Few-Layer Graphene-Based Nanofluids with Enhanced Thermal Conductivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
3. Results and Discussion
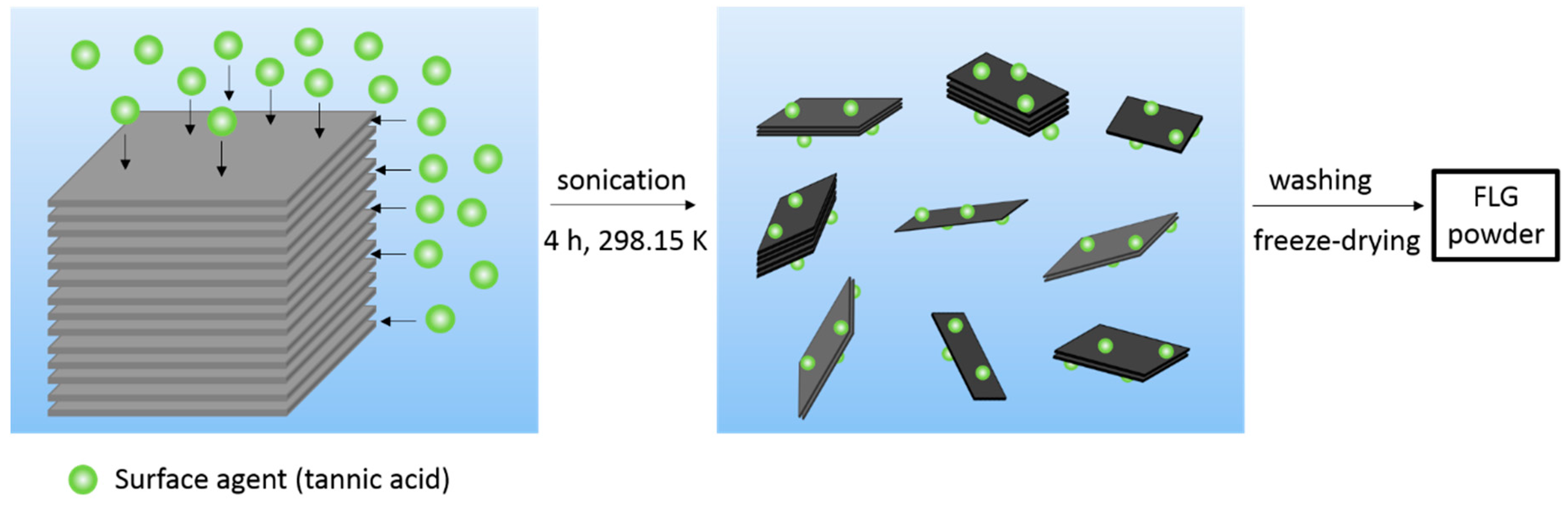
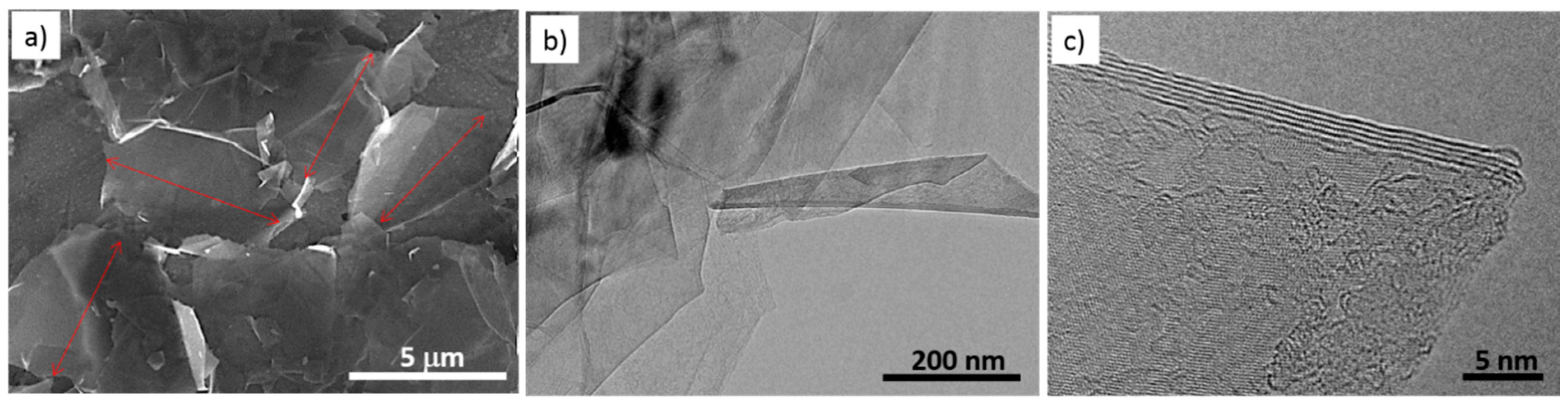
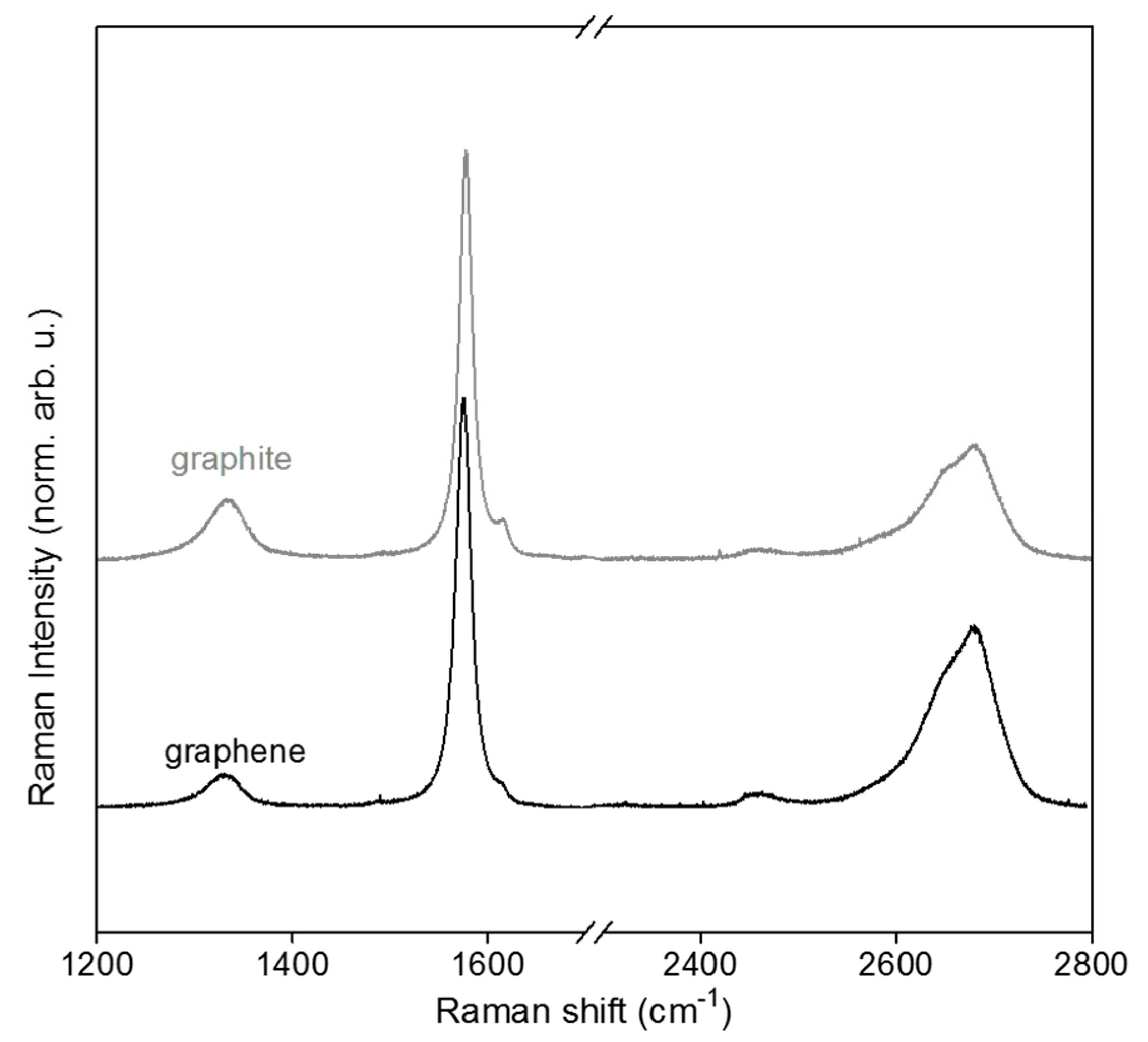
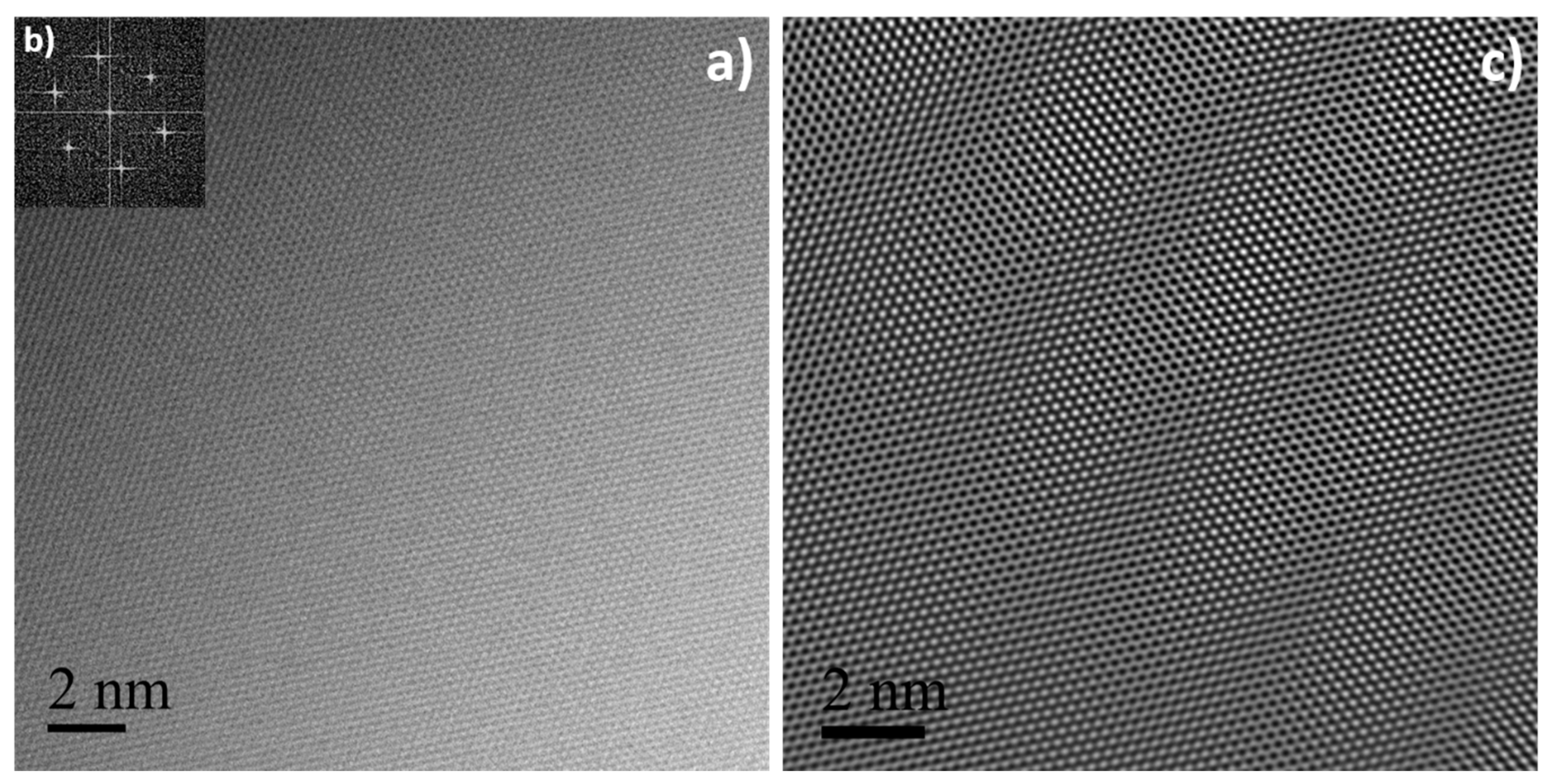
3.1. Few-Layer Graphene Synthesis and Characterization
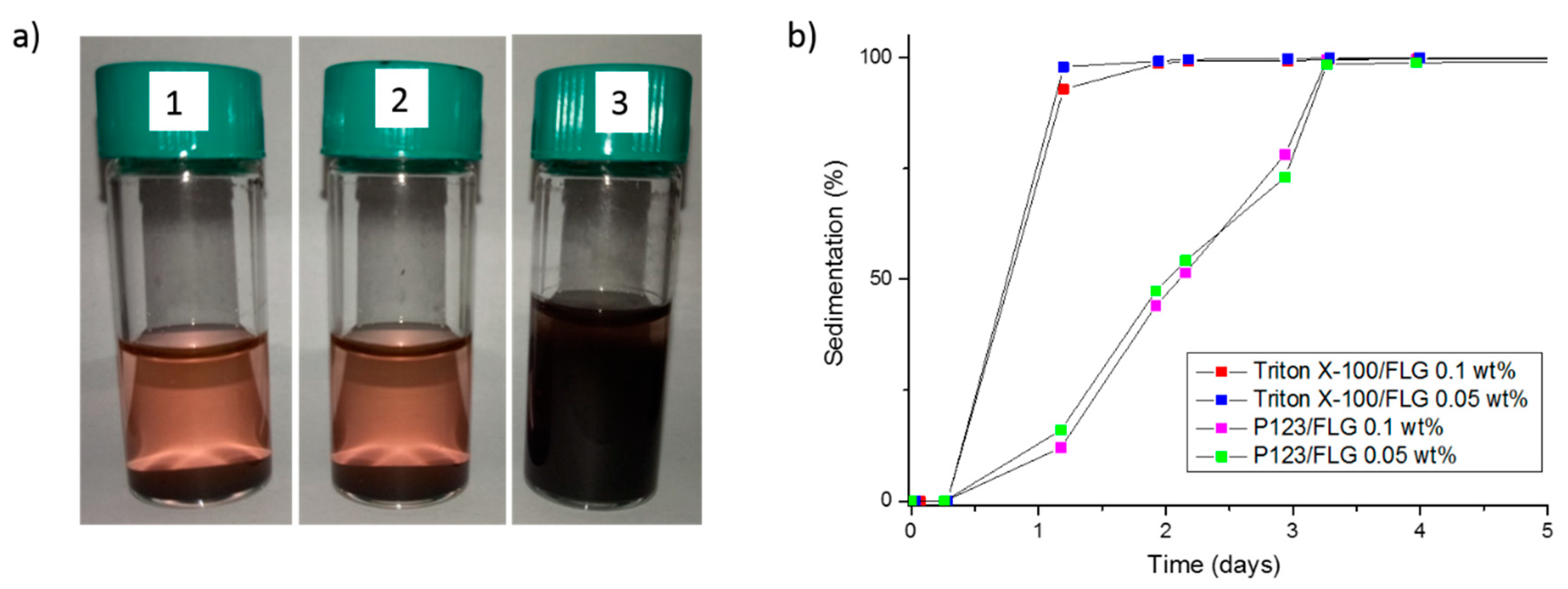
3.2. Stability at Rest of the Prepared FLG-Based Nanofluids
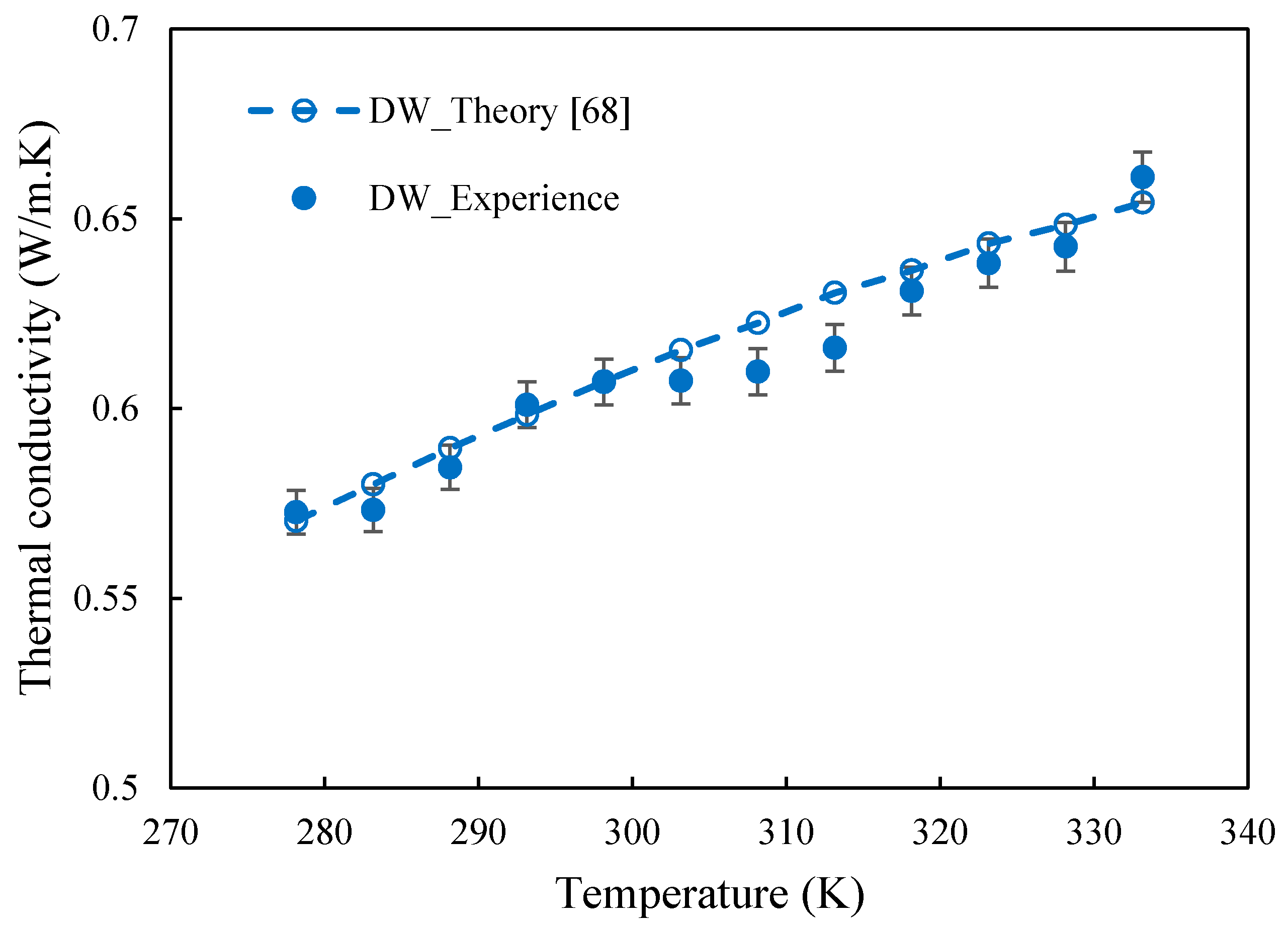
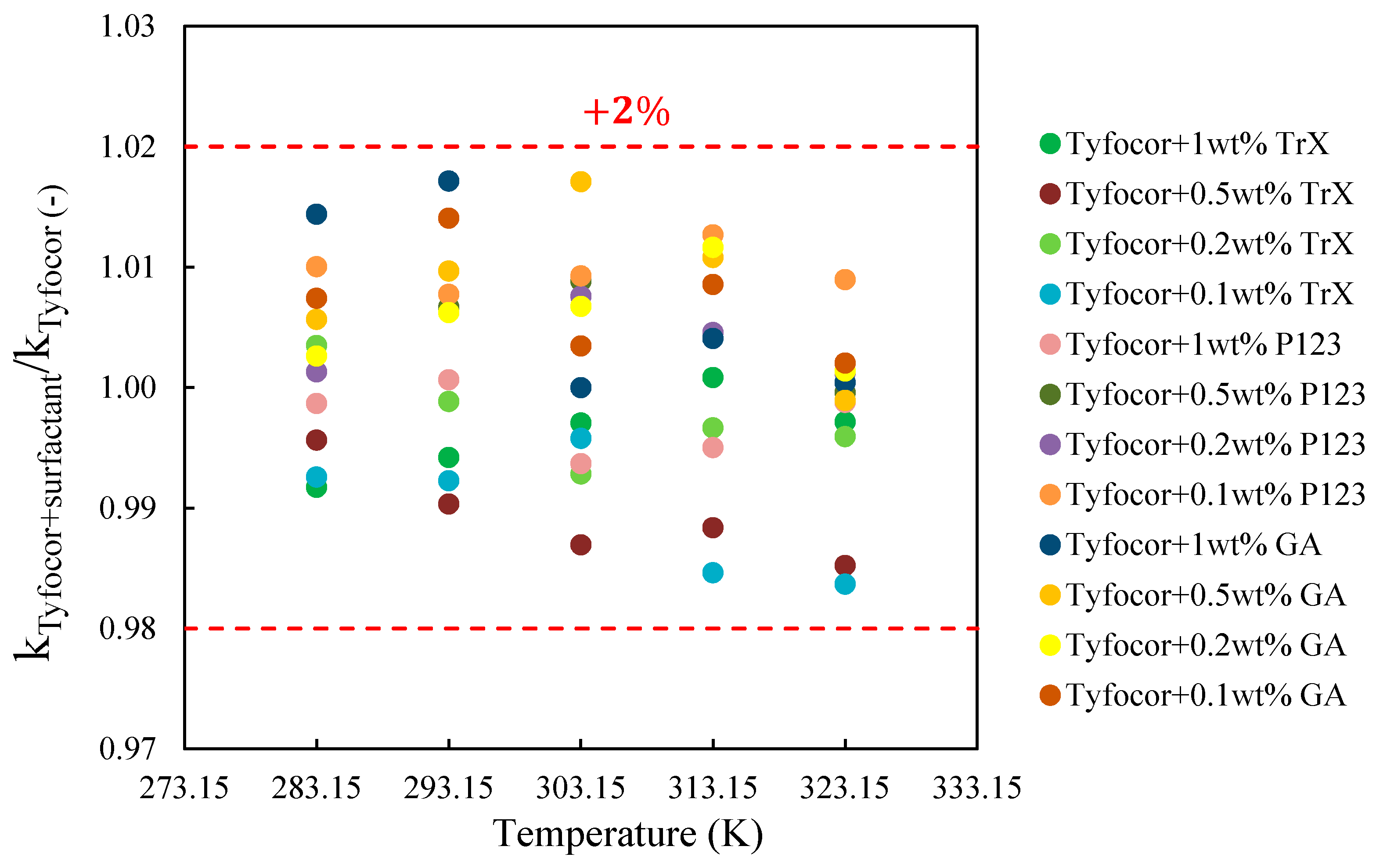
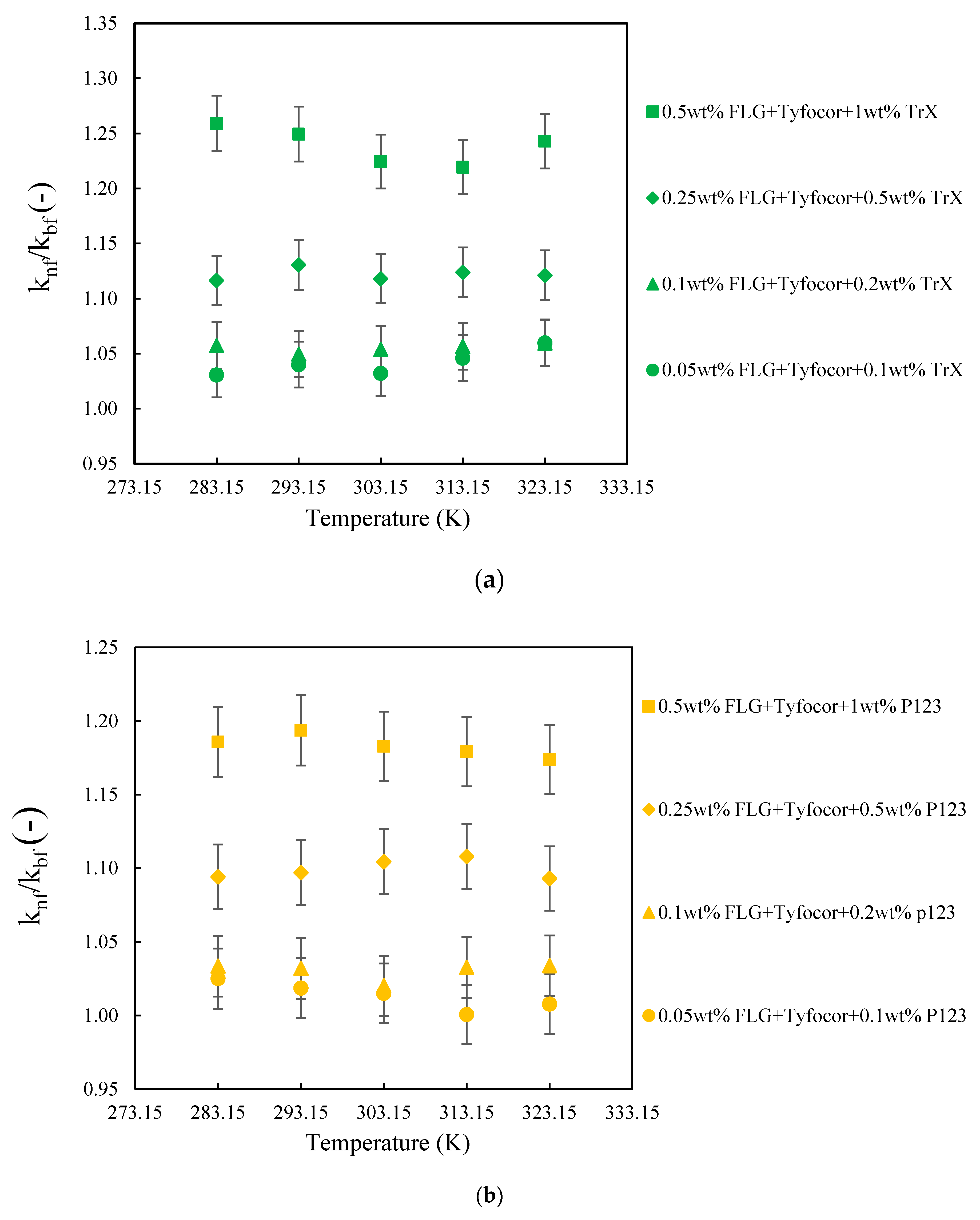
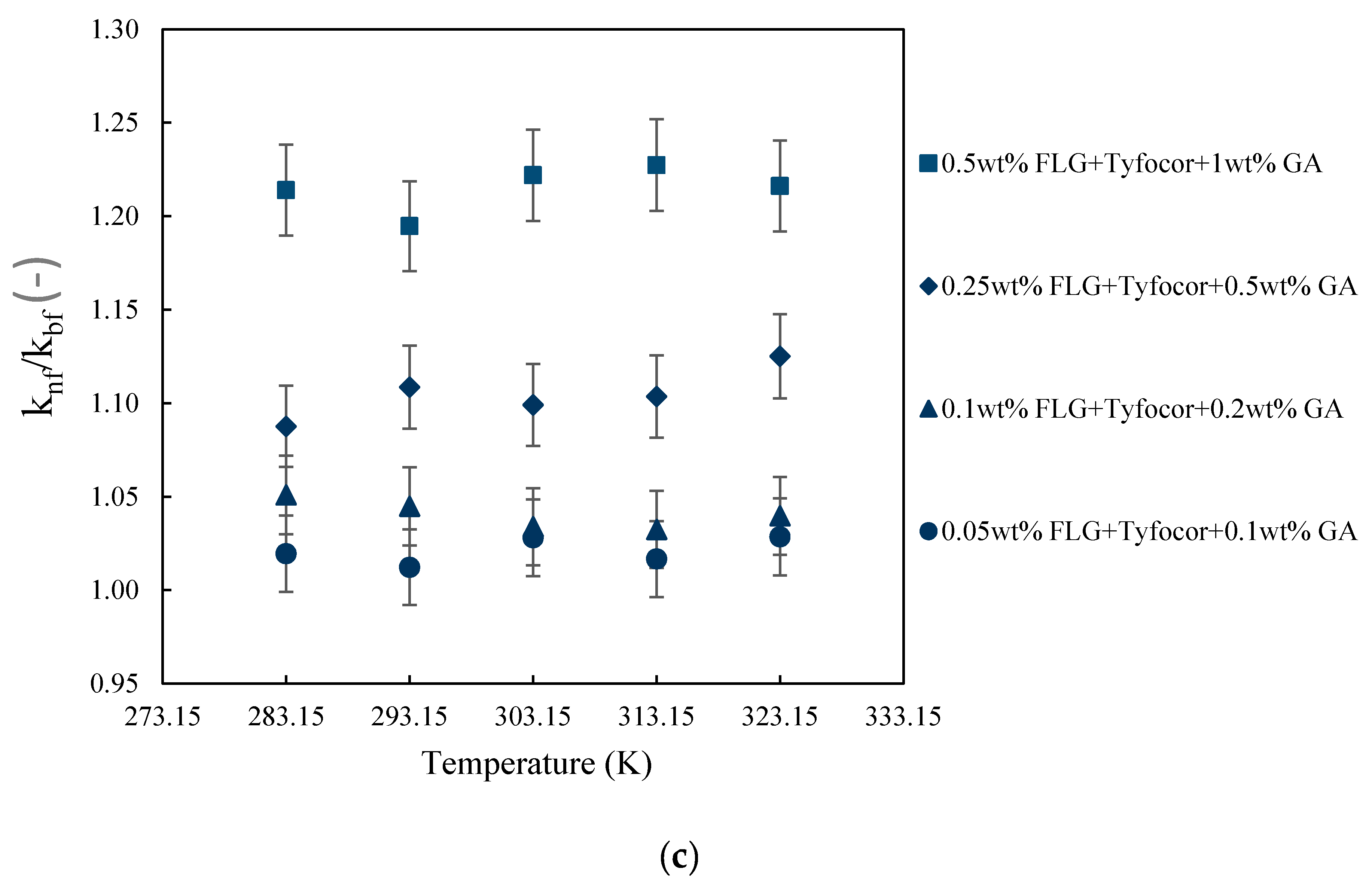
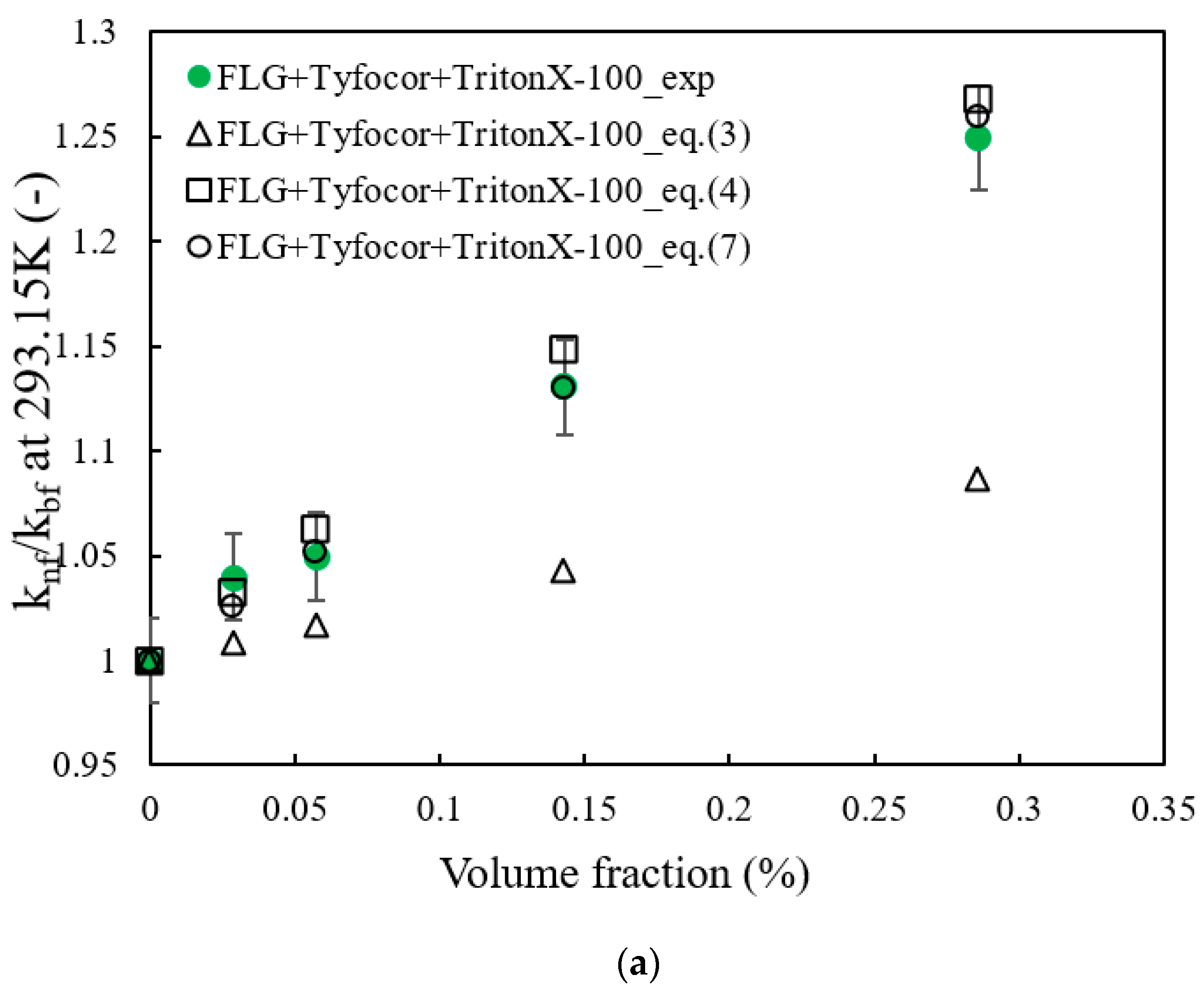
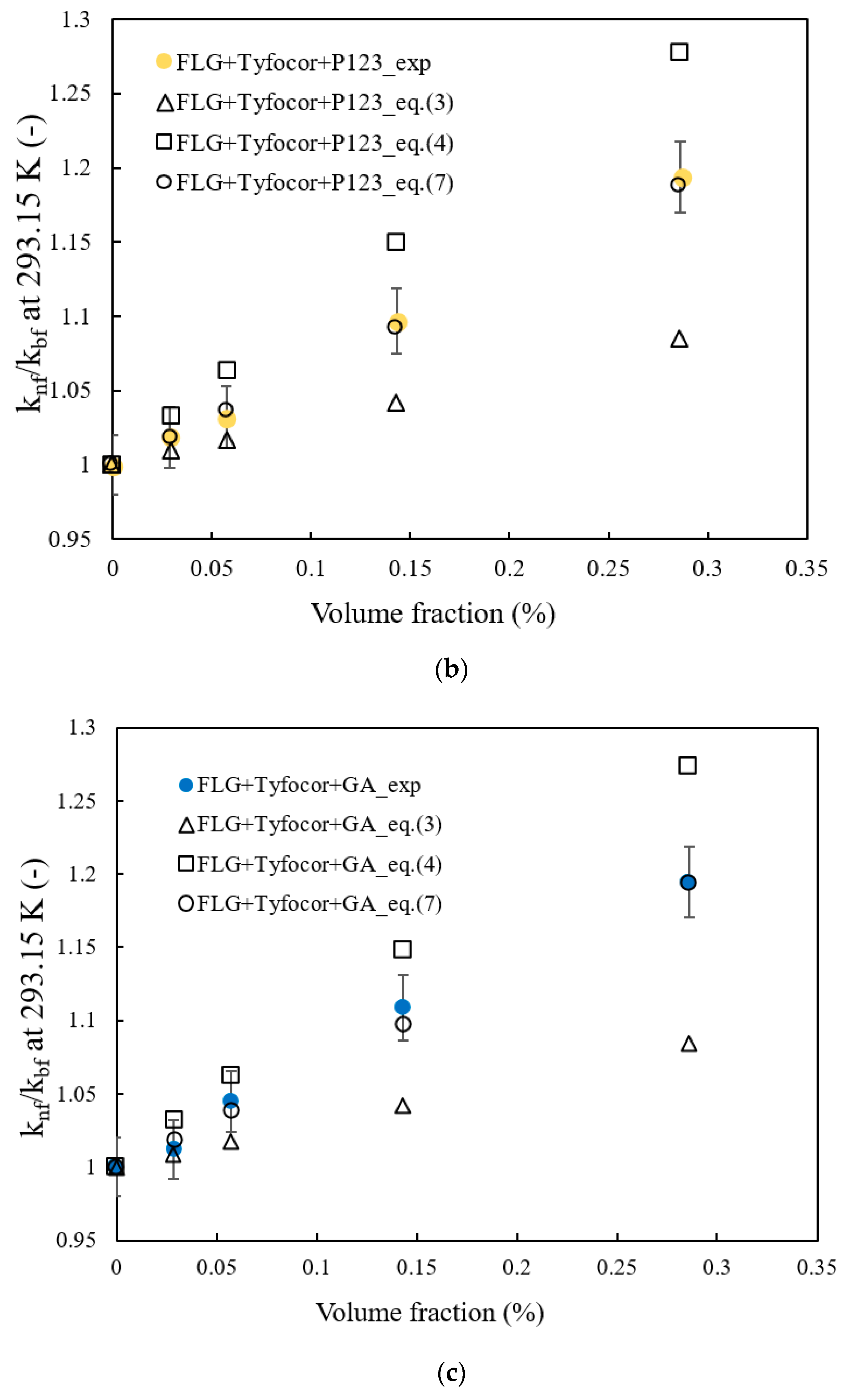
3.3. Thermal Conductivity of FLG and Nanofluids
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| ABS | Transmitted intensity |
| AAD | absolute average deviation [%] |
| DW | Deionized Water |
| η | flatness ratio effect of graphene |
| FFT | Fast Fourier TransformGum |
| GA | Gum Arabic |
| HFLG | highly crumpled few layer graphene |
| HRTEM | high-resolution transmission electron microscopy |
| FLG | few layer graphene |
| IFFT | Inverse FFT |
| L | Average length of graphene |
| PG | Propylene glycol |
| P123 | Pluronic® P123 |
| ρ | Density |
| Interfacial thermal resistance | |
| SEM | scanning electron microscopy |
| St. Dev. | standard deviation |
| TEM | transmission electron microscopy |
| T | temperature [K] |
| t | graphene thickness |
| t0 | particles dispersion |
| TrX | Triton X-100 |
| mass fraction | |
| apparent mass fraction | |
| volume fraction | |
| Subscripts/superscripts | |
| eff | effective |
| nf | nanofluid |
| np | nanoparticles |
| bf | base fluid |
References
- Nižetić, S.; Djilali, N.; Papadopoulos, A.; Rodrigues, J.J.P.C. Smart technologies for promotion of energy efficiency, utilization of sustainable resources and waste management. J. Clean. Prod. 2019, 231, 565–591. [Google Scholar] [CrossRef]
- Riffat, S.B.; Ma, X. Thermoelectrics: A review of present and potential applications. Appl. Therm. Eng. 2003, 23, 913–935. [Google Scholar] [CrossRef]
- Park, S.; Kang, H.; Yoon, H.J. Structure–thermopower relationships in molecular thermoelectrics. J. Mater. Chem. A 2019, 7, 14419–14446. [Google Scholar] [CrossRef]
- Wang, H.; Yu, C. Organic Thermoelectrics: Materials Preparation, Performance Optimization, and Device Integration. Joule 2019, 3, 53–80. [Google Scholar] [CrossRef] [Green Version]
- Hajatzadeh Pordanjani, A.; Aghakhani, S.; Afrand, M.; Mahmoudi, B.; Mahian, O.; Wongwises, S. An updated review on application of nanofluids in heat exchangers for saving energy. Energy Convers. Manag. 2019, 198, 111886. [Google Scholar] [CrossRef]
- Tawfik, M.M. Experimental studies of nanofluid thermal conductivity enhancement and applications: A review. Renew. Sustain. Energy Rev. 2017, 75, 1239–1253. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.U.S.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles, International Mechanical Engineering Congress and Exhibition; Argonne National Lab: Lemont, IL, USA, 1995; pp. 99–105.
- Haddad, Z.; Abid, C.; Oztop, H.F.; Mataoui, A. A review on how the researchers prepare their nanofluids. Int. J. Therm. Sci. 2014, 76, 168–189. [Google Scholar] [CrossRef]
- Puliti, G.; Paolucci, S.; Sen, M. Nanofluids and Their Properties. Appl. Mech. Rev. 2011, 64. [Google Scholar] [CrossRef]
- Bahiraei, M.; Heshmatian, S. Graphene family nanofluids: A critical review and future research directions. Energy Convers. Manag. 2019, 196, 1222–1256. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C. Investigation of a nanofluid-based concentrating thermal photovoltaic with a parabolic reflector. Energy Convers. Manag. 2019, 180, 171–182. [Google Scholar] [CrossRef]
- Yen, P.-H.; Wang, J.-C. Power generation and electric charge density with temperature effect of alumina nanofluids using dimensional analysis. Energy Convers. Manag. 2019, 186, 546–555. [Google Scholar] [CrossRef]
- Nazari, S.; Safarzadeh, H.; Bahiraei, M. Experimental and analytical investigations of productivity, energy and exergy efficiency of a single slope solar still enhanced with thermoelectric channel and nanofluid. Renew. Energy 2019, 135, 729–744. [Google Scholar] [CrossRef]
- Karimipour, A.; Bagherzadeh, S.A.; Goodarzi, M.; Alnaqi, A.A.; Bahiraei, M.; Safaei, M.R.; Shadloo, M.S. Synthesized CuFe2O4/SiO2 nanocomposites added to water/EG: Evaluation of the thermophysical properties beside sensitivity analysis & EANN. Int. J. Heat Mass Transf. 2018, 127, 1169–1179. [Google Scholar] [CrossRef]
- Safaei, M.R.; Ranjbarzadeh, R.; Hajizadeh, A.; Bahiraei, M.; Afrand, M.; Karimipour, A. Effects of cobalt ferrite coated with silica nanocomposite on the thermal conductivity of an antifreeze: New nanofluid for refrigeration condensers. Int. J. Refrig. 2019, 102, 86–95. [Google Scholar] [CrossRef]
- Sardarabadi, H.; Zeinali Heris, S.; Ahmadpour, A.; Passandideh-Fard, M. Experimental investigation of a novel type of two-phase closed thermosyphon filled with functionalized carbon nanotubes/water nanofluids for electronic cooling application. Energy Convers. Manag. 2019, 188, 321–332. [Google Scholar] [CrossRef]
- Qi, C.; Liu, M.; Tang, J. Influence of triangle tube structure with twisted tape on the thermo-hydraulic performance of nanofluids in heat-exchange system based on thermal and exergy efficiency. Energy Convers. Manag. 2019, 192, 243–268. [Google Scholar] [CrossRef]
- Bahiraei, M.; Ahmadi, A.A. Thermohydraulic performance analysis of a spiral heat exchanger operated with water–alumina nanofluid: Effects of geometry and adding nanoparticles. Energy Convers. Manag. 2018, 170, 62–72. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Estelle, P. A state of the art review on viscosity of nanofluids. Renew. Sustain. Energy Rev. 2017, 76, 1134–1152. [Google Scholar] [CrossRef]
- Afrand, M.; Hemmat Esfe, M.; Abedini, E.; Teimouri, H. Predicting the effects of magnesium oxide nanoparticles and temperature on the thermal conductivity of water using artificial neural network and experimental data. Phys. E Low Dimens. Syst. Nanostruct. 2017, 87, 242–247. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Hajmohammad, M.H. Thermal conductivity and viscosity optimization of nanodiamond-Co3O4/EG (40:60) aqueous nanofluid using NSGA-II coupled with RSM. J. Mol. Liq. 2017, 238, 545–552. [Google Scholar] [CrossRef]
- Li, C.H.; Peterson, G.P. The effect of particle size on the effective thermal conductivity of Al2O3-water nanofluids. J. Appl. Phys. 2007, 101, 044312. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Languri, E.M.; Nunna, M.R. Effect of particle size and viscosity on thermal conductivity enhancement of graphene oxide nanofluid. Int. Commun. Heat Mass Transf. 2016, 76, 308–315. [Google Scholar] [CrossRef]
- Sarviya, R.M.; Fuskele, V. Review on Thermal Conductivity of Nanofluids. Mater. Today-Proc. 2017, 4, 4022–4031. [Google Scholar] [CrossRef]
- Esfahani, N.N.; Toghraie, D.; Afrand, M. A new correlation for predicting the thermal conductivity of ZnO–Ag (50%–50%)/water hybrid nanofluid: An experimental study. Powder Technol. 2018, 323, 367–373. [Google Scholar] [CrossRef]
- Xia, G.; Jiang, H.; Liu, R.; Zhai, Y. Effects of surfactant on the stability and thermal conductivity of Al2O3/de-ionized water nanofluids. Int. J. Therm. Sci. 2014, 84, 118–124. [Google Scholar] [CrossRef]
- Estellé, P.; Halelfadl, S.; Maré, T. Lignin as dispersant for water-based carbon nanotubes nanofluids: Impact on viscosity and thermal conductivity. Int. Commun. Heat Mass Transf. 2014, 57, 8–12. [Google Scholar] [CrossRef]
- Yasinskiy, A.; Navas, J.; Aguilar, T.; Alcántara, R.; Gallardo, J.J.; Sánchez-Coronilla, A.; Martín, E.I.; De Los Santos, D.; Fernández-Lorenzo, C. Dramatically enhanced thermal properties for TiO2-based nanofluids for being used as heat transfer fluids in concentrating solar power plants. Renew. Energy 2018, 119, 809–819. [Google Scholar] [CrossRef]
- Yiamsawas, T.; Dalkilic, A.S.; Mahian, O.; Wongwises, S. Measurement and Correlation of the Viscosity of Water-Based Al2O3 and TiO2 Nanofluids in High Temperatures and Comparisons with Literature Reports. J. Dispers. Sci. Technol. 2013, 34, 1697–1703. [Google Scholar] [CrossRef]
- Angayarkanni, S.A.; Philip, J. Review on thermal properties of nanofluids: Recent developments. Adv. Colloid Interface Sci. 2015, 225, 146–176. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.U.-S.; Li, S.; Eastman, J.A. Measuring Thermal Conductivity of Fluids Containing Oxide Nanoparticles. J. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Mahian, O.; Kianifar, A.; Heris, S.Z.; Wongwises, S. Natural convection of silica nanofluids in square and triangular enclosures: Theoretical and experimental study. Int. J. Heat Mass Transf. 2016, 99, 792–804. [Google Scholar] [CrossRef]
- Gómez-Villarejo, R.; Martín, E.I.; Navas, J.; Sánchez-Coronilla, A.; Aguilar, T.; Gallardo, J.J.; Alcántara, R.; De los Santos, D.; Carrillo-Berdugo, I.; Fernández-Lorenzo, C. Ag-based nanofluidic system to enhance heat transfer fluids for concentrating solar power: Nano-level insights. Appl. Energy 2017, 194, 19–29. [Google Scholar] [CrossRef]
- Navas, J.; Sánchez-Coronilla, A.; Martín, E.I.; Teruel, M.; Gallardo, J.J.; Aguilar, T.; Gómez-Villarejo, R.; Alcántara, R.; Fernández-Lorenzo, C.; Piñero, J.C.; et al. On the enhancement of heat transfer fluid for concentrating solar power using Cu and Ni nanofluids: An experimental and molecular dynamics study. Nano Energy 2016, 27, 213–224. [Google Scholar] [CrossRef]
- Seong, H.; Kim, G.; Jeon, J.; Jeong, H.; Noh, J.; Kim, Y.; Kim, H.; Huh, S. Experimental Study on Characteristics of Grinded Graphene Nanofluids with Surfactants. Materials 2018, 11, 950. [Google Scholar] [CrossRef] [Green Version]
- Naddaf, A.; Zeinali Heris, S. Experimental study on thermal conductivity and electrical conductivity of diesel oil-based nanofluids of graphene nanoplatelets and carbon nanotubes. Int. Commun. Heat Mass Transf. 2018, 95, 116–122. [Google Scholar] [CrossRef]
- Kamatchi, R.; Kannan, K.G. An aqua based reduced graphene oxide nanofluids for heat transfer applications: Synthesis, characterization, stability analysis, and thermophysical properties. Int. J. Renew. Energy Res. 2018, 8, 313–319. [Google Scholar]
- Bahaya, B.; Johnson, D.W.; Yavuzturk, C.C. On the Effect of Graphene Nanoplatelets on Water—Graphene Nanofluid Thermal Conductivity, Viscosity, and Heat Transfer Under Laminar External Flow Conditions. J. Heat Transf. 2018, 140. [Google Scholar] [CrossRef] [Green Version]
- Hussien, A.A.; Abdullah, M.Z.; Yusop, N.M.; Al-Nimr, M.A.; Atieh, M.A.; Mehrali, M. Experiment on forced convective heat transfer enhancement using MWCNTs/GNPs hybrid nanofluid and mini-tube. Int. J. Heat Mass Transf. 2017, 115, 1121–1131. [Google Scholar] [CrossRef]
- Manasrah, A.D.; Al-Mubaiyedh, U.A.; Laui, T.; Ben-Mansour, R.; Al-Marri, M.J.; Almanassra, I.W.; Abdala, A.; Atieh, M.A. Heat transfer enhancement of nanofluids using iron nanoparticles decorated carbon nanotubes. Appl. Therm. Eng. 2016, 107, 1008–1018. [Google Scholar] [CrossRef]
- Ettefaghi, E.; Ghobadian, B.; Rashidi, A.; Najafi, G.; Khoshtaghaza, M.H.; Pourhashem, S. Preparation and investigation of the heat transfer properties of a novel nanofluid based on graphene quantum dots. Energy Convers. Manag. 2017, 153, 215–223. [Google Scholar] [CrossRef]
- Novoselov, K.S. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Sen Gupta, S.; Manoj Siva, V.; Krishnan, S.; Sreeprasad, T.S.; Singh, P.K.; Pradeep, T.; Das, S.K. Thermal conductivity enhancement of nanofluids containing graphene nanosheets. J. Appl. Phys. 2011, 110, 084302. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Wang, H.; Sasmito, A.P.; Mujumdar, A.S. Measurement and modeling of thermal conductivity of graphene nanoplatelet water and ethylene glycol base nanofluids. Int. J. Heat Mass Transf. 2018, 123, 97–109. [Google Scholar] [CrossRef]
- Vallejo, J.P.; Pérez-Tavernier, J.; Cabaleiro, D.; Fernández-Seara, J.; Lugo, L. Potential heat transfer enhancement of functionalized graphene nanoplatelet dispersions in a propylene glycol-water mixture. Thermophysical profile. J. Chem. Thermodyn. 2018, 123, 174–184. [Google Scholar] [CrossRef]
- Sun, Z.; Pöller, S.; Huang, X.; Guschin, D.; Taetz, C.; Ebbinghaus, P.; Masa, J.; Erbe, A.; Kilzer, A.; Schuhmann, W.; et al. High-yield exfoliation of graphite in acrylate polymers: A stable few-layer graphene nanofluid with enhanced thermal conductivity. Carbon 2013, 64, 288–294. [Google Scholar] [CrossRef]
- Amiri, A.; Ahmadi, G.; Shanbedi, M.; Etemadi, M.; Zubir, M.N.M.; Chew, B.T.; Kazi, S.N. Heat transfer enhancement of water-based highly crumpled few-layer graphene nanofluids. RSC Adv. 2016, 6, 105508–105527. [Google Scholar] [CrossRef]
- Amiri, A.; Shanbedi, M.; Rafieerad, A.R.; Rashidi, M.M.; Zaharinie, T.; Zubir, M.N.M.; Kazi, S.N.; Chew, B.T. Functionalization and exfoliation of graphite into mono layer graphene for improved heat dissipation. J. Taiwan Inst. Chem. Eng. 2017, 71, 480–493. [Google Scholar] [CrossRef]
- Alawi, O.A.; Sidik, N.A.C.; Kazi, S.N.; Najafi, G. Graphene nanoplatelets and few-layer graphene studies in thermo-physical properties and particle characterization. J. Therm. Anal. Calorim. 2019, 135, 1081–1093. [Google Scholar] [CrossRef]
- Alawi, O.A.; Mallah, A.R.; Kazi, S.N.; Sidik, N.A.C.; Najafi, G. Thermophysical properties and stability of carbon nanostructures and metallic oxides nanofluids. J. Therm. Anal. Calorim. 2019, 135, 1545–1562. [Google Scholar] [CrossRef]
- Berrada, N.; Hamze, S.; Desforges, A.; Ghanbaja, J.; Gleize, J.; Maré, T.; Vigolo, B.; Estellé, P. Surface tension of functionalized MWCNT-based nanofluids in water and commercial propylene-glycol mixture. J. Mol. Liquids 2019, 293, 111473. [Google Scholar] [CrossRef] [Green Version]
- Borode, A.O.; Ahmed, N.A.; Olubambi, P.A. Surfactant-aided dispersion of carbon nanomaterials in aqueous solution. Phys. Fluids 2019, 31, 071301. [Google Scholar] [CrossRef]
- Guardia, L.; Fernández-Merino, M.J.; Paredes, J.I.; Solís-Fernández, P.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. High-throughput production of pristine graphene in an aqueous dispersion assisted by non-ionic surfactants. Carbon 2011, 49, 1653–1662. [Google Scholar] [CrossRef]
- Rasheed, A.K.; Khalid, M.; Rashmi, W.; Gupta, T.C.S.M.; Chan, A. Graphene based nanofluids and nanolubricants—Review of recent developments. Renew. Sustain. Energy Rev. 2016, 63, 346–362. [Google Scholar] [CrossRef]
- Keklikcioglu Cakmak, N. The impact of surfactants on the stability and thermal conductivity of graphene oxide de-ionized water nanofluids. J. Therm. Anal. Calorim. 2020, 139, 1895–1902. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Tahan Latibari, S.; Mehrali, M.; Togun, H.; Zubir, M.N.M.; Kazi, S.N.; Metselaar, H.S.C. Preparation, characterization, viscosity, and thermal conductivity of nitrogen-doped graphene aqueous nanofluids. J. Mater. Sci. 2014, 49, 7156–7171. [Google Scholar] [CrossRef]
- Qamar, S.; Yasin, S.; Ramzan, N.; Iqbal, T.; Akhtar, M.N. Preparation of stable dispersion of graphene using copolymers: Dispersity and aromaticity analysis. Soft Mater. 2019, 17, 190–202. [Google Scholar] [CrossRef]
- Akbari, A.; Alavi Fazel, S.A.; Maghsoodi, S.; Shahbazi Kootenaei, A. Thermo-physical and stability properties of raw and functionalization of graphene nanoplatelets-based aqueous nanofluids. J. Dispers. Sci. Technol. 2019, 40, 17–24. [Google Scholar] [CrossRef]
- Sarsam, W.S.; Amiri, A.; Kazi, S.N.; Badarudin, A. Stability and thermophysical properties of non-covalently functionalized graphene nanoplatelets nanofluids. Energy Convers. Manag. 2016, 116, 101–111. [Google Scholar] [CrossRef]
- Azizi, M.; Hosseini, M.; Zafarnak, S.; Shanbedi, M.; Amiri, A. Experimental Analysis of Thermal Performance in a Two-Phase Closed Thermosiphon Using Graphene/Water Nanofluid. Ind. Eng. Chem. Res. 2013, 52, 10015–10021. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, Y.; Qi, H.; Cai, W. Experimental study on heat transfer performance of pulsating heat pipes with hybrid working fluids. Int. J. Heat Mass Transf. 2020, 157, 119727. [Google Scholar] [CrossRef]
- Shazali, S.S.; Amiri, A.; Mohd Zubir, M.N.; Rozali, S.; Zabri, M.Z.; Mohd Sabri, M.F.; Soleymaniha, M. Investigation of the thermophysical properties and stability performance of non-covalently functionalized graphene nanoplatelets with Pluronic P-123 in different solvents. Mater. Chem. Phys. 2018, 206, 94–102. [Google Scholar] [CrossRef]
- E37 Committee. Test Method for Evaluating the Resistance to Thermal Transmission of Materials by the Guarded Heat Flow Meter Technique; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Cabaleiro, D.; Nimo, J.; Pastoriza-Gallego, M.J.; Piñeiro, M.M.; Legido, J.L.; Lugo, L. Thermal conductivity of dry anatase and rutile nano-powders and ethylene and propylene glycol-based TiO2 nanofluids. J. Chem. Thermodyn. 2015, 83, 67–76. [Google Scholar] [CrossRef]
- Banisharif, A.; Aghajani, M.; Van Vaerenbergh, S.; Estellé, P.; Rashidi, A. Thermophysical properties of water ethylene glycol (WEG) mixture-based Fe3O4 nanofluids at low concentration and temperature. J. Mol. Liquids 2020, 302, 112606. [Google Scholar] [CrossRef]
- Zeroual, S.; Estellé, P.; Cabaleiro, D.; Vigolo, B.; Emo, M.; Halim, W.; Ouaskit, S. Ethylene glycol based silver nanoparticles synthesized by polyol process: Characterization and thermophysical profile. J. Mol. Liquids 2020, 310, 113229. [Google Scholar] [CrossRef]
- Sengers, J.; Watson, J. Improved International Formulations for the Viscosity and Thermal-Conductivity of Water Substance. J. Phys. Chem. Ref. Data 1986, 15, 1291–1314. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kairi, M.I.; Dayou, S.; Kairi, N.I.; Abu Bakar, S.; Vigolo, B.; Mohamed, A.R. Toward high production of graphene flakes—A review on recent developments in their synthesis methods and scalability. J. Mater. Chem. A 2018, 6, 15010–15026. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Ershova, O.V.; Lillestolen, T.C.; Bichoutskaia, E. Study of polycyclic aromatic hydrocarbons adsorbed on graphene using density functional theory with empirical dispersion correction. Phys. Chem. Chem. Phys. 2010, 12, 6483–6491. [Google Scholar] [CrossRef] [Green Version]
- Schmaltz, B.; Weil, T.; Müllen, K. Polyphenylene-Based Materials: Control of the Electronic Function by Molecular and Supramolecular Complexity. Adv. Mater. 2009, 21, 1067–1078. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuinstra, F.; Koenig, J. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Navas, H.; Desforges, A.; Ghanbaja, J.; Vigolo, B.; Estellé, P. Long-term stability of graphene based nanofluids. IJMERR 2017, 6, 529–533. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Gavrilov, A.N.; McCloskey, J.M.; Tolmachev, Y.V.; Sprunt, S.; Lopatina, L.M.; Selinger, J.V. Thermal conductivity and particle agglomeration in alumina nanofluids: Experiment and theory. Phys. Rev. E 2007, 76, 061203. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, J.P.; Álvarez-Regueiro, E.; Cabaleiro, D.; Fernández-Seara, J.; Fernández, J.; Lugo, L. Functionalized graphene nanoplatelet nanofluids based on a commercial industrial antifreeze for the thermal performance enhancement of wind turbines. Appl. Therm. Eng. 2019, 152, 113–125. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Colla, L.; Barison, S.; Lugo, L.; Fedele, L.; Bobbo, S. Heat Transfer Capability of (Ethylene Glycol + Water)-Based Nanofluids Containing Graphene Nanoplatelets: Design and Thermophysical Profile. Nanoscale Res. Lett. 2017, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Agromayor, R.; Cabaleiro, D.; Pardiñas, Á.; Vallejo, J.; Fernández-Seara, J.; Lugo, L. Heat Transfer Performance of Functionalized Graphene Nanoplatelet Aqueous Nanofluids. Materials 2016, 9, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Laguna, M.D.; Castro-Alvarez, A.; Sledzinska, M.; Maire, J.; Costanzo, F.; Ensing, B.; Pruneda, M.; Ordejon, P.; Torres, C.M.S.; Gomez-Romero, P.; et al. Mechanisms behind the enhancement of thermal properties of graphene nanofluids. Nanoscale 2018, 10, 15402–15409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, C.-W.; Birringer, R.; Clarke, D.R.; Gleiter, H. Effective thermal conductivity of particulate composites with interfacial thermal resistance. J. Appl. Phys. 1997, 81, 6692–6699. [Google Scholar] [CrossRef]
- Estellé, P.; Halelfadl, S.; Maré, T. Thermal conductivity of CNT water based nanofluids: Experimental trends and models overview. J. Therm. Eng. 2015. [Google Scholar] [CrossRef]
- Chu, K.; Li, W.; Tang, F. Flatness-dependent thermal conductivity of graphene-based composites. Phys. Lett. A 2013, 377, 910–914. [Google Scholar] [CrossRef]
- Zhong, W.-R.; Zhang, M.-P.; Ai, B.-Q.; Zheng, D.-Q. Chirality and thickness-dependent thermal conductivity of few-layer graphene: A molecular dynamics study. Appl. Phys. Lett. 2011, 98, 113107. [Google Scholar] [CrossRef]
- Alexeev, D.; Chen, J.; Walther, J.H.; Giapis, K.P.; Angelikopoulos, P.; Koumoutsakos, P. Kapitza Resistance between Few-Layer Graphene and Water: Liquid Layering Effects. Nano Lett. 2015, 15, 5744–5749. [Google Scholar] [CrossRef] [PubMed]
- Selvam, C.; Lal, D.M.; Harish, S. Thermal conductivity enhancement of ethylene glycol and water with graphene nanoplatelets. Thermochim. Acta 2016, 642, 32–38. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamze, S.; Berrada, N.; Cabaleiro, D.; Desforges, A.; Ghanbaja, J.; Gleize, J.; Bégin, D.; Michaux, F.; Maré, T.; Vigolo, B.; et al. Few-Layer Graphene-Based Nanofluids with Enhanced Thermal Conductivity. Nanomaterials 2020, 10, 1258. https://doi.org/10.3390/nano10071258
Hamze S, Berrada N, Cabaleiro D, Desforges A, Ghanbaja J, Gleize J, Bégin D, Michaux F, Maré T, Vigolo B, et al. Few-Layer Graphene-Based Nanofluids with Enhanced Thermal Conductivity. Nanomaterials. 2020; 10(7):1258. https://doi.org/10.3390/nano10071258
Chicago/Turabian StyleHamze, Samah, Nawal Berrada, David Cabaleiro, Alexandre Desforges, Jaafar Ghanbaja, Jérôme Gleize, Dominique Bégin, Florentin Michaux, Thierry Maré, Brigitte Vigolo, and et al. 2020. "Few-Layer Graphene-Based Nanofluids with Enhanced Thermal Conductivity" Nanomaterials 10, no. 7: 1258. https://doi.org/10.3390/nano10071258
APA StyleHamze, S., Berrada, N., Cabaleiro, D., Desforges, A., Ghanbaja, J., Gleize, J., Bégin, D., Michaux, F., Maré, T., Vigolo, B., & Estellé, P. (2020). Few-Layer Graphene-Based Nanofluids with Enhanced Thermal Conductivity. Nanomaterials, 10(7), 1258. https://doi.org/10.3390/nano10071258






