N, Migliori A, Zhao K, Pasquini L, Züttel A. CO2 Hydrogenation over Unsupported Fe-Co Nanoalloy Catalysts. Nanomaterials. 2020; 10(7):1360. https://doi.org/10.3390/nano10071360
Chicago/Turabian StyleCalizzi, Marco, Robin Mutschler, Nicola Patelli, Andrea Migliori, Kun Zhao, Luca Pasquini, and Andreas Züttel. 2020. "CO2 Hydrogenation over Unsupported Fe-Co Nanoalloy Catalysts" Nanomaterials 10, no. 7: 1360. https://doi.org/10.3390/nano10071360
APA StyleCalizzi, M., Mutschler, R., Patelli, N., Migliori, A., Zhao, K., Pasquini, L., & Züttel, A. (2020). CO2 Hydrogenation over Unsupported Fe-Co Nanoalloy Catalysts. Nanomaterials, 10(7), 1360. https://doi.org/10.3390/nano10071360