RETRACTED: Manufacturing of Conductive, Wear-Resistant Nanoreinforced Cu-Ti Alloys Using Partially Oxidized Electrolytic Copper Powder
Abstract
1. Introduction
2. Materials and Methods
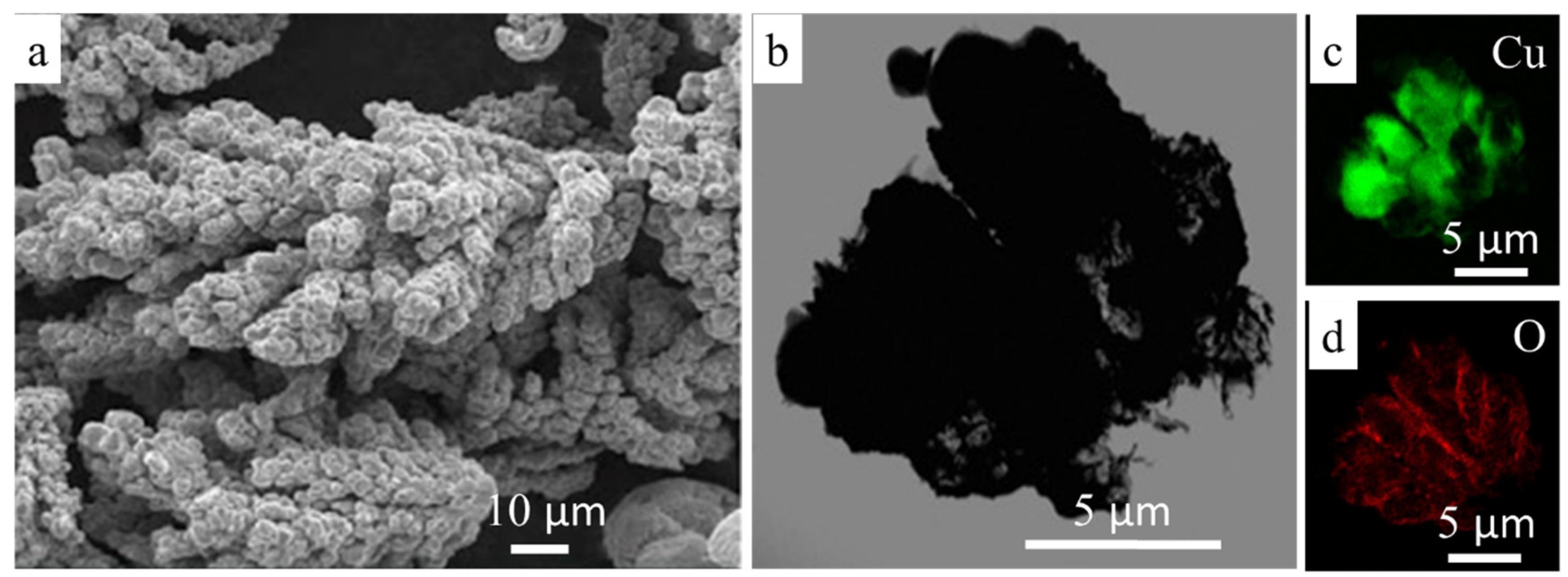
2.1. Preparation of Reactive Composite Powders
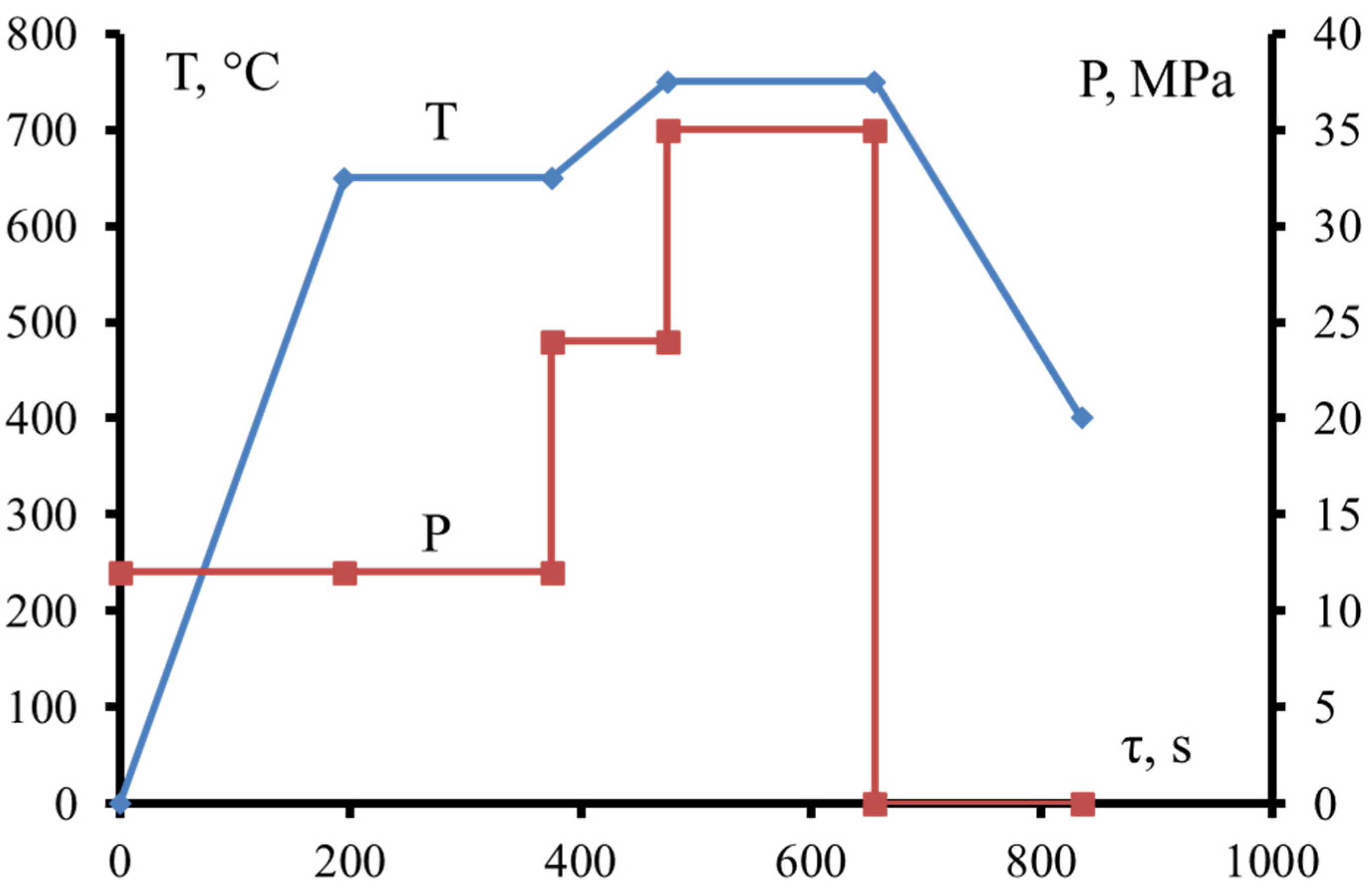
2.2. Hot Pressing of Reactive Composites
2.3. Tensile and Bending Testing
2.4. Measurements of Hardness and Relative Density
2.5. Microstructural and XRD Investigations
2.6. Tribological Testing
2.7. Measurements of Electrical Conductivity
3. Results
3.1. Single-Stage and Two-Stage HEBM
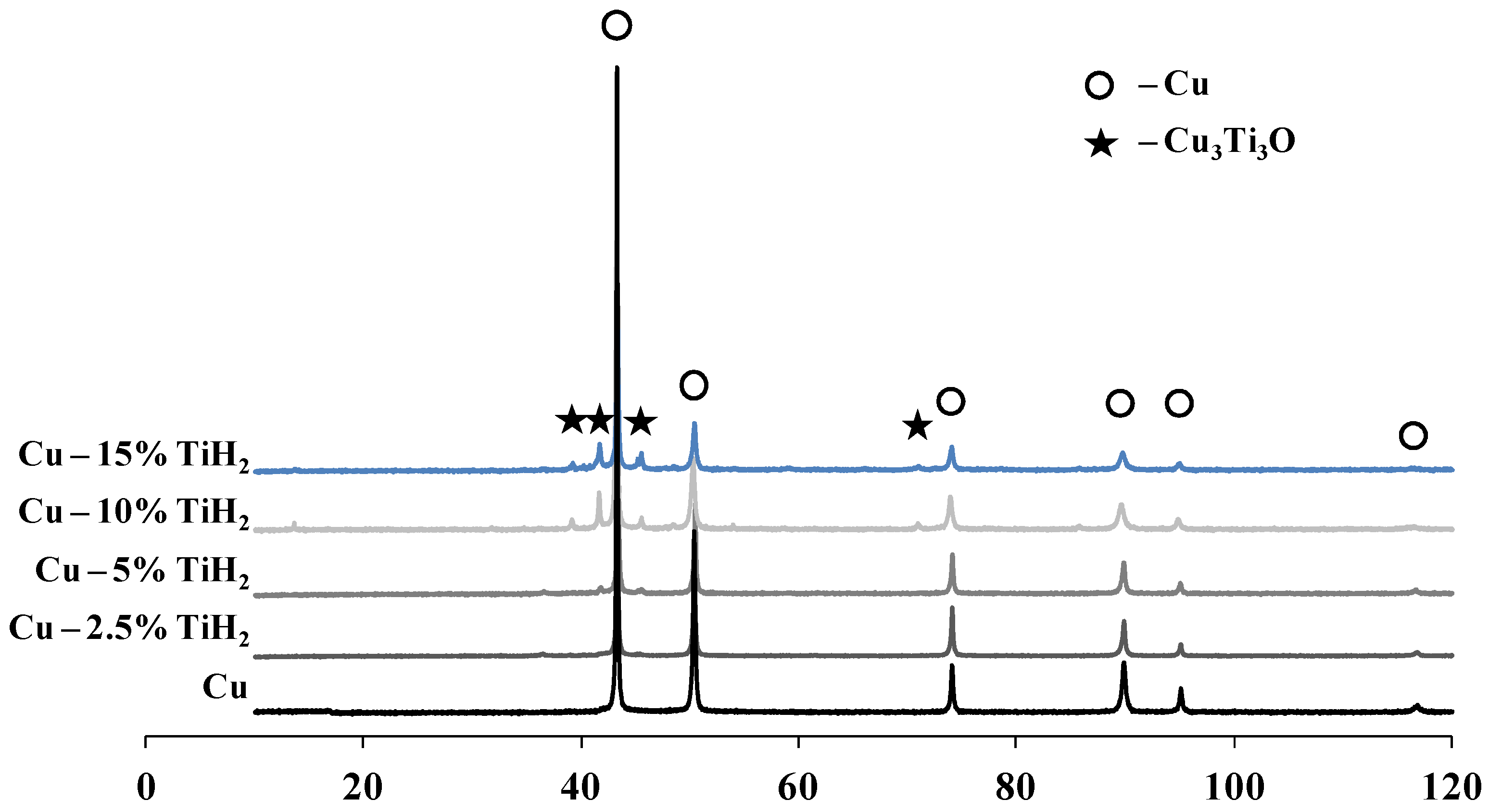
3.2. Reactive Hot Pressing of Ball-Milled Cu-(0–15)% TiH2 Mixtures
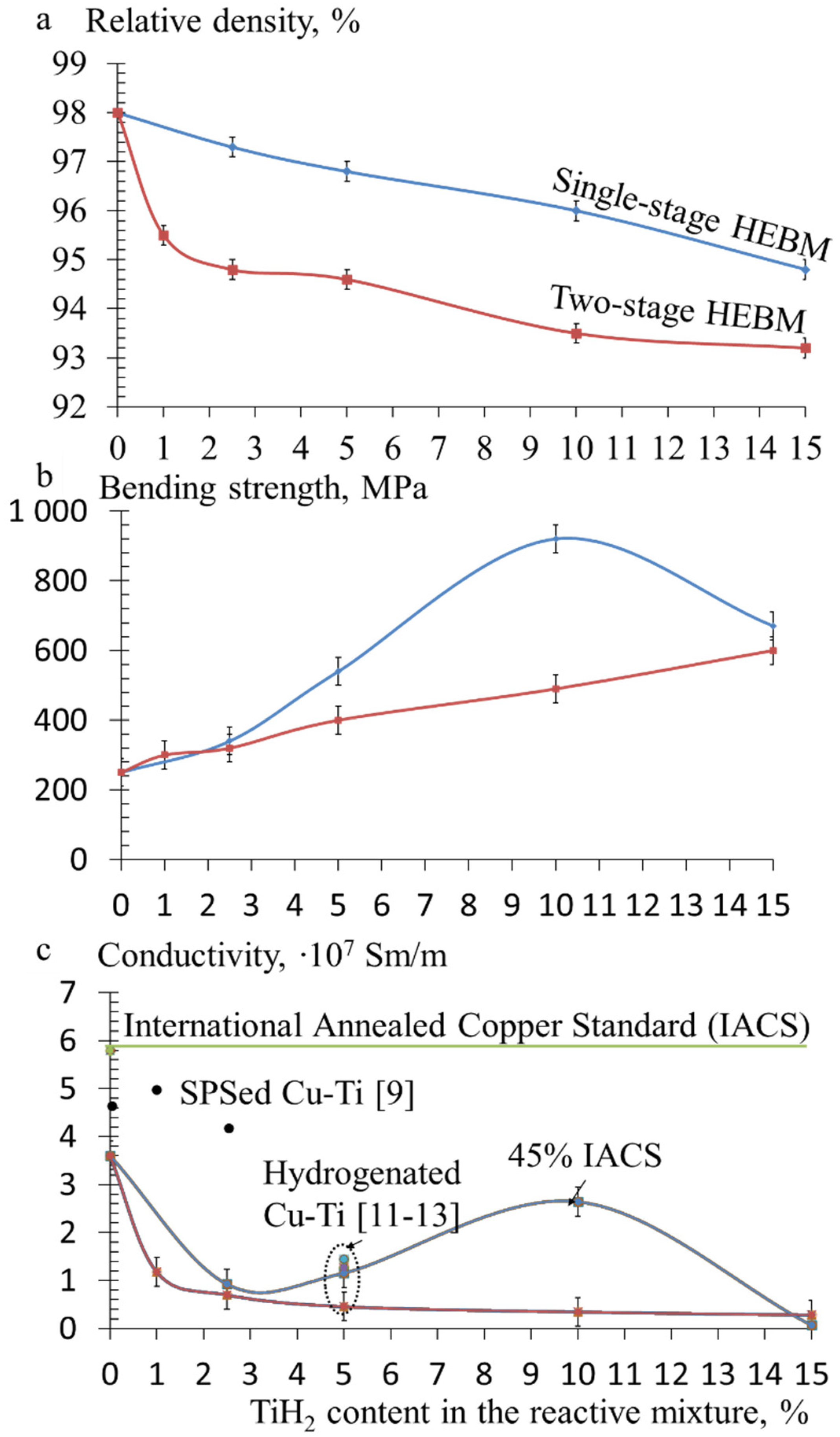
3.3. Mechanical Properties and Electrical Conductivity of Hot-Pressed Cu-TiH2 Samples
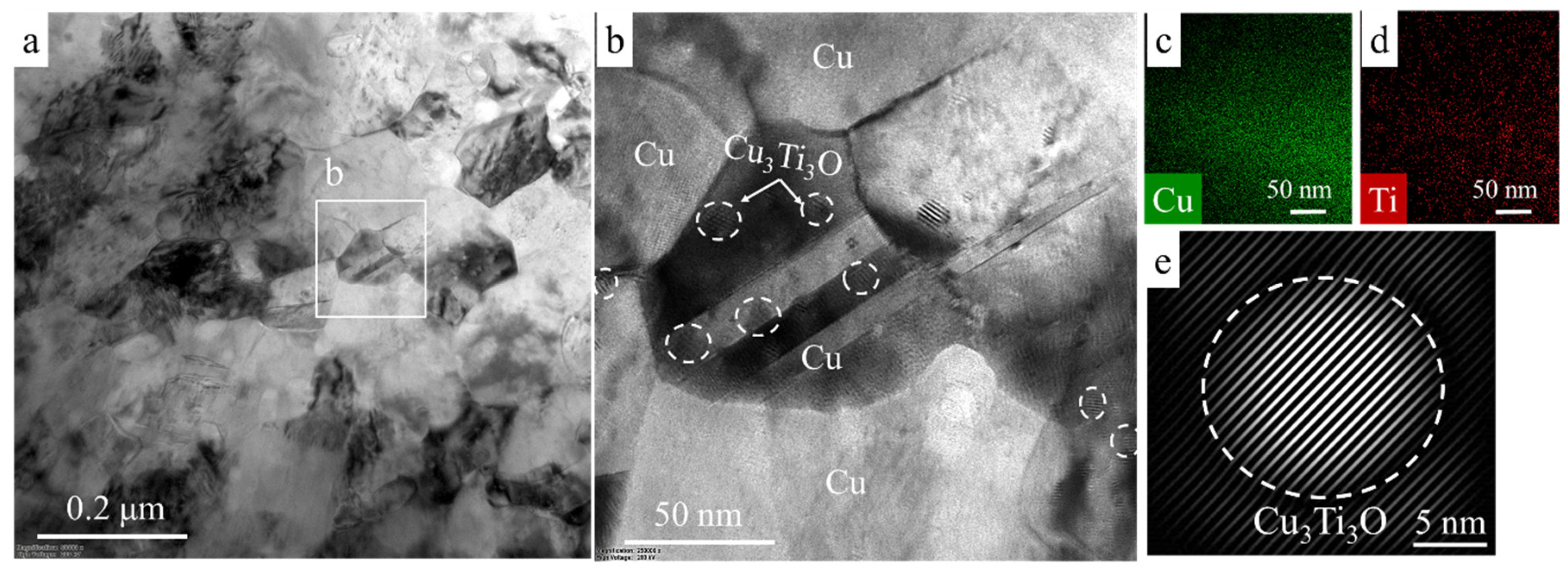
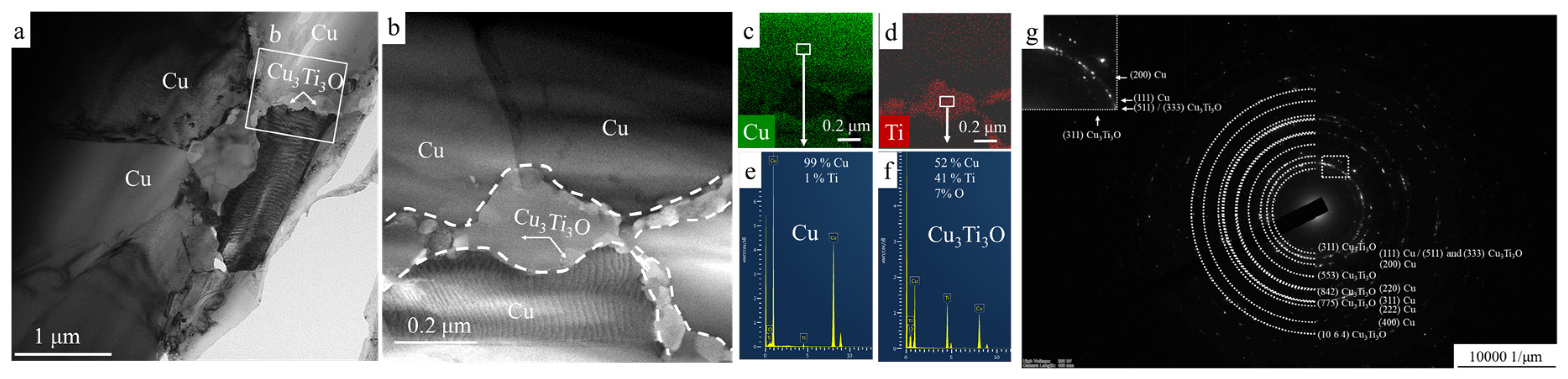
3.4. TEM Investigation of Hot-Pressed Cu-TiH2 Samples
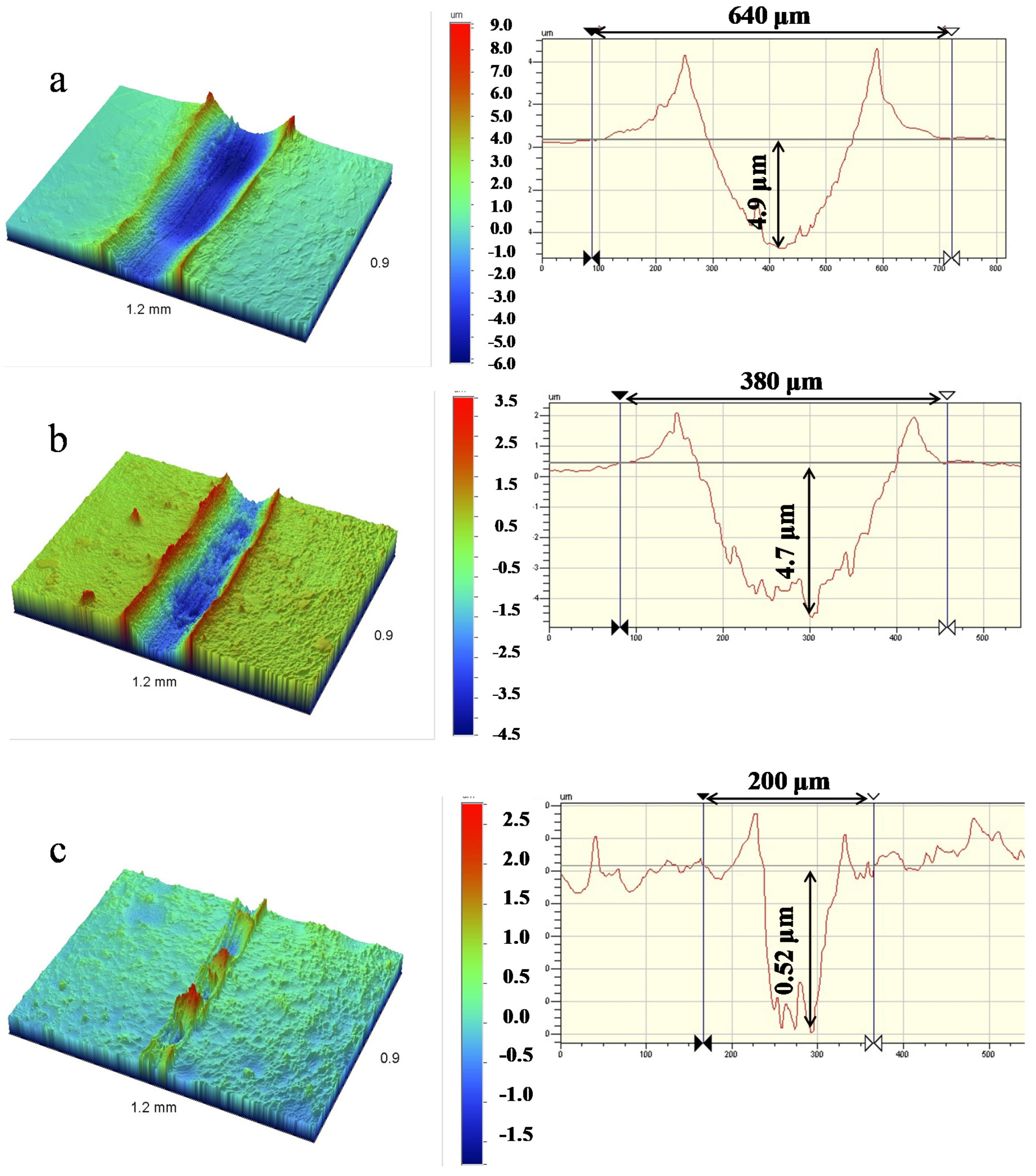
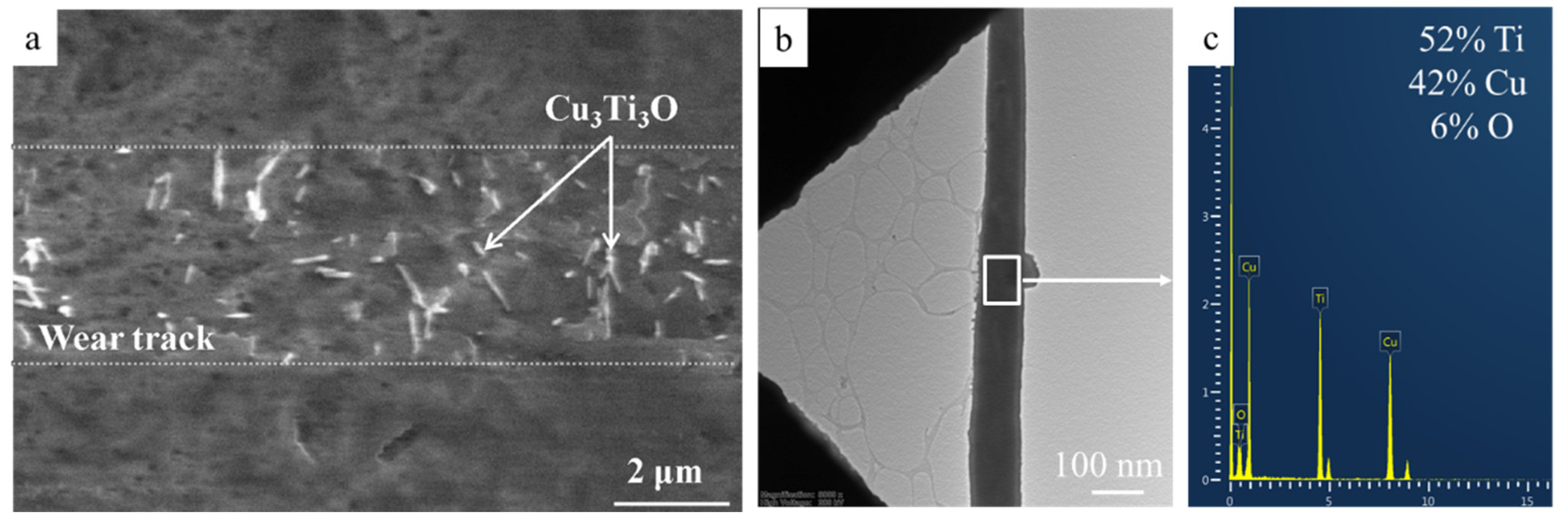
3.5. Tribological Performance of Hot-Pressed Cu-TiH2 Alloys
4. Discussion
4.1. Diffusion of Oxygen and Hydrogen in Copper
4.2. Diffusion of Titanium in Copper
4.3. Reproducibility of the Manufacturing Process
4.4. Structural Heterogeneity and the Mechanical Properties of Hot-Pressed Alloys
5. Conclusions
- Two high-energy ball-milling (HEBM) protocols were employed for the fabrication of Cu-Ti alloys: single-stage and two-stage ball milling, resulting in an order of magnitude refinement of TiH2 particles in the reactive mixtures.
- Single-stage HEBM processing led to the partial retention of Ti in the microstructure of hot-pressed specimens as the α-Ti phase, whereas the two-stage HEBM led to the complete conversion of TiH2 into the Cu3Ti3O phase during the hot pressing.
- In the HP samples prepared using single-stage HEBM processing, the Cu3Ti3O phase formed 10–20 nm precipitates distributed uniformly throughout the copper matrix, whereas two-stage processing led to the formation of 0.1–0.2 μm wide polycrystalline Cu3Ti3O layers on the boundaries of Cu grains. As compared to nanosized precipitates, grain-boundary Cu3Ti3O layers provided a considerably higher contribution to the resistivity of the alloy, resulting in a lower electrical conductivity (2.6 × 107 Sm/m in case of single-stage HEBM and 0.4 × 107 Sm/m in case of two-stage HEBM).
- Nanosized Cu3Ti3O precipitates provided more effective pinning of the Cu grain boundaries, promoting the formation of finer Cu grains (100–200 nm) in the alloys produced using single-stage HEBM as compared to the alloys produced using two-stage HEBM (1–2 μm Cu grains), which resulted in higher bending strength (up to 950 MPa) and tensile strength (920 MPa).
- The HP Cu-Ti alloy produced using single-stage HEBM Cu-10% TiH2 of mixture demonstrated 10 times lower specific wear as compared to copper and 3.5 times lower wear as compared to the alloy produced by the two-stage HEBM (1.1 × 10−5 and 3.8 × 10−5 mm3/N/m, correspondingly). This effect was caused by the higher strength of the alloy and formation of Cu3Ti3O microfibers in the wear debris, which decreased the friction coefficient against the Al2O3 counter-body.
Author Contributions
Funding
Conflicts of Interest
References
- Datta, A.; Soffa, W.A. The structure and properties of age hardened Cu–Ti alloys. Acta Metall. 1976, 24, 987–1001. [Google Scholar] [CrossRef]
- Hameda, A.A.; Blaz, L. Microstructure of hot-deformed Cu–3.45 wt.% Ti alloy. Mater. Sci. Eng. A 1998, 254, 83–89. [Google Scholar] [CrossRef]
- Laughlin, D.E.; Cahn, J.W. The crystal structure of the metastable precipitate in copper-based copper–titanium alloys. Scr. Metall. 1974, 8, 75–78. [Google Scholar] [CrossRef]
- Soffa, W.A.; Laughlin, D.E. High-strength age hardening copper–titanium alloys: Redivivus. Prog. Mater. Sci. 2004, 49, 347–366. [Google Scholar] [CrossRef]
- Semboshi, S.; Sato, S.; Ishikuro, M.; Wagatsuma, K.; Iwase, A.; Takasugi, T. Investigation of precipitation behavior in age-hardenable Cu–Ti alloys by an extraction-based approach. Metall. Mater. Trans. A 2014, 45, 3401–3411. [Google Scholar] [CrossRef]
- Semboshi, S.; Nishida, T.; Numakura, H.; Al-Kassaab, T.; Kirchheim, R. Effects of aging temperature on electrical conductivity and hardness of Cu–3 at.% Ti alloy aged in a hydrogen atmosphere. Metall. Mater. Trans. A 2011, 42, 2136–2143. [Google Scholar] [CrossRef]
- Suzuki, S.; Hirabayashi, K.; Shibata, H.; Mimura, K.; Isshiki, M.; Waseda, Y. Electrical and thermal conductivities in quenched and aged high-purity Cu–Ti alloys. Scr. Mater. 2003, 48, 431–435. [Google Scholar] [CrossRef]
- Nagarjuna, S.; Balasubramanian, K.; Sarma, D.S. Effect of Ti additions on the electrical resistivity of copper. Mater. Sci. Eng. A 1997, 225, 118–124. [Google Scholar] [CrossRef]
- Eze, A.A.; Jamiru, T.; Sadiku, E.R.; Durowoju, M.Ọ.; Kupolati, W.K.; Ibrahim, I.D.; Obadele, B.A.; Olubambi, P.A.; Diouf, S. Effect of titanium addition on the microstructure, electrical conductivity and mechanical properties of copper by using SPS for the preparation of Cu-Ti alloys. J. Alloys Compd. 2018, 736, 163–171. [Google Scholar] [CrossRef]
- Semboshi, S.; Numakura, H.; Gao, W.L.; Suda, H.; Sugawara, A. Effect of Prior Cold-Working on Strength and Electrical Conductivity of Cu-Ti Dilute Alloy Aged in a Hydrogen Atmosphere. Mater. Sci. Forum 2010, 654–656, 1315–1318. [Google Scholar] [CrossRef]
- Semboshi, S.; Nishida, T.; Numakura, H. Microstructure and mechanical properties of Cu–3 at.% Ti alloy aged in a hydrogen atmosphere. Mater. Sci. Eng. A 2009, 517, 105–113. [Google Scholar] [CrossRef]
- Semboshi, S.; Orimo, S.; Suda, H.; Gao, W.; Sugawara, A. Aging of Copper-Titanium Dilute Alloys in Hydrogen Atmosphere: Influence of Prior-Deformation on Strength and Electrical Conductivity. Mater. Trans. 2011, 52, 2137–2142. [Google Scholar] [CrossRef]
- Semboshi, S.; Takasugi, T. Fabrication of high-strength and high-conductivity Cu–Ti alloy wire by aging in a hydrogen atmosphere. J. Alloys Compd. 2013, 580 (Suppl. 1), S397–S400. [Google Scholar] [CrossRef]
- Olson, G.B. Computational Design of Hierarchically Structured Materials. Science 1997, 277, 1237–1242. [Google Scholar] [CrossRef]
- Ming, K.; Bi, X.; Wang, J. Strength and ductility of CrFeCoNiMo alloy with hierarchical microstructures. Int. J. Plast. 2019, 113, 255–268. [Google Scholar] [CrossRef]
- Thompson, B.R.; Horozov, T.S.; Stoyanov, S.D.; Paunov, V.N. Hierarchically structured composites and porous materials from soft templates: Fabrication and applications. J. Mater. Chem. A 2019, 7, 8030–8049. [Google Scholar] [CrossRef]
- Song, G.; Hong, S.J.; Lee, J.K.; Song, S.H.; Hong, S.H.; Kim, K.B.; Liaw, P.K. Optimization of B2/L21 hierarchical precipitate structure to improve creep resistance of a ferritic Fe-Ni-Al-Cr-Ti superalloy via thermal treatments. Scr. Mater. 2019, 161, 18–22. [Google Scholar] [CrossRef]
- Hasan, M.N.; Liu, Y.F.; An, X.H.; Gu, J.; Song, M.; Cao, Y.; Li, Y.S.; Zhu, Y.T.; Liao, X.Z. Simultaneously enhancing strength and ductility of a high-entropy alloy via gradient hierarchical microstructures. Int. J. Plast. 2019, 123, 178–195. [Google Scholar] [CrossRef]
- Olson, G.B. Systems design of hierarchically structured materials: Advanced steels. J. Comput.-Aided Mater. Des. 1998, 4, 143. [Google Scholar] [CrossRef]
- Potanin, A.Y.; Vorotilo, S.; Pogozhev, Y.S.; Rupasov, S.I.; Lobova, T.A.; Levashov, E.A. Influence of mechanical activation of reactive mixtures on the microstructure and properties of SHS-ceramics MoSi2–HfB2–MoB. Ceram. Int. 2019, 45, 20354–20361. [Google Scholar] [CrossRef]
- Vorotilo, S.; Potanin, A.Y.; Pogozhev, Y.S.; Levashov, E.A.; Kochetov, N.A.; Kovalev, D.Y. Self-propagating high-temperature synthesis of advanced ceramics MoSi2–HfB2–MoB. Ceram. Int. 2019, 45, 96–107. [Google Scholar] [CrossRef]
- Potanin, A.Y.; Vorotilo, S.; Pogozhev, Y.S.; Rupasov, S.I.; Loginov, P.A.; Shvyndina, N.V.; Sviridova, T.A.; Levashov, E.A. High-temperature oxidation and plasma torch testing of MoSi2–HfB2–MoB ceramics with single-level and two-level structure. Corros. Sci. 2019, 158, 108074. [Google Scholar] [CrossRef]
- Vorotilo, S.; Levashov, E.A.; Kurbatkina, V.V.; Kovalev, D.Y.; Kochetov, N.A. Self-propagating high-temperature synthesis of nanocomposite ceramics TaSi2-SiC with hierarchical structure and superior properties. J. Eur. Ceram. Soc. 2018, 38, 433–443. [Google Scholar] [CrossRef]
- Vorotilo, S.; Loginov, P.A.; Kovalev, D.Y.; Levashov, E.A. DFT–Driven design of hierarchically structured, strong and highly conductive alloys in Cu–Ti system via in situ hydration-re-oxidation. J. Alloys Compd. 2020, 832, 154823. [Google Scholar] [CrossRef]
- Sawa, K. Sliding Electrical Contacts and Materials. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: Boston, MA, USA, 2013. [Google Scholar]
- Bateni, M.R.; Ashrafizadeh, F.; Szpunar, J.A.; Drew, R.A.L. Improving the tribological behavior of copper through novel Ti–Cu intermetallic coatings. Wear 2002, 253, 626–639. [Google Scholar] [CrossRef]
- Sadeghi, N.; Aghajani, H.; Akbarpour, M.R. Microstructure and tribological properties of in-situ TiC-C/Cu nanocomposites synthesized using different carbon sources (graphite, carbon nanotube and graphene) in the Cu-Ti-C system. Ceram. Int. 2018, 44, 22059–22067. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zhou, Y.C.; Wang, J.Y. Tribological behavior of Ti2SnC particulate reinforced copper matrix composites. Mater. Sci. Eng. A 2006, 422, 266–271. [Google Scholar] [CrossRef]
- Lei, Q.; Li, S.; Zhu, J.; Xiao, Z.; Zhang, F.; Li, Z. Microstructural evolution, phase transition, and physics properties of a high strength Cu–Ni–Si–Al alloy. Mater. Charact. 2019, 147, 315–323. [Google Scholar] [CrossRef]
- Li, J.; Huang, G.; Mi, X.; Peng, L.; Xie, H.; Kang, Y. Microstructure evolution and properties of a quaternary Cu–Ni–Co–Si alloy with high strength and conductivity. Mater. Sci. Eng. A 2019, 766, 138390. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Li, B.; Shi, J.; Liu, Y.; Xiao, P. Effect of cold rolling and aging treatment on the microstructure and properties of Cu–3Ti–2Mg alloy. J. Alloys Compd. 2019, 818, 152915. [Google Scholar] [CrossRef]
- Xiong, N.; Bao, R.; Yi, J.; Tao, J.; Liu, Y.; Fang, D. Interface evolution and its influence on mechanical properties of CNTs/Cu-Ti composite. Mater. Sci. Eng. A 2019, 755, 75–84. [Google Scholar] [CrossRef]
- Caskey, G.R.; Dexter, A.H.; Holzworth, M.L.; Louthan, M.R.; Derrick, R.G. The Effect of Oxygen on Hydrogen Transport in Copper. Corrosion 1976, 32, 370–374. [Google Scholar] [CrossRef]
- Ishikawa, T.; McLellan, R.B. The diffusivity of hydrogen in copper at low temperatures. J. Phys. Chem. Solids 1985, 46, 445–447. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Takao, K. The electrochemical determination of diffusivity and solubility of hydrogen in copper. J. Jpn. Inst. Met. 1982, 46, 285–290. [Google Scholar] [CrossRef]
- Perkins, W.G.; Begeal, D.R. Permeation and diffusion of hydrogen in ceramvar, copper and ceramvar-copper laminates. Berichte der Bunsengesellschaft für Physikalische Chemie 1972, 76, 863. [Google Scholar]
- Magnusson, H.; Frisk, K. Thermodynamic evaluation of Cu-H-O-S-P system. In Phase Stabilities and Solubilities for OFP-Copper; SKB TR-13-11; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2013. [Google Scholar]
- Albert, E.; Kirchheim, R. Diffusivity of oxygen in copper. Scr. Metall. 1981, 15, 673–677. [Google Scholar] [CrossRef]
- Pastorek, R.L.; Rapp, R.A. The solubility and diffusivity of oxygen in solid copper from electrochemical measurements. Trans. Metall. Soc. AIME 1969, 245, 1711–1720. [Google Scholar]
- Ramana Rao, A.V.; Tare, V.B. Diffusion coefficient of oxygen in solid copper by the steady state current method using a solid electrolyte. Zeitschrift für Metallkunde 1972, 63, 70–73. [Google Scholar]
- Országh, J.; Bouillon, F. Diffusion de l’oxygène dans le cuivre monocristallin. Mémoires Scientifiques de la Revue de Métallurgie 1973, 70, 319–325. (In French) [Google Scholar]
- Horinouchi, H.; Shinohara, M.; Otsuka, T.; Hashizume, K.; Tanabe, T. Determination of hydrogen diffusion and permeation coefficients in pure copper at near room temperature by means of tritium tracer techniques. J. Alloys Compd. 2013, 580, S73–S75. [Google Scholar] [CrossRef]
- Magnusson, H.; Frisk, K. Self-Diffusion and Impurity Diffusion of Hydrogen, Oxygen, Sulphur and Phosphorus in Copper; Technical Report TR-13-24; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 2013. [Google Scholar]
- Jelić, D.; Tomić-Tucaković, B.; Mentus, S. A kinetic study of copper(II) oxide powder reduction with hydrogen, based on thermogravimetry. Thermochim. Acta 2011, 521, 211–217. [Google Scholar] [CrossRef]
- Tilliander, U.; Ragnhild, A.E.; Seetharaman, S. Kinetics studies of hydrogen reduction of Cu2O. Zeitschrift für Metallkunde 2006, 97, 72–78. [Google Scholar]
- Iijima, Y.; Hoshino, K.; Hirano, K. Diffusion of titanium in copper. Metall. Trans. A 1977, 8, 997–1001. [Google Scholar] [CrossRef]
- Korotayev, A.D.; Tsinenko, O.V.; Protasov, A.T. Kinetics of continuous and discontinuous decomposition in a copper-titanium alloy. Pt. 1. Fiz Met. Metalloved+. 1968, 26, 789. [Google Scholar]
- Kosyanov, D.Y.; Yavetskiy, R.P.; Parkhomenko, S.V.; Doroshenko, A.G.; Vorona, I.O.; Zavjalov, A.P.; Zakharenko, A.M.; Vornovskikh, A.A. A new method for calculating the residual porosity of transparent materials. J. Alloys Compd. 2019, 781, 892–897. [Google Scholar] [CrossRef]
- Bidulská, J.; Bidulský, R.; Actis Grande, M.; Kvačkaj, T. Different Formation Routes of Pore Structure in Aluminum Powder Metallurgy Alloy. Materials 2019, 12, 3724. [Google Scholar] [CrossRef] [PubMed]
- Bidulský, R.; Grande, M.A.; Bidulská, J.; Vlado, M.; Kvačkaj, T. Wear Mechanism of Chromium Pre-Alloyed Sintered Steel. High Temp. Mater. Process. 2009, 28, 175–180. [Google Scholar] [CrossRef]





| Specimen | Cu | Cu3Ti3O | α-Ti | |||
|---|---|---|---|---|---|---|
| H, GPa | E, GPa | H, GPa | E, GPa | H, GPa | E, GPa | |
| Cu | 1.1 ± 0.1 | 135 ± 10 | - | - | - | - |
| Cu-10% TiH2 two-stage HEBM | 5.5 ± 0.3 | 132 ± 9 | 6.9 ± 0.5 | 165 ± 15 | - | - |
| Cu-10% TiH2 single-stage HEBM | 5.2 ± 0.4 | 140 ± 12 | 5.8 ± 0.6 | 158 ± 15 | 3.0 ± 0.2 | 110 ± 8 |
| Sample | Normalized Wear, 10−5·mm3/N/m | Coefficient of Friction (CoF) |
|---|---|---|
| Cu | 9.3 | 0.86 |
| Cu-10% TiH2 (single-stage HEBM) | 1.1 | 0.6 |
| Cu-10% TiH2 (two-stage HEBM) | 3.8 | 0.74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorotilo, S.; Loginov, P.A.; Churyumov, A.Y.; Prosviryakov, A.S.; Bychkova, M.Y.; Rupasov, S.I.; Orekhov, A.S.; Kiryukhantsev-Korneev, P.V.; Levashov, E.A. RETRACTED: Manufacturing of Conductive, Wear-Resistant Nanoreinforced Cu-Ti Alloys Using Partially Oxidized Electrolytic Copper Powder. Nanomaterials 2020, 10, 1261. https://doi.org/10.3390/nano10071261
Vorotilo S, Loginov PA, Churyumov AY, Prosviryakov AS, Bychkova MY, Rupasov SI, Orekhov AS, Kiryukhantsev-Korneev PV, Levashov EA. RETRACTED: Manufacturing of Conductive, Wear-Resistant Nanoreinforced Cu-Ti Alloys Using Partially Oxidized Electrolytic Copper Powder. Nanomaterials. 2020; 10(7):1261. https://doi.org/10.3390/nano10071261
Chicago/Turabian StyleVorotilo, Stepan, Pavel Alexandrovich Loginov, Alexandr Yuryevich Churyumov, Alexey Sergeevich Prosviryakov, Marina Yakovlevna Bychkova, Sergey Ivanovich Rupasov, Anton Sergeevich Orekhov, Philipp Vladimirovich Kiryukhantsev-Korneev, and Evgeny Alexandrovich Levashov. 2020. "RETRACTED: Manufacturing of Conductive, Wear-Resistant Nanoreinforced Cu-Ti Alloys Using Partially Oxidized Electrolytic Copper Powder" Nanomaterials 10, no. 7: 1261. https://doi.org/10.3390/nano10071261
APA StyleVorotilo, S., Loginov, P. A., Churyumov, A. Y., Prosviryakov, A. S., Bychkova, M. Y., Rupasov, S. I., Orekhov, A. S., Kiryukhantsev-Korneev, P. V., & Levashov, E. A. (2020). RETRACTED: Manufacturing of Conductive, Wear-Resistant Nanoreinforced Cu-Ti Alloys Using Partially Oxidized Electrolytic Copper Powder. Nanomaterials, 10(7), 1261. https://doi.org/10.3390/nano10071261









