Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress
Abstract
1. Introduction
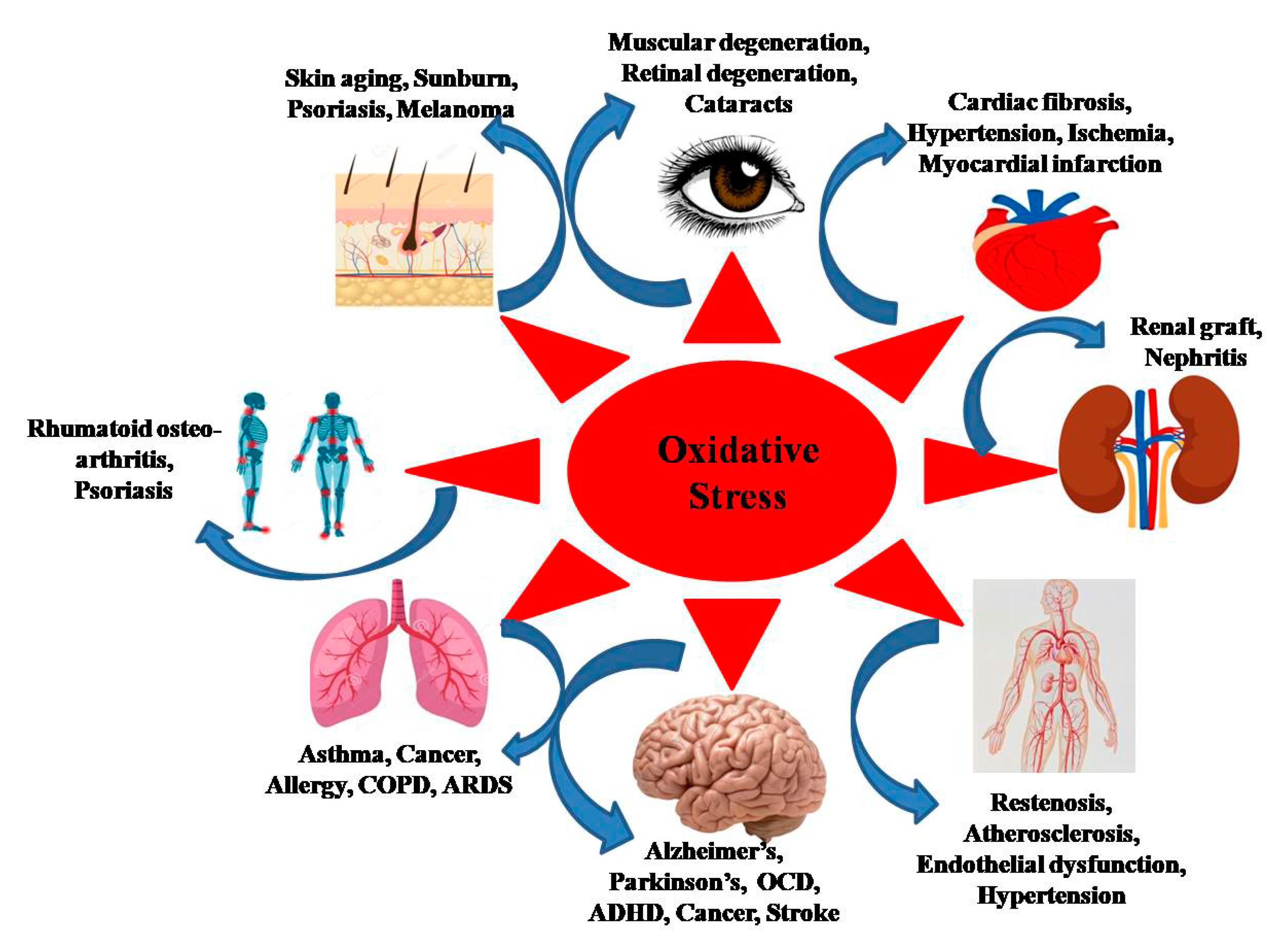
2. Synergism between ROS and Age-Related Diseases
2.1. Cancer
2.2. Cardiovascular Disease
2.3. Neurodegenerative Diseases
2.4. Respiratory Disorders
3. Antioxidants
4. Sources of Antioxidants
5. Nano-Antioxidants
6. Antioxidant Functionalized Nanoparticles
6.1. Silver Nanoparticles (AgNPs)
6.2. Gold Nanoparticles (AuNPs)
6.3. Copper Oxide Nanoparticles (Cu2ONPs)
6.4. Iron Nanoparticles (INPs)
6.5. Zinc Oxide (ZnONPs), Selenium (SeNPs) and Nickel Oxide Nanoparticles (NiONPs)
7. Challenges
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dabhade, P.; Kotwal, S. Tackling the aging process with biomolecules: A possible role for caloric restriction, food-derived nutrients, vitamins, amino acids, peptides, and minerals. J. Nutr. Gerontol. Geriatr. 2013, 32, 24–40. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Shokolenko, I.N.; Wilson, G.L.; Alexeyev, M.F. Aging: A mitochondrial DNA perspective, critical analysis and an update. World J. Exp. Med. 2014, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lee, K.Y.; Shim, Y.H. Normal aging: Definition and physiologic changes. J. Korean Med. Assoc. 2017, 60, 358–363. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; De Lourdes Bastos, M. Antioxidant activity of Hypericum androsaemum infusion: Scavenging activity against superoxide radical, hydroxyl radical and hypochlorous acid. Biol. Pharm. Bull. 2002, 25, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinson’s Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Clarendon Press: Oxford, UK, 2007. [Google Scholar]
- Bahorun, T.; Soobrattee, M.A.; Luximon-Ramma, V.; Aruoma, O.I. Free radicals and antioxidants in cardiovascular health and disease. Internet J. Med. Update 2006, 1, 25–41. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Free radicals: Health implications and their mitigation by herbals. Br. J. Med. Med. Res. 2015, 7, 438–457. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncola, J.; Cronin, M.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Genestra, M. Oxyl radicals, redox-sensitive signaling cascades and antioxidants. Cell. Signal. 2007, 19, 1807–1819. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Ricordi, C.; Garcia-Contreras, M.; Farnetti, S. Diet and inflammation: Possible effects on immunity, chronic diseases, and life span. J. Am. Coll. Nutr. 2015, 34, 10–13. [Google Scholar] [CrossRef]
- Sharma, P.; Mehta, M.; Dhanjal, D.S.; Kaur, S.; Gupta, G.; Singh, H.; Thangavelu, L.; Rajeshkumar, S.; Tambuwala, M.; Bakshi, H.A.; et al. Emerging trends in the novel drug delivery approaches for the treatment of lung cancer. Chem. Biol. Interact. 2019, 309, 108720. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.; Sethi, G. Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Chen, W.; Cao, Y.; Cao, Q.; Cui, Y.; Li, Y.; Wu, J. Targeting reactive oxygen species in cancer via Chinese herbal medicine. Oxid. Med. Cell. Longev. 2019, 2019, 9240426. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Badana, A.K.; Murali, M.G.; Shailender, G.; Malla, R. Reactive oxygen species: A key constituent in cancer survival. Biomark. Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Mehta, M.; Dhanjal, D.S.; Paudel, K.R.; Singh, B.; Gupta, G.; Rajeshkumar, S.; Thangavelu, L.; Tambuwala, M.M.; Bakshi, H.A.; Chellappan, D.K.; et al. Cellular signalling pathways mediating the pathogenesis of chronic inflammatory respiratory diseases: An update. Inflammopharmacology 2020, 1–23. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- He, F.; Zuo, L. Redox roles of reactive oxygen species in cardiovascular diseases. Int. J. Mol. Sci. 2015, 16, 27770–27780. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive oxygen species: A key hallmark of cardiovascular disease. Adv. Med. 2016, 2016, 9152732. [Google Scholar] [CrossRef]
- Sag, C.M.; Santos, C.X.; Shah, A.M. Redox regulation of cardiac hypertrophy. J. Mol. Cell Cardiol. 2014, 73, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Prather, E.R.; Garrison, D.E.; Zuo, L. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle. Int. J. Mol. Sci. 2018, 19, 417. [Google Scholar] [CrossRef] [PubMed]
- Van der Pol, A.; Van Gilst, W.H.; Voors, A.A.; Van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Khaltaev, N.; Axelrod, S. Chronic respiratory diseases global mortality trends, treatment guidelines, life style modifications, and air pollution: Preliminary analysis. J. Thorac. Dis. 2019, 11, 2643–2655. [Google Scholar] [CrossRef]
- Boukhenouna, S.; Wilson, M.A.; Bahmed, K.; Kosmider, B. Reactive oxygen species in chronic obstructive pulmonary disease. Oxid. Med. Cell. Longev. 2018, 2018, 5730395. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Chattopadhyay, I.; Rajasekaran, S. Oxidative stress mechanisms in the pathogenesis of environmental lung diseases. In Oxidative Stress in Lung Diseases; Chakraborti, S., Chakraborti, T., Ghosh, R., Ganguly, N.K., Parinandni, N.L., Eds.; Springer: Singapore, 2020; Volume 2, pp. 103–137. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef]
- Matill, H.A. Antioxidants. Annu. Rev. Biochem. 1947, 16, 177–192. [Google Scholar] [CrossRef] [PubMed]
- German, J. Food processing and lipid oxidation. Adv. Exp. Med. Biol. 1999, 459, 23–50. [Google Scholar] [PubMed]
- Jacob, R. Three eras of vitamin C discovery. Subcell. Biochem. 1996, 25, 1–16. [Google Scholar]
- Knight, J. Free radicals: Their history and current status in aging and disease. Ann. Clin. Lab. Sci. 1998, 28, 331–346. [Google Scholar]
- Halliwell, B. How to characterize an antioxidant- An update. Biochem. Soc. Symp. 1995, 61, 73–101. [Google Scholar]
- Shi, H.L.; Noguchi, N.; Niki, N. Comparative study on dynamics of antioxidative action of α- tocopheryl hydroquinone, ubiquinoland α- tocopherol, against lipid peroxidation. Free Radic. Biol. Med. 1999, 27, 334–346. [Google Scholar] [CrossRef]
- Levine, M.; Ramsey, S.C.; Daruwara, R. Criteria and recommendation for vitamin C intake. JAMA 1991, 281, 1415–1423. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Z.; Wang, W.; Zhou, Q.; Shi, G.; Wei, F.; Jiang, G. Developmental toxicity of synthetic phenolic antioxidants to the early life stage of zebrafish. Sci. Total. Environ. 2018, 643, 559–568. [Google Scholar] [CrossRef]
- Aguilera, Y.; Martin-Cabrejas, M.A.; González de Mejia, E. Phenolic compounds in fruits and beverages consumed as part of the mediterranean diet: Their role in prevention of chronic diseases. Phytochem. Rev. 2016, 15, 405–423. [Google Scholar] [CrossRef]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-food byproducts as a new source of natural food additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020, 129, 108849. [Google Scholar] [CrossRef]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Niksic, M.M.; Griensven, L.V. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Barros, L.; Abreu, R.M.V. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef]
- Munir, N.; Sharif, N.; Naz, S.; Manzoor, F. Algae: A potent antioxidant source. Sky J. Microbiol. Res. 2013, 1, 22–31. [Google Scholar]
- Venkatesan, J.; Kim, S.K.; Shim, S.K. Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae Ecklonia cava. Nanomaterials 2016, 6, 235. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Gómez-Serranillos, M.P.; Crespo, A. Antioxidant potential of lichen species and their secondary metabolites. A systematic review. Pharm. Biol. 2015, 54, 1–17. [Google Scholar] [CrossRef]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug. Anal 2017, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Ahmadian, E.; Panahi-Azar, V.; Hosseini, H.; Tabibiazar, M.; Dizaj, S.M. Hepatoprotective and free radical scavenging actions of quercetin nanoparticles on aflatoxin B1-induced liver damage: In vitro/in vivo studies. Artif. Cells Nanomed. Biotechnol. 2017, 46, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Dizaj, S.M.; Chodari, L.; Sunar, S.; Hasanzadeh, A.; Ahmadian, E.; Hasanzadeh, M. The promising future of nano-antioxidant therapy against environmental pollutants induced-toxicities. Biomed. Pharmacother. 2018, 103, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.; Tal, A.A.; Skallberg, A.; Brommesson, C.; Hu, Z.; Boyd, R.D.; Olovsson, W.; Fairley, N.; Abrikosov, I.A.; Zhang, X.; et al. Cerium oxide nanoparticles with antioxidant capabilities and gadolinium integration for MRI contrast enhancement. Sci. Rep. 2018, 8, 6999. [Google Scholar] [CrossRef]
- Das, S.; Dowding, J.M.; Klump, K.E.; McGinnis, J.F.; Self, W.; Seal, S. Cerium oxide nanoparticles: Applications and prospects in nanomedicine. Nanomedicine 2013, 8, 1483–1508. [Google Scholar] [CrossRef]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.N.T.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Kim, C.K.; Kim, T.; Choi, I.Y.; Soh, M.; Kim, D.; Kim, Y.J.; Jang, H.; Yang, H.S.; Kim, J.Y.; Park, H.K.; et al. Ceria nanoparticles that can protect against ischemic stroke. Angew. Chem. Int. Ed. Engl. 2012, 51, 11039–11043. [Google Scholar] [CrossRef] [PubMed]
- Hirst, S.M.; Karakoti, A.; Singh, S.; Self, W.; Tyler, R.; Seal, S.; Reilly, C.M. Bio-distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice. Envrion. Toxicol. 2013, 28, 107–118. [Google Scholar] [CrossRef]
- Caputo, F.; Nicola, M.D.; Sienkiewicz, A.; Giovanetti, A.; Bejarano, I.; Licoccia, S.; Traversa, E.; Ghibelli, L. Cerium oxide nanoparticles, combining antioxidant and UV shielding properties, prevent UV-induced cell damage and mutagenesis. Nanoscale 2015, 7, 15643–15656. [Google Scholar] [CrossRef]
- Sonaje, K.; Italia, J.L.; Sharma, G.; Bhardwaj, V.; Tikoo, K.; Kumar, M.N.V.R. Development of biodegradable nanoparticles for oral delivery of ellagic acid and evaluation of their antioxidant efficacy against cyclosporine A-induced nephrotoxicity in rats. Pharm. Res. 2007, 24, 899–908. [Google Scholar] [CrossRef]
- Yun, X.; Maximov, V.D.; Yu, J.; Zhu, H.; Vertegel, A.A.; Kindly, M.S. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J. Cereb. Blood Flow. Metab. 2013, 33, 583–592. [Google Scholar] [CrossRef]
- Chorny, M.; Hood, E.; Levy, R.J.; Muzykantov, V.R. Endothelial delivery of antioxidant enzymes loaded into non-polymeric magnetic nanoparticles. J. Control. Release 2010, 146, 144–151. [Google Scholar] [CrossRef]
- Reddy, M.K.; Labhasetwar, V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: An effective strategy to reduce ischemia-reperfusion injury. FASEB J. 2009, 23, 1384–1395. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Zhang, Y.; Yokoyama, W. Fabrication of curcumin-loaded bovine serum albumin (BSA)-dextran nanoparticles and the cellular antioxidant activity. Food Chem. 2018, 239, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Elle, R.E.; Rahmani, S.; Lauret, C.; Morena, M.; Bidel, L.P.R.; Boulahtouf, A.; Balaguer, P.; Cristol, J.P.; Durand, J.O.; Charnay, C.; et al. Functionalized mesoporous silica nanoparticle with antioxidants as a new carrier that generates lower oxidative stress impact on cells. Mol. Pharm. 2016, 13, 2647–2660. [Google Scholar] [CrossRef] [PubMed]
- Tzankova, V.; Aluani, D.; Kondeva-Burdina, M.; Yordanov, Y.; Odzhakov, F.; Apostolov, A.; Yoncheva, K. Hepatoprotective and antioxidant activity of quercetin loaded chitosan/alginate particles in vitro and in vivo in a model of paracetamol-induced toxicity. Biomed. Pharmacother. 2017, 92, 569–579. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Ferrarelli, T.; Barone, E.; Picci, N.; Mancuso, C. Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes. Colloids Surf. B Biointerfaces 2013, 109, 273–279. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Chen, C.; Liu, Y. Nanocarrier: A potential tool for future antioxidant therapy. Free Radic. Res. 2014, 48, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.; Lowman, A. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Vila, A.; Sanchez, A.; Tobıo, M.; Calvo, P.; Alonso, M. Design of biodegradable particles for protein delivery. J. Control. Release 2002, 78, 15–24. [Google Scholar] [CrossRef]
- Shah, B.R.; Zhang, C.; Li, Y.; Li, B. Bioaccessibility and antioxidant activity of curcumin after encapsulated by nano and pickering emulsion based on chitosan-tripolyphosphate nanoparticles. Food Res. Int. 2016, 89, 399–407. [Google Scholar] [CrossRef]
- Pu, H.L.; Chiang, W.L.; Maiti, B.; Liao, Z.X.; Ho, Y.C.; Shim, M.S.; Chuang, E.Y.; Xia, Y.; Sung, H.W. Nanoparticles with dual responses to oxidative stress and reduced pH for drug release and anti-inflammatory applications. ACS Nano 2014, 8, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Kumbhar, S.T. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem. Biophys. Rep. 2017, 10, 76–81. [Google Scholar]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. 2018, 25, 10392–10406. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Abbas, Q.; Ali, A.; Raza, H.; Kim, S.J.; Zia, M.; Haq, I.U. Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Future J. Pharm. Sci. 2016, 2, 31–36. [Google Scholar] [CrossRef]
- Sriranjani, R.; Srinithya, B.; Vellingiri, V.; Brindha, P.; Anthony, S.P.; Sivasubramanian, A.; Muthuraman, M.S. Silver nanoparticle synthesis using Clerodendrum phlomidis leaf extract and preliminary investigation of its antioxidant and anticancer activities. J. Mol. Liq. 2016, 220, 926–930. [Google Scholar] [CrossRef]
- Kalaiyarasan, T.; Bharti, V.K.; Chaurasia, O.P. One pot green preparation of Seabuckthorn silver nanoparticles (SBT@AgNPs) featuring high stability and longevity, antibacterial, antioxidant potential: A nano disinfectant future perspective. RSC Adv. 2017, 7, 51130–51141. [Google Scholar] [CrossRef]
- Sharma, B.; Deswal, R. Single pot synthesized gold nanoparticles using Hippophae rhamnoides leaf and berry extract showed shape-dependent differential nanobiotechnological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 408–418. [Google Scholar] [CrossRef]
- Das, D.; Ghosh, R.; Mandal, P. Biogenic synthesis of silver nanoparticles using S1 genotype of Morus alba leaf extract: Characterization, antimicrobial and antioxidant potential assessment. SN Appl. Sci. 2019, 1, 498. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Jha, P.K.; Vignesh, V.; Rajkuberan, C.; Jeyaraj, M.; Selvakumar, M.; Jha, R.; Sivaramakrishnan, S. Cannonball fruit (Couroupita guianensis, Aubl.) extract mediated synthesis of gold nanoparticles and evaluation of its antioxidant activity. J. Mol. Liq. 2016, 215, 229–236. [Google Scholar]
- Patra, J.K.; Das, G.; Baek, K.H. Phyto-mediated biosynthesis of silver nanoparticles using the rind extract of watermelon (Citrullus lanatus) under photo-catalyzed condition and investigation of its antibacterial, anticandidal and antioxidant efficacy. J. Photochem. Photobiol. B 2016, 161, 200–210. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Jayabalan, R.; Sharma, N.; Bastia, A.K.; Mohanta, T.K. Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.). Front. Mol. Biosci. 2017, 4, 14. [Google Scholar] [CrossRef]
- Hamelian, M.; Zangeneh, M.M.; Amisama, A.; Varmira, K.; Veisi, H. Green synthesis of silver nanoparticles using Thymus kotschyanus extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Appl. Organomet. Chem. 2018, 32, e4458. [Google Scholar] [CrossRef]
- Chandra, H.; Patel, D.; Kumari, P.; Jangwan, J.S.; Yadav, S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 212–220. [Google Scholar] [CrossRef]
- Veena, S.; Devasena, T.; Sathak, S.S.M.; Yasasve, M.; Vishal, L.A. Green synthesis of gold nanoparticles from Vitex negundo leaf extract: Characterization and in vitro evaluation of antioxidant-antibacterial activity. J. Clust. Sci. 2019, 30, 1591–1597. [Google Scholar] [CrossRef]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Subbaiy, R.; Selvam, M.M. Green synthesis of copper nanoparticles from Hibicus rosasinensis and their antimicrobial, antioxidant activities. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1183–1190. [Google Scholar]
- Ghosh, S.; More, P.; Nitnavare, R.; Jagtap, S.; Chippalkatti, R.; Derle, A.; Kitture, R.; Asok, A.; Kale, S.; Singh, S.; et al. Antidiabetic and antioxidant properties of copper nanoparticles synthesized by medicinal plant Dioscorea bulbifera. J. Nanomed. Nanotechnol. 2015, S6, 007. [Google Scholar]
- Sarkar, J.; Chakraborty, N.; Chatterjee, A.; Bhattacharjee, A.; Dasgupta, D.; Acharya, K. Green synthesized copper oxide nanoparticles ameliorate defence and antioxidant enzymes in Lens culinaris. Nanomaterials 2020, 10, 312. [Google Scholar] [CrossRef]
- Dobrucka, R. Antioxidant and catalytic activity of biosynthesized CuO nanoparticles using extract of Galeopsidis herba. J. Inorg. Organomet. Polym. Mater. 2018, 28, 812–819. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Menon, S.; Kumar, S.V.; Tambuwala, M.M.; Bakshi, H.A.; Mehta, M.; Satija, S.; Gupta, G.; Chellappan, D.K.; Thangavelu, L.; et al. Antibacterial and antioxidant potential of biosynthesized copper nanoparticles mediated through Cissus arnotiana plant extract. J. Photochem. Photobiol. 2019, 197, 111531. [Google Scholar] [CrossRef]
- Harshiny, M.; Iswarya, C.N.; Matheswaran, M. Biogenic synthesis of iron nanoparticles using Amaranthus dubius leaves extract as reducing agents. Powder Technol. 2015, 286, 744–749. [Google Scholar] [CrossRef]
- Muthukumar, H.; Manickam, M. Amaranthus spinosus leaf extract mediated FeO nanoparticles: Physicochemical traits, photocatalytic and antioxidant activity. ACS Sustain. Chem. Eng. 2015, 3, 3149–3156. [Google Scholar] [CrossRef]
- Tuzun, B.S.; Fafal, T.; Tastan, P.; Kivcak, B.; Yelken, B.O.; Kayabasi, C.; Susluer, S.Y.; Gunduz, C. Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract. Green Process. Synth. 2020, 9, 153–163. [Google Scholar] [CrossRef]
- Srihasam, S.; Thyagarajan, K.; Korivi, M.; Lebaka, V.R.; Mallem, S.P.R. Phytogenic generation of NiO nanoparticles using Stevia leaf extract and evaluation of their in-vitro antioxidant and antimicrobial properties. Biomolecules 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Markus, J.; Mathiyalagan, R.; Kim, Y.J.; Abbai, R.; Singh, P.; Ahn, S.; Perez, Z.E.J.; Hurh, J.; Yang, D.C. Intracellularsynthesis of goldnanoparticles with antioxidantactivity by probiotic Lactobacillus kimchicus DCY51T isolated from Koreankimchi. Enzym. Microb. Technol. 2016, 95, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Baygar, T.; Ugur, A. Biosynthesis of silver nanoparticles by Streptomyces griseorubens isolated from soil and their antioxidant activity. IET Nanobiotechnol. 2017, 11, 286–291. [Google Scholar] [CrossRef]
- Oladipo, I.C.; Lateef, A.; Elegbede, J.A.; Azeez, M.A.; Asafa, T.M.; Yekeen, T.A.; Akinboro, A.; Gueguim-Kana, E.B.; Beukes, L.S.; Oluyide, T.O.; et al. Enterococcus species for the one-pot biofabrication of gold nanoparticles: Characterization and nanobiotechnological applications. J. Photochem. Photobiol. B 2017, 173, 250–257. [Google Scholar] [CrossRef]
- Veeraapandian, S.; Sawant, S.N.; Doble, M. Antibacterial and antioxidant activity of protein capped silver and gold nanoparticles synthesized with Escherichia coli. J. Bimed. Nanotechnol. 2012, 8, 140–148. [Google Scholar] [CrossRef]
- Shanmugasundaram, T.; Radhakrishnan, M.; Gopikrishnan, V.; Pazhanimurugan, R.; Balagurunathan, R. A study of the bactericidal, anti-biofouling, cytotoxic and antioxidant properties of actinobacterially synthesised silver nanoparticles. Colloids Surf. B Biointerfaces 2013, 111, 680–687. [Google Scholar] [CrossRef]
- Ramya, S.; Shanmugasundaram, T.; Balagurunathan, R. Biomedical potential of actinobacterially synthesised selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J. Trace Elem. Med. Biol. 2015, 32, 30–39. [Google Scholar] [CrossRef]
- Torres, S.K.; Campos, V.L.; León, C.G.; Rodríguez-Llamazares, S.M.; Rajos, S.M.; González, M.; Smith, C.; Mondaca, M.A. Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J. Nanopart Res. 2012, 14, 1236. [Google Scholar] [CrossRef]
- Sowani, H.; Mohite, P.; Munot, H.; Shouche, Y.; Bapat, T.; Kumar, A.R.; Kulkarni, M.; Zinjarde, S. Green synthesis of gold and silver nanoparticles by an Actinomycete Gordonia amicalis HS-11: Mechanistic aspects and biological application. Process Biochem. 2016, 51, 374–383. [Google Scholar] [CrossRef]
- Sivasankar, P.; Seedevi, P.; Poongodi, S.; Sivakumar, M.; Murugan, T.; Sivakumar, L.; Sivakumar, K. Characterization, antimicrobial and antioxidant property of exopolysaccharide mediated silver nanoparticles synthesized by Streptomyces violaceus MM72. Carbohydr. Polym. 2018, 181, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.B.; Moniri, M.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Saad, W.Z.; Namvar, F.; Navaderi, M.; Mohamad, R. Biosynthesis of ZnO nanoparticles by a new Pichia kudriavzevii yeast strain and evaluation of their antimicrobial and antioxidant activities. Molecules 2017, 22, 872. [Google Scholar] [CrossRef] [PubMed]
- Netala, V.R.; Bethu, M.S.; Pushpalatha, B.; Baki, V.B.; Aishwarya, S.; Rao, J.V.; Tartte, V. Biogenesis of silver nanoparticles using endophytic fungus Pestalotiopsis microspora and evaluation of their antioxidant and anticancer activities. Int. J. Nanomed. 2016, 11, 5683–5696. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, H.M.; Joshi, C.G.; Danagoudar, A.; Poyya, J.; Kudva, A.K.; Dhananjaya, B.L. Biogenic synthesis of gold nanoparticles by marine endophytic fungus-Cladosporium cladosporioides isolated from seaweed and evaluation of their antioxidant and antimicrobial properties. Process Biochem. 2017, 63, 137–144. [Google Scholar]
- Manjunath, H.M.; Joshi, C.G. Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus- Cladosporium cladosporioides. Process Biochem. 2019, 82, 199–204. [Google Scholar] [CrossRef]
- Netala, V.R.; Kotakadi, V.S.; Bobbu, P.; Gaddam, S.A.; Tartte, V. Endophytic fungal isolate mediated biosynthesis of silver nanoparticles and their free radical scavenging activity and anti microbial studies. 3Biotech 2016, 6, 132. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Wang, M.H. Trichoderma based synthesis of anti-pathogenic silver nanoparticles and their characterization, antioxidant and cytotoxicity properties. Microb. Pathog. 2018, 114, 269–273. [Google Scholar] [CrossRef]
- Gao, Y.; Anand, M.A.V.; Ramachandran, V.; Karthikkumar, V.; Shalini, V.; Vijayalakshmi, S.; Ernest, D. Biofabrication of zinc oxide nanoparticles from Aspergillus niger, their antioxidant, antimicrobial and anticancer activity. J. Clust. Sci. 2019, 30, 937–946. [Google Scholar] [CrossRef]
- Govindappa, M.; Farheen, H.; Chandrappa, C.P.; Rai, R.V.; Raghavendra, V.B. Mycosynthesis of silver nanoparticles using extract of endophytic fungi, Penicillium species of Glycosmis mauritiana, and its antioxidant, antimicrobial, anti-inflammatory and tyrokinase inhibitory activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035014. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Sreekanth, T.V.M.; Lee, J.I.; Lee, K.D. Mycosynthesis: Antibacterial, antioxidant and antiproliferative activities of silver nanoparticles synthesized from Inonotus obliquus (Chaga mushroom) extract. J. Photochem. Photobiol. B 2014, 130, 299–304. [Google Scholar] [CrossRef]
- Popli, D.; Anil, V.; Subramanyam, A.B.; Namratha, M.N.; Ranjitha, V.R.; Rao, S.N.; Rai, R.V.; Govindappa, M. Endophyte fungi, Cladosporium species-mediated synthesis of silver nanoparticles possessing in vitro antioxidant, anti-diabetic and anti-Alzheimer activity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Sriramulu, M.; Sumathi, S. Photocatalytic, antioxidant, antibacterial and anti-inflammatory activity of silver nanoparticles synthesised using forest and edible mushroom. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 045012. [Google Scholar] [CrossRef]
- Lee, K.D.; Nagajyothi, P.C.; Sreekanth, T.V.M.; Park, S. Eco-friendly synthesis of gold nanoparticles (AuNPs) using Inonotus obliquus and their antibacterial, antioxidant and cytotoxic activities. J. Ind. Eng. Chem. 2015, 26, 67–72. [Google Scholar] [CrossRef]
- Aygün, A.; Özdemir, S.; Gülcan, M.; Cellat, K.; Şen, F. Synthesis and characterization of Reishi mushroom-mediated green synthesis of silver nanoparticles for the biochemical applications. J. Pharm. Bimed. Anal. 2020, 178, 112970. [Google Scholar] [CrossRef]
- Poudel, M.; Pokharel, R.; Sudip, K.C.; Awal, S.C.; Pradhananga, R. Biosynthesis of silver nanoparticles using Ganoderma Lucidum and assessment of antioxidant and antibacterial activity. Int. J. Appl. Sci. Biotechnol. 2017, 5, 523–531. [Google Scholar] [CrossRef]
- Naveena, B.E.; Prakash, S. Biological synthesis of gold nanoparticles using marine algae Gracilaria corticata and its application as a potent antimicrobial and antioxidant agent. Asian J. Pharm. Clin. Res. 2013, 6, 179–182. [Google Scholar]
- Sharma, B.; Purkayastha, D.D.; Hazra, S.; Thajamanbi, M.; Bhattacharjee, C.R.; Ghosh, N.N.; Rout, J. Biosynthesis of fluorescent gold nanoparticles using an edible freshwater red alga, Lemanea fluviatilis (L.) C.Ag. and antioxidant activity of biomatrix loaded nanoparticles. Bioprocess Biosyst. Eng. 2014, 37, 2559–2565. [Google Scholar] [CrossRef]
- Dasari, S.; Suresh, K.A.; Rajesh, M.; Reddy, C.S.S.; Hemalatha, C.S.; Wudayagiri, R.; Valluru, L. Biosynthesis, characterization, antibacterial and antioxidant activity of silver nanoparticles produced by lichens. J. Bionanosci. 2013, 7, 237–244. [Google Scholar] [CrossRef]
- Debnath, R.; Purkayastha, D.D.; Hazra, S.; Ghosh, N.N.; Bhattacharjee, C.R.; Rout, J. Biogenic synthesis of antioxidant, shape selective gold nanomaterials mediated by high altitude lichens. Mater. Lett. 2016, 169, 58–61. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Kuča, K.; Kalia, A.; Nepovimova, E.; Verma, R.; Kumar, D. Flower-basedgreensynthesis of metallicnanoparticles: Applicationsbeyondfragrance. Nanomaterials 2020, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Guajardo-Pachecoa, M.J.; Morales-Sanchz, J.E.; González-Hernándezc, J.; Ruiz, F. Synthesis of copper nanoparticles using soybeans as a chelant agent. Mater. Lett. 2010, 64, 1361–1364. [Google Scholar] [CrossRef]
- Xi, Y.; Hu, C.; Gao, P.; Yang, R.; He, X.; Wang, X.; Wan, B. Morphology and phase selective synthesis of CuxO (x = 1, 2) nanostructures and their catalytic degradation activity. Mater. Sci. Eng. B 2010, 166, 113–117. [Google Scholar] [CrossRef]
- He, Y. A novel solid-stabilized emulsion approach to CuO nanostructures microspheres. Mater. Res. Bull. 2007, 42, 190–195. [Google Scholar] [CrossRef]
- Motogoshi, R.; Oku, T.; Suzuki, A.; Kikuchi, K.; Kikuchi, S.; Jeyadevan, B.; Cuya, J. Fabrication and characterization of cupprious oxide: Fullerene solar cells. Synth. Met. 2010, 160, 1219–1222. [Google Scholar] [CrossRef]
- Herlekar, M.; Barve, S.; Kumar, R. Plant-mediated green synthesis of iron nanoparticles. J. Nanopart. Res. 2014, 2014, 140614. [Google Scholar] [CrossRef]
- Huber, D.L. Synthesis, properties, and applications of iron nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef]
- Guo, J.; Wang, R.; Tjiu, W.W.; Pan, J.; Liu, T. Synthesis of Fe nanoparticles@ graphene composites for environmental applications. J. Hazard. Mater. 2012, 225, 63–73. [Google Scholar] [CrossRef]
- Babay, S.; Mhiri, T.; Toumi, M. Synthesis, structural and spectroscopic characterizations of maghemite γ-Fe2O3 prepared by one-step coprecipitation route. J. Mol. Struct. 2015, 1085, 286–293. [Google Scholar] [CrossRef]
- Saleh, N.; Kim, H.J.; Phenrat, T.; Matyjaszewski, K.; Tilton, R.D.; Lowry, G.V. Ionic strength and composition affect the mobility of surface-modified Fe0 nanoparticles in water-saturated sand columns. Environ. Sci. Technol. 2008, 42, 3349–3355. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, D.G.; Yoon, H.; Choi, Y.S.; Yoon, J.; Lee, J.C. Polyphenol/FeIII complex coated membranes having multifunctional properties prepared by a one-step fast assembly. Adv. Mater. Interfaces 2015, 2, 1500298. [Google Scholar] [CrossRef]
- Yang, L.; Cao, Z.; Sajja, H.K.; Mao, H.; Wang, L.; Geng, H.; Xu, H.; Jiang, T.; Wood, W.C.; Nie, S.; et al. Development of receptor targeted magnetic iron oxide nanoparticles for efficient drug delivery and tumor imaging. J. Biomed. Nanotechnol. 2008, 4, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Ebrahiminezhad, A.; Zare-Hoseinabadi, A.; Sarmah, A.K.; Taghizadeh, S.; Ghasemi, Y.; Berenjian, A. Plant-mediated synthesis and applications of iron nanoparticles. Mol. Biotechnol. 2018, 60, 154–168. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles -An eco-friendly approach. Res. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Kirthi, A.V.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Guarav, K.; Karthik, L.; Rao, K.V. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 90, 78–84. [Google Scholar] [CrossRef]
- Pulit-prociak, J.; Chwastowski, J.; Kucharski, A.; Banach, M. Applied surface science functionalization of textiles with silver and zinc oxide nanoparticles. Appl. Surf. Sci. 2016, 385, 543–553. [Google Scholar] [CrossRef]
- Wodka, D.; Bielaníska, E.; Socha, R.P.; Elzbieciak-Wodka, M.; Gurgul, J.; Nowak, P.; Warszyński, P.; Kumakiri, I. Photocatalytic activity of titanium dioxide modified by silver nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1945–1953. [Google Scholar] [CrossRef]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zheng, W.; Fan, C.; Zhang, Y.; Chen, T. Functionalized selenium nanoparticles with nephroprotective activity, the important roles of ROS mediated signaling pathways. J. Mater. Chem. 2013, 1, 6365–6372. [Google Scholar] [CrossRef]
- Din, M.I.; Rani, A. Recent advances in the synthesis and stabilization of nickel and nickel oxide nanoparticles: A green adeptness. Int. J. Anal. Chem. 2016, 2016, 3512145. [Google Scholar]
- Saxena, A.; Kumar, K.; Mozumdar, S. Ni-nanoparticles: An efficient green catalyst for chemo-selective oxidative couplingof thiols. J. Mol. Catal. A Chem. 2007, 269, 35–40. [Google Scholar] [CrossRef]
- Alonso, F.; Riente, P.; Yus, M. Hydrogen-transfer reduction of carbonyl compounds promoted by nickel nanoparticles. Tetrahedron 2008, 64, 1847–1852. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Pitchumani, K. Clay entrapped nickel nanoparticles as efficient and recyclable catalysts forhydrogenation of olefins. Tetrahedron Lett. 2008, 49, 1818–1823. [Google Scholar] [CrossRef]
- Alonso, F.; Riente, P.; Yus, M. Wittig-type olefination of alcohols promoted by nickel nanoparticles: Synthesis ofpolymethoxylated and polyhydroxylated stilbenes. Eur. J. Org. Chem. 2009, 2009, 6034–6042. [Google Scholar] [CrossRef]
- Alonso, F.; Riente, P.; Yus, M. Alcohols for the α-alkylationof methyl ketones and indirect aza-wittig reaction promoted bynickel nanoparticles. Eur. J. Org. Chem. 2008, 2008, 4908–4914. [Google Scholar] [CrossRef]
- Li, X.K.; Ji, W.J.; Zhao, J.; Wang, S.J.; Au, C.T. Ammonia decomposition over Ru and Ni catalysts supported on fumed SiO2, MCM-41, and SBA-15. J. Catal. 2005, 236, 181–189. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Xie, X.; Liu, J.; Xu, Y.; Shen, W. Novel Nicatalysts for methane decomposition to hydrogen and carbonnanofibers. J. Catal. 2006, 238, 412–424. [Google Scholar] [CrossRef]
- Al-Rawi, M.; Diabaté, S.; Weiss, C. Uptake and intracellular localization of submicron and nano-sized SiO₂ particles in HeLa cells. Arch Toxicol. 2011, 85, 813–826. [Google Scholar] [CrossRef]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Clift, M.J.D.; Rothen-Rutishauser, B.; Brown, D.M.; Duffin, R.; Ronaldson, K.; Proudfoot, L.; Guy, K.; Stone, V. The impact of different nanoparticle surface chemistry and size on uptake and toxicity in a murine macrophage cell line. Toxicol. Appl. Pharmacol. 2008, 232, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Rabolli, V.; Thomassen, L.C.; Uwambayinema, F.; Martens, J.A.; Lison, D. The cytotoxic activity of amorphous silica nanoparticles is mainly influenced by surface area and not by aggregation. Toxicol. Lett. 2011, 206, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.; Soares, M.E.; Duarte, J.A.; Soares, L.; Maia, S.; Gomes, P.; Pereira, E.; Fraga, S.; Carmo, H.; De Lourdes Bastos, M. Effect of surface coating on the biodistribution profile of gold nanoparticles in the rat. Eur. J. Pharm. Biopharm. 2012, 80, 185–193. [Google Scholar] [CrossRef]
- Cho, W.S.; Cho, M.; Jeong, J.; Choi, M.; Cho, H.Y.; Han, B.S.; Kim, S.H.; Kim, H.O.; Lim, Y.T.; Chung, B.H.; et al. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2009, 236, 16–24. [Google Scholar] [CrossRef]
- Knaapen, A.M.; Borm, P.J.; Albrecht, C.; Schins, R.P. Inhaled particles and lung cancer. Part A: Mechanisms. Int. J. Cancer 2004, 109, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
| Source | Antioxidants | Ref. |
|---|---|---|
| Bacteria | Thiazostatins A, 5-(2,4-Dimethylbenzyl) Pyrrolidin-2-One, Phenazoviridin, Benthophoenin, Benthocyanins A, B and C, Benzastatins C, Benzastatins A, (Z)-1-((1-Hydroxypenta-2,4-Dien-1-Yl)Oxy)Anthracene-9,10-Dione | [53] |
| Plants | Gallic acid, Protocatechuic acid, p-Coumaric acid, Caffeic acid, Rosmarinic acid, Carnosol, Carnosic acid, Rosmanol, Rutin, Epicatechin gallate, Epigallocatechin gallate, Epicatechin, Quercetin (flavanol), Eugenol, Carvacrol, Safrole, Thymol, Myristicin, Menthol, 1,8-Cineol, α-Terpineol, p-Cymene, Cinnamaldehyde, Piperine, Flavone, Flavonol, Chalcone, Flavanone, Anthocyanin, Anthocyanidin-3,5-glucoside, Alpha tocopherol, Gamma tocopherol, Ascorbic acid, Ascorbyl palmitate, Propyl gallate, Resveratrol | [55] |
| Fungi | Isopestacin, Pestacin, Atrovenetin, 2-Acetonyl-2,4,9,-Trihydroxy-6-Methoxy-7-Methyl-1HPhenalene-1,3(2H)-Dione, Graphislactone, 4,6-dihydroxy-5-methoxy-7-methyl-1,3-dihydroisobenzofuran, 4,5,6-trihydroxy-7-methyl-1,3-dihydroisobenzofuran, 4,6-dihydroxy-5-methoxy-7-methylphthalide, Kojic acid, Phomapyrone C, Versicolone A, Terremutin, Terreic Acid, Neoechinulin A, Candidusin B, 3″-Dihydroxyterphenyllin and 3-Hydroxyterphenyllin, p-Hydroxybenzoic acid, Protocatechuic acid, Gallic acid, Gentisic acid, Vanillic acid, 5-Sulfosalicylic acid, Syringic acid¸ Veratric acid, Vanillin, Cinnamic acid, p-Coumaric acid, o-Coumaric acid, Caffeic acid, Ferulic acid, 3-O-Caffeoylquinic acid, 4-O-Caffeoylquinic acid, 5-O-Caffeoylquinic acid, Quercetin, Rutin, Kaempferol, Myricetin, Chrysin, Catechin, Hesperetin, Naringenin, Naringin, Formometin, Biochanin, Pyrogallol, Resveratrol, Ellagic acid, Tannic acid, Sinapic acid, Flavonols, Flavones, Isoflavones, Flavanones, Anthocyanidins, Flavanols, Vitamin C, Vitamin E, Homogentisic acid | [53,56,57] |
| Algae | β-carotene, Lutein, Bromophenol, Carrageenan, Fucophlorethols, Fucoxanthin, Galactan sulphate, Phlorotannins, Phycoerythrin, Porphyran, Shinorine, Catechin, Epicatechin, Gallate, Alginic acid, Laminaran, Vitamin A, Phloroglucinol, Eckol, Fucodiphlorethol G, Phlorofucofuroeckol A, 7-phloroeckol, Dieckol, 6,6′-bieckol, Triphlorethol-A, 2,7′-phloroglucinol-6,6′-bieckol | [58,59] |
| Lichens | 1-Chloropannarin, 2-O-Methylsekikaic acid, Atranol, Atranorin, Barbatic acid, Boninic acid, Chloroatranol, Chloroatranorin, Chlorohematommic acid, Cryptostictinolide, Divaricatic acid, Ergosterol peroxide, Ethyl chlorohematommate, Evernic acid, Fumarprotocetraric acid, Gyrophoric acid, Hematommic acid, Lecanoric acid, Lecanoric acid, Methyl orsellinate, Orcinol, Physodic acid, Protrocetraric acid, Sekikaic acid, Umbilicaric acid | [60] |
| Nanoparticles | Delivered Antioxidant/Enzymes | Method of Preparation | Characterization | Size | Test System | Biological Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Poly(lactide-co glycolide) (PLGA) and Polycaprolactone (PCL) | Ellagic acid | Emulsion -diffusion-evaporation | DLS, Zeta potential | ND | Overnight fasted male Sprague Dawley (SD) rats | Prevent cyclosporine A (CyA)-induced nephrotoxicity | [71] |
| Polybutylcyanoacrylate, Liposomes, Poly(Lactide-co-Glycolide) | Superoxide dismutase (SOD) | Emulsion solvent evaporation | DLS, Zeta potential | ND | C57BL/6 mice | Nanoparticles displayed protection against reperfusion injury and ischemia when applied after injury reduced in infarct volume with a 50% to 60%, lowered inflammatory markers, and improved in mice behavior | [72] |
| Iron oxide (magnetite) | Catalase and SOD | Nanoprecipitation | Zeta potential, TEM | 303 ± 38 nm (Catalase loaded), 350 ± 10 nm (SOD loaded) | Bovine aortic endothelial cells (BAEC), Primary human umbilical vein endothelial cells (HUVEC) | Cultured endothelial cells rapidly take magnetically responsive nanoparticles (MNP) under magnetic guidance catalase-loaded providing increased resistance to oxidative stress (62 ± 12% cells rescued from hydrogen peroxide induced cell death vs. 10 ± 4% under non-magnetic conditions) | [73] |
| Poly(lactide-co glycolide) (PLGA) | SOD | Emulsion solvent evaporation | TEM, DLS, Zeta potential | 81 ± 4 nm | Male Sprague-Dawley rats | NPs encapsulated by superoxide dismutase helps in reduction of cerebral injury and promote neurological recovery in a rat cerebral ischemia-reperfusion model | [74] |
| Bovine serum albumin (BSA)-dextran | Curcumin | Self-assembly | TEM, DLS, Zeta potential | 115 nm | Caco-2 cells | At 5 μg/mL curcumin in BSA-dextran nanoparticle the CAA (cellular antioxidant activity) value was 65.35, significantly more that of free curcumin (48.61) at the same concentration, showing that nanoencapsulation increased the uptake of curucmin (p < 0.05). Curucmin-loaded BSA-dextran nanoparticle EC50 values was 3.27 μg/mL, indicating the CAA of curcumin was enhanced by nanoparticle-based delivery systems | [75] |
| Mesoporous silica | Caffeic acid, Rutin | ND | TEM, Zeta potential | 200 nm | Caco-2 and the epidermal HaCaT cell lines | After 24 h incubation of cells with grafted nanoparticles the best results were given by Rutin in terms of antioxidant capacities preservation during coupling procedures, decrease of ROS level and cellular toxicity alleviation. Rutin protective effects were found more apparated in HaCaT than in Caco-2 cells, revealing much cellular specificity towards defense against oxidative stress; MSN-RUT has ability to stimulate a strong Nrf2 protective response in HaCaT cells, accompanied by a comparable induction of HO-1 mRNA. These responses level in Caco-2 cells was again less important. | [76] |
| Chitosan/alginate | Quercetin | Gelation | Zeta potential | ND | Human hepatocellular carcinoma HepG2 cells and Male Wistar rats (paracetamol-induced liver injury) | Pretreatment of HepG2 cells with (10 µg/mL) encapsulated quercetin significantly reduction in cell viability in H2O2-induced oxidative stress (0.1 mM H2O2), thus showing an efficacious in vitro protection; oral pretreatment with encapsulated quercetin (0.18 mg/kg b.w., 7 days) significantly reduced the increased serum transaminases ALT and AST levels, reduced the lipid peroxidation and restored the gluthation (a marker of cell antioxidant defence system) levels | [77] |
| Stearic acid- and stearyl ferulate-based solid lipid | Trans-ferulic acid | Microemulsion | DLS, TEM | 505 ± 8.2 (SLN-FA), 600 ± 3.4 nm (SLN-SF-FA) | Male rats | Both SLN-SF-FA and SLN-FA dose-dependently reduced lipid peroxidation induced by the three oxidants (NADPH/ADP-Fe3+, AAPH and SIN-1). SLN-SF-FA showed high efficiency (EC50) and potency (maximal activity) against NADPH/ADPFe3+ and AAPH-induced lipid peroxidation | [78] |
| Antioxidant Source | Types of Nanoparticles | Biological Extract | Temperature | Reaction Time | Characterizations | Morphology | Size | Stability | Antioxidant Activity | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Plant | Silver | Lantana camara L. leaves extract (terpenes rich) | RT | 24 h | UV-Vis, XRD, Zeta potential; SEM | Sphere | 425 nm | Nd | A10 μL of AgNP (2 mg/mL), spot intensity was found good and comparable with ascorbic acid | [84] |
| Silver | Taraxacum officinale leaf extract | RT | 15 min | UV-Vis, XRD, FTIR, HRTEM | Sphere | 15 nm | 4 months | The efficiency of AgNPs were found against ABTS radicals, displayed an IC50 value of 45.6 μg/mL; scavenging potential of Nitric Oxide is 72.1% at 100 μg/mL concentration with IC50 value of 55.2 μg/mL | [85] | |
| Silver | Bergenia ciliate crude extract | RT | 3 h | UV-Vis, SEM, FTIR, | Sphere | 35 nm | Nd | Results of DPPH activity showed the effective free radical % scavenging potential of Bergenia ciliate AgNPs is 59.31% | [86] | |
| Silver | Clerodendrum phlomidis L. leaves extract | RT | 10 min | UV-Vis, SEM, TEM, EDAX, FT-IR | Sphere | 23–42 nm | Nd | AgNPs exhibited remarkable antioxidant activity than the crude extract using phosphomolybdate assay, ferric reducing power, superoxide radical scavenging activity and DPPH assay | [87] | |
| Silver | Hippophae rhamnoides L. leaves extract | RT | 24 h | UV-Vis, TEM, HRTEM, FTIR | Sphere | 10–40 nm | 1 year | The SBT@AgNPs showed excellent DPPH radical scavenging capacity. The results also revealed that the antioxidant properties of the samples depends on dose as their concentrations (5–25 µg mL−1) increase their percentage DPPH radical scavenging abilities also increased | [88] | |
| Gold | Hippophae rhamnoides ssp. Turkestanica leaves and berries extract | RT | 2 min (LE), 15 min (BE) | UV-Vis, HRTEM, FTIR, XRD | Triangles, hexagon and sphere (BE AuNPs), Sphere (LE AuNPs) | 55 nm (BE AuNPs), 27 nm (LE AuNPs) | 5 months | Colorimetric DPPH assay at (80 µg/mL) concentration showed, free radical scavenging activity was found maximum in LE AuNPs (81%) and BE (70%) AuNPs. LE AuNPs nanospheres (IC50 49 µg) revealed a little better (14%) antioxidant capacity as compared to BE nanotriangles (IC50 57 µg) | [89] | |
| Silver | Morus alba leaf extract | RT | 10 min | UV-Vis, FTIR, SEM, FESEM, EDX, HRTEM, XRD, DLS | Sphere | 12–39 nm | Nd | Dose dependent antioxidant activity against free radicals like DPPH, ABTS+, superoxide and nitric oxide | [90] | |
| Gold | Couroupita guianensis Aubl. fruit extract | 70 °C | 60 min | UV-Vis, FTIR, TEM, XRD, DLS, Zeta potential | Cubic | 26 nm | 45 days | For DPPH assay, CGAuNPs IC50 was 37 μg/mL; CGAuNPs were potent in scavenging the hydroxyl radicals with IC50 values of 30 and 36 μg/mL respectively; CGAuNPs superoxide scavenging activity increased with increasing concentrations and was observed as 89.8% inhibition rate | [91] | |
| Silver | Citrullus lanatus rind extract | RT | 24 h | UV-Vis, SEM, EDX, FTIR, XRD | Sphere | 109.97 nm | Nd | AgNPs DPPH free radical scavenging activity at 20–100 μg/mL ranged from 21.65% to 60.97%; AgNPs ABTS radical scavenging activity was 11.25% to 55.26% at a concentration of 20–100 μg/mL; AgNPs Nitric oxide scavenging activity was 9.05% to 54.15% at a concentration of 20–100 μg/mL | [92] | |
| Silver | Erythrina suberosa (Roxb.) leaf extract | RT | Over night | UV-Vis, ATR-FTIR, DLS, TEM, | Sphere | 12–115 nm | Nd | AgNPs antioxidant potential was estimated by DPPH radical scavenging assay having IC50 30.04 μg/mL | [93] | |
| Silver | Thymus kotschyanus extract | RT | 30 min | UV-Vis, FTIR, XRD, EDS, SEM, AFM, HRTEM | Sphere | 50–60 nm | Nd | AgNPs DPPH free radical scavenging activities demonstrate effective inhibition as compared to BHT as the standard antioxidant | [94] | |
| Zinc Oxide | Berberis aristata leaf extract | 70 °C | ND | UV-Vis, XRD, FTIR, SEM, EDX, DLS | Needle | 90–110 nm | Nd | B. aristata leaves extract ZnO nanoparticles showed percent inhibition of 32.06% at the concentration of 1 μg/mL and for 5 μg/mL it was to be 61.63% | [95] | |
| Gold | Vitex negundo leaf extract | RT | ND | UV-Vis, XRD, FTIR, TEM | Sphere | 20–70 nm | Nd | Radical scavenging activity of DPPH shown that at a 120 µg/mL concentration, the scavenging activity NPs reached 84.64% and the IC50 of the NPs was found to be 62.18 µg; The nitric oxide assay results revealed that the antioxidant property of NPs at a concentration of 120 µg/mL, the NPs scavenging activity reached 69.79% with IC50 estimated at 70.45 µg | [96] | |
| Silver | Cestrum nocturnum leaf extract | RT | 1 week | XRD, TEM, EDS, SEM, FTIR, | Sphere | 20 nm | Nd | AgNPs antioxidant activity for DPPH method was 29.55% | [97] | |
| Copper Oxide | Hibicus rosasinensis leaf extract | RT | 48 h | UV-Vis, FTIR, TEM | ND | ND | Nd | Good antioxidant activity from FRAP assay | [98] | |
| Copper Oxide | Dioscorea bulbifera tuber extract | 40 °C | 5 h | UV-Vis, TEM, EDS, XRD, DLS | Sphere | 86–126 nm | Nd | Showed 40.81 ± 1.44%, 79.06 ± 1.02% and 48.39 ± 1.46% scavenging activity against DPPH, nitric oxide and superoxide radicals respectively | [99] | |
| Copper Oxide | Adiantum lunulatum whole plant extract | RT | 1 h | UV-Vis, DLS, TEM, EDX, XRD, FTIR | Sphere | 6.5 nm | Nd | CAT, APX, and SOD activities have steadily increased according to the increasing concentration of copper nanoparticles treatment to Lens culinaris | [100] | |
| Copper Oxide | Galeopsidis herba. G. herba extract | 25 °C | 24 h | UV-Vis, SEM, FTIR, TEM | Sphere | 10 nm | Nd | Showed good scavenging activity against DPPH | [101] | |
| Copper Oxide | Cissus arnotiana leaf extract | RT | 4 h | UV-Vis, XRD, SEM, TEM | Sphere | 80–90 nm | Nd | The antioxidant property observed was comparatively equal with the standard antioxidant agent ascorbic acid at a maximum concentration of 40 μg/mL DPPH assay | [102] | |
| Iron | Amaranthus dubius leaf extract | 60 °C | 90 min | UV-Vis, FTIR, XRD, SEM | Sphere | 43–220 nm | Nd | Showed high antioxidant activity against DPPH | [103] | |
| Iron | Amaranthus spinosus leaf extract | RT | 90 min | UV-Vis, FTIR, TEM, EDX, XRD | Sphere | ND | Nd | Antioxidant efficiency was observed to be 93% against DPPH | [104] | |
| Iron | Asphodelus aestivus Brot. extract | 50–60 °C | 20 min | UV-Vis, FTIR, TEM, EDX, SEM, XRD | NS | 20–25 nm | Nd | Antioxidant activity against DPPH (IC50: 3.48 µg/mL) and ABTS (60.52%) | [105] | |
| Nickel Oxide | Stevia rebaudiana Bertoni leaf extract | 100 °C | 2 h | UV-Vis, XRD, SEM, TEM, FTIR | Sphere | 20–50 nm | Nd | Antioxidant efficiency was observed to be 70% against DPPH | [106] | |
| Bacteria | Gold | Lactobacillus kimchicus DCY51T biomass | RT | 12 h | UV-Vis, FE-TEM, XRD, DLS, FTIR | Sphere | 13 nm | NS | Lowest concentration of the biosynthesized AuNps DPPH percentage scavenging ability was 15.85 ± 0.49 and when concentration was increased to 500 μg/mL this scavenging ability was increased to 60 ± 1.82 | [107] |
| Silver | Streptomyces griseorubens AU2 cell free supernatant | RT | 48 h | UV-Vis, FTIR, TEM, SEM, XRD | Sphere | 5–20 nm | Nd | DPPH free radical scavenging activity of AgNPs showed at various concentrations viz. 9.66, 14.27, 15.59, 23.46 and 54.99% | [108] | |
| Gold | Enterococcus species cell free extract | RT | 30 min | UV-Vis, TEM, EDX, FTIR | Sphere | 8–50 nm | Nd | AuNPs have ability to scavenge DPPH at all the investigated concentrations (1–40 μg/mL), yielding activities of 33.24–51.47% | [109] | |
| Gold and Silver | Escherichia coli cell protein | RT | ND | UV-Vis, XRD, FTIR, TEM | Triangular, circular, hexagonal (AuNPs), Sphere (AgNPs) | 10–100 nm (AuNPs), 10–50 nm (AgNPs) | 3 months | EC75 (for scavenging 75% effective concentration) of protein capped gold nanoparticles is 916 µg/mL | [110] | |
| Silver | Streptomyces naganishii MA7 biomass | RT | 72 h | UV-Vis, FTIR, XRD, EDX, AFM, SEM, TEM, HRTEM | Sphere | 5–50 nm | Nd | At a concentration of 1000 μg/mL, AgNPs showed good reducing power comparatively than ascorbic acid (vitamin C) | [111] | |
| Selenium | Streptomyces minutiscleroticus M10A62 biomass | RT | 72 h | UV-Vis, XRD, HRTEM, FTIR, EDX | Sphere | 10–250 nm | Nd | SeNPs actinobacterially synthesized were found strong free radical scavenging activity compared with standard ascorbic acid was proved by positive DPPH activity. Free radical scavenging activity depends on concentration as increases with increased concentration of SeNPs | [112] | |
| Selenium | Pantoea agglomerans UC-32 | RT | 24 h | TEM, EDS, SEM | Sphere | 100 nm | Nd | High antioxidant activity in human umbilical vein endothelial cells | [113] | |
| Gold and Silver | Gordonia amicalis HS-11 cell free supernatant | 100 °C | 10 min | UV-Vis, XRD, TEM, FTIR | Grain | 5–25 nm | Nd | CFS synthesized AgNPs and AuNPs respectively, showed 88.5 and 87.75% inhibition towards hydroxyl radicals; CFS mediated AuNPs inhibited nitric oxide radicals by 67.5%and with AgNPs the inhibited by 61.5% | [114] | |
| Silver | Streptomyces violaceus MM72 exopolysaccharides | RT | 1 h | TEM, EDX, XRD | ND | 30 nm | Nd | The DPPH radical scavenging activity shown by SNPs of 89.5% at 50 μg/mL concentration; SNPs exhibited the more total antioxidant activity of 0.730 at 50 μg/mL concentration; H2O2 scavenging activity of SNPs was evaluated at different concentrations, 50 μg/mL exhibited a higher activity of 72.5% which was significantly higher than that of the standard L-ascorbic acid; SNPs (50 μg/mL) had a nitric oxide scavenging activity of 60.1%; Ferric reducing power of the SNPs was estimated by the reduction of Fe/ferricyanide and the inhibition was observed at 0.390 AU at 50 μg/mL | [115] | |
| Fungi | Zinc Oxide | Pichia kudriavzevii cell free extract | RT | 36 h | UV-Vis, XRD, TEM, Zeta potential | Hexagonal | 10–61 nm | Nd | DPPH radical scavenging activities IC50 values were 10 ± 0.52, 5.26 ± 0.42 and 25.46 ± 0.35 µg/mL for ZnO/T1, ZnO/T2 and ZnO/T3 respectively | [116] |
| Silver | Pestalotiopsis microspora filtrate | RT | 24 h | UV-Vis, FTIR, XRD, TEM, DLS | Sphere | 2–10 nm | Nd | IC50concentrations (the concentration of the sample required to scavenge 50% radicals) of the biosynthesized AgNPs were found to be 76.95 ± 2.96 μg/mL; Biosynthesized AgNPs also exhibited effective scavenging activity against H2O2 radicals and the maximum scavenging activity of 51.14% ± 1.78% was observed at the highest concentration of 100 μg/mL | [117] | |
| Gold | Cladosporium cladosporioides filtrate | RT | ND | UV-Vis, FESEM, EDX, XRD, FTIR, DLS, AFM | Cubic | 100 nm | 6 months | DPPH radical scavenging capacity of the AuNPs was found to be dose dependent; AuNPs was subjected to reducing power assay where it showed moderate activity of 1.51 ± 0.03 mg of AAE/g sample | [118] | |
| Silver | Cladosporium cladosporioides filtrate | RT | ND | UV-Vis, FESEM, XRD, FTIR, DLS, AFM | Sphere | 100 nm | Nd | AgNPs showed potent antioxidant potential and their radical scavenging ability was increasing with increment in their concentration | [119] | |
| Silver | Aspergillus versicolor ENT7 filtrate | RT | 24 h | UV-Vis, TEM, XRD, FTIR | Sphere | 3–40 nm | Nd | The radical scavenging activity for the AgNPs at 100 µg/mL was determined as 60.04% which is close to 68.52% obtained for the standard ascorbic acid at the same concentration. IC50 value for the AgNPs is found to be 60.64 µg/mL | [120] | |
| Silver | Trichoderma atroviride cell free filtrate | 40 °C | ND | UV-Vis, TEM, EDS, FTIR | Variables | 15–25 nm | Nd | AgNPs exhibited quite higher DPPH scavenging activity at concentration dependent manner with IC50 of 45.6 μg/mL | [121] | |
| Zinc Oxide | Aspergillus niger cell free filtrate | RT | 24 h | UV-Vis, FTIR, XRD, DLS, SEM | Rod and cluster | 80–130 nm | Nd | Maximum DPPH scavenging of 57.74% was obtained at 100 µg/mL concentration of ZnONPs; ABTS assay scavenging of 73.58% was obtained at 100 µg/mL concentration of ZnONPs | [122] | |
| Silver | Penicillium species extract | RT | 10 min | UV-Vis, SEM, XRD | Sphere | 18 nm | Nd | FRAP reducing ability potentially reduced by PsAgNPs was 1109.41 where the standard ascorbic acid shows 1648.52 μm | [123] | |
| Silver | Inonotus obliquus extract | RT | 80 min | UV-Vis, SEM, EDX, TEM, XRD, FTIR | Sphere | 14.2–35.2 nm | 8 months | Free radical scavenging activity of the AgNPs on ABTS radicals was found to increase with increase in the concentration, showing maximum inhibition (76.57%) at 1 mM and minimum inhibition (60.98%) at 0.125 mM solution | [124] | |
| Silver | Cladosporium species extract | RT | 1 h | UV-Vis, SEM, XRD, FTIR | Sphere | 24 nm | Nd | CsAgNPs exhibited potent antioxidant potential and as the concentration increases the radical scavenging ability also increases | [125] | |
| Silver | Ganoderma lucidium and Agaricus Bisporus extract | RT and 60 °C | 12 h (RT), 5 h (60 °C) | UV-Vis, SEM, XRD, FTIR | Rod | 10–80 nm | Nd | EHT synthesized AgNPs shows higher antioxidant activity (75% ± 0.24%) in comparison of standard (70.34% ± 0.03%) | [126] | |
| Gold | Inonotus obliquus extract | RT | 30 min | UV-Vis, SEM, EDX, TEM, AFM, XRD, FTIR | Sphere, triangle, hexagonal and rod | 23 nm | Nd | ABTS scavenging effect increased with increasing concentration of AuNPs. The ABTS radical scavenging activity showed a maximum and minimum at 1 mM and 0.125 mM, respectively | [127] | |
| Silver | Ganoderma lucidium extract | RT | ND | UV-Vis, TEM, XRD, FTIR, XPS | Sphere | 15–22 nm | Nd | DPPH free radicals scavenging activity of AgNPs raised from 32.57% to 54.16% when concentration raised from 50 mg/L to 100 mg/L | [128] | |
| Silver | Ganoderma lucidium extract | 60 °C | 24 h | UV-Vis, XRD, SEM, FTIR | Sphere | 10–30 nm | Nd | Percentage of inhibition by silver nanoparticles showing maximum with 73.49% and minimum with 55.34% at 100 and 10 μg/mL respectively | [129] | |
| Algae | Gold | Gracilaria corticata extract | 40 °C | 4 h | UV-Vis, SEM | ND | 45–57 nm | Nd | Synthesized AuNPs revealed a good capacity of DPPH free radical scavenging | [130] |
| Gold | Lemanea fluviatilis (L.) C.Ag. extract | RT | 12 h | UV-Vis, XRD, TEM, HRTEM, DLS, FTIR | Sphere | 5–15 nm | Up to 3 months | DPPH scavenging (%) vs. different weights of the sample was found to be 18.10 mg | [131] | |
| Silver | Ecklonia cava extract | RT | 72 h | UV-Vis, FTIR, XRD, TEM, DLS | Sphere | 43 nm | Nd | 250 µg/mL of Ecklonia cava extract or biosynthesized AgNPs was mixed with DPPH solution, ca. 50% of scavenging activity was achieved. DPPH radical scavenging activities of Ecklonia cava extract and biosynthesized AgNPs were similar at the same concentrations (e.g., 100, 250, and 500 µg/mL) | [59] | |
| Lichens | Silver | Parmeliopsis ambigua, Punctelia subrudecta, Evernia mesomorpha, Xanthoparmelia plitti mycelia mats | RT | 24 h | UV-Vis, SEM, FTIR | Variables | 150–250 nm | Nd | SNPs samples total antioxidant capacity are 2.55 ± 0.05, 3.35 ± 0.04, 2.90 ± 0.01, 2.22 ± 0.01 µg AA/g respectively. Punctelia subrudecta displayed a higher activity (3.35 ± 0.04 µg AA/g) than the remaining samples; The hydroxyl radical scavenging activity showed that values were 7.75 ± 0.10, 34.48 ± 1.19, 31.97 ± 1.87 and 27.21 ± 1.39 µg/mL for all four samples respectively; Among the tested lichen SNPs, Punctelia subrudecta (Punctelia subrudecta) gave highest reducing power | [132] |
| Gold | Acroscyphus sphaerophoroides Lev, Sticta nylanderiana extract | RT | 12 h | UV-Vis, FTIR, XRD, TEM | Quasi-spherical and prismatic (Acroscyphus sp.), Twinned (Sticta sp.) | 5–35 nm (Acroscyphus sp.), 20–50 nm (Sticta sp.) | Up to 3 months | 1.66 and 4.48 mg sample concentration (SC50) were found | [133] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Singh Dhanjal, D.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress. Nanomaterials 2020, 10, 1334. https://doi.org/10.3390/nano10071334
Kumar H, Bhardwaj K, Nepovimova E, Kuča K, Singh Dhanjal D, Bhardwaj S, Bhatia SK, Verma R, Kumar D. Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress. Nanomaterials. 2020; 10(7):1334. https://doi.org/10.3390/nano10071334
Chicago/Turabian StyleKumar, Harsh, Kanchan Bhardwaj, Eugenie Nepovimova, Kamil Kuča, Daljeet Singh Dhanjal, Sonali Bhardwaj, Shashi Kant Bhatia, Rachna Verma, and Dinesh Kumar. 2020. "Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress" Nanomaterials 10, no. 7: 1334. https://doi.org/10.3390/nano10071334
APA StyleKumar, H., Bhardwaj, K., Nepovimova, E., Kuča, K., Singh Dhanjal, D., Bhardwaj, S., Bhatia, S. K., Verma, R., & Kumar, D. (2020). Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress. Nanomaterials, 10(7), 1334. https://doi.org/10.3390/nano10071334












