Translating Scientific Advances in the AOP Framework to Decision Making for Nanomaterials
Abstract
1. Introduction
2. Materials and Methods
3. Overview of the AOP Framework and Current Status
3.1. Overview
- AOPs are not chemical-specific. Specificity limits the predictive utility of AOPs for new substances.
- AOPs are designed with modular units. These components should be reusable to enhance flexibility, and they should be designed to accommodate differing levels of detail based on evidence.
- AOPs are a unit of development. An individual AOP is defined as a single, nonbranching sequence of KEs, linked by KERs, connecting a single MIE to a single AO. This structure reduces the complexity, and is a practical unit for development and evaluation.
- AOPs form networks. Multiple AOPs, sharing one or more common KE or KER, form networks that more realistically represent the complexity of biological systems needed to make accurate biological predictions of adverse toxicological outcomes.
- AOPs should be continuously updated. New research should be used to inform and refine existing AOPs.
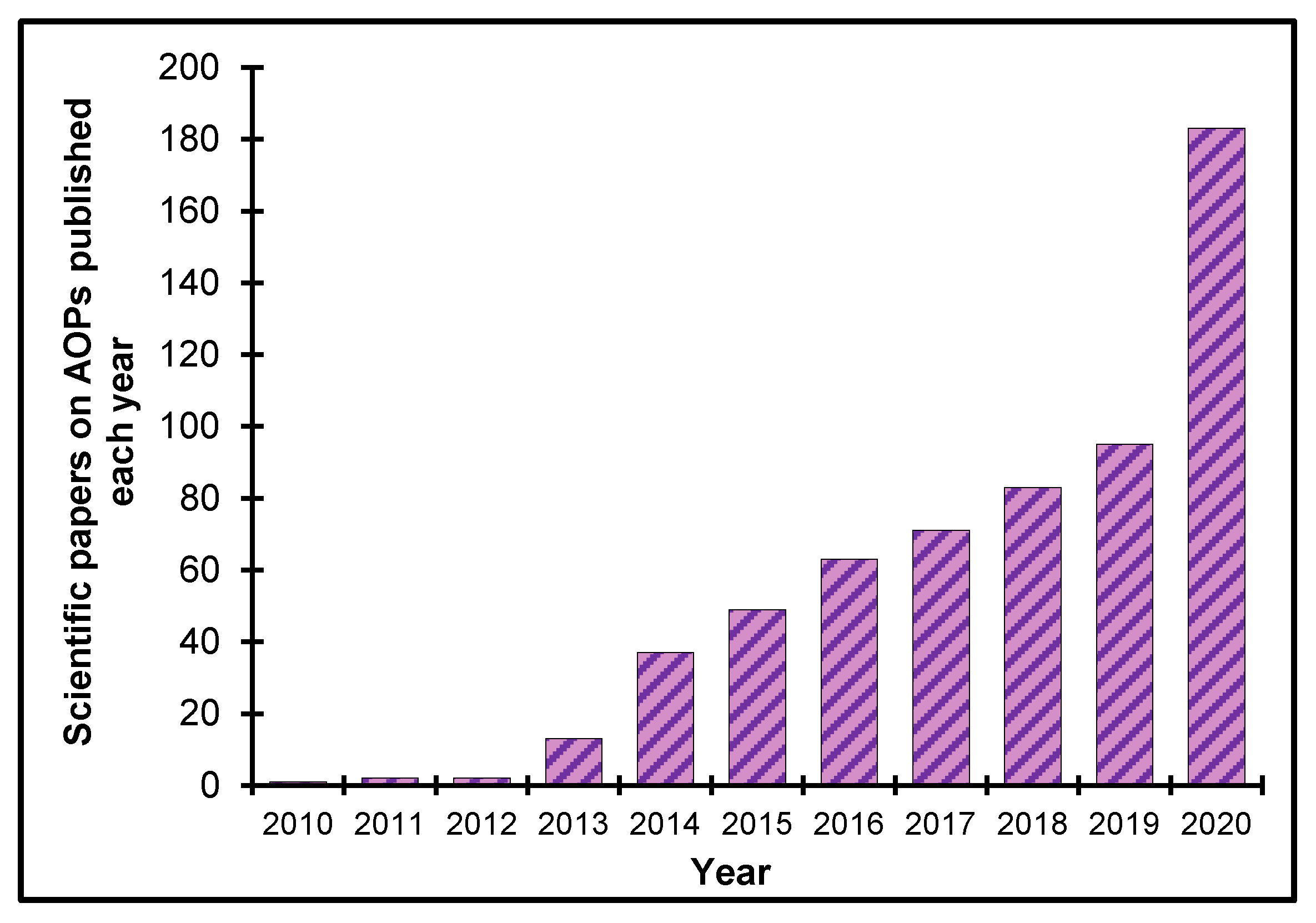
3.2. Current Status
4. Potential Applications of AOPs
- A structured framework to evaluate existing information available for a chemical of interest; potential sources include in chemico, in silico, in vitro, ex vivo, in vivo and ‘omics’ data;
- A way to identify data gaps and efficiently generate missing information to increase confidence in decision making and assessments of risk;
- A framework to apply an iterative approach until sufficient information is gathered for decision making.
5. Recent Progress with AOPs for Nanosafety
5.1. Progress in AOP Development for Manufactured Nanomaterials
5.2. Progress in the Application and Use of AOPs
6. Challenges in the Development and Application of AOPs
6.1. Nanomaterial-Specific Challenges
6.1.1. Limitations of Current Literature
| SmartNanoTox Conducting in vivo, in vitro and in silico research to develop AOPs for adverse pulmonary effects following MN exposure. Research is being used to develop simplified in vitro or in silico tests for the AOPs developed in the project, targeting identified MIEs and KEs for adverse respiratory outcomes from MN inhalation. | PATROLS Developing mechanism-based, nonanimal methods, models and computational tools for MN hazard characterization, targeting the KEs in established AOPs. This includes in silico hazard testing systems, in vitro human tissue models, ecotoxicology models and methods for MN characterization in biological systems. |
| OECD WPMN NanoAOP Developed a methodology and approach to use existing nanotoxicology literature to support MN-relevant AOP development and its use in decision making. | NanoSolveIT Developing: (i) innovative modeling techniques and tools for nanoinformatics; (ii) an IATA to identify the specific characteristics of MNs that are responsible for adverse effects on human health or the environment; and (iii) in silico methods, models and tools which are useful for AOP development using toxicogenomics data and linked to nanomaterial “fingerprints”. See also [19]. |
| NanoCommons Developing a nanoinformatics research infrastructure including a knowledge base to facilitate the reuse of existing nanosafety data, tools to support Open and FAIR data curation and annotation, and in silico tools for analysis and prediction of MN environmental and human health impacts. | GRACIOUS Developing a grouping strategy for MNs that can be incorporated into an IATA. |
| COSMOS Identifying common sets of metadata objects with standard definitions and methods to build better metadata repositories. | DaNa A database with important and generally understandable information on health and the environment as they relate to the application of nanomaterials, as well as data on the safety of manufactured nanomaterials. |
| RiskGONE Developing science-based risk governance of MNs based on an understanding of risks and risk management practices. The project is developing new tools and/or modifying existing ones to identify the environmental and human health impacts of MNs. AOPs for human and environmental end-points are being developed. These tools will be integrated into the work of a European Risk Governance Council to provide governance decisions on the safety of the specific materials. | NanoReg2 Aimed to couple ‘safe-by-design’ (SbD) to the regulatory process, using value chain implementation studies to establish SbD as a fundamental pillar in the validation of a novel NMs. Grouping concepts developed by NanoReg2 were prepared as guidance documents to support industries or regulatory agencies. |
6.1.2. Assays and Methods to Assess MIEs and KEs
6.1.3. Influence of MN Physical and Chemical Properties
6.2. Technical Challenges
6.2.1. AOP Networks
6.2.2. Exposure and Dose
6.2.3. Individual and Interspecies Differences
6.2.4. Repair Mechanisms
6.3. Barriers to Adoption
6.3.1. Lack of Guidance for Risk Assessors
6.3.2. Engagement of Multiple Stakeholders
6.3.3. Communication
7. Summary
8. The Way Forward
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kennedy, A.; Brame, J.; Rycroft, T.; Wood, M.; Zemba, V.; Weiss, C.; Hull, M.; Hill, C.; Geraci, C.; Linkov, I. A Definition and Categorization System for Advanced Materials: The Foundation for Risk-Informed Environmental Health and Safety Testing. Risk Anal. 2019, 39, 1783–1795. [Google Scholar] [CrossRef]
- Carusi, A.; Davies, M.R.; De Grandis, G.; Escher, B.I.; Hodges, G.; Leung, K.M.; Whelan, M.; Willett, C.; Ankley, G.T. Harvesting the promise of AOPs: An assessment and recommendations. Sci. Total. Environ. 2018, 628–629, 1542–1556. [Google Scholar] [CrossRef]
- European Commission. Animals Used for Scientific Purposes. 2020. Available online: https://ec.europa.eu/environment/chemicals/lab_animals/index_en.htm (accessed on 8 September 2019).
- US EPA. Alternative Test Methods and Strategies to Reduce Vertebrate Animal Testing. 2019. Available online: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/alternative-test-methods-and-strategies-reduce (accessed on 6 August 2019).
- Burden, N.; Aschberger, K.; Chaudhry, Q.; Clift, M.J.D.; Fowler, P.; Johnston, H.; Landsiedel, R.; Rowland, J.; Stone, V.; Doak, S.H. Aligning nanotoxicology with the 3Rs: What is needed to realise the short, medium and long-term opportunities? Regul. Toxicol. Pharmacol. 2017, 91, 257–266. [Google Scholar] [CrossRef]
- Stone, V.; Pozzi-Mucelli, S.; Tran, L.; Aschberger, K.; Sabella, S.; Vogel, U.; Poland, C.; Balharry, D.; Fernandes, T.F.; Gottardo, S.; et al. ITS-NANO—Prioritising nanosafety research to develop a stakeholder driven intelligent testing strategy. Part. Fibre Toxicol. 2014, 11, 9. [Google Scholar] [CrossRef]
- Allen, T.E.H.; Goodman, J.M.; Gutsell, S.; Russell, P.J. Defining Molecular Initiating Events in the Adverse Outcome Pathway Framework for Risk Assessment. Chem. Res. Toxicol. 2014, 27, 2100–2112. [Google Scholar] [CrossRef]
- Gerloff, K.; Landesmann, B.; Worth, A.P.; Munn, S.; Palosaari, T.; Whelan, M. The Adverse Outcome Pathway approach in nanotoxicology. Comput. Toxicol. 2017, 1, 3–11. [Google Scholar] [CrossRef]
- Halappanavar, S.; Brule, S.V.D.; Nymark, P.; Gaté, L.; Seidel, C.; Valentino, S.; Zhernovkov, V.; Danielsen, P.H.; De Vizcaya, A.; Wolff, H.; et al. Adverse outcome pathways as a tool for the design of testing strategies to support the safety assessment of emerging advanced materials at the nanoscale. Part. Fibre Toxicol. 2020, 17, 16. [Google Scholar] [CrossRef]
- European Food Safety Authority. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, 5327. [Google Scholar]
- Halappanavar, S.; Ede, J.D.; Shatkin, J.A.; Krug, H.F. A systematic process for identifying key events for advancing the development of nanomaterial relevant adverse outcome pathways. NanoImpact 2019, 15, 100178. [Google Scholar] [CrossRef]
- Delrue, N.; Sachana, M.; Sakuratani, Y.; Gourmelon, A.; Leinala, E.; Diderich, R. The Adverse Outcome Pathway Concept: A Basis for Developing Regulatory Decision-making Tools. Altern. Lab. Anim. 2016, 44, 417–429. [Google Scholar] [CrossRef]
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, D.L.; Crump, U.; Garcia-Reyero, N.; Hecker, M.; Hutchinson, T.H.; Lalone, C.A.; Landesmann, B.; Lettieri, T.; Munn, S.; Nepelska, M.; et al. Adverse Outcome Pathway (AOP) Development I: Strategies and Principles. Toxicol. Sci. 2014, 142, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Langley, G. Adverse Outcome Pathways: Will they Deliver a Superior Alternative to Animal Testing? Lush Prize: 2017. Available online: https://lushprize.org/wp/wp-content/uploads/Lush-Prize-AOP-paper-for-WC10-final.pdf (accessed on 8 September 2019).
- Organisation for Economic Cooperation and Development. Revised Guidance Document on Developing and Assessing Adverse Outcome Pathways; Series on Testing and Assessment; OECD: Paris, France, 2017. [Google Scholar]
- Fadeel, B.; Farcas, L.; Hardy, B.; Vazquez-Campos, S.; Hristozov, D.; Marcomini, A.; Lynch, I.; Valsami-Jones, E.; Alenius, H.; Savolainen, K. Advanced tools for the safety assessment of nanomaterals. Nat. Nanotechnol. 2018, 13, 537–543. [Google Scholar] [CrossRef]
- Horizon Europe. Orientations towards the First Strategic Plan Implementing the Research and Innovation Framework Programme Horizon Europe. 2019. Available online: https://clepa.eu/wp-content/uploads/2019/07/Horizon-Europe-Strategic-Planning-Summer-2019 (accessed on 8 September 2019).
- Afantitis, A.; Melagraki, G.; Isigonis, P.; Tsoumanis, A.; Varsou, D.D.; Valsami-Jones, E.; Papadiamantis, A.; Ellis, L.-J.A.; Sarimveis, H.; Doganis, P.; et al. NanoSolveIT Project: Driving nanoinformatics research to develop innovative and integrated tools for in silico nanosafety assessment. Comput. Struct. Biotechnol. J. 2020, 18, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Ede, J.D.; Ong, K.J.; Goergen, M.; Rudie, A.; Pomeroy-Carter, C.A.; Shatkin, J.A. Risk analysis of cellulose nanomaterails by inhalation: Current state of science. Nanomaterials 2019, 9, 337. [Google Scholar] [CrossRef]
- Kasai, T.; Umeda, Y.; Ohnishi, M.; Mine, T.; Kondo, H.; Takeuchi, T.; Matsumoto, M.; Fukushima, S. Lung carcinogenicity of inhaled multi-walled carbon nanotube in rats. Part. Fibre Toxicol. 2015, 13, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Zhernovkov, V.; Jacobsen, N.R.; Voss, C.; Strunz, M.; Ansari, M.; Schiller, H.B.; Halappanavar, S.; Poulsen, S.S.; Kholodenko, B.; et al. Acute Phase Response as a Biological Mechanism-of-Action of (Nano)particle-Induced Cardiovascular Disease. Small 2020, 16, e1907476. [Google Scholar] [CrossRef]
- Danielsen, P.H.; Knudsen, K.B.; Štrancar, J.; Umek, P.; Koklič, T.; Garvas, M.; Vanhala, E.; Savukoski, S.; Ding, Y.; Madsen, A.M.; et al. Effects of physicochemical properties of TiO2 nanomaterials for pulmonary inflammation, acute phase response and alveolar proteinosis in intratracheally exposed mice. Toxicol. Appl. Pharmacol. 2020, 386, 114830. [Google Scholar] [CrossRef]
- Rahman, L.; Williams, A.; Gelda, K.; Nikota, J.; Wu, D.; Vogel, U.; Halappanavar, S. 21st century tools for nanotoxicology: Transcriptomic biomarker panel and precision-cut lung slice organ mimic system for the assessment of nanomaterial-induced lung fibrosis. Small 2000, 29, e2000272. [Google Scholar] [CrossRef]
- Gaté, L.; Knudsen, K.B.; Seidel, C.; Berthing, T.; Chézeau, L.; Jacobsen, N.R.; Valentino, S.; Wallin, H.; Bau, S.; Wolff, H.; et al. Pulmonary toxicity of two different multi-walled carbon nanotubes in rat: Comparison between intratracheal instillation and inhalation exposure. Toxicol. Appl. Pharmacol. 2019, 375, 17–31. [Google Scholar] [CrossRef]
- Yang, L.; Gradl, R.; Dierolf, M.; Möller, W.; Kutschke, D.; Feuchtinger, A.; Hehn, L.; Donnelley, M.; Günther, B.; Achterhold, K.; et al. Multimodal Precision Imaging of Pulmonary Nanoparticle Delivery in Mice: Dynamics of Application, Spatial Distribution, and Dosimetry. Small 2019, 15, e1904112. [Google Scholar] [CrossRef] [PubMed]
- Mech, A.; Rasmussen, K.; Jantunen, P.; Aicher, L.; Alessandrelli, M.; Bernauer, U.; Bleeker, E.A.J.; Bouillard, J.; Fanghella, P.D.P.; Draisci, R.; et al. Insights into possibilities for grouping and read-across for nanomaterials in EU chemicals legislation. Nanotoxicology 2019, 13, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Papadiamantis, A.G.; Klaessig, F.C.; Exner, T.E.; Hofer, S.; Hofstaetter, N.; Himly, M.; Williams, M.A.; Doganis, P.; Hoover, M.D.; Afantis, A. Metadata stewardship in nanosafety research: Community-driven organisation of metadata schemas to support FAIR nanoscience data. Nanomaterials 2020, in press. [Google Scholar]
- Lalone, C.; Ankley, G.T.; Belanger, S.E.; Embry, M.R.; Hodges, G.; Knapen, D.; Munn, S.; Perkins, E.J.; Rudd, M.; Villeneuve, D.L.; et al. Advancing the adverse outcome pathway framework-An international horizon scanning approach. Environ. Toxicol. Chem. 2017, 36, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Albin, M.; Alenius, H.; Bhattacharya, K.; Carlander, U.; Gliga, A.; Grafström, R.; Gustavsson, P.; Johanson, G.; Julander, A.; et al. Nanotoxicology—State-of-the-Art and Future Research Needs. IMM Rapport Nr. 1; Institute of Environmental Medicine, Karolinska Institutet: Solna, Sweden, 2018. [Google Scholar]
- Krug, H.F.; Nau, K. Reliability for Nanosafety research—Considerations on the basis of a comprehensive literature review. ChemBioEng Rev. 2017, 4, 331–338. [Google Scholar] [CrossRef]
- Krug, H.F. The uncertainty with nanosafety: Validity and reliability of published data. Colloids Surf. B Biointerfaces 2018, 172, 113–117. [Google Scholar] [CrossRef]
- Faria, M.; Björnmalm, M.; Thurecht, K.J.; Kent, S.J.; Parton, R.; Kavallaris, M.; Johnston, A.P.R.; Gooding, J.J.; Corrie, S.R.; Boyd, B.J.; et al. Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol. 2018, 13, 777–785. [Google Scholar] [CrossRef]
- Ellis, L.-J.A.; Valsami-Jones, E.; Lynch, I. Exposure medium and particle ageing moderate the toxicological effects of nanomaterials to Daphnia magna over multiple generations: A case for standard test review? Environ. Sci. Nano 2020, 7, 1136–1149. [Google Scholar] [CrossRef]
- Nasser, F.; Lynch, I. Updating traditional regulatory tests for use with novel materials: Nanomaterial toxicity testing with Daphnia magna. Saf. Sci. 2019, 118, 497–504. [Google Scholar] [CrossRef]
- Potthoff, A.; Weil, M.; Meißner, T.; Kühnel, D. Towards sensible toxicity testing for nanomaterials: Proposal for the specification of test design. Sci. Technol. Adv. Mater. 2015, 16, 65006. [Google Scholar] [CrossRef]
- Stone, V.; Johnston, H.; Schins, R.P. Development of in vitro systems for nanotoxicology: Methodological considerations. Crit. Rev. Toxicol. 2009, 39, 613–626. [Google Scholar] [CrossRef] [PubMed]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Pirela, S.V.; Pal, A.; Liu, J.; Srebric, J.; Demokritou, P. Advanced computational modeling for in vitro nanomaterial dosimetry. Part. Fibre Toxicol. 2015, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Labib, S.; Williams, A.; Yauk, C.L.; Nikota, J.; Wallin, H.; Vogel, U.; Halappanavar, S. Nano-risk Science: Application of toxicogenomics in an adverse outcome pathway framework for risk assessment of multi-walled carbon nanotubes. Part. Fibre Toxicol. 2016, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.T.; Poulsen, S.S.; Hadrup, N.; Jacobsen, N.R.; Vogel, U. Commentary: The chronic inhalation study in rats for assessing lung cancer risk may be better than its reputation. Part. Fibre Toxicol. 2019, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Vogel, U.; Cassee, F.R. Editorial: Dose-dependent ZnO particle-induced acute phase response in humans warrants re-evaluation of occupational exposure limits for metal oxides. Part. Fibre Toxicol. 2018, 15, 7. [Google Scholar] [CrossRef]
- Nikota, J.; Banville, A.; Goodwin, L.R.; Wu, D.; Williams, A.; Yauk, C.L.; Wallin, H.; Vogel, U.; Halappanavar, S. Stat-6 signaling pathway and not Interleukin-1 mediates multi-walled carbon nanotube-induced lung fibrosis in mice: Insights from an adverse outcome pathway framework. Part. Fibre Toxicol. 2017, 14, 37. [Google Scholar] [CrossRef]
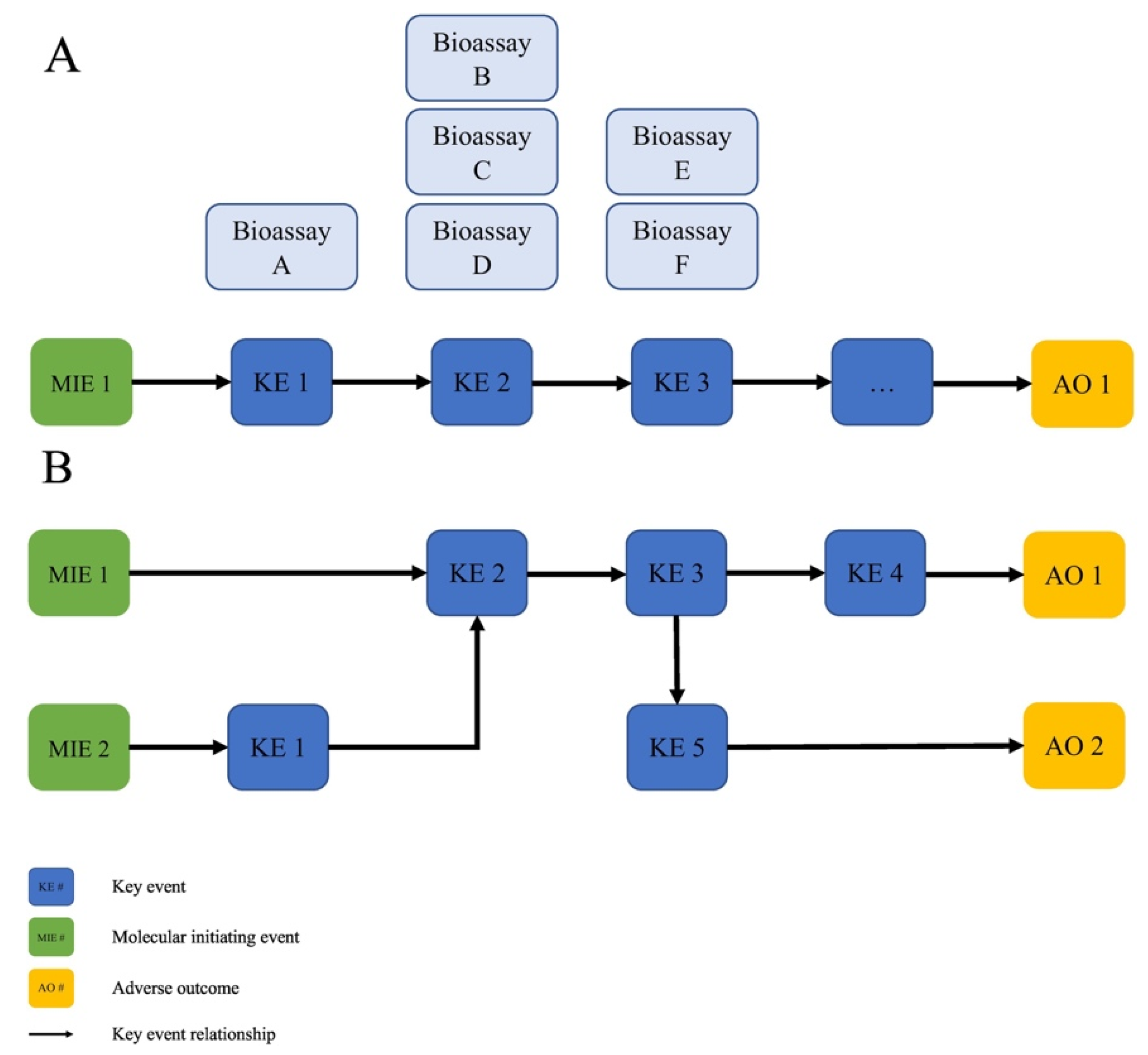

| 1. How can the use of the AOP framework be advanced for decision making about the safety of MNs? |
| 2. What is needed for the future development of MN-relevant AOPs and supporting data? |
| 3. How can the development and adoption of in vitro assays be targeted to KEs to enable the use of AOPs as part of an IATA for MN decision making? |
| 1. Evaluation of existing information |
|
|
|
| 2. Identification of data gaps and generation of new data |
|
|
|
|
|
| 3. Iterative decision making |
|
|
| 1. Advance MN-relevant and Advanced Material Considerations in AOP Development The AOP framework requires the continued development of predictive pathways, building on the efforts of the toxicology community via the AOP Wiki. Needs include updates to toxicological mechanisms within AOPs that identify processes and considerations relevant to MN toxicity. Further, the path for emerging advanced and hybrid materials to use the AOP Framework for early-stage safety decisions can be outlined from these efforts. Recommendations include: | ||
| Short-term Actions | Medium-term Actions | Long-term Actions |
| 1. Establish the types of data required to develop AOPs for MNs and identify existing NM-relevant AOPs; 2. Compare molecular initiating events (MIEs), key events (KEs), key event relationships (KERs) and adverse outcomes (AOs) identified for MNs to AOPs in the AOP Wiki; 3. Identify MN-relevant MIEs, KEs and KERs. Research should include identifying MN-relevant mechanisms and MIEs, which are often based upon physical interactions with MNs instead of molecular ones (e.g., frustrated phagocytosis and particle-surface-induced reactive oxygen species [9]). | 1. Identify similarities and differences in MIEs, KEs and KERs between other emerging advanced materials and MNs [9]; 2. Conduct targeted research on MNs to elucidate the effect of interspecies variability on AOPs and the development of related testing strategies [40,41]. This can include side-by-side testing of in vitro cell lines and 3D models from a number of species exposed to a suite of MNs to examine conserved mechanisms and potencies; 3. Use ‘omics’ approaches to identify gene, protein and metabolite markers of MN exposure (e.g., using heatmaps) and their implications for AOP development [24,39,42]. | 1. Develop data sets for quantitative AOPs (qAOPs) that include consideration of the exposure conditions necessary for MN risk assessment. This includes adopting formal definitions and structures for qAOPs and developing case studies outlining the development and use of qAOPs for MNs; 2. Develop a testing strategy for advanced and hybrid materials (smart and responsive materials) to identify and quantify MIEs, KEs, KERs and AOs [9]; 3. Convene experts to discuss how AOPs can account for individual (e.g., sex or life-stage) and interspecies variations which can be then used to reduce the associated uncertainty for decision making. |
| 2. Utilize Existing Data from the Literature and Previous Projects A diverse set of data has been developed that may be useful for furthering the development, application and use of AOPs for MN risk assessment. So far as possible, these data should be taken advantage of to advance knowledge and identify opportunities for additional AOP development. This requires extensive expert-driven curation efforts. Recommendations include: | ||
| Short-term Actions | Medium-term Actions | Long-term Actions |
| 1. Evaluate data quality from the peer review literature and current suite of in vitro assays based on identified KEs; 2. Encourage researchers (and publishers) to make their raw toxicology data from peer-reviewed literature available publicly; 3. Harmonize formats for reporting toxicology data (including negative results) to facilitate the development of databases; 4. Build searchable databases for priority MNs that includes funded research and literature (e.g., available data developed under NanoCommons, eNanoMapper, NanoReg2, GRACIOUS and DaNa projects), as well as traditional chemical databases (e.g., TOXCAST). Efforts should include collecting negative data. | 1. Develop guidance on how the existing nanotoxicity literature, despite its documented limitations (e.g., minimal reporting of physical and chemical characteristics), can be used for AOP development and decision making; 2. Broaden access and use of existing data sources (e.g., Nanomaterial-Biological Interactions Knowledgebase, Nanomaterials Knowledge Informatics Commons (NIKC); eNanoMapper, NanoCommons KnowledgeBase) and other resources; 3. Evaluate publicly available REACH data for MNs in terms of use in AO and predictive modeling; 4. Identify novel biomarkers for hazard evaluation; 5. Develop research projects to fill data gaps for identified endpoints. | Create processes to continually update publicly available databases as new data is developed. |
| 3. Promote Reliable and Quantitative MN Data Generation and Management High quality data are essential to ensure that MN-relevant AOPs can be developed and used in decision making. Guidance is needed on the types of data and reporting standards to enable the use of AOP in regulatory decision making. Coordinated efforts among stakeholders will improve efficiency and limit additional testing. Recommendations include: | ||
| Short-term Actions | Medium-term Actions | Long-term Actions |
| 1. Identify priority AOs observed with MNs and initiate research into AOP development for these AOs; 2. Standardize the endpoints and reporting elements of assays evaluating MIEs, KEs and KERs so that high quality, comparable data is generated, published and added to databases, including negative data; 3. Develop guidance on the types of data that need to be generated and reported by the research community for their work to be useful in regulatory decision making. | 1. Generate data to allow for grouping – data collection/mining to determine the mode of action using MNs that can represent groups of MNs/functionalizations; 2. Develop MN-specific resources (for the AOP Wiki) to encourage the coordination and cooperation among stakeholders which is needed for efficient, high-quality AOP development. | 1. Advance modeling and QSAR databases and link to the physical and chemical attributes of MNs; 2. Adopt iterative decision making, including increased confidence in nontraditional methods and use of nontraditional data and methods to improve weight-of-evidence in decision making. |
| 4. Advance Knowledge of the Quantitative Relationships Between MN Physical and Chemical Characteristics and AOP Elements A better understanding of the quantitative relationships between MN physical and chemical characteristics and toxicological outcomes is required. It is recommended to: | ||
| Short-term Actions | Medium-term Actions | Long-term Actions |
| 1. Review findings of existing data and research on the relationships between physical and chemical properties and MN KEs, including MIEs, AOs and KERs; 2. Develop hypotheses of predictive relationships between MN physical and chemical properties and biological outcomes. | 1. Test predictive physical and chemical relationships of MNs to biological outcomes, using carefully controlled changes within and across materials (furthering the work of the projects which inaugurated this effort, such as SmartNanoTox and NanoMILE); 2. Assess the importance of using alternative dose metrics to mass (e.g., surface area, particle number) in predicting toxicological outcomes for MNs; 3. Where appropriate, incorporate alternative dose metrics into developed benchmark levels for MNs for screening and risk assessment. | 1. Develop quantitative structure–activity relationships (QSAR) as predictive tools for KEs. |
| 5. Identify Current Applications of the AOP Framework for MN Decision Making Current applications of the AOP framework (e.g., prioritization, grouping and read-across) can be adopted into decision making. It is recommended to: | ||
| Short-term Actions | Medium-term Actions | Long-term Actions |
| 1. Identify screening-level MN safety decisions that are fit-for-purpose/can rely on AOPs; 2. Incorporate AOP elements into grouping and read-across decision trees for MNs. | 1. Adopt a testing scheme/decision tree for MN grouping and read-across; 2. Develop guidance and case studies for use of AOPs in regulatory decision making (e.g., MN prioritization; grouping, categorization and read-across; and hazard identification and ranking). | 1. Develop guidance and case studies for the use of AOPs in product development decision making, and the implementation of a safe-by-design approach. |
| 6. Establish Test Methods and Protocols which are Useful for MN Decision Making Test methods to accurately measure MN-relevant MIEs and KEs are required to advance the use of AOPs as part of an IATA for MN decision making. The development (and verification) of harmonized and standardized MN-relevant test methods is needed: | ||
| Short-term Actions | Medium-term Actions | Long-term Actions |
| 1. Evaluate, advance or develop physical and chemical characterization protocols for MNs and determine how they can be used to identify MIE, KE and AO portions of the AOP framework; 2. Prioritize KEs and the assays/methods to characterize them for development, with KEs closer to an AO being prioritized to ensure relevance for regulatory decision making as part of an IATA; 3. Evaluate, advance or develop in silico, in chemico, in vitro and ex vivo assays for MNs and determine how they can be used to characterize MIE, KE and AO portions of the AOP framework [24]; 4. Initiate Test Guideline development for assays tailored to MNs that address considerations such as physical and chemical characterization, dispersion and dosing relevant to AOPs; 5. Develop guidance on the minimum level of validation required for a given in vitro assay or method for regulatory decision making. | 1. Create voluntary standard methods for IATA; 2. Consider the formal adoption of IATA for certain MN hazard or risk decisions; 3. Advance new in vitro test development to screen for MIEs and KEs; 4. Identify test methods (including in silico) for high throughput screening. | 1. Develop OECD test guidelines for MNs that relate to MIEs, KEs and AOs; 2. Where appropriate, formally adopt IATA for MNs; 3. Adopt harmonized, standardized tests for high throughput screening. |
| 7. Demonstrate Predictive Capability of AOPs and In Vitro Test Methods A coordinated effort is needed to ensure alternative testing strategies are predictive of adverse outcomes of regulatory relevance. Recommendations include: | ||
| Short-term Actions | Medium-term Actions | Long-term Actions |
| 1. Assess the strength of evidence for considering dose-response relationships in AOPs as predictive tools for MN risk assessment. | 1. Compare the predictive capability of in vitro assays for MIEs and KEs with in vivo observations or epidemiological data; 2. Design and conduct side-by-side in vitro and in vivo testing for representative MNs to compare toxicity mechanisms and potency across MNs and assays (furthering the work of the projects that inaugurated this effort, such as PATROLS). | 1. Develop and test predictive alternative testing models; 2. Validate predictive alternative testing models. |
| 8. Guidance to Facilitate Adoption of MN-relevant AOPs for MN Decision Making The science required to address the technical challenges of transitioning to alternative (i.e., nonanimal) toxicity testing is progressing, but efforts are needed to translate and incorporate these developments into decision making about the safety of MNs. Recommendations include: | ||
| Short-term Actions | Medium-term Actions | Long-term Actions |
| 1. Identify MN-relevant and MN-specific AOPs, KEs and KERs, including assessments of the AOPs which have been officially OECD-endorsed, approved or are under review and under active development for MN-relevance (e.g., https://aopwiki.org/aops/173) | 1. Develop and validate an IATA based on KEs, KERs and AOPs that can be used in risk assessments of new nanoscale materials. This includes identifying and prioritizing which KEs are critical for testing as part of an IATA, building on the work currently ongoing in NanoSolveIT; 2. Develop guidance for risk assessors on developing an IATA based on AOP frameworks for MN safety assessments. This should include how to pick critical KEs, or a suite of KEs, for testing. | 1. Incorporate technical developments into specific regulatory guidance/policy documents. |
| 9. Stakeholder Communication and Engagement on the Use of AOPs for MN Decision Making To facilitate the development, adoption and use of the AOP framework for MN decision making, the engagement of multiple stakeholders with a broad range of expertise is essential, and coordination and cooperation are needed. Recommendations include: | ||
| Short-term Actions | Medium-term Actions | Long-term Actions |
| 1. Develop communication and educational materials on the use of the AOP framework for MN decision making for nontechnical stakeholders; 2. Organize additional workshops which seek to encourage participation from various stakeholders with a vested interest in AOP development and application for MN decision making, including academics, policy-makers, regulators and industry. | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ede, J.D.; Lobaskin, V.; Vogel, U.; Lynch, I.; Halappanavar, S.; Doak, S.H.; Roberts, M.G.; Shatkin, J.A. Translating Scientific Advances in the AOP Framework to Decision Making for Nanomaterials. Nanomaterials 2020, 10, 1229. https://doi.org/10.3390/nano10061229
Ede JD, Lobaskin V, Vogel U, Lynch I, Halappanavar S, Doak SH, Roberts MG, Shatkin JA. Translating Scientific Advances in the AOP Framework to Decision Making for Nanomaterials. Nanomaterials. 2020; 10(6):1229. https://doi.org/10.3390/nano10061229
Chicago/Turabian StyleEde, James D., Vladimir Lobaskin, Ulla Vogel, Iseult Lynch, Sabina Halappanavar, Shareen H. Doak, Megan G. Roberts, and Jo Anne Shatkin. 2020. "Translating Scientific Advances in the AOP Framework to Decision Making for Nanomaterials" Nanomaterials 10, no. 6: 1229. https://doi.org/10.3390/nano10061229
APA StyleEde, J. D., Lobaskin, V., Vogel, U., Lynch, I., Halappanavar, S., Doak, S. H., Roberts, M. G., & Shatkin, J. A. (2020). Translating Scientific Advances in the AOP Framework to Decision Making for Nanomaterials. Nanomaterials, 10(6), 1229. https://doi.org/10.3390/nano10061229







