Soft Actuated Hybrid Hydrogel with Bioinspired Complexity to Control Mechanical Flexure Behavior for Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of HAp Nanorods
- Step 1. Micellar solution preparation. First, proper amount of commercial CTAB was weighed and dissolved in 35 mL of distilled water until reaching a 3.13 mM solution. Then, 2 mL of polypropylene glycol (PPG) were added to the mixture, put in an autoclavable bottle and stirred for 10 min; the addition of CTAB and PPG creates a micellar solution with a high degree of ionization. Above the critical micellar concentration, CTAB turns spherical micelles into rod-like shapes. As the nucleation of HAp occurs at the interface of the micelelles, the mixture serves as a template which give to the nanoparticles their final size and shape.
- Step 2. Chemical Reaction. Next, an aqueous solution of 2 M NaNO2 and 0.22 g of CaCl2 were added and stirred until complete dissolution, obtaining the calcium precursor. After that, 20 mL of Na3PO4 140 mM were added drop by drop to the shaking solution. At this point, phosphate groups interact with the calcium ions at the outer part of the micelles, starting the chemical reaction and nucleation. After all the reagents were combined, the stirring was maintained for 60 min.
- Step 3. Filtration, thermal processing and purification. After the reaction, the solution was autoclaved at 120 °C for 24 h. Following, the mixture was filtered through a moistened, fat-free, double filter paper, and washed with 200 mL of distilled water. After that, in order to eliminate the excessive moisture of the samples, they were dried in an oven at 50 °C for 24 h. The final step consisted in a thermal treatment to eliminate completely the possible impurities that the sample could present, igniting it in the muffle furnace at 400 °C for 3 h.
2.3. Preparation of the Crosslinked Films
- Step 1. Preparation of the protein solutions. To synthesize the hydrogels, each one of the protein solutions was prepared separately. First, a known amount of the alginic acid sodium salt powder was dissolved in phosphate buffered saline (PBS) to obtain a 5% (w/v) solution. The mixture was then kept under gentle agitation at 250 rpm during 3 h at 37 °C. Next, and similarly, a 2% (w/v) gelatin solution was prepared by dissolving the gelatin powder in PBS. The solution was magnetically stirred at 300 rpm and 58 °C for 1 h. Meanwhile, both fibrinogen and bovine serum albumin 0.5% (w/v) solutions were prepared by dissolving the proper amount of each one of the materials in the buffer and agitated until complete dissolution.
- Step 2. Obtention of the protein mixtures. When all the solutions were ready, alginate was combined with fibrinogen or bovine serum albumin, respectively, at a 1:1 ratio, to obtain the initial Alg/Fib and Alg/BSA mixtures. Then, the gelatin was added to both mixtures at a volume ratio of 2:1:1 (gelatin: alginate: Fib/BSA) and stirred gently until getting the homogeneous viscous solutions Gel/Alg/Fib and Gel/Alg/BSA.
- Step 3. Formation and drying of the films. Once the final protein mixtures were obtained, aliquots of 4.5 mL were added to 35 mm diameter Petri dishes and left to dry for 24 h at 45 °C. When the protein mixtures are appropriately dried, as a consequence of the great loss of liquid they suffer in the oven, they become fragile films that lay in the bottom of the Petri dishes (Gel/Alg/Fib and Gel/Alg/BSA films).
- Step 4. Preparation of the crosslinking solutions. Meanwhile, two different fresh crosslinking solutions (C1 and C2) were prepared in Falcon tubes. The first crosslinking solution C1 was composed of 3.48 mL of a 110 mM aqueous solution of CaCl2, 7.08 mL of 11 mM HAp solution, and 7.44 mL of water. Crosslinking solution C2 was prepared similarly but without the presence of HAp: 3.48 mL of 110 mM of CaCl2 and 14.52 mL of water. The addition of CaCl2 assured the alginic acid polymerization and the crosslinking by calcium diffusion through the gels.
- Step 5. Crosslinking process and obtention of the final hydrogels. At this point, Falcon tubes containing the crosslinking solutions were sonicated for 10 min and vortexed. Finally, 1.5 mL of the crosslinking solutions C1 and C2 were added into empty Petri dishes. Dried protein films were subsequently placed on top of the solutions using tweezers, carefully enough to avoid wrinkles and bubbles in the membranes. Next, films were left on an orbital shaker at 150 rpm at room temperature (RT) for 3 h, to ensure a complete crosslinking. Following this procedure, each of the films crosslinked with the C1 solution contained a final amount of HAp of 6.525 mg.
2.4. Characterization of the Polymeric Hydrogels
2.5. Rheological Characterization
3. Results and Discussion
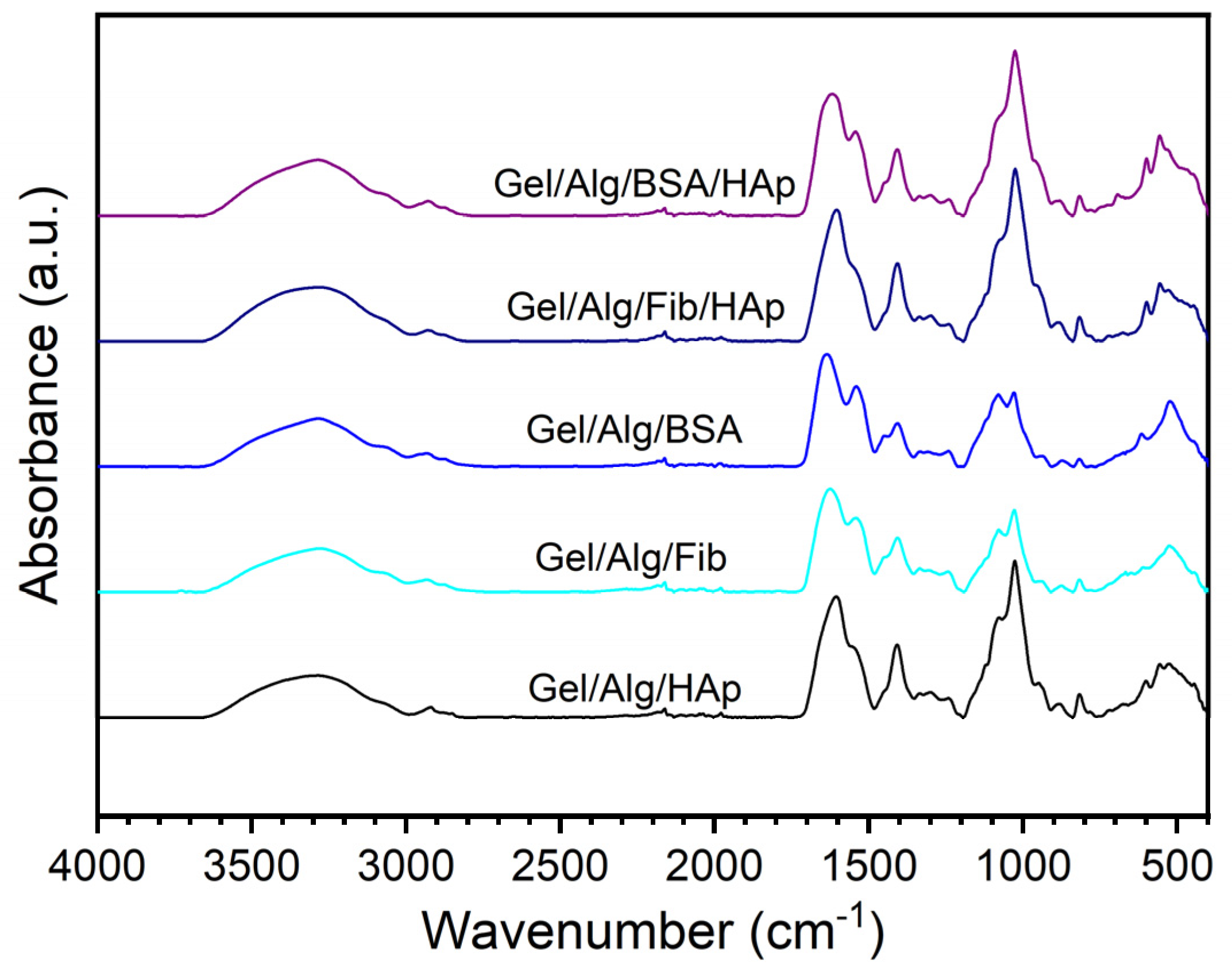
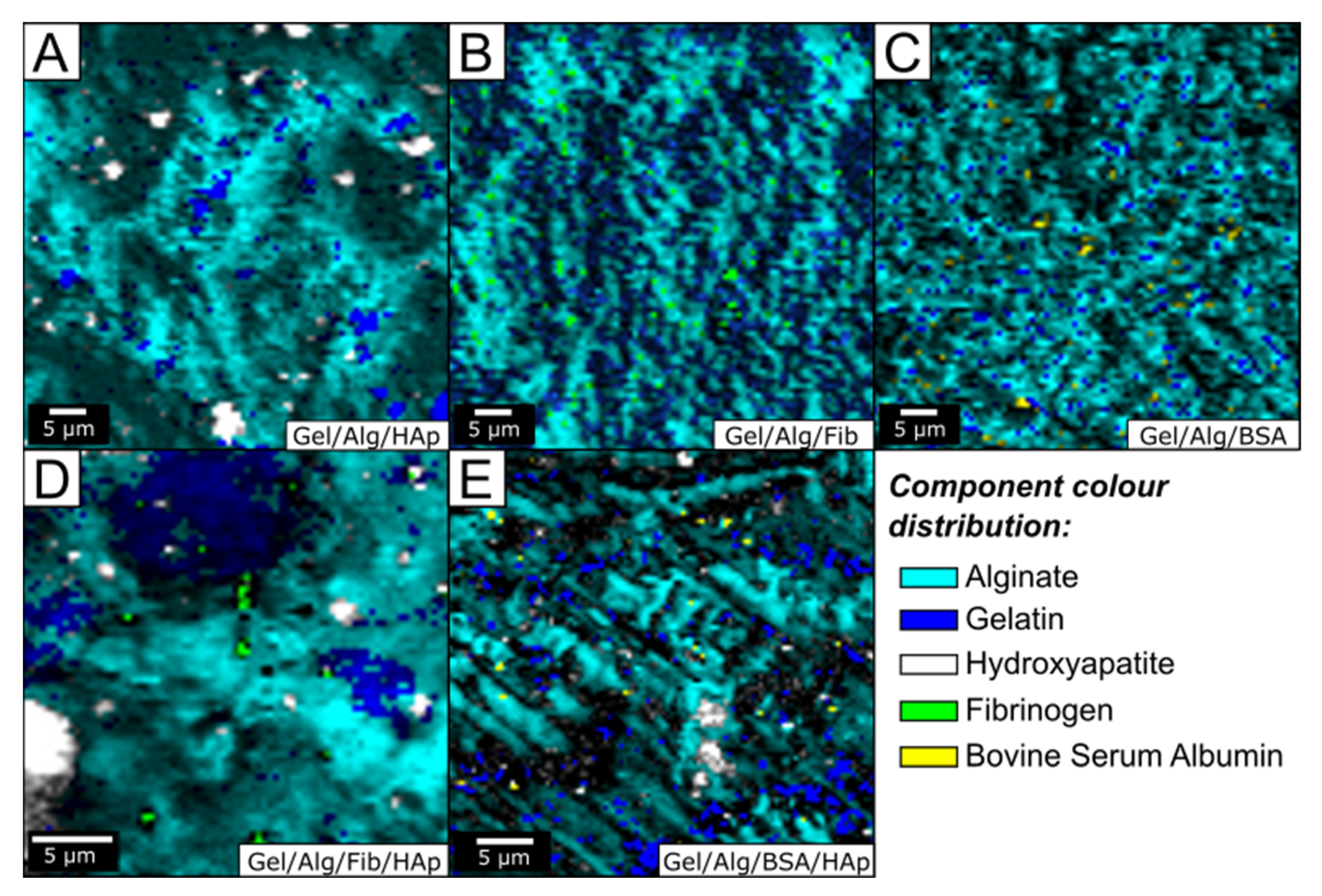
3.1. Morphological and Structural Analysis
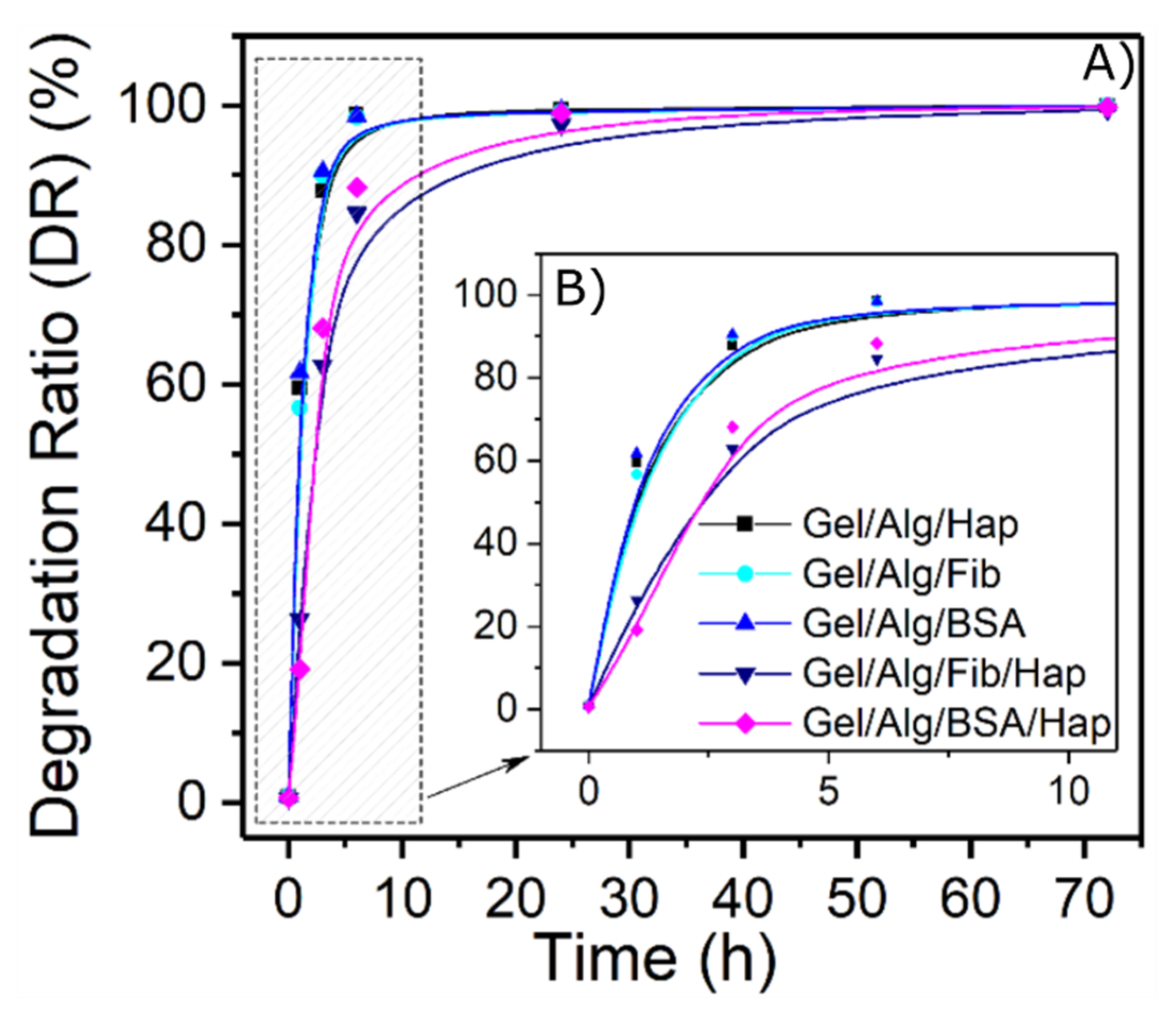
3.2. Swelling and Degradation Behavior
3.3. Viscoelastic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brandl, F.; Sommer, F.; Goepferich, A. Rational design of hydrogels for tissue engineering: Impact of physical factors on cell behavior. Biomaterials 2007, 28, 134–146. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, L.-J.; Yang, G.; Sun, B.; Wang, X.; Jiang, B.; Yin, G.-Q.; Zhang, L.; Li, X.; Liu, M. Construction of smart supramolecular polymeric hydrogels cross-linked by discrete organoplatinum (II) metallacycles via post-assembly polymerization. J. Am. Chem. Soc. 2016, 138, 4927–4937. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wu, R.T.; Long, L.; Ke, X.S.; Guo, C.; Ghang, Y.J.; Lynch, V.M.; Huang, F.; Sessler, J.L. Encoding, reading, and transforming information using multifluorescent supramolecular polymeric hydrogels. Adv. Mater. 2018, 30, 1705480. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Messina, P.V.; Dodero, V.I.; Ruso, J.M. Rheological properties of ovalbumin hydrogels as affected by surfactants addition. Int. J. Biol. Macromol. 2011, 48, 495–500. [Google Scholar] [CrossRef]
- Rial, R.; Soltero, J.F.A.; Verdes, P.V.; Liu, Z.; Ruso, J.M. Mechanical Properties of Composite Hydrogels for Tissue Engineering. Curr. Top. Med. Chem. 2018, 18, 1214–1223. [Google Scholar] [CrossRef]
- Beattie, G.M.; Montgomery, A.M.P.; Lopez, A.D.; Hao, E.; Perez, B.; Just, M.L.; Lakey, J.R.T.; Hart, M.E.; Hayek, A. A novel approach to increase human islet cell mass while preserving β-cell function. Diabetes 2002, 51, 3435–3439. [Google Scholar] [CrossRef]
- Ruso, J.M.; Messina, P.V. Modelling and Simulation of Biological Systems in Medical Applications. In Biopolymers for Medical Applications; CRC Press: Boca Raton, FL, USA, 2017; pp. 241–276. [Google Scholar]
- Rial, R.n.; Tichnell, B.; Latimer, B.; Liu, Z.; Messina, P.V.; Ruso, J.M. Structural and kinetic visualization of the protein corona on bioceramic nanoparticles. Langmuir ACS J. Surf. Colloids 2018, 34, 2471–2480. [Google Scholar] [CrossRef] [PubMed]
- Messina, P.V.; Prieto, G.; Ruso, J.M.; Sarmiento, F. Conformational changes in human serum albumin induced by sodium perfluorooctanoate in aqueous solutions. J. Phys. Chem. B 2005, 109, 15566–15573. [Google Scholar] [CrossRef]
- Taboada, P.; Mosquera, V.; Ruso, J.M.; Sarmiento, F.; Jones, M.N. Interaction between penicillins and human serum albumin: A thermodynamic study of micellar-like clusters on a protein. Langmuir ACS J. Surf. Colloids 2000, 16, 934–938. [Google Scholar] [CrossRef]
- Sabín, J.; Prieto, G.; González-Pérez, A.; Ruso, J.M.; Sarmiento, F. Effects of fluorinated and hydrogenated surfactants on human serum albumin at different pHs. Biomacromolecules 2006, 7, 176–182. [Google Scholar] [CrossRef]
- D’Elía, N.L.; Gravina, A.N.; Ruso, J.M.; Laiuppa, J.A.; Santillán, G.E.; Messina, P.V. Manipulating the bioactivity of hydroxyapatite nano-rods structured networks: Effects on mineral coating morphology and growth kinetic. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 5014–5026. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Raeber, G.P.; Zisch, A.H.; Tirelli, N.; Hubbell, J.A. Cell-responsive synthetic hydrogels. Adv. Mater. 2003, 15, 888–892. [Google Scholar] [CrossRef]
- Gjorevski, N.; Ranga, A.; Lutolf, M.P. Bioengineering approaches to guide stem cell-based organogenesis. Development 2014, 141, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Mir, S.H.; Nagahara, L.A.; Thundat, T.; Mokarian-Tabari, P.; Furukawa, H.; Khosla, A. Review—Organic-Inorganic Hybrid Functional Materials: An Integrated Platform for Applied Technologies. J. Electrochem. Soc. 2018, 165, B3137–B3156. [Google Scholar] [CrossRef]
- Saveleva, M.S.; Eftekhari, K.; Abalymov, A.; Douglas, T.E.L.; Volodkin, D.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of Hybrid Materials—The Place of Inorganics-in-Organics in it, Their Composition and Applications. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X. Fluid and cell behaviors along a 3D printed alginate/gelatin/fibrin channel. Biotechnol. Bioeng. 2015, 112, 1683–1695. [Google Scholar] [CrossRef]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- D’Elia, N.L.; Gravina, N.; Ruso, J.M.; Marco-Brown, J.L.; Sieben, J.M.; Messina, P.V. Albumin-mediated deposition of bone-like apatite onto nano-sized surfaces: Effect of surface reactivity and interfacial hydration. J. Colloid Interface Sci. 2017, 494, 345–354. [Google Scholar] [CrossRef]
- Eyrich, D.; Brandl, F.; Appel, B.; Wiese, H.; Maier, G.; Wenzel, M.; Staudenmaier, R.; Goepferich, A.; Blunk, T. Long-term stable fibrin gels for cartilage engineering. Biomaterials 2007, 28, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Grassl, E.D.; Oegema, T.R.; Tranquillo, R.T. A fibrin-based arterial media equivalent. J. Biomed. Mater. Res. Part A 2003, 66A, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Jockenhoevel, S.; Zund, G.; Hoerstrup, S.P.; Chalabi, K.; Sachweh, J.S.; Demircan, L.; Messmer, B.J.; Turina, M. Fibrin gel–advantages of a new scaffold in cardiovascular tissue engineering. Eur. J. Cardio-Thorac. Surg. 2001, 19, 424–430. [Google Scholar] [CrossRef]
- Bhakta, G.; Lee, K.H.; Magalhães, R.; Wen, F.; Gouk, S.S.; Hutmacher, D.W.; Kuleshova, L.L. Cryoreservation of alginate–fibrin beads involving bone marrow derived mesenchymal stromal cells by vitrification. Biomaterials 2009, 30, 336–343. [Google Scholar] [CrossRef]
- Shikanov, A.; Xu, M.; Woodruff, T.K.; Shea, L.D. Interpenetrating fibrin–alginate matrices for in vitro ovarian follicle development. Biomaterials 2009, 30, 5476–5485. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, H.H.K. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials 2011, 32, 7503–7513. [Google Scholar] [CrossRef]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef]
- Shapiro, L.; Cohen, S. Novel alginate sponges for cell culture and transplantation. Biomaterials 1997, 18, 583–590. [Google Scholar] [CrossRef]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Mazzitelli, S.; Luca, G.; Mancuso, F.; Calvitti, M.; Calafiore, R.; Nastruzzi, C.; Johnson, S.; Badylak, S.F. Production and characterization of engineered alginate-based microparticles containing ECM powder for cell/tissue engineering applications. Acta Biomater. 2011, 7, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Gillette, B.M.; Jensen, J.A.; Tang, B.; Yang, G.J.; Bazargan-Lari, A.; Zhong, M.; Sia, S.K. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nat. Mater. 2008, 7, 636. Available online: https://www.nature.com/articles/nmat2203#supplementary-information (accessed on 2 July 2020). [CrossRef]
- Chung, J.H.Y.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Yan, Y.; Zheng, W.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R. Rapid Prototyping Three-Dimensional Cell/Gelatin/Fibrinogen Constructs for Medical Regeneration. J. Bioact. Compat. Polym. 2007, 22, 363–377. [Google Scholar] [CrossRef]
- Montalbano, G.; Toumpaniari, S.; Popov, A.; Duan, P.; Chen, J.; Dalgarno, K.; Scott, W.E.; Ferreira, A.M. Synthesis of bioinspired collagen/alginate/fibrin based hydrogels for soft tissue engineering. Mater. Sci. Eng. C 2018, 91, 236–246. [Google Scholar] [CrossRef]
- Grzesiak, J.; Kolankowski, J.; Marycz, K. Osteogenic Differentiation of Canine Adipose Stem Cells Cultured in Alginate-Fibrin-Based Hydrogel. J. Biomater. Tissue Eng. 2015, 5, 703–710. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef]
- Sakai, S.; Yamaguchi, S.; Takei, T.; Kawakami, K. Oxidized alginate-cross-linked alginate/gelatin hydrogel fibers for fabricating tubular constructs with layered smooth muscle cells and endothelial cells in collagen gels. Biomacromolecules 2008, 9, 2036–2041. [Google Scholar] [CrossRef]
- Rial, R.; Tahoces, P.G.; Hassan, N.; Cordero, M.; Liu, Z.; Ruso, J.M. Noble microfluidic system for bioceramic nanoparticles engineering. Mater. Sci. Eng. C 2019, 102, 221–227. [Google Scholar] [CrossRef]
- Li, W.; Xu, H.; Han, X.; Sun, S.; Chai, Q.; Xu, X.; Man, Z. Simultaneously promoting adhesion and osteogenic differentiation of bone marrow-derived mesenchymal cells by a functional electrospun scaffold. Colloids Surf. B Biointerfaces 2020, 192, 111040. [Google Scholar] [CrossRef]
- Abduljauwad, S.N.; Ahmed, H.-u.-R. Enhancing cancer cell adhesion with clay nanoparticles for countering metastasis. Sci. Rep. 2019, 9, 5935. [Google Scholar] [CrossRef]
- Abalymov, A.; Van der Meeren, L.; Saveleva, M.; Prikhozhdenko, E.; Dewettinck, K.; Parakhonskiy, B.; Skirtach, A.G. Cells-Grab-on Particles: A Novel Approach to Control Cell Focal Adhesion on Hybrid Thermally Annealed Hydrogels. ACS Biomater. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Liu, H.S.; Chin, T.S.; Lai, L.S.; Chiu, S.Y.; Chung, K.H.; Chang, C.S.; Lui, M.T. Hydroxyapatite synthesized by a simplified hydrothermal method. Ceram. Int. 1997, 23, 19–25. [Google Scholar] [CrossRef]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul-E-Noor, F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detsch, R.; Boccaccini, A.R. Fabrication of alginate–gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B 2014, 2, 1470–1482. [Google Scholar] [CrossRef]
- Navarra, G.; Peres, C.; Contardi, M.; Picone, P.; San Biagio, P.L.; Di Carlo, M.; Giacomazza, D.; Militello, V. Heat- and pH-induced BSA conformational changes, hydrogel formation and application as 3D cell scaffold. Arch. Biochem. Biophys. 2016, 606, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Simon, V.; Banica, F.; Deleanu, C. Fibrinogen adsorption onto bioglass aluminosilicates. Rom. J. Biophys. 2007, 17, 237–245. [Google Scholar]
- Dainiak, M.B.; Allan, I.U.; Savina, I.N.; Cornelio, L.; James, E.S.; James, S.L.; Mikhalovsky, S.V.; Jungvid, H.; Galaev, I.Y. Gelatin–fibrinogen cryogel dermal matrices for wound repair: Preparation, optimisation and in vitro study. Biomaterials 2010, 31, 67–76. [Google Scholar] [CrossRef]
- De Moura, M.R.; Ahmad Aouada, F.; Favaro, S.L.; Radovanovic, E.; Forti Rubira, A.; Muniz, E.C. Release of BSA from porous matrices constituted of alginate–Ca2+ and PNIPAAm-interpenetrated networks. Mater. Sci. Eng. C 2009, 29, 2319–2325. [Google Scholar] [CrossRef]
- Nishinari, K. Rheological and DSC study of sol-gel transition in aqueous dispersions of industrially important polymers and colloids. Colloid Polym. Sci. 1997, 275, 1093. [Google Scholar] [CrossRef]
- Sarvestani, A.S.; Jabbari, E. Modeling and Experimental Investigation of Rheological Properties of Injectable Poly(lactide ethylene oxide fumarate)/Hydroxyapatite Nanocomposites. Biomacromolecules 2006, 7, 1573–1580. [Google Scholar] [CrossRef]
- Chae, B.S.; Lee, Y.S.; Jhon, M.S. The scaling behavior of a highly aggregated colloidal suspension microstructure and its change in shear flow. Colloid Polym. Sci. 2004, 282, 236–242. [Google Scholar] [CrossRef]
- Chynoweth, S.; Michopoulos, Y. Generic properties of rheological flow curves. J. Non-Newton. Fluid Mech. 1997, 69, 1–14. [Google Scholar] [CrossRef]
- Parhi, P.; Ramanan, A.; Ray, A. Preparation and Characterization of Alginate and Hydroxyapatite-Based Biocomposite. J. Appl. Polym. Sci. 2006, 102, 5162–5165. [Google Scholar] [CrossRef]
- Rajkumar, M.N.M.; Venkatachalam, R. Development of Nanocomposites Based on Hydroxyapatite/Sodium Alginate: Synthesis and Characterisation. Mater. Charact. 2011, 62, 469–479. [Google Scholar] [CrossRef]
- Guesmi, Y.; Agougui, H.; Lafi, R.; Jabli, M.; Amor, H. Synthesis of Hydroxyapatite-Sodium Alginate via a co-Precipitation Technique for Efficient Adsorption of Methylene Blue Dye. J. Mol. Liq. 2017, 249, 912–920. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, R.; Cheng, Y.; Fu, J. Super-tough double-network hydrogels reinforced by covalently compositing with silica-nanoparticles. Soft Matter 2012, 8, 6048–6056. [Google Scholar] [CrossRef]
- Achayuthakan, P.; Suphantharika, M. Pasting and rheological properties of waxy corn starch as affected by guar gum and xanthan gum. Carbohydr. Polym. 2008, 71, 9–17. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Y.; Chen, X.; Zhao, B.; Zhang, J. Flow behavior, thixotropy and dynamical viscoelasticity of sodium alginate aqueous solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, X.; Xiong, S.; Liu, R.; Gu, Z.; You, J.; Yin, T.; Hu, Y. Insights into the rheological behaviors evolution of alginate dialdehyde crosslinked collagen solutions evaluated by numerical models. Mater. Sci. Eng. C 2017, 78, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Martinez, A.W.; Caves, J.M.; Naik, N.; Haller, C.A.; Chaikof, E.L. Microablation of collagen-based substrates for soft tissue engineering. Biomed. Mater. 2014, 9, 011002. [Google Scholar] [CrossRef] [PubMed]


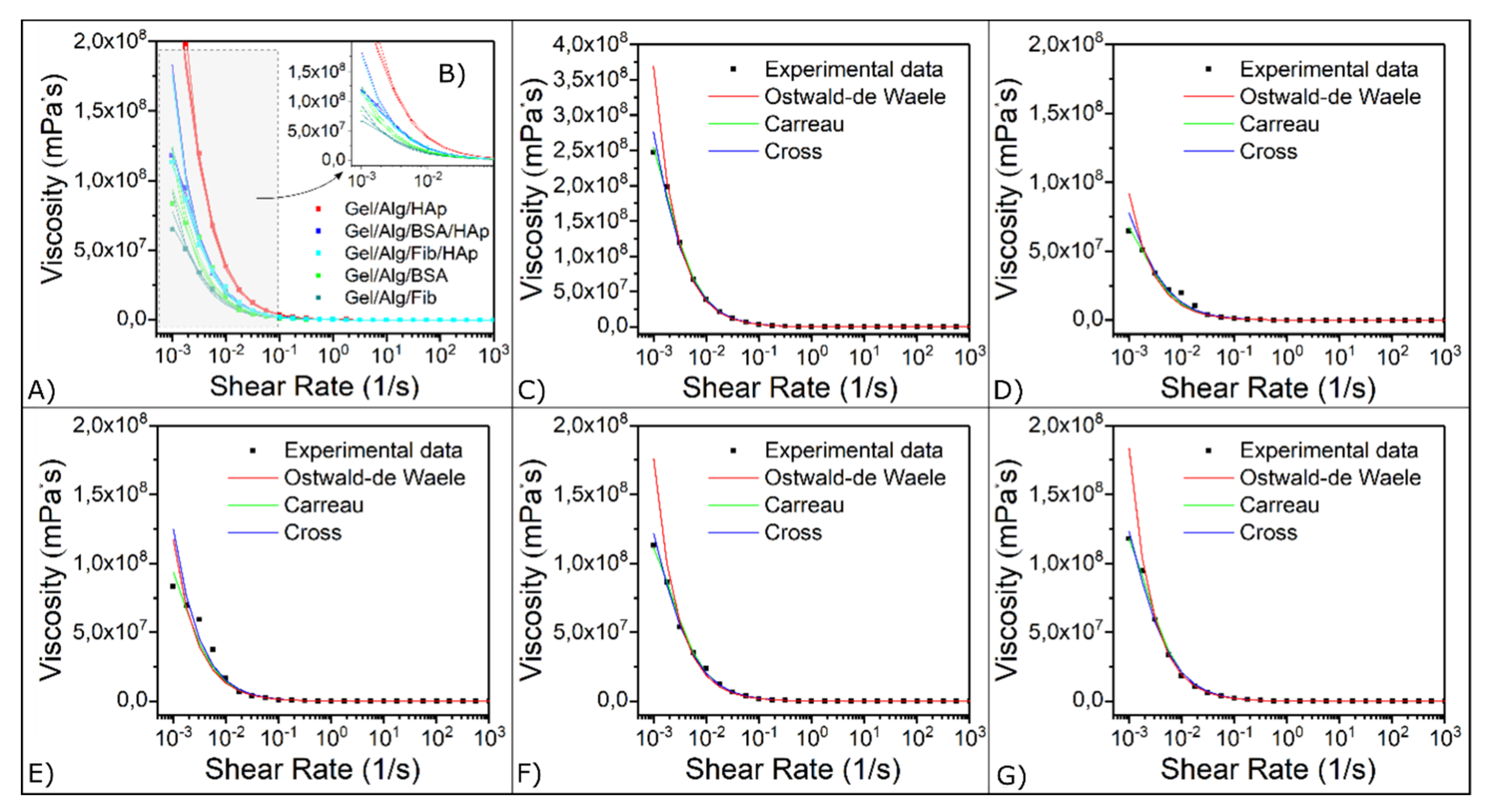

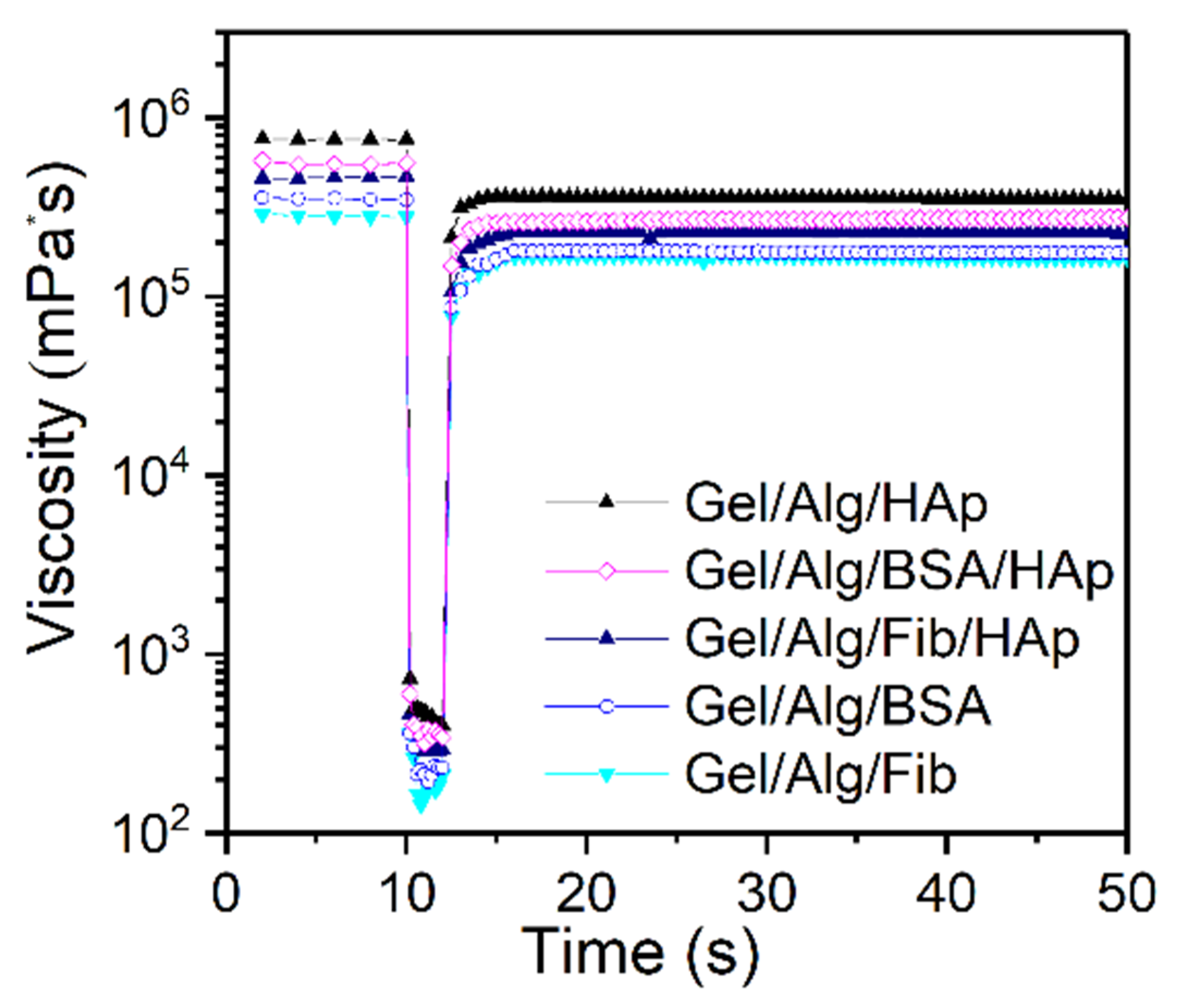


| Samples | ||||||
|---|---|---|---|---|---|---|
| Parameters | Gel/Alg/Fib | Gel/Alg/BSA | Gel/Alg/Fib/HAp | Gel/Alg/BSA/HAp | Gel/Alg/HAp | |
| Ostwald-de Waele | K | 148.31 | 170.09 | 207.17 | 212.11 | 351.63 |
| n | 0.0692 | 0.0542 | 0.0240 | 0.0213 | 0.0068 | |
| r2 | 0.88 | 0.87 | 0.80 | 0.81 | 0.85 | |
| Carreau | η0 | 8.44 × 107 | 1.29 × 108 | 1.32 × 108 | 1.41 × 108 | 3.25 × 108 |
| η∞ | 57.688 | 83.806 | 91.906 | 95.432 | 98.690 | |
| λ | 784.61 | 968.68 | 641.72 | 666.37 | 787.3 | |
| n | 0.4753 | 0.4830 | 0.4960 | 0.4969 | 0.5096 | |
| r2 | 0.98 | 0.95 | 0.99 | 0.99 | 0.99 | |
| Cross | η0 | 2.00 × 108 | 2.14 × 108 | 2.78 × 108 | 2.68 × 108 | 7.87 × 108 |
| η∞ | 69.151 | 85.956 | 96.405 | 92.515 | 99.621 | |
| K1 | 1606.6 | 6396.6 | 1294.2 | 1176.9 | 1837.0 | |
| d | 0.9631 | 0.9727 | 0.9951 | 0.9978 | 1.0188 | |
| r2 | 0.96 | 0.94 | 0.99 | 0.99 | 0.98 | |
| G0 × 10−3 (Pa) | η0 × 10−4 (Pa·s) | G1 × 10−3 (Pa) | η1 × 10−4 (Pa·s) | r2 | |
|---|---|---|---|---|---|
| Gel/Alg/BSA | 5.62 ± 0.21 | 53.95 ± 2.22 | 6.52 ± 0.34 | 3.32 ± 0.49 | 0.986 |
| Gel/Alg/Fib | 4.64 ± 0.19 | 56.99 ± 2.81 | 5.97 ± 0.35 | 2.86 ± 0.47 | 0.980 |
| Gel/Alg/BSA/HAp | 9.29 ± 0.11 | 165.94 ± 5.32 | 12.21 ± 0.29 | 12.45 ± 6.95 | 0.997 |
| Gel/Alg/Fib/HAp | 7.75 ± 0.10 | 169.31 ± 8.17 | 12.01 ±0.40 | 10.51 ± 8.27 | 0.992 |
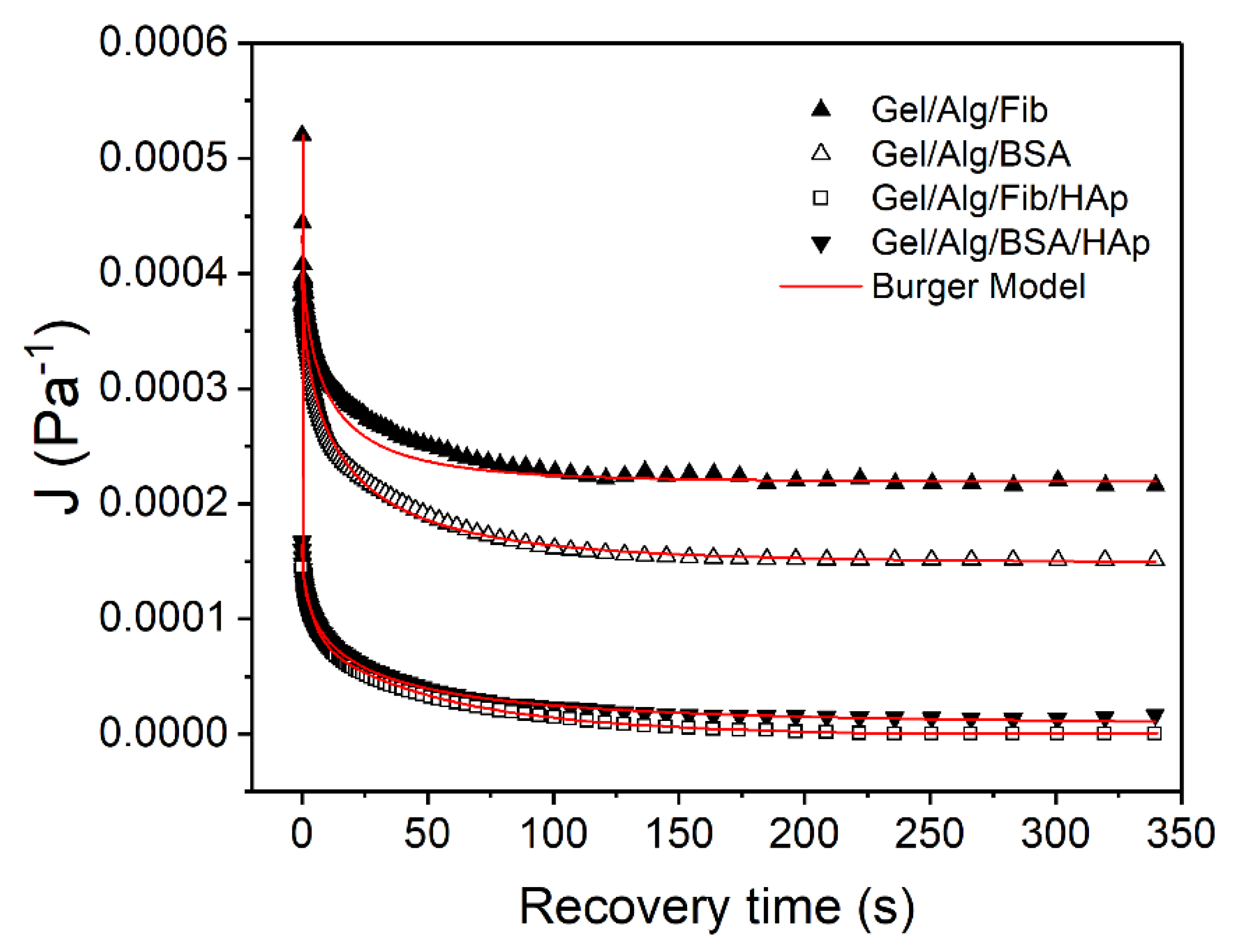
| J∞ × 105 (Pa−1) | JKV × 105 (Pa−1) | α (s−1) | β | r2 | |
|---|---|---|---|---|---|
| Gel/Alg/BSA | 14.88 ± 0.13 | 25.08± 0.34 | 0.21 ± 0.01 | 0.56 ± 0.01 | 0.997 |
| Gel/Alg/Fib | 21.92 ± 0.37 | 22.69 ± 1.35 | 0.31 ± 0.05 | 0.54 ± 0.05 | 0.953 |
| Gel/Alg/BSA/HAp | 0.79 ± 0.08 | 14.63 ± 0.16 | 0.25 ± 0.01 | 0.48 ± 0.01 | 0.999 |
| Gel/Alg/Fib/HAp | 0.87 ± 0.03 | 13.88 ± 0.49 | 0.16 ± 0.02 | 0.59 ± 0.03 | 0.989 |
| JMAX × 105 (Pa−1) | JSM × 105 (Pa−1) | |
|---|---|---|
| Gel/Alg/BSA | 56.965 ± 0.01 | 17.006 ± 0.51 |
| Gel/Alg/Fib | 66.094 ± 0.01 | 21.490 ± 1.71 |
| Gel/Alg/BSA/HAp | 28.726 ± 0.01 | 13.309 ± 0.23 |
| Gel/Alg/Fib/HAp | 30.624 ± 0.01 | 15.875 ± 0.61 |
| JSM (%) | JKV (%) | J∞ (%) | RG (%) | |
|---|---|---|---|---|
| Gel/Alg/BSA | 29.85 | 44.03 | 26.12 | 73.88 |
| Gel/Alg/Fib | 32.51 | 34.32 | 33.16 | 66.84 |
| Gel/Alg/BSA/HAp | 46.33 | 50.92 | 2.75 | 97.25 |
| Gel/Alg/Fib/HAp | 51.84 | 45.33 | 2.83 | 97.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rial, R.; Liu, Z.; Ruso, J.M. Soft Actuated Hybrid Hydrogel with Bioinspired Complexity to Control Mechanical Flexure Behavior for Tissue Engineering. Nanomaterials 2020, 10, 1302. https://doi.org/10.3390/nano10071302
Rial R, Liu Z, Ruso JM. Soft Actuated Hybrid Hydrogel with Bioinspired Complexity to Control Mechanical Flexure Behavior for Tissue Engineering. Nanomaterials. 2020; 10(7):1302. https://doi.org/10.3390/nano10071302
Chicago/Turabian StyleRial, Ramón, Zhen Liu, and Juan M. Ruso. 2020. "Soft Actuated Hybrid Hydrogel with Bioinspired Complexity to Control Mechanical Flexure Behavior for Tissue Engineering" Nanomaterials 10, no. 7: 1302. https://doi.org/10.3390/nano10071302
APA StyleRial, R., Liu, Z., & Ruso, J. M. (2020). Soft Actuated Hybrid Hydrogel with Bioinspired Complexity to Control Mechanical Flexure Behavior for Tissue Engineering. Nanomaterials, 10(7), 1302. https://doi.org/10.3390/nano10071302





