Nickel-Containing Ceria-Zirconia Doped with Ti and Nb. Effect of Support Composition and Preparation Method on Catalytic Activity in Methane Dry Reforming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Catalyst Characterization
2.2.1. N2 Adsorption
2.2.2. XRD
2.2.3. Raman
2.2.4. UV–Vis DR Spectroscopy
2.2.5. TEM
2.2.6. TPR
2.2.7. Metallic Surface Area Measurements by Hydrogen Adsorption
2.3. Catalytic Activity Measurement
3. Results and Discussion
3.1. Catalyst Characterization
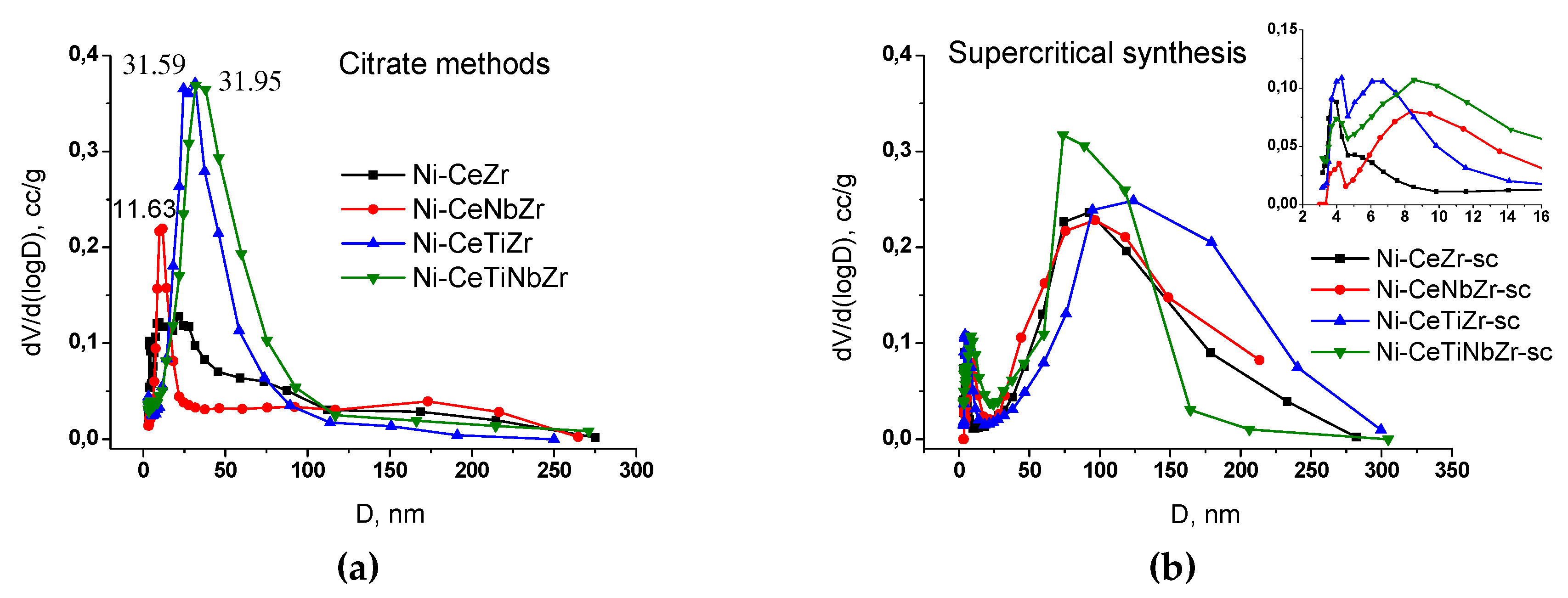
3.1.1. Study of Texture and Surface Area by N2 Adsorption
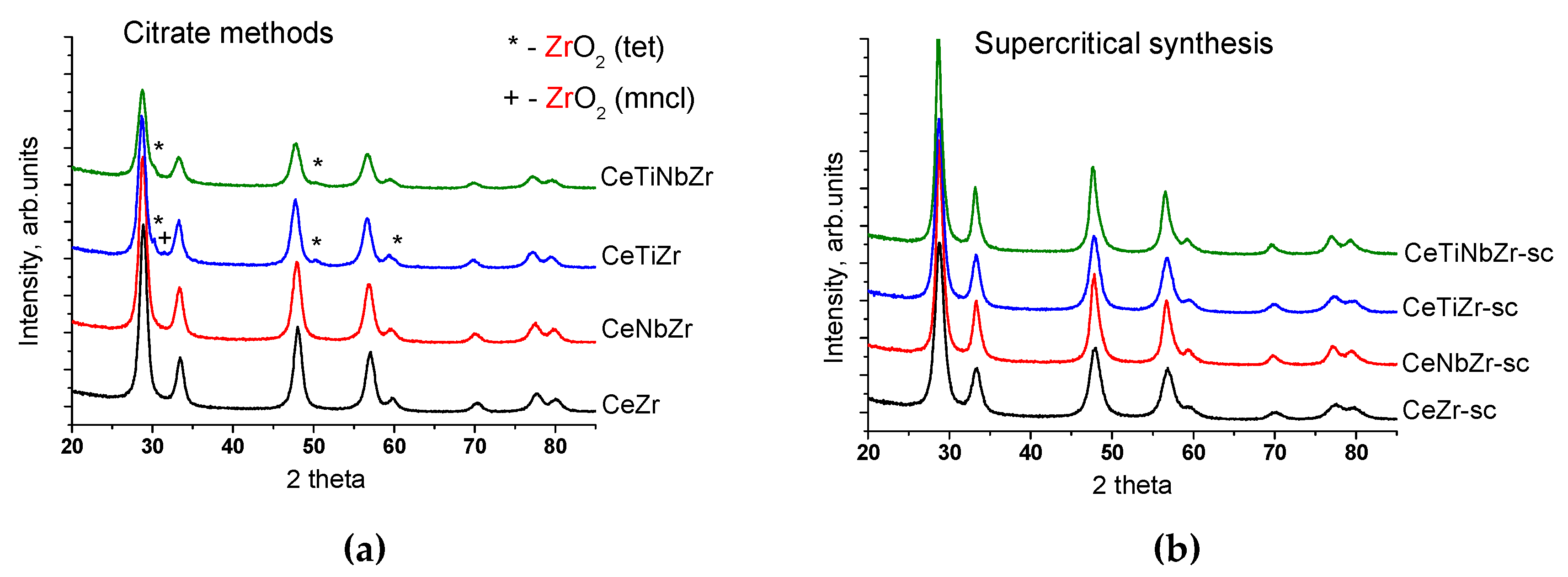
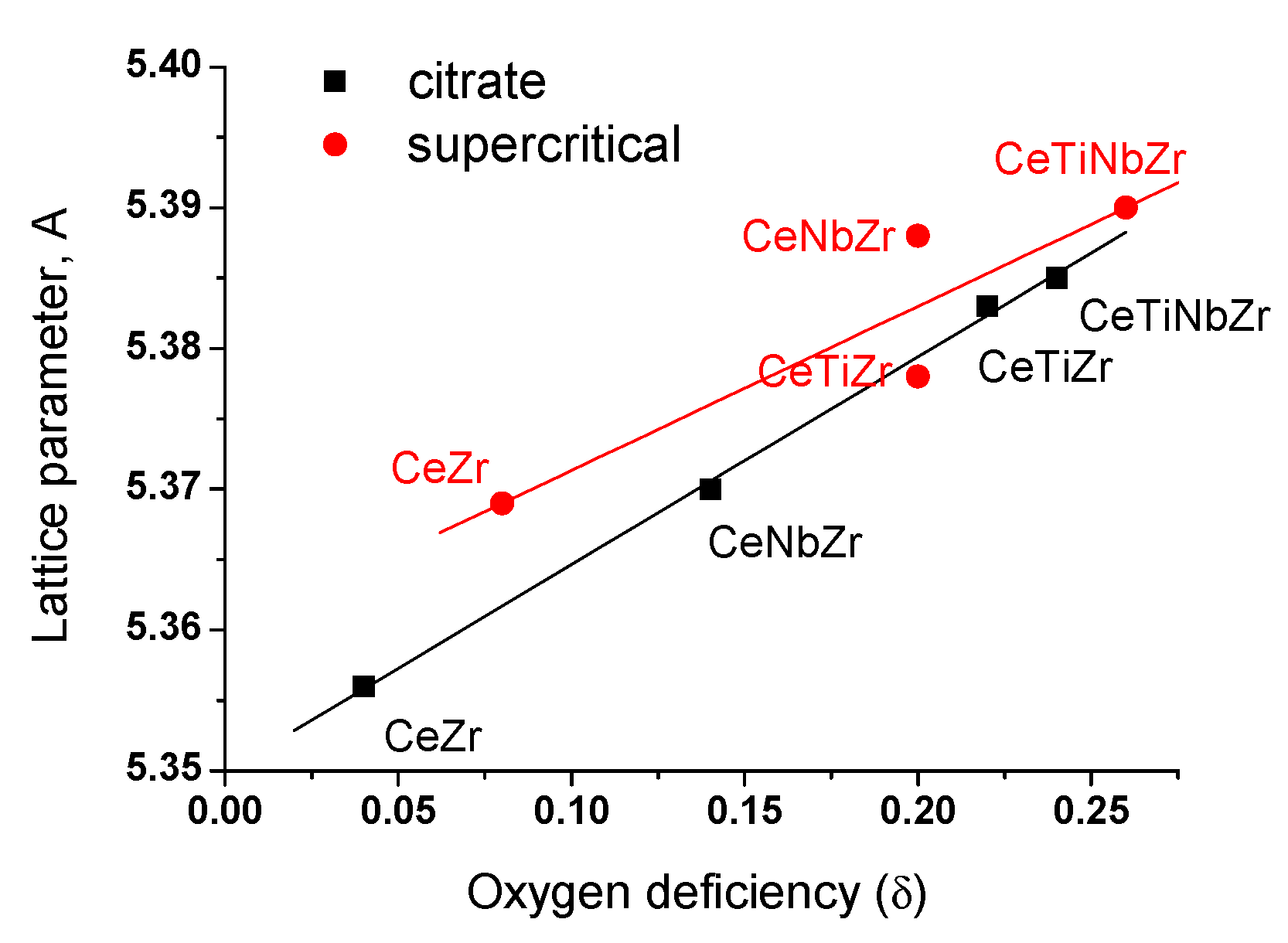
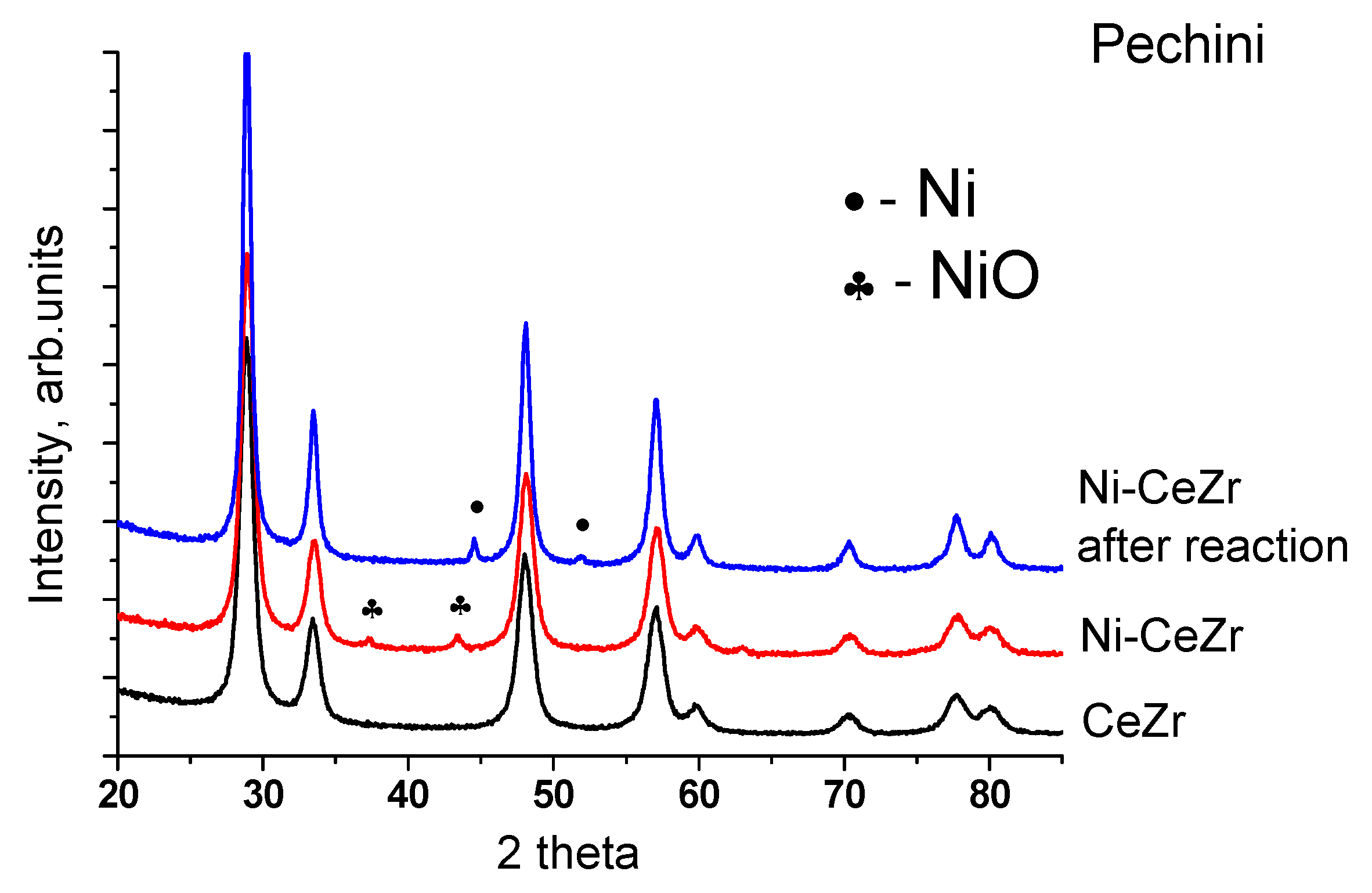
3.1.2. Structural Analysis by X-ray Diffraction
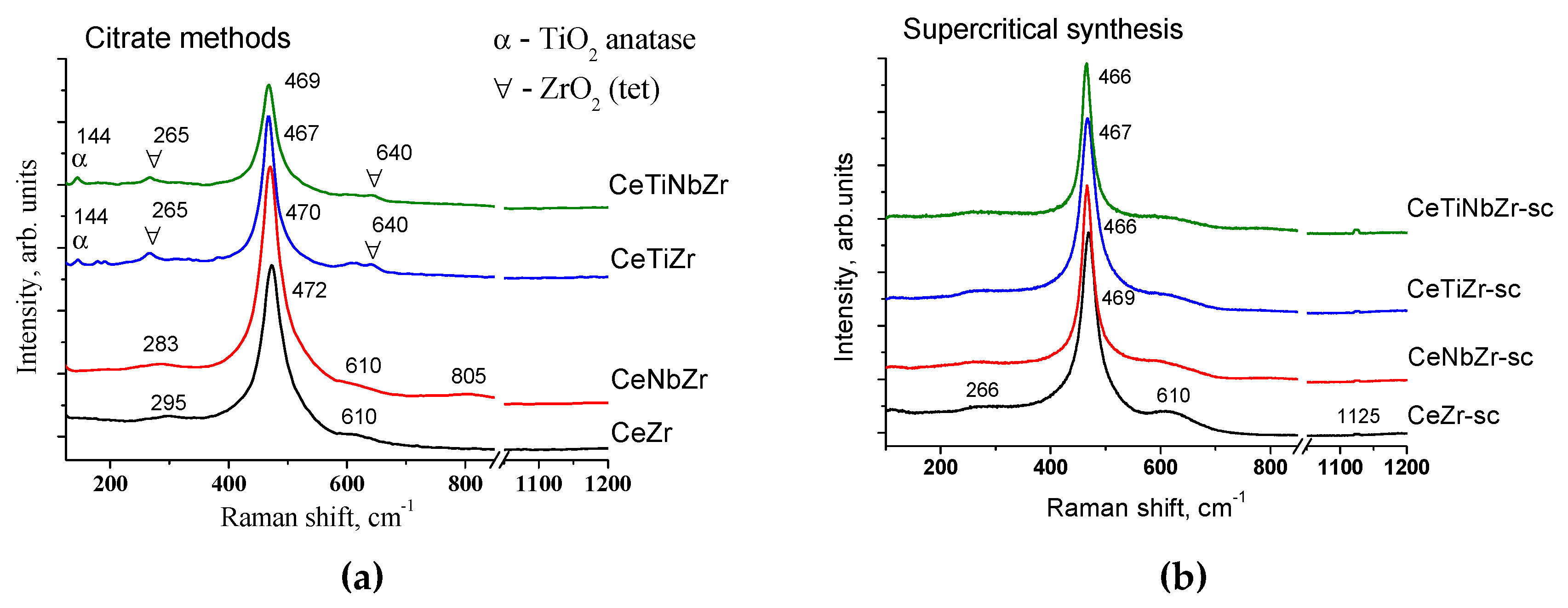
3.1.3. Structure Characterization by Raman Spectroscopy
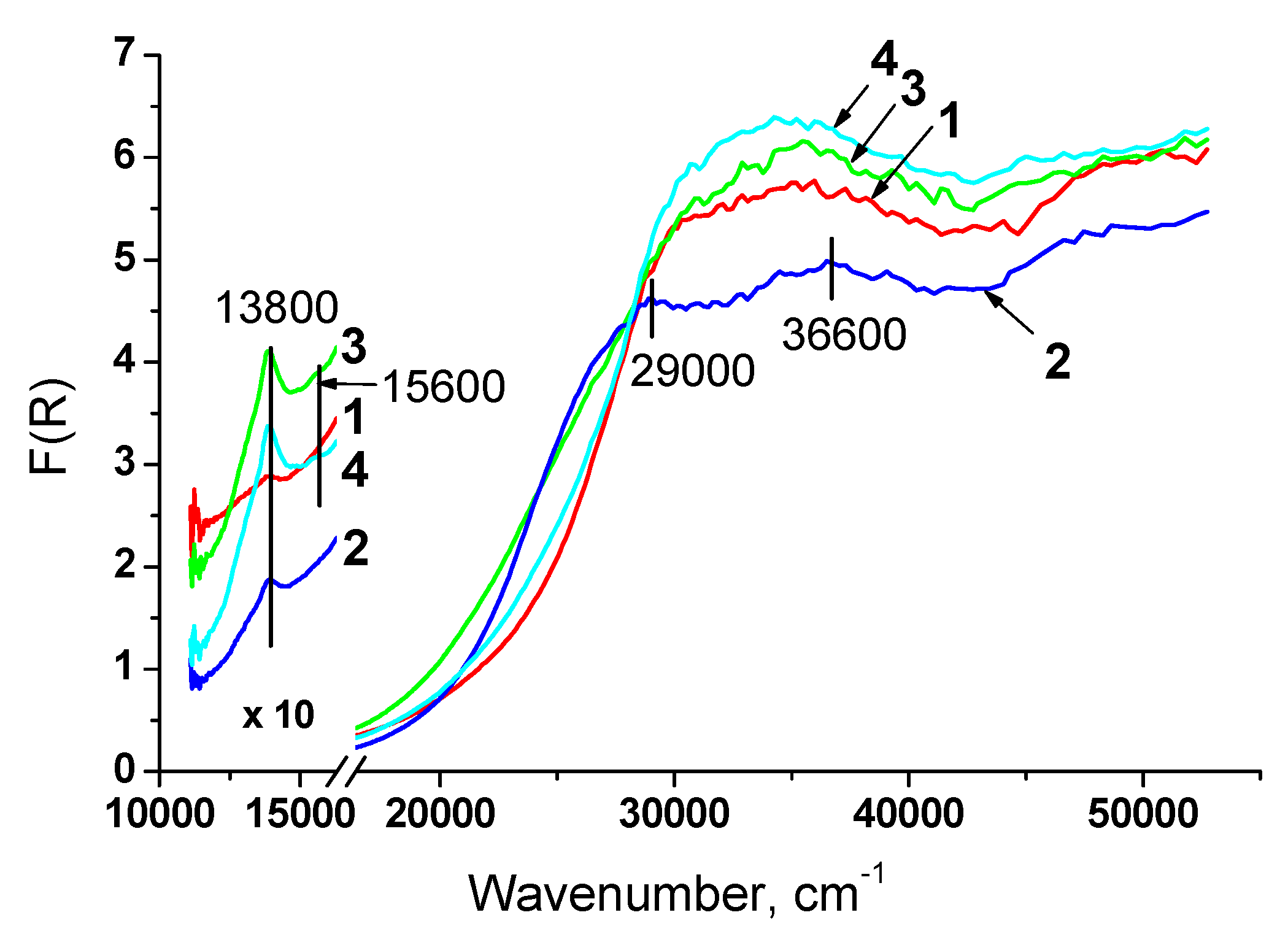
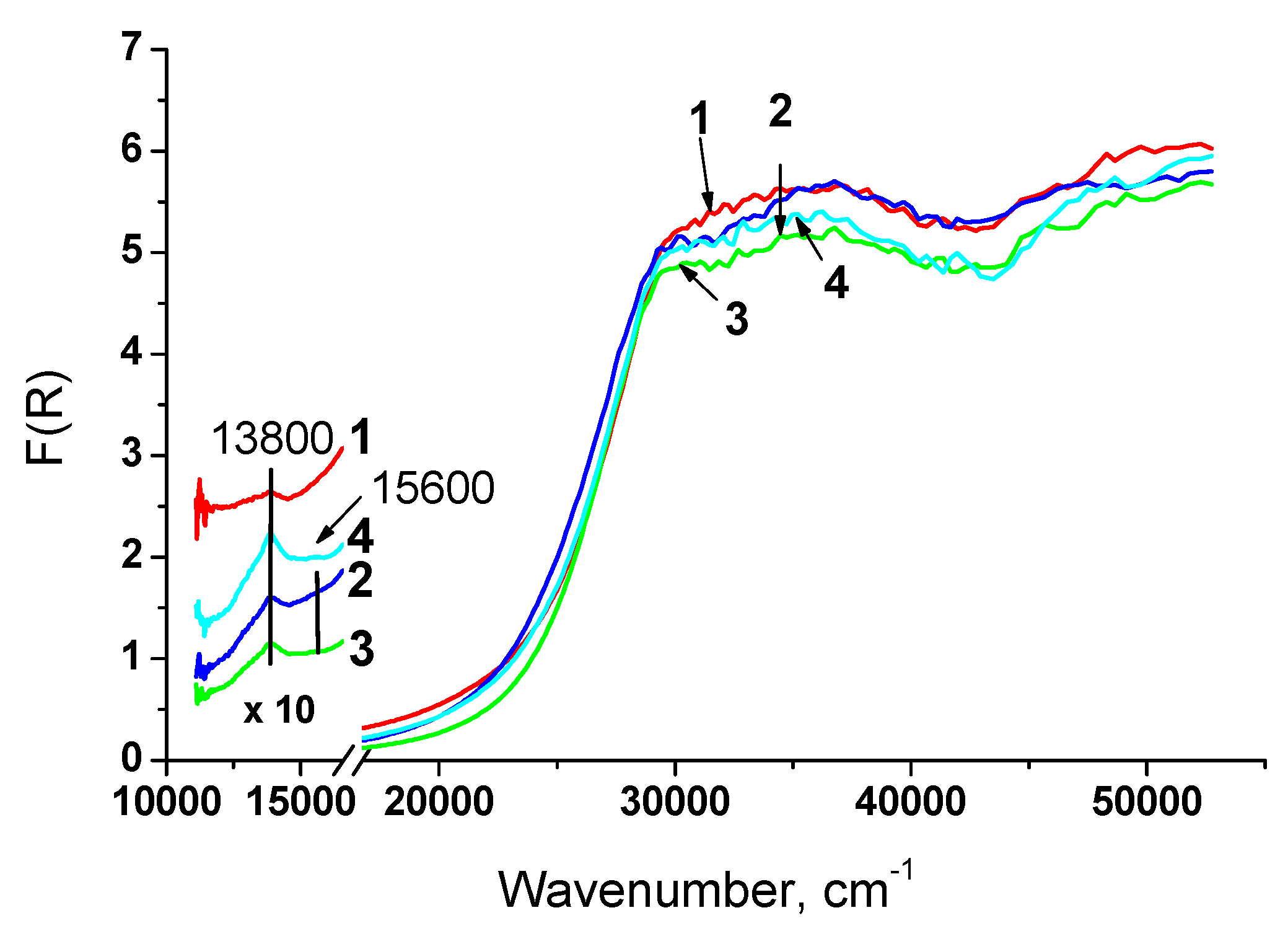
3.1.4. Structure characterization by UV-Vis DR spectroscopy
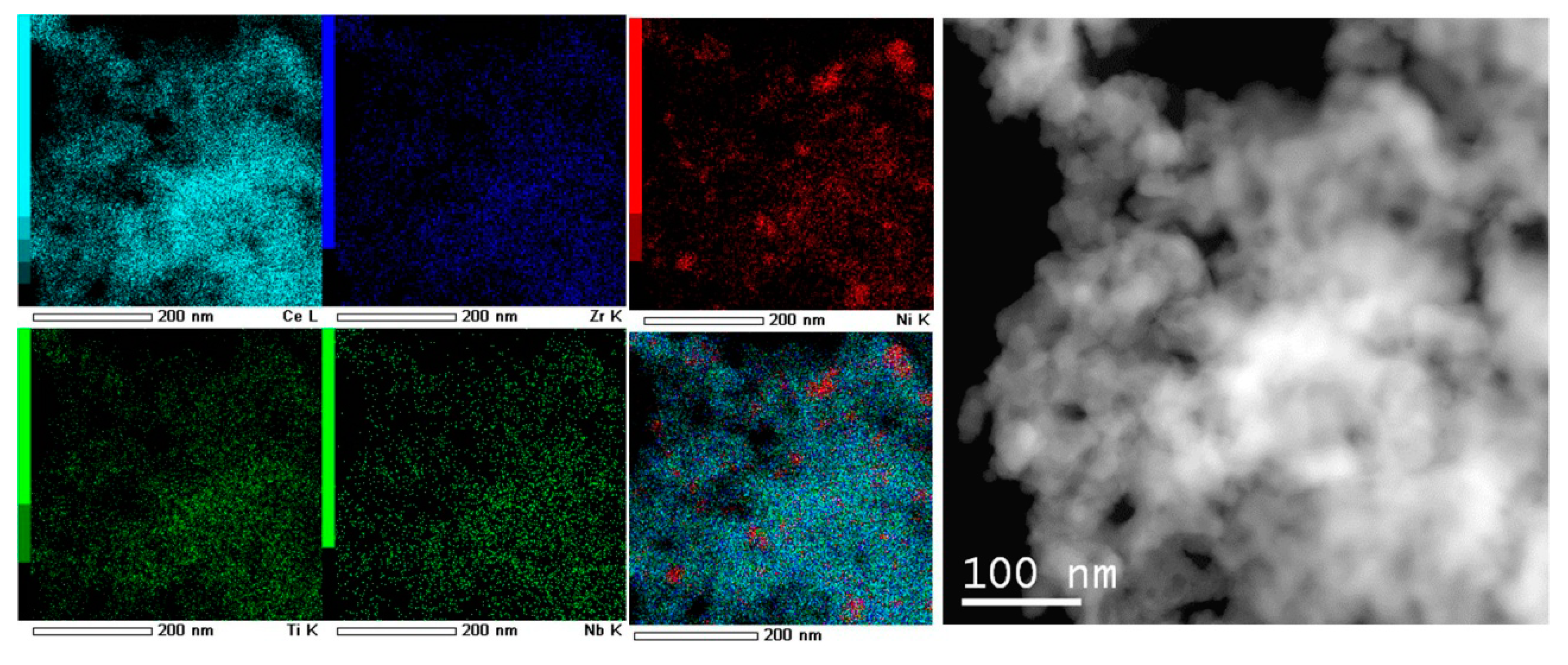
3.1.5. Morphology Characterization by TEM
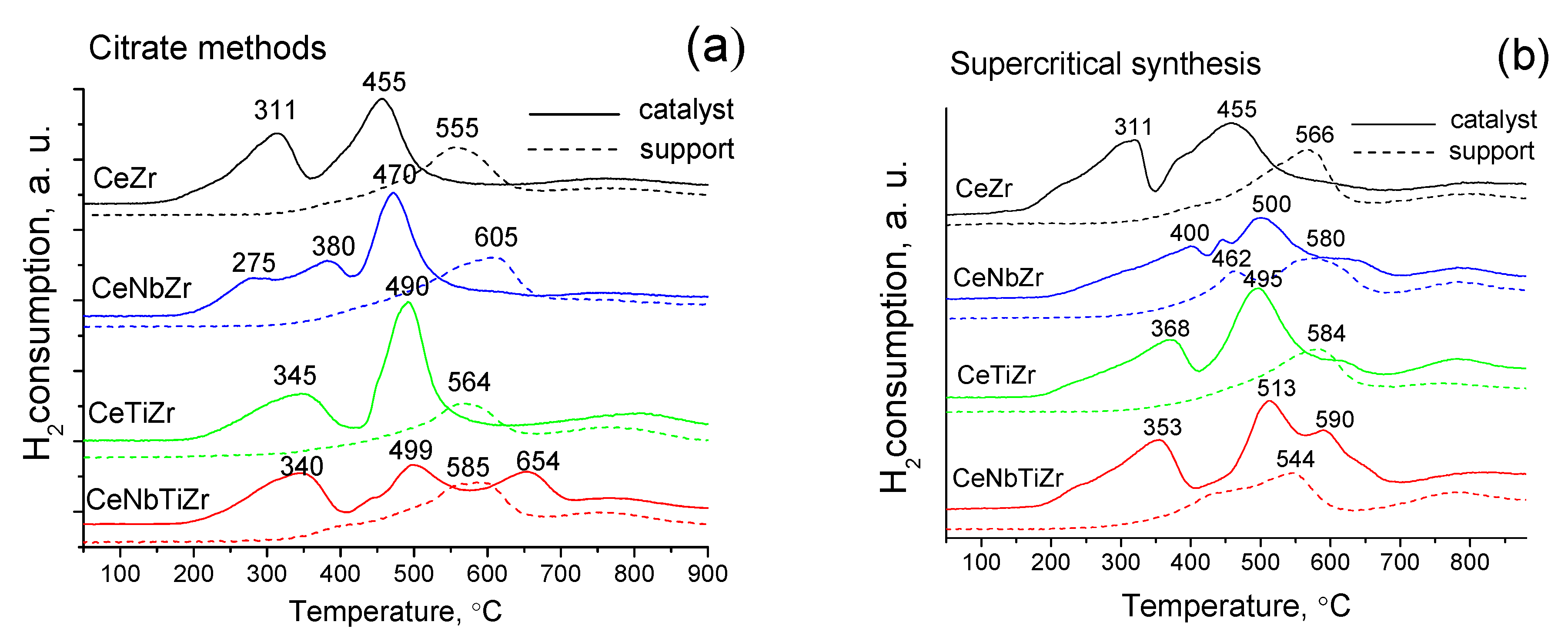
3.1.6. Study of Reducibility by H2 TPR
3.2. Catalytic Studies in Methane Dry Reforming
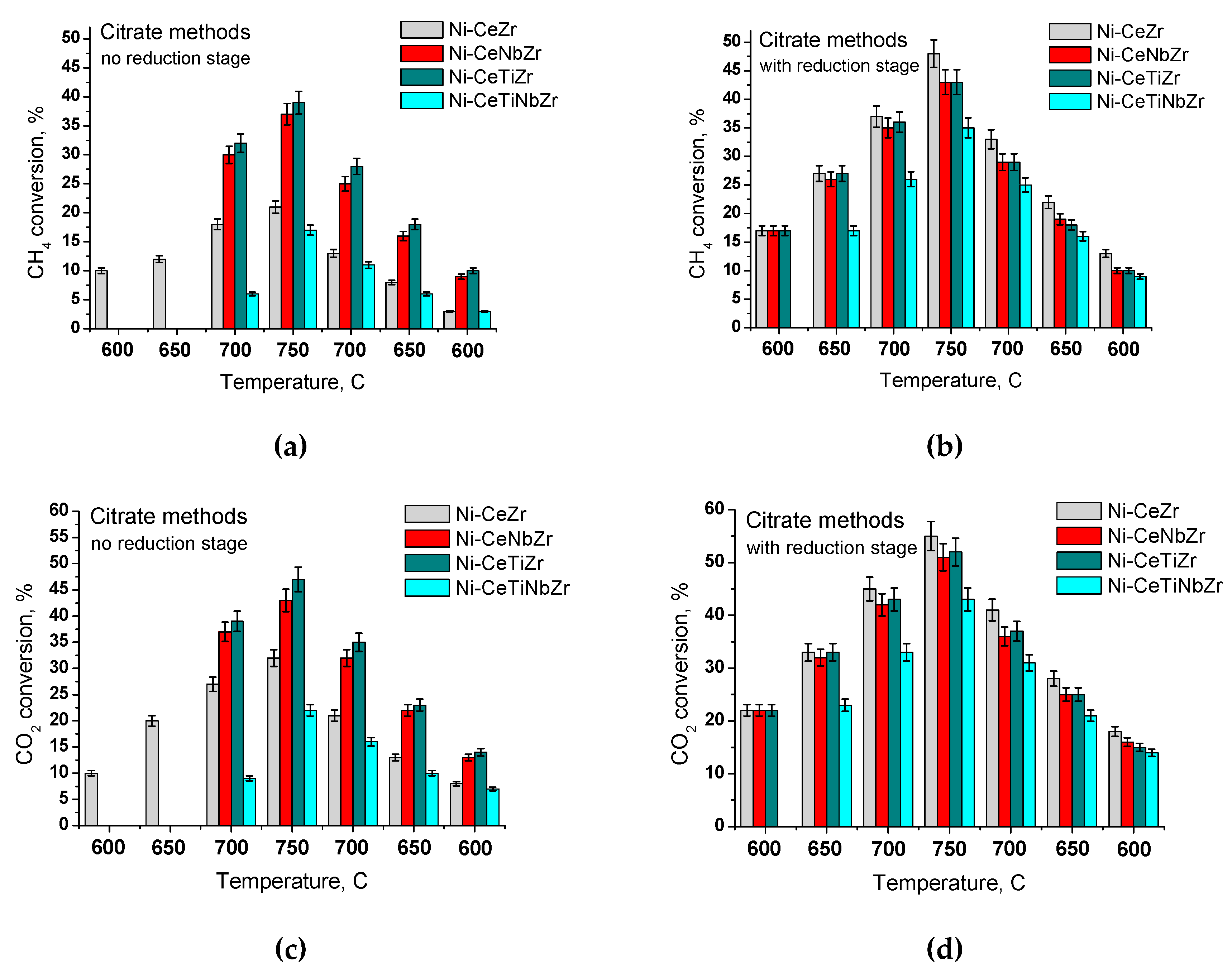
3.2.1. Effect of Temperature and Reducing Pretreatment of Citrate Samples
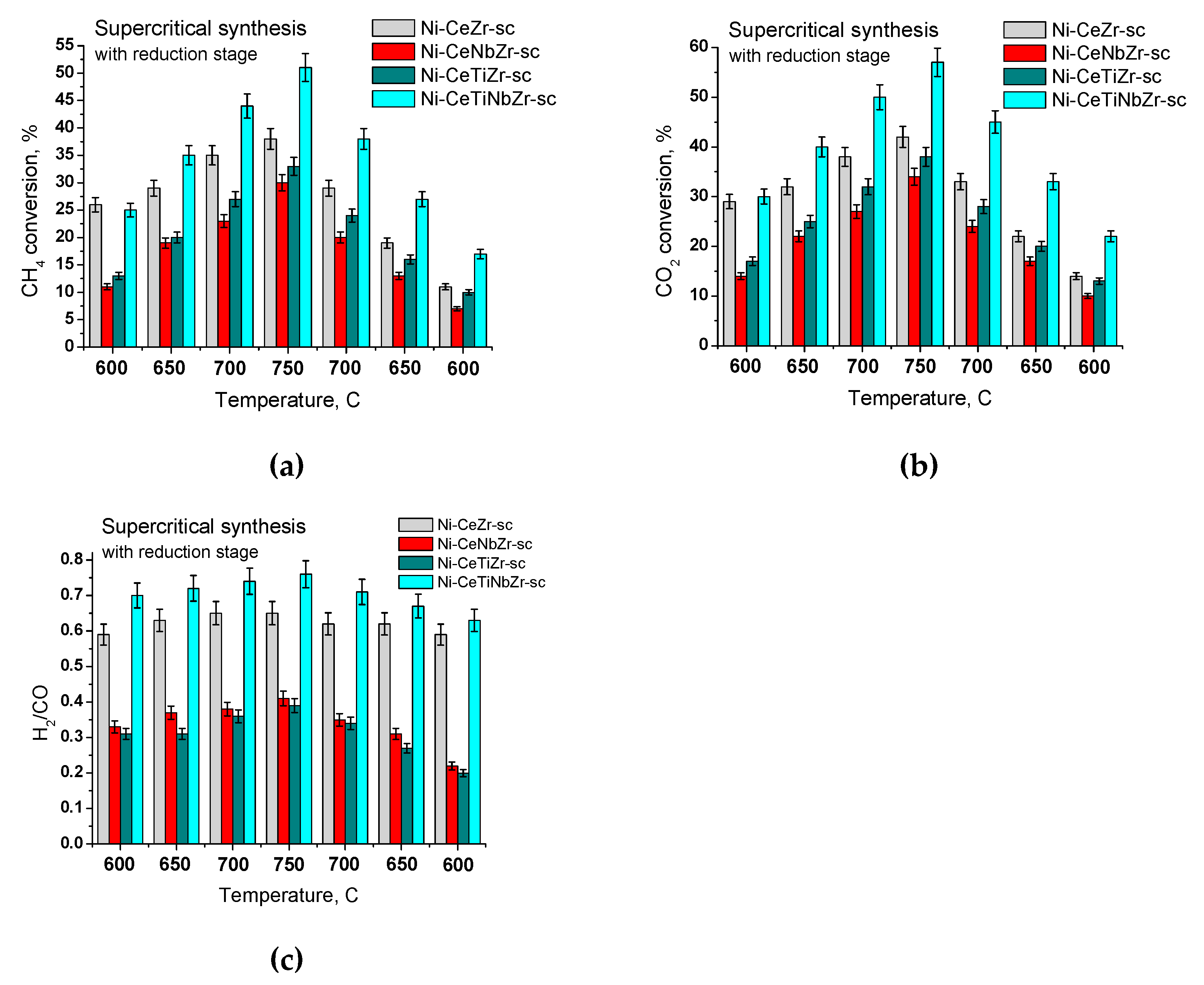
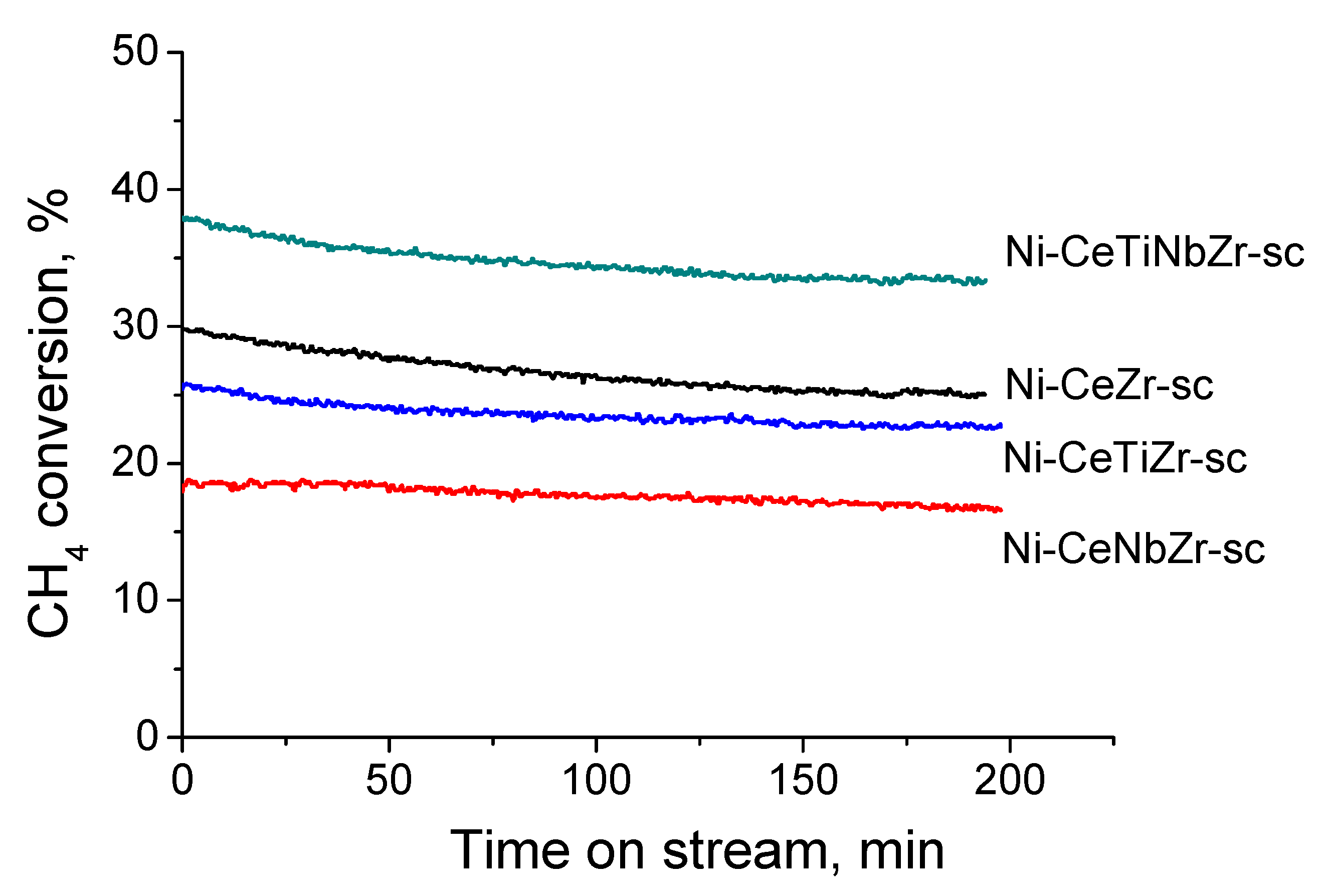
3.2.2. Samples Prepared in Supercritical Media
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, G.; Liu, J.; Xu, Y.; Sun, Y. A review of CH4–CO2 reforming to synthesis gas over Ni-based catalysts in recent years (2010–2017). Int. J. Hydrog. Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di, E.; Kopula, J.; Senthil, S.; Selvakumar, K. Dry reforming of methane over Ni supported on doped CeO2: New insight on the role of dopants for CO2 activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Takano, A.; Tagawa, T.; Goto, S. Carbon dioxide reforming of methane on supported nickel catalysts. J. Chem. Eng. Jpn. 1994, 27, 727–731. [Google Scholar] [CrossRef] [Green Version]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Li, P.; Chen, I.; Penner-hahn, J.E.; Tien, T.; Lewandowska, A.E.; Ban, M.A.; Wachs, I.E.; Jehng, J.; Hardcastle, F.D.; Ali, S.; et al. The Journal of Supercritical Fluids Relationship between size distribution of synthesized nanoparticles and flow and thermal fields in a flow-type reactor for supercritical hydrothermal synthesis. J. Supercrit. Fluids 2016, 109, 43–50. [Google Scholar]
- Trovarelli, A. Catalysis by Ceria and Related Materials; Hutchings, G.J., Ed.; Catalytic Science Series; Imperial College Press: London, UK, 2002; Volume 2. [Google Scholar]
- Makri, M.M.; Vasiliades, M.A.; Petallidou, K.C.; Efstathiou, A.M. Effect of support composition on the origin and reactivity of carbon formed during dry reforming of methane over 5 wt% Ni/Ce1-xMxO2-δ (M = Zr4+, Pr3+) catalysts. Catal. Today 2016, 259, 150–164. [Google Scholar] [CrossRef]
- Dutta, G.; Waghmare, U.V.; Baidya, T.; Hegde, M.S.; Priolkar, K.R.; Sarode, P.R. Reducibility of Ce1-xZrxO2: Origin of enhanced oxygen storage capacity. Catal. Lett. 2006, 108, 165–172. [Google Scholar] [CrossRef]
- Ozawa, M.; Kimura, M.; Isogai, A. The application of CeZr oxide solid solution to oxygen storage promoters in automotive catalysts. J. Alloys Compd. 1993, 193, 73–75. [Google Scholar] [CrossRef]
- Safavinia, B.; Wang, Y.; Jiang, C.; Roman, C.; Darapaneni, P.; Larriviere, J.; Cullen, D.A.; Dooley, K.M.; Dorman, J.A. Enhancing CexZr1−xO2 Activity for Methane Dry Reforming Using Subsurface Ni Dopants. ACS Catal. 2020, 10, 4070–4079. [Google Scholar] [CrossRef]
- Rao, G.R.; Kašpar, J.; Meriani, S.; di Monte, R.; Graziani, M. NO decomposition over partially reduced metallized CeO2-ZrO2 solid solutions. Catal. Lett. 1994, 24, 107–112. [Google Scholar] [CrossRef]
- Adschiri, T.; Kanazawa, K.; Arai, K. Rapid and Continuous Hydrothermal Crystallization of Metal Oxide Particles in Supercritical Water. J. Am. Ceram. Soc. 1992, 75, 1019–1022. [Google Scholar] [CrossRef]
- Liotta, L.F.; Pantaleo, G.; Macaluso, A.; Marcì, G.; Gialanella, S.; Deganello, G. Ceria-Zirconia Nanostructured Materials for Catalytic Applications: Textural Characteristics and Redox Properties. J. Sol-Gel. Sci. Technol. 2003, 28, 119–132. [Google Scholar] [CrossRef]
- Mamontov, E.; Egami, T.; Brezny, R.; Koranne, M.; Tyagi, S. Lattice defects and oxygen storage capacity of nanocrystalline ceria and ceria-zirconia. J. Phys. Chem. B 2000, 104, 11110–11116. [Google Scholar] [CrossRef]
- Darr, J.A.; Zhang, J.; Makwana, N.M.; Weng, X. Continuous Hydrothermal Synthesis of Inorganic Nanoparticles: Applications and Future Directions. Chem. Rev. 2017, 117, 11125–11238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.R.; Lee, K.Y.; Suh, M.J.; Ihm, S.K. Ceria-zirconia mixed oxide prepared by continuous hydrothermal synthesis in supercritical water as catalyst support. Catal. Today 2012, 185, 25–34. [Google Scholar] [CrossRef]
- Slostowski, C.; Marre, S.; Babot, O.; Toupance, T.; Aymonier, C. Near- and supercritical alcohols as solvents and surface modifiers for the continuous synthesis of cerium oxide nanoparticles. Langmuir 2012, 28, 16656–16663. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, E.K.C.; Habu, T.; Tooriyama, H.; Ohtani, M.; Kobiro, K. Ultra-simple synthetic approach to the fabrication of CeO2-ZrO2 mixed nanoparticles into homogeneous, domain, and core-shell structures in mesoporous spherical morphologies using supercritical alcohols. J. Supercrit. Fluids 2015, 97, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Laosiripojana, N.; Assabumrungrat, S. Methane steam reforming over Ni/Ce-ZrO2 catalyst: Influences of Ce-ZrO2 support on reactivity, resistance toward carbon formation, and intrinsic reaction kinetics. Appl. Catal. A Gen. 2005, 290, 200–211. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Kuznetsova, T.G.; Veniaminov, S.A.; Kochubey, D.I.; Novgorodov, B.N.; Burgina, E.B.; Moroz, E.M.; Paukshtis, E.A.; Ivanov, V.P.; Trukhan, S.N.; et al. Cation/anion modified ceria-zirconia solid solutions promoted by Pt as catalysts of methane oxidation into syngas by water in reversible redox cycles. React. Kinet. Catal. Lett. 2002, 76, 83–92. [Google Scholar] [CrossRef]
- Velu, S.; Gangwal, S.K. Synthesis of alumina supported nickel nanoparticle catalysts and evaluation of nickel metal dispersions by temperature programmed desorption. Solid State Ionics 2006, 177, 803–811. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies ofinteratomic distances in halides and chalcogenides. Acta. Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, X.; Si, Z.; Weng, D.; Ma, J.; Xu, T. Impacts of niobia loading on active sites and surface acidity in NbOx/CeO2–ZrO2 NH3–SCR catalysts. Appl. Catal. B Environ. 2015, 179, 380–394. [Google Scholar] [CrossRef] [Green Version]
- Dutta, G.; Waghmare, U.V.; Baidya, T.; Hegde, M.S.; Priolkar, K.R.; Sarode, P.R. Origin of Enhanced Reducibility/Oxygen Storage Capacity of Ce1-xTixO2 Compared to CeO2 or TiO2. Chem. Mater. 2006, 18, 3249–3256. [Google Scholar] [CrossRef]
- Lu, F.; Jiang, B.; Wang, J.; Huang, Z.; Liao, Z.; Yang, Y.; Zheng, J. Promotional effect of Ti doping on the ketonization of acetic acid over a CeO2 catalyst. RSC Adv. 2017, 7, 22017–22026. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, D.; Wei, S.; Zhang, Y. Determination of active site densities and mechanisms for soot combustion with O2 on Fe-doped CeO2 mixed oxides. J. Catal. 2010, 276, 16–23. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Ce3+ and oxygen vacancy mediated tuning of structural and optical properties of CeO2 nanoparticles. Mater. Chem. Phys. 2012, 131, 666–671. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, Q.; Jiang, Z. Nanosized CuO–ZrxCe1−xOy aerogel catalysts prepared by ethanol supercritical drying for catalytic deep oxidation of benzene. Powder Technol. 2009, 194, 109–114. [Google Scholar] [CrossRef]
- McBride, J.R.; Hass, K.C.; Poindexter, B.D.; Weber, W.H. Raman and xray studies of Ce1−xRExO2−y, where RE=La, Pr, Nd, Eu, Gd, and Tb. J. Appl. Phys. 1994, 76, 2435–2441. [Google Scholar] [CrossRef]
- Kim, D.-J.; Jung, H.-J. Raman Spectroscopy of Tetragonal Zirconia Solid Solutions. J. Am. Ceram. Soc. 1993, 76, 2106–2108. [Google Scholar] [CrossRef]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Ma, W.; Lu, Z.; Zhang, M. Investigation of structural transformations in nanophase titanium dioxide by Raman spectroscopy. Appl. Phys. A 1998, 66, 621–627. [Google Scholar] [CrossRef]
- Nowak, I.; Ziolek, M. Niobium Compounds: Preparation, Characterization, and Application in Heterogeneous Catalysis. Chem. Rev. 1999, 99, 3603–3624. [Google Scholar] [CrossRef] [PubMed]
- Becerra, A.M.; Castro-Luna, A.E. An investigation on the presence of NiAl2O4 in a stable Ni on alumina catalyst for dry reforming. J. Chil. Chem. Soc. 2005, 50, 465–469. [Google Scholar] [CrossRef]
- Scheffer, B.; Heijeinga, J.J.; Moulijn, J.A. An Electron Spectroscopy and X-ray Dlffraction Study of NiO/Al2O3 and NiO-WO3/Al2O3 Catalysts. J. Phys. Chem. 1987, 91, 4752–4759. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Kuznetsova, T.G.; Alikina, G.M.; Frolova, Y.V.; Lukashevich, A.I.; Muzykantov, V.S.; Rogov, V.A.; Batuev, L.C.; Kriventsov, V.V.; Kochubei, D.I.; et al. Ceria-based fluorite-like oxide solid solutions promoted by precious metals as catalysts of methane transformation into syngas. In New Topics in Catalysis Research; McReynolds, D.K., Ed.; Nova Science Publishers: New York, NY, USA, 2007; pp. 97–197. [Google Scholar]
- Phoka, S.; Laokul, P.; Swatsitang, E.; Promarak, V.; Seraphin, S.; Maensiria, S. Synthesis, structural and optical properties of CeO2 nanoparticles synthesized by a simple polyvinyl pyrrolidone (PVP) solution route. Mater. Chem. Phys. 2009, 115, 423–428. [Google Scholar] [CrossRef]
- Patsalas, P.; Logothetidis, S.; Sygellou, L.; Kennou, S. Structure-dependent electronic properties of nanocrystalline cerium oxide films. Phys. Rev. B Condens. Matter. 2003, 68, 035104. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Kalathil, S.; Lee, J.; Cho, M.H. Band Gap Engineering of CeO2 Nanostructure by Electrochemically Active Biofilm for Visible Light Applications. RSC Adv. 2014, 4, 16782–16791. [Google Scholar] [CrossRef]
- Danilova, I.G.; Slavinskaya, E.M.; Zaikovskii, V.I.; Ivanova, A.S.; Boronin, A.I.; Gulyaev, R.V.; Amosov, Y.I. Effect of Preparation Procedure on the Properties of CeO2. Kinet. Catal. 2010, 51, 143–148. [Google Scholar] [CrossRef]
- Bensalem, A.; Muller, J.C.; BozonVerduraz, F. Faraday communications. From bulk CeO2 to supported cerium–oxygen clusters: A diffuse reflectance approach. J. Chem. Soc. Faraday Trans. 1992, 85, 153–154. [Google Scholar] [CrossRef]
- Rao, G.R.; Sahu, H.R. XRD and UV-Vis diffuse reflectance analysis of CeO2–ZrO2 solid solutions synthesized by combustion method. Proc. Indian Acad. Sci. (Chem. Sci.) 2001, 113, 651–658. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Smirnova, M.Y.; Pavlova, S.N.; Krieger, T.A.; Bespalko, Y.N.; Anikeev, V.I.; Chesalov, Y.A.; Kaichev, V.V.; Mezentseva, N.V.; Sadykov, V.A. The Synthesis of Ce1–xZrxO2 Oxides in Supercritical Alcohols and Catalysts for Carbon Dioxide Reforming of Methane on Their Basis. Russ. J. Phys. Chem. B 2017, 11, 1312–1321. [Google Scholar] [CrossRef]
- Slostowski, C.; Marre, S.; Babot, O.; Toupance, T.; Aymonier, C. Effect of Thermal Treatment on the Textural Properties of CeO2 Powders Synthesized in Near- and Supercritical Alcohols. ChemPhysChem 2015, 16, 3493–3499. [Google Scholar] [CrossRef]
- Auxéméry, A.; Frias, B.B.; Smal, E.; Dziadek, K.; Philippot, G.; Legutko, P.; Simonov, M.; Thomas, S.; Adamski, A.; Sadykov, V.; et al. Continuous supercritical solvothermal preparation of nanostructured ceria-zirconia as supports for dry methane reforming catalysts. J. Supercrit. Fluids 2020, 162, 104855. [Google Scholar] [CrossRef]
- López, J.M.; Gilbank, A.L.; García, T.; Solsona, B.; Agouram, S.; Torrente-Murciano, L. The prevalence of surface oxygen vacancies over the mobility of bulk oxygen in nanostructured ceria for the total toluene oxidation. Appl. Catal. B Environ. 2015, 174–175, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Bruce, L.A.; Hoang, M.; Hughes, A.E.; Turney, W.T. Surface area control during the synthesis and reduction of high area ceria catalyst supports. Appl. Catal. A Gen. 1996, 134, 351–362. [Google Scholar] [CrossRef]
- Kim, J.R.; Myeong, W.J.; Ihm, S.K. Characteristics in oxygen storage capacity of ceria-zirconia mixed oxides prepared by continuous hydrothermal synthesis in supercritical water. Appl. Catal. B Environ. 2007, 71, 57–63. [Google Scholar] [CrossRef]
- Montoya, J.A.; Romero-Pascual, E.; Gimon, C.; Del Angel, P.; Monzón, A. Methane reforming with CO2 over Ni/ZrO2-CeO2 catalysts prepared by sol-gel. Catal. Today 2000, 63, 71–85. [Google Scholar] [CrossRef]
- Romero-Núñez, A.; Díaz, G. High oxygen storage capacity and enhanced catalytic performance of NiO/NixCe1-xO2-δ nanorods: Synergy between Ni-doping and 1D morphology. RSC Adv. 2015, 5, 54571–54579. [Google Scholar] [CrossRef]


| № | Composition | Synthesis Method | |
|---|---|---|---|
| Citrate | Supercritical | ||
| 1 | 5%Ni/Ce0.75Zr0.25O2 | Ni-CeZr | Ni-CeZr-sc |
| 2 | 5%Ni/Ce0.75Nb0.1Zr0.15O2 | Ni-CeNbZr | Ni-CeNbZr-sc |
| 3 | 5%Ni/Ce0.75Ti0.1Zr0.15O2 | Ni-CeTiZr | Ni-CeTiZr-sc |
| 4 | 5%Ni/Ce0.75Ti0.05Nb0.05Zr0.15O2 | Ni-CeTiNbZr | Ni-CeTiNbZr-sc |
| Sample | Citrate | Supercritical | |||
|---|---|---|---|---|---|
| Oxide | Ni/Oxide | Ni/oxide after DRM | Oxide | Ni/Oxide | |
| SBET, m2 g−1 | |||||
| CeZr | 45 | 35 | 22 | 29 | 21 |
| CeNbZr | 36 | 29 | 28 | 19 | 17 |
| CeTiZr | 30 | 26 | 36 | 28 | 23 |
| CeTiNbZr | 37 | 28 | 30 | 22 | 26 |
| Vtotal, cm3 g−1 | |||||
| CeZr | 0.159 | 0.143 | 0.150 | 0.150 | 0.144 |
| CeNbZr | 0.135 | 0.112 | 0.131 | 0.186 | 0.190 |
| CeTiZr | 0.177 | 0.187 | 0.190 | 0.165 | 0.184 |
| CeTiNbZr | 0.213 | 0.208 | 0.240 | 0.217 | 0.184 |
| Sample | Parameter Cell of Fluorite Phase, Å | Crystallite Size, nm | ||||||
|---|---|---|---|---|---|---|---|---|
| Citrate | Supercritical | Citrate | Supercritical | |||||
| Oxide | Ni/Oxide | Oxide | Ni/Oxide | Oxide | Ni/Oxide | Oxide | Ni/Oxide | |
| CeZr | 5.356 | 5.357 | 5.369 | 5.368 | 10.4 | 10.7 | 8.8 | 9.4 |
| CeNbZr | 5.370 | 5.369 | 5.388 | 5.392 | 10.3 | 10.8 | 12.0 | 14 |
| CeTiZr | 5.385 | 5.389 | 5.378 | 5.379 | 10.3 | 10.8 | 10.0 | 10.4 |
| CeTiNbZr | 5.383 | 5.384 | 5.390 | 5.392 | 9.5 | 10.7 | 13.5 | 14.5 |
| Ni-CeZr | Ni-CeTiZr | Ni-CeNbZr | Ni-CeTiNbZr | |
|---|---|---|---|---|
| citrate route | 3.20 ± 0.02 | 3.10 ± 0.02 | 2.84 ± 0.02 | 3.22 ± 0.02 |
| supercritical synthesis | 3.25 ± 0.02 | 3.21 ± 0.02 | 3.17 ± 0.02 | 3.22 ± 0.02 |
| Sample | H2 Consumption, mmol H2 g−1cat | |||
|---|---|---|---|---|
| Citrate | Supercritical | |||
| Oxide | Ni/Oxide | Oxide | Ni/Oxide | |
| CeZr | 1.50 | 2.18 | 1.36 | 2.21 |
| CeNbZr | 1.59 | 2.18 | 1.39 | 1.84 |
| CeTiZr | 1.33 | 1.98 | 1.20 | 2.32 |
| CeTiNbZr | 1.48 | 1.90 | 1.30 | 2.06 |
| Sample | NNi, mol g−1cat | keff, s−1 | W0, M−1 s−1 | TOF, s−1 |
|---|---|---|---|---|
| Ni-CeZr-sc | 7.09 × 10−5 | 45.67 | 0.299 | 3.7 |
| Ni-CeTiZr-sc | 4.98 × 10−5 | 38.36 | 0.245 | 7.6 |
| Ni-CeNbZr-sc | 3.37 × 10−5 | 26.46 | 0.166 | 8.2 |
| Ni-CeTiNbZr-sc | 3.39 × 10−5 | 63.74 | 0.407 | 9.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonov, M.; Bespalko, Y.; Smal, E.; Valeev, K.; Fedorova, V.; Krieger, T.; Sadykov, V. Nickel-Containing Ceria-Zirconia Doped with Ti and Nb. Effect of Support Composition and Preparation Method on Catalytic Activity in Methane Dry Reforming. Nanomaterials 2020, 10, 1281. https://doi.org/10.3390/nano10071281
Simonov M, Bespalko Y, Smal E, Valeev K, Fedorova V, Krieger T, Sadykov V. Nickel-Containing Ceria-Zirconia Doped with Ti and Nb. Effect of Support Composition and Preparation Method on Catalytic Activity in Methane Dry Reforming. Nanomaterials. 2020; 10(7):1281. https://doi.org/10.3390/nano10071281
Chicago/Turabian StyleSimonov, Mikhail, Yulia Bespalko, Ekaterina Smal, Konstantin Valeev, Valeria Fedorova, Tamara Krieger, and Vladislav Sadykov. 2020. "Nickel-Containing Ceria-Zirconia Doped with Ti and Nb. Effect of Support Composition and Preparation Method on Catalytic Activity in Methane Dry Reforming" Nanomaterials 10, no. 7: 1281. https://doi.org/10.3390/nano10071281
APA StyleSimonov, M., Bespalko, Y., Smal, E., Valeev, K., Fedorova, V., Krieger, T., & Sadykov, V. (2020). Nickel-Containing Ceria-Zirconia Doped with Ti and Nb. Effect of Support Composition and Preparation Method on Catalytic Activity in Methane Dry Reforming. Nanomaterials, 10(7), 1281. https://doi.org/10.3390/nano10071281







