Synthesis and Surface Engineering of Inorganic Nanomaterials Based on Microfluidic Technology
Abstract
1. Introduction
2. Progress of Microfluidic Technology in the Synthesis of Inorganic Nanomaterials
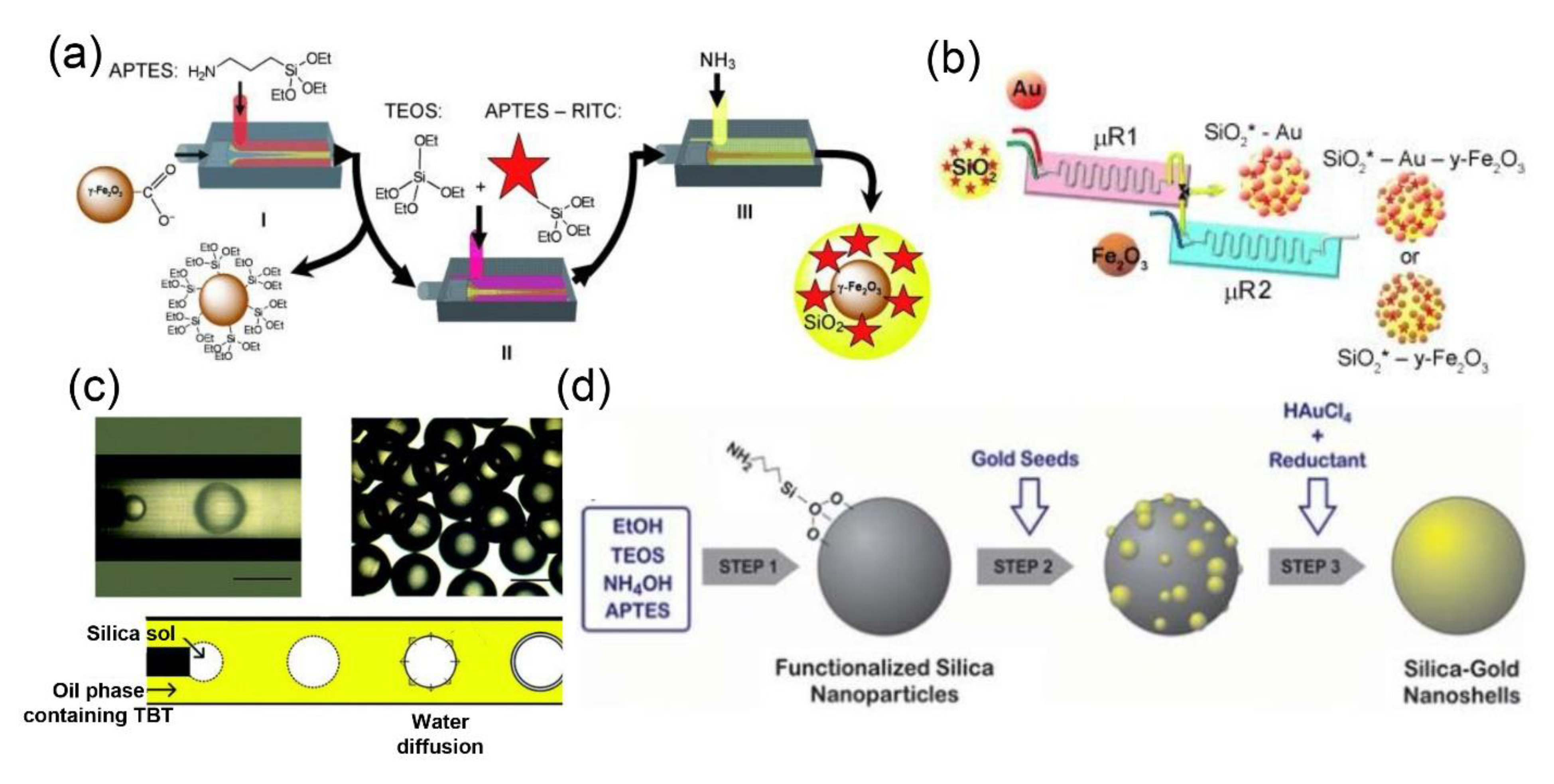
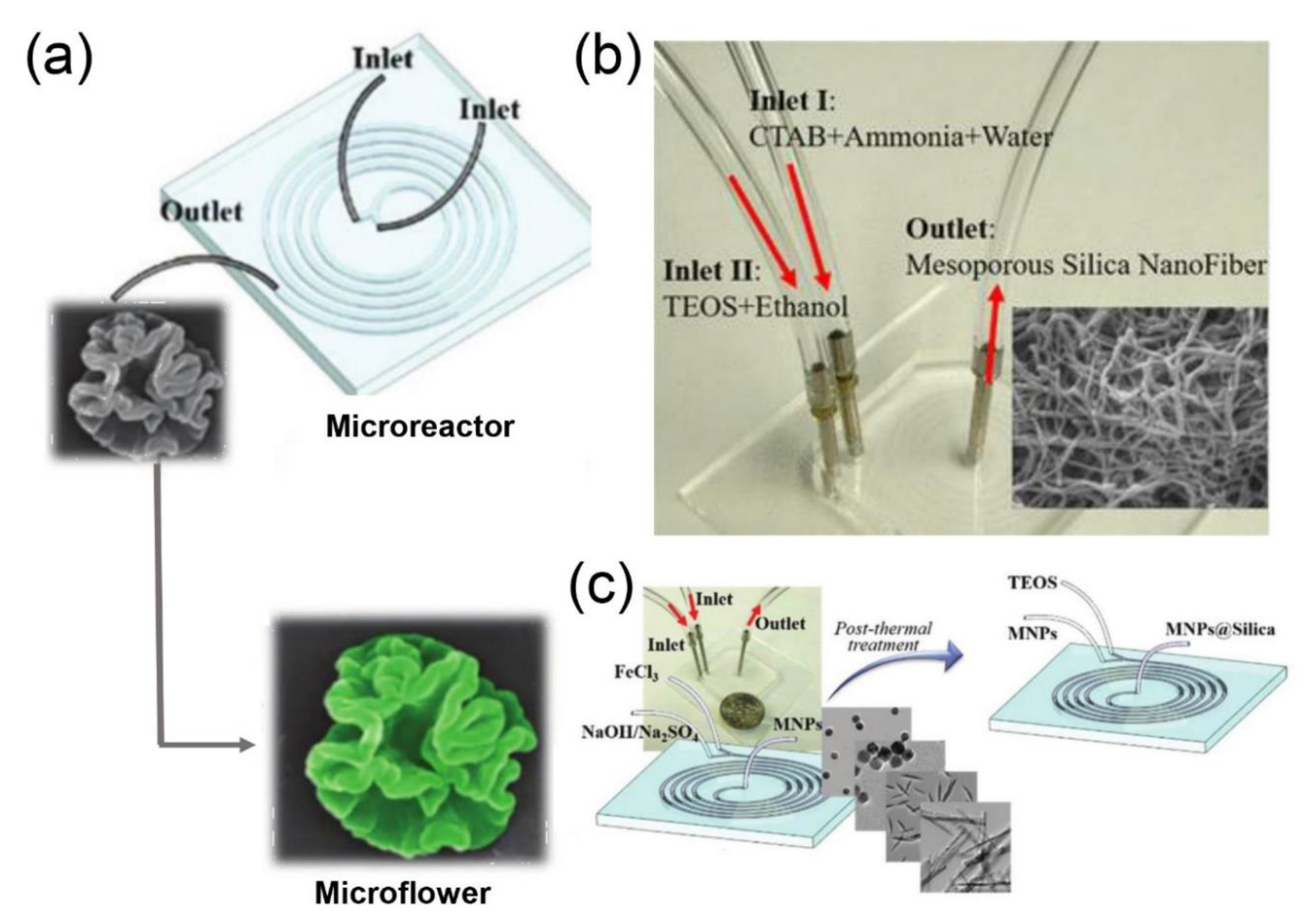
2.1. Silica Nanomaterials
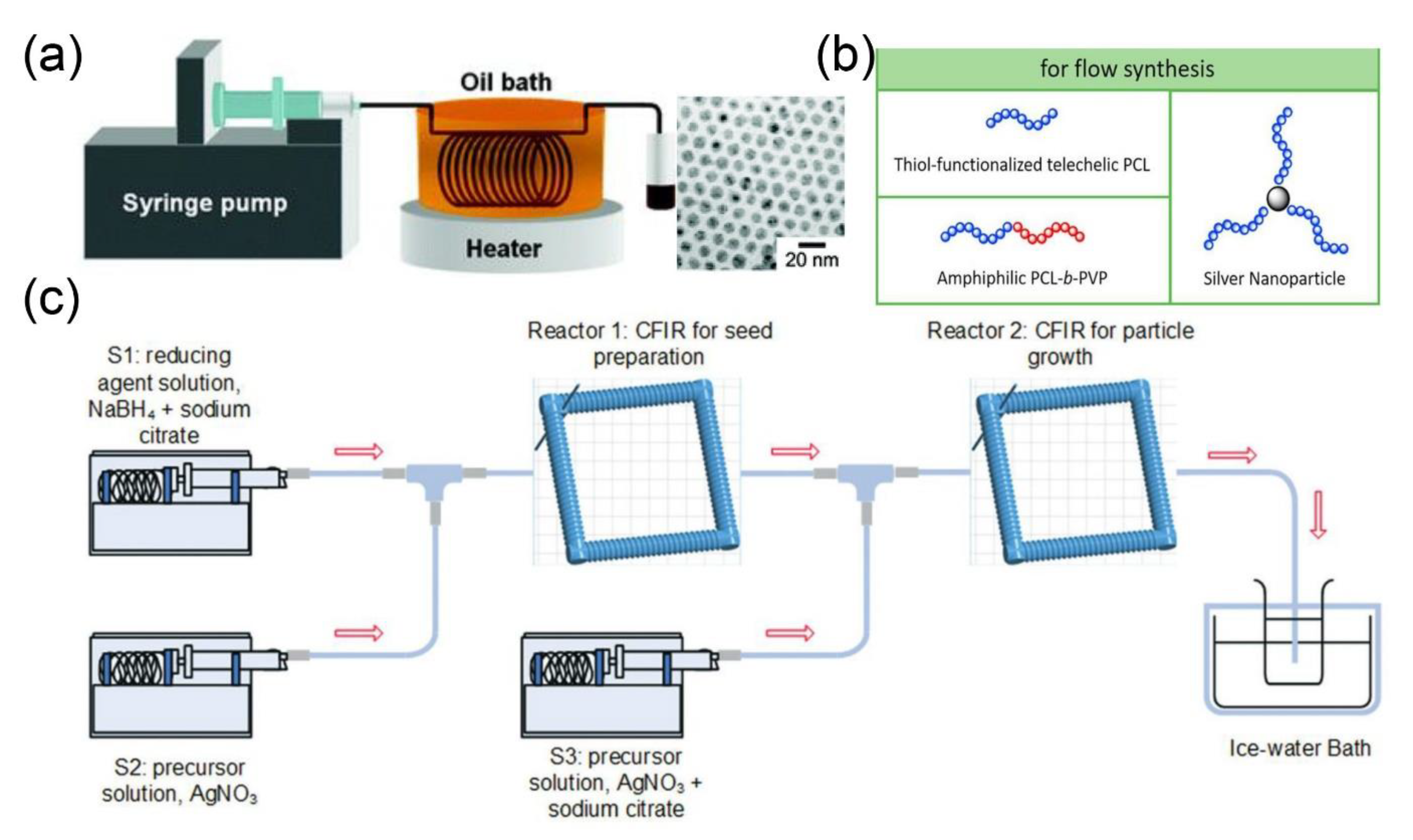
2.2. Metal and Metal Composite Nanomaterials
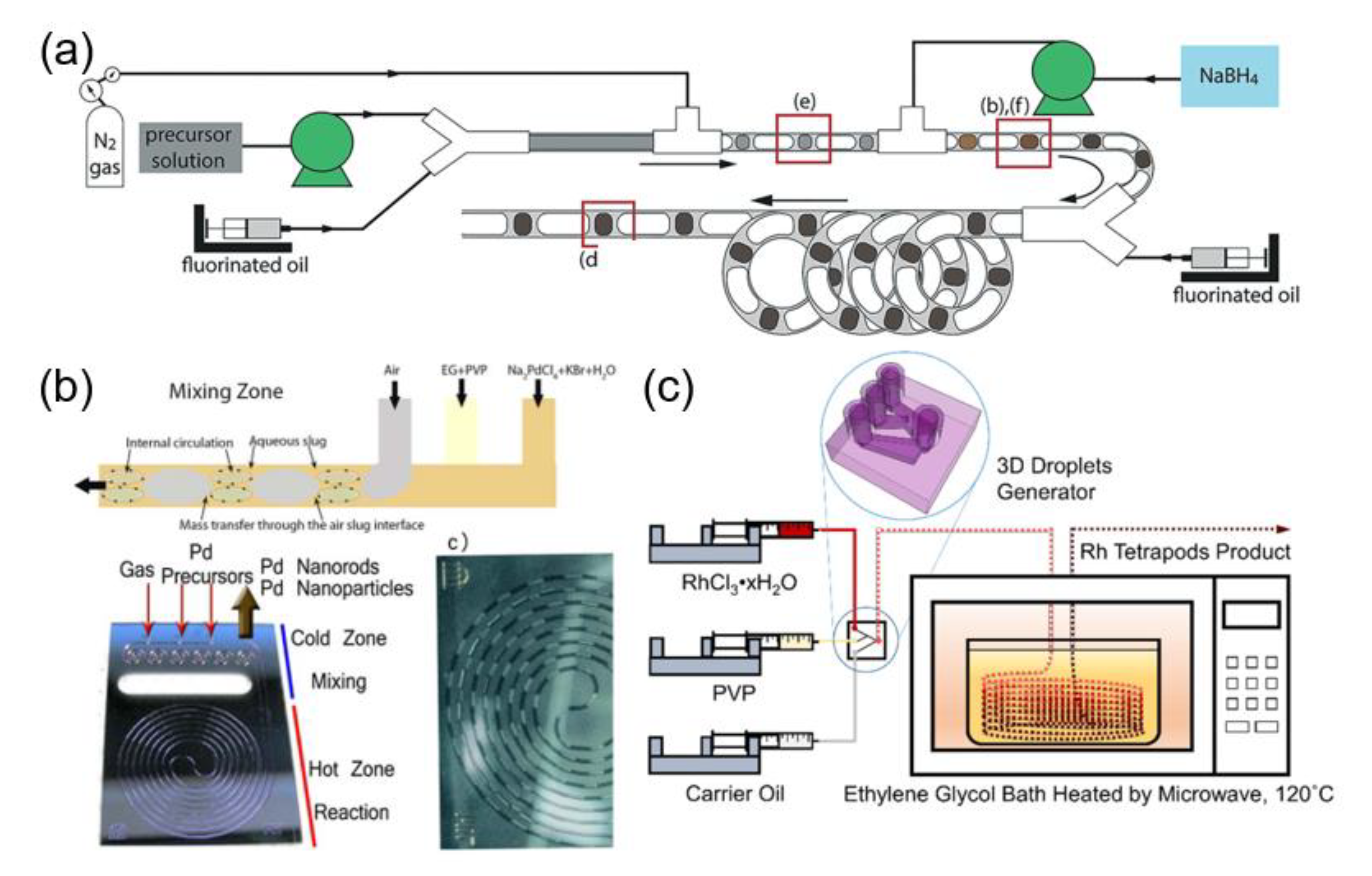
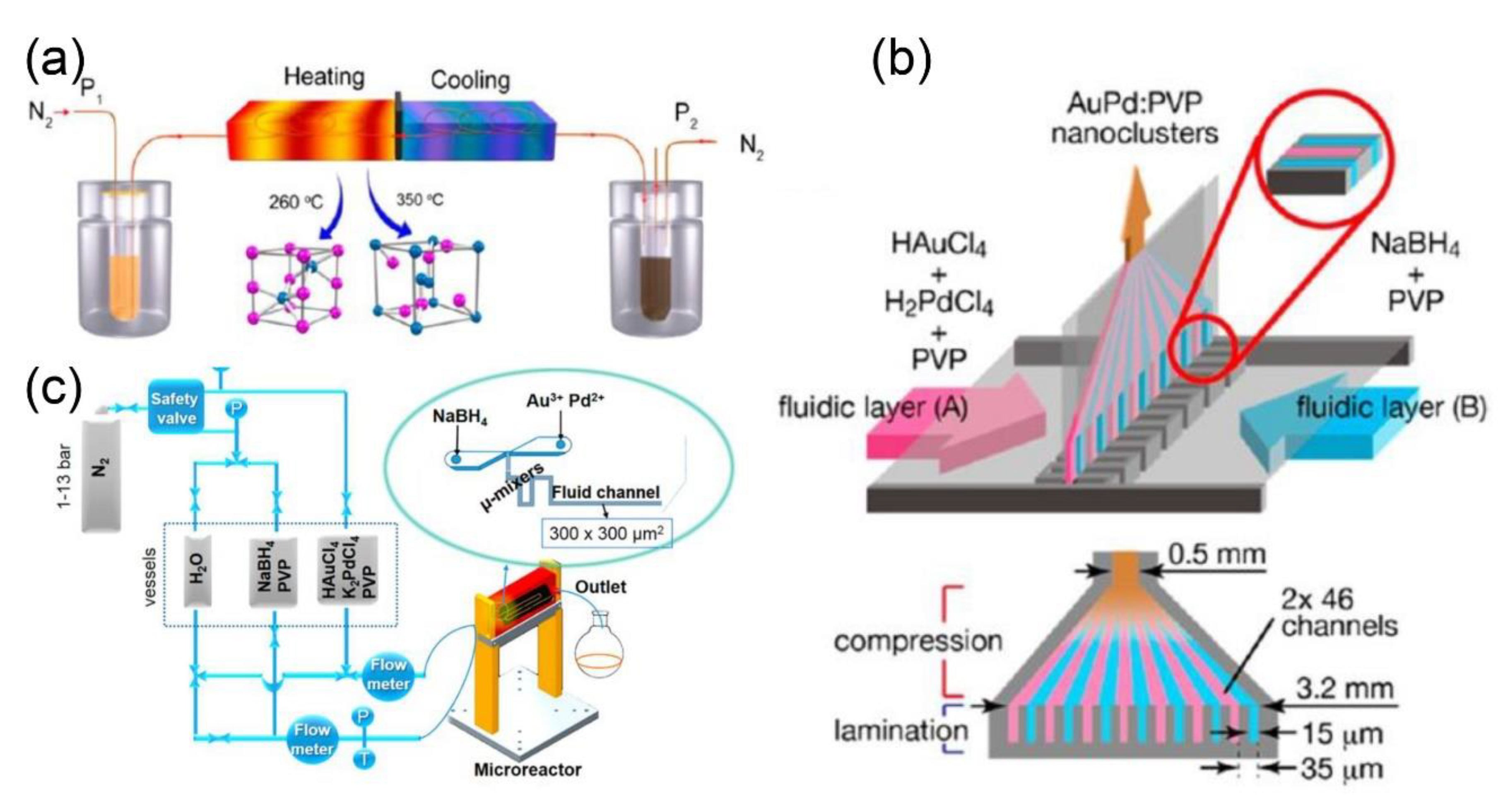
2.2.1. Microfluidic Synthesis of Metal Nanoparticles
2.2.2. Microfluidic Synthesis of Metal Composite Nanoparticles
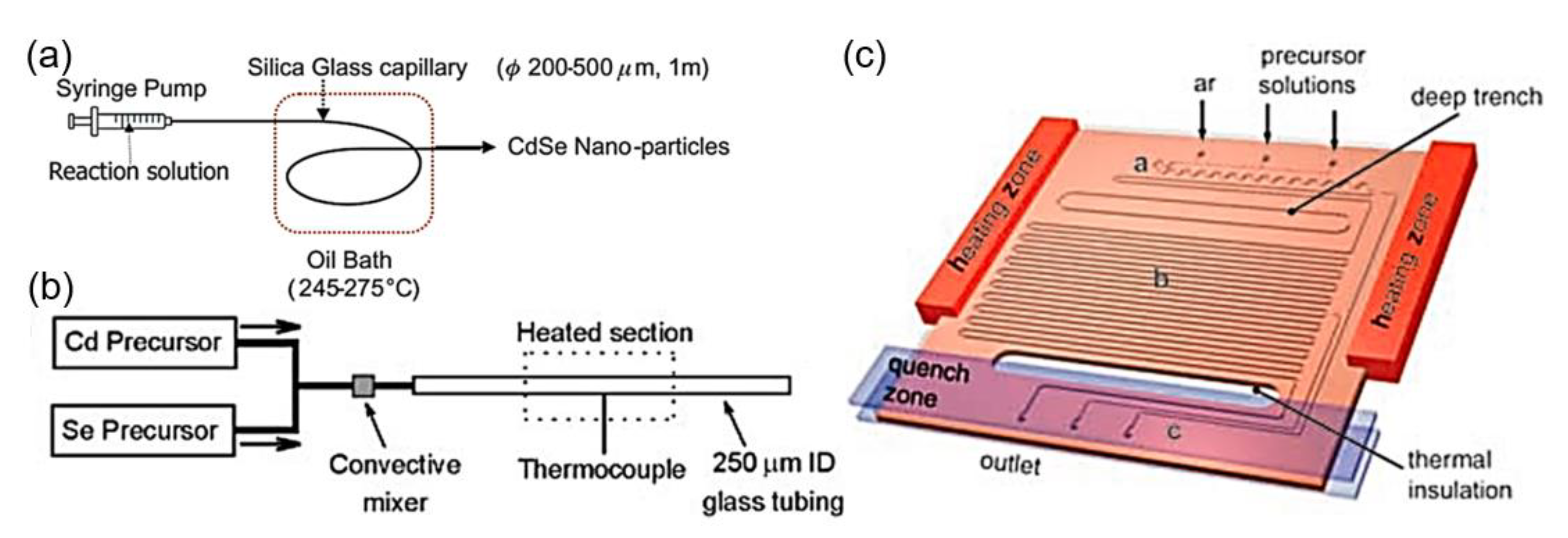
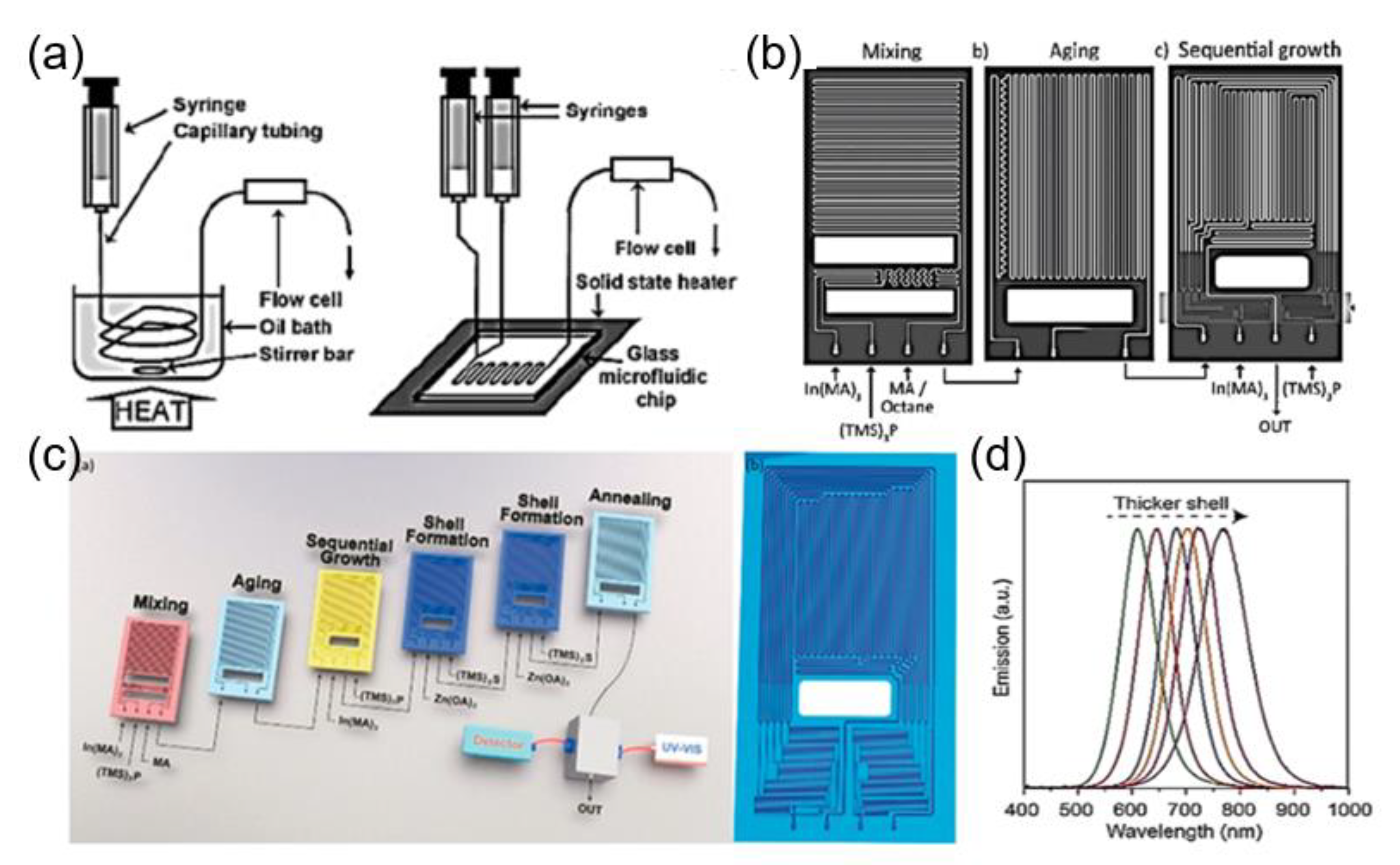
2.2.3. Microfluidic Synthesis of QDs
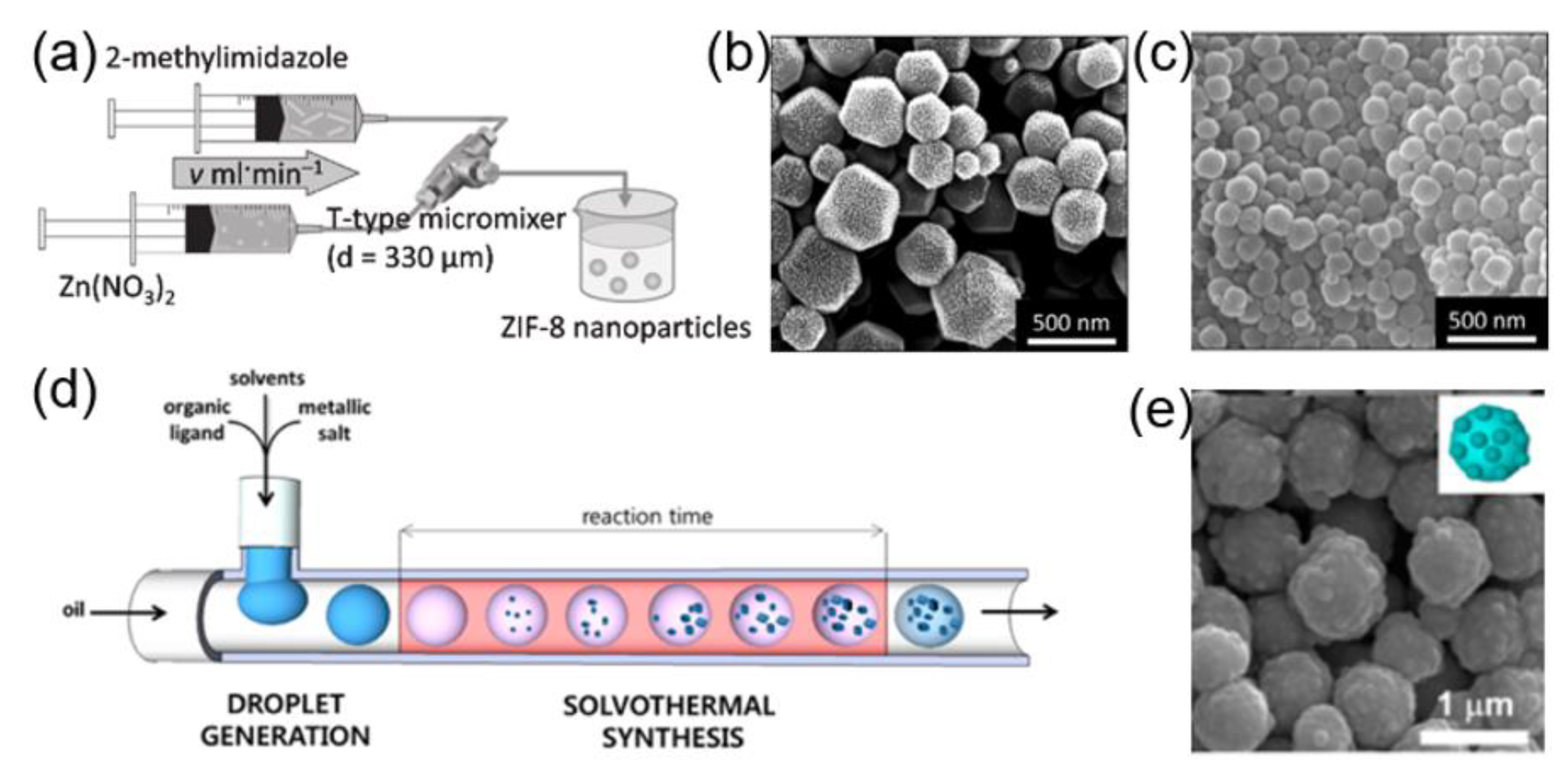
2.2.4. Microfluidic Synthesis of MOFs
3. Progress of Microfluidic Technology for the Surface Modification of Inorganic Nanomaterials
3.1. Surface Modification of Solid Particles
3.2. Surface Modification of Porous Materials
4. Conclusions and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Sun, X.; Cai, W.; Chen, X. Positron emission tomography imaging using radiolabeled inorganic nanomaterials. Acc. Chem. Res. 2015, 48, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lu, Y.; Liu, J.; Yang, X. Production and photoelectric activity of P and Al Co-doped ZnO nanomaterials. Eur. J. Inorg. Chem. 2015, 22, 3708–3714. [Google Scholar] [CrossRef]
- Caminade, A.M. Inorganic dendrimers: Recent advances for catalysis, nanomaterials, and nanomedicine. Chem. Soc. Rev. 2016, 45, 5174–5186. [Google Scholar] [CrossRef]
- Hu, B.; Chen, W.; Zhou, J. High performance flexible sensor based on inorganic nanomaterials. Sens. Actuators B Chem. 2013, 176, 522–533. [Google Scholar] [CrossRef]
- Sao, R.; Vaish, R.; Sinha, N. Multifunctional drug delivery systems using inorganic nanomaterials: A review. J. Nanosci. Nanotechnol. 2015, 15, 1960–1972. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Song, S.; Lee, J.; Kim, J.-M. Size-controlled fabrication of supramolecular vesicles for the construction of conjugated polymer sensors with enhanced optical properties. Langmuir 2010, 26, 17840–17842. [Google Scholar] [CrossRef]
- Liu, Z.; Fontana, F.; Python, A.; Hirvonen, J.; Santos, H.A. Microfluidics for production of particles: Mechanism, methodology, and applications. Small 2019, 16, 1904673. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Valencia1, P.M.; Farokhzad, O.C.; Karnik, R.; Langer, R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat. Nanotechnol. 2012, 7, 623–629. [Google Scholar] [CrossRef]
- Gu, F.X.; Karnik, R.; Wang, A.Z.; Alexis, F.; Levy-Nissenbaum, E.; Hong, S.; Langer, R.S.; Farokhzad, O.C. Targeted nanoparticles for cancer therapy. Nano Today 2007, 2, 14–21. [Google Scholar] [CrossRef]
- Ma, J.; Lee, S.M.-Y.; Yi, C.; Li, C.-W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications. Lab Chip 2017, 17, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Baah, D.; Tigner, J.; Bean, K.; Walker, N.; Britton, B.; Floyd-Smith, T. Microfluidic synthesis and post processing of non-spherical polymeric microparticles. Microfluid. Nanofluid. 2012, 12, 657–662. [Google Scholar] [CrossRef][Green Version]
- Abate, A.R.; Krummel, A.T.; Lee, D.; Marquez, M.; Holtze, C.; Weitz, D.A. Photoreactive coating for high-contrast spatial patterning of microfluidic device wettability. Lab Chip 2008, 8, 2157. [Google Scholar] [CrossRef]
- Sarma, P.; Patowari, P.K. Design and Analysis of passive Y-type micromixers for enhanced mixing performance for biomedical and microreactor application. J. Adv. Manuf. Syst. 2016, 15, 161–172. [Google Scholar] [CrossRef]
- Thorsen, T.; Roberts, R.W.; Arnold, F.H.; Quake, S.R. Dynamic pattern formation in a vesicle-generating microfluidic device. Phys. Rev. Lett. 2001, 86, 4163–4166. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Helgeson, M.E.; Myerson, A.S.; Hatton, T.A.; Doyle, P.S.; Trout, B.L. Controlled nucleation from solution using polymer microgels. J. Am. Chem. Soc. 2011, 133, 3756–3759. [Google Scholar] [CrossRef]
- Wannasarit, S.; Wang, S.; Figueiredo, P.; Trujillo, C.; Eburnea, F.; Simón-Gracia, L.; Correia, A.; Ding, Y.; Teesalu, T.; Liu, D.; et al. A virus-mimicking pH-responsive acetalated dextran-based membrane-active polymeric nanoparticle for intracellular delivery of antitumor therapeutics. Adv. Funct. Mater. 2019, 29, 1905352. [Google Scholar] [CrossRef]
- Larrea, A.; Clemente, A.; Luque-Michel, E.; Sebastian, V. Efficient production of hybrid bio-nanomaterials by continuous microchannel emulsification: Dye-doped SiO2 and Au-PLGA nanoparticles. Chem. Eng. J. 2017, 316, 663–672. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Zhang, J.X.J. Microfluidics for silica biomaterials synthesis: Opportunities and challenges. Biomater. Sci. 2019, 7, 2218–2240. [Google Scholar] [CrossRef]
- Roberts, E.J.; Karadaghi, L.R.; Wang, L.; Malmstadt, N.; Brutchey, R.L. Continuous flow methods of fabricating catalytically active metal nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 27479–27502. [Google Scholar] [CrossRef]
- Kubendhiran, S.; Bao, Z.; Dave, K.; Liu, R.-S. Microfluidic synthesis of semiconducting colloidal QDs and their applications. ACS Appl. Mater. Interfaces 2019, 2, 1773–1790. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y. Microfluidic synthesis of nanohybrids. Small 2017, 13, 1604084. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Gunther, A.; Schmidt, M.A.; Jensen, K.F. Microfluidic synthesis of colloidal silica. Langmuir 2004, 20, 8604–8611. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Gomez, L.; Irusta, S.; Arruebo, M.; Santamaria, J. Comparative study of the synthesis of silica nanoparticles in micromixer–microreactor and batch reactor systems. Chem. Eng. J. 2011, 171, 674–683. [Google Scholar] [CrossRef]
- He, P.; Greenway, G.; Haswell, S.J. Microfluidic synthesis of silica nanoparticles using polyethylenimine polymers. Chem. Eng. J. 2011, 167, 694–699. [Google Scholar] [CrossRef]
- Nie, Y.; Hao, N.; Zhang, J.X.J. Ultrafast synthesis of multifunctional submicrometer hollow silica spheres in microfluidic spiral channels. Sci. Rep. 2017, 7, 12616. [Google Scholar] [CrossRef]
- Shiba, K.; Kambara, K.; Ogawa, M. Size-controlled syntheses of nanoporous silica spherical particles through a microfluidic approach. Ind. Eng. Chem. Res. 2010, 49, 8180–8183. [Google Scholar] [CrossRef]
- Ng, T.N.; Chen, X.Q.; Yeung, K.L. Direct manipulation of particle size and morphology of ordered mesoporous silica by flow synthesis. RSC Adv. 2015, 5, 13331–13340. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Closson, A.B.; Zhang, J.X.J. Microfluidic synthesis and on-chip enrichment application of two-dimensional hollow sandwich-like mesoporous silica nanosheet with water ripple-like surface. J. Colloid. Interface Sci. 2019, 539, 87–94. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Zhang, J.X.J. Microfluidic flow synthesis of functional mesoporous silica nanofibers with tunable aspect ratios. ACS Sustain. Chem. Eng. 2018, 6, 1522–1526. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Tadimety, A.; Closson, A.B.; Zhang, J.X.J. Microfluidics-mediated self-template synthesis of anisotropic hollow ellipsoidal mesoporous silica nanomaterials. Mater. Res. Lett. 2017, 5, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Nie, Y.; Shen, T.; Zhang, J.X.J. Microfluidics-enabled rational design of immunomagnetic nanomaterials and their shape effect on liquid biopsy. Lab Chip 2018, 18, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Yoo, Y.; Cheng, Z.; Jeong, H.-K. Generation of monodisperse mesoporous silica microspheres with controllable size and surface morphology in a microfluidic device. Adv. Funct. Mater. 2008, 18, 4014–4021. [Google Scholar] [CrossRef]
- Duraiswamy, S.; Khan, S.A. Plasmonic nanoshell synthesis in microfluidic composite foams. Nano Lett. 2010, 10, 3757–3763. [Google Scholar] [CrossRef] [PubMed]
- Wacker, J.B.; Lignos, I.; Parashar, V.K.; Gijs, M.A.M. Controlled synthesis of fluorescent silica nanoparticles inside microfluidic droplets. Lab Chip 2012, 12, 3111–3116. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Li, S.; Xu, J.; Luo, G. A one-step microfluidic approach for controllable preparation of nanoparticle-coated patchy microparticles. Microfluid. Nanofluid. 2012, 13, 491–498. [Google Scholar] [CrossRef]
- Rahman, M.T.; Krishnamurthy, P.G.; Parthiban, P.; Jain, A.; Park, C.P.; Kim, D.-P.; Khan, S.A. Dynamically tunable nanoparticle engineering enabled by short contact-time microfluidic synthesis with a reactive gas. RSC Adv. 2013, 3, 2897–2900. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Makila, E.; Fan, J.; Herranz-Blanco, B.; Wang, C.-F.; Rosa, R.; Ribeiro, A.J.; Salonen, J.; Hirvonen, J.; et al. Microfluidic assisted one-step fabrication of porous silicon@acetalated dextran nanocomposites for precisely controlled combination chemotherapy. Biomaterials 2015, 39, 249–259. [Google Scholar] [CrossRef]
- Ferraro, D.; Lin, Y.; Teste, B.; Talbot, D.; Malaquin, L.; Descroix, S.; Abou-Hassan, A. Continuous chemical operations and modifications on magnetic gamma-Fe2O3 nanoparticles confined in nanoliter droplets for the assembly of fluorescent and magnetic SiO2@gamma-Fe2O3. Chem. Commun. 2015, 51, 16904–16907. [Google Scholar] [CrossRef][Green Version]
- Hassan, N.; Stocco, A.; Abou-Hassan, A. Droplet liquid/liquid interfaces generated in a microfluidic device for assembling Janus inorganic nanohybrids. J. Phys. Chem. C 2015, 119, 10758–10765. [Google Scholar] [CrossRef]
- Hou, L.; Jiang, H.; Lee, D. Bubble-filled silica microfibers from multiphasic flows for lightweight composite fabrication. Chem. Eng. J. 2016, 288, 539–545. [Google Scholar] [CrossRef]
- Wagner, J.; Kirner, T.; Mayer, G.; Albert, J.; Köhler, J.M. Generation of metal nanoparticles in a microchannel reactor. Chem. Eng. J. 2003, 101, 251–260. [Google Scholar] [CrossRef]
- Fu, Q.; Rana, G.; Xu, W. A microfluidic-based controllable synthesis of rolled or rigid ultrathin gold nanoplates. RSC Adv. 2015, 5, 37512–37516. [Google Scholar] [CrossRef]
- Uson, L.; Sebastian, V.; Arruebo, M.; Santamaria, J. Continuous microfluidic synthesis and functionalization of gold nanorods. Chem. Eng. J. 2016, 285, 286–292. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, C.; Wang, F.; Zhang, Y.; Zhang, L. A novel vapor-liquid segmented flow based on solvent partial vaporization in microstructured reactor for continuous synthesis of nickel nanoparticles. Chem. Eng. J. 2012, 204, 48–53. [Google Scholar] [CrossRef]
- Roberts, E.J.; Habas, S.E.; Wang, L.; Ruddy, D.A.; White, E.A.; Baddour, F.G.; Griffin, M.B.; Schaidle, J.A.; Malmstadt, N.; Brutchey, R.L. High-throughput continuous flow synthesis of nickel nanoparticles for the catalytic hydrodeoxygenation of guaiacol. ACS Sustain. Chem. Eng. 2017, 5, 632–639. [Google Scholar] [CrossRef]
- Lin, X.Z.; Terepka, A.D.; Yang, H. Synthesis of silver nanoparticles in a continuous flow tubular microreactor. Nano Lett. 2004, 4, 2227–2232. [Google Scholar] [CrossRef]
- Thiele, M.; Knauer, A.; Csáki, A.; Mallsch, D.; Henkel, T.; Köhler, J.M.; Fritzsche, W. High-throughput synthesis of uniform silver seed particles by a continuous microfluidic synthesis platform. Chem. Eeg. Technol. 2015, 38, 1131–1137. [Google Scholar] [CrossRef]
- Wu, K.J.; Torrente-Murciano, L. Continuous synthesis of tuneable sized silver nanoparticles via a tandem seed-mediated method in coiled flow inverter reactors. React. Chem. Eng. 2018, 3, 267–276. [Google Scholar] [CrossRef]
- Wong, W.K.; Yap, S.K.; Lim, Y.C.; Khan, S.A.; Pelletier, F.; Corbos, E.C. Robust, non-fouling liters-per-day flow synthesis of ultra-small catalytically active metal nanoparticles in a single-channel reactor. React. Chem. Eng. 2017, 2, 636–641. [Google Scholar] [CrossRef]
- Sebastian, V.; Basak, S.; Jensen, K.F. Continuous synthesis of palladium nanorods in oxidative segmented flow. AlChE J. 2016, 62, 373–380. [Google Scholar] [CrossRef]
- Kunal, P.; Roberts, E.J.; Riche, C.T.; Jarvis, K.; Malrnstadt, N.; Brutchey, R.L.; Humphrey, S.M. Continuous flow synthesis of Rh and RhAg alloy nanoparticle catalysts enables scalable production and improved morphological control. Chem. Mater. 2017, 29, 4341–4350. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, F.; Peng, M.; Wang, X.; Xia, D.; Guo, G. One-Step, Facile and ultrafast synthesis of phase- and size-controlled Pt-Bi intermetallic nanocatalysts through continuous-flow microfluidics. J. Am. Chem. Soc. 2015, 137, 6263–6269. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Sakai, Y.; Tsunoyama, H.; Nakajima, A. Development of ultrafine multichannel microfluidic mixer for synthesis of bimetallic nanoclusters: Catalytic application of highly monodisperse AuPd nanoclusters stabilized by poly (N-vinylpyrrolidone). Langmuir 2014, 30, 10539–10547. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, G.; Gaur, A.; Doronkin, D.E.; Lichtenberg, H.; Wang, W.; Wang, D.; Rinke, G.; Ewinger, A.; Dittmeyer, R.; Grunwaldt, J.-D. Microfluidic synthesis of ultrasmall AuPd nanoparticles with a homogeneously mixed alloy structure in fast continuous flow for catalytic applications. J. Phys. Chem. C 2018, 122, 1721–1731. [Google Scholar] [CrossRef]
- Niu, G.; Zhou, M.; Yang, X.; Park, J.; Lu, N.; Wang, J.; Kim, M.J.; Wang, L.; Xia, Y. Synthesis of Pt-Ni octahedra in continuous-flow droplet reactors for the scalable production of highly active catalysts toward oxygen reduction. Nano Lett. 2016, 16, 3850–3857. [Google Scholar] [CrossRef]
- Wang, H.; Niu, G.; Zhou, M.; Wang, X.; Park, J.; Bao, S.; Chi, M.; Cai, Z.; Xia, Y. Scalable synthesis of palladium icosahedra in plug reactors for the production of oxygen reduction reaction catalysts. ChemCatChem 2016, 8, 1658–1664. [Google Scholar] [CrossRef]
- Nakamura, H.; Yamaguchi, Y.; Miyazaki, M.; Maeda, H.; Uehara, M.; Mulvaney, P. Preparation of CdSe nanocrystals in a micro-flow-reactor. Chem. Commun. 2002, 23, 2844–2845. [Google Scholar] [CrossRef]
- Yen, B.K.H.; Stott, N.E.; Jensen, K.F.; Bawendi, M.G. A continuous-flow microcapillary reactor for the preparation of a size series of CdSe nanocrystals. Adv. Mater. 2003, 15, 1858–1862. [Google Scholar] [CrossRef]
- Yen, B.K.H.; Gunther, A.; Schmidt, M.A.; Jensen, K.F.; Bawendi, M.G. A microfabricated gas-liquid segmented flow reactor for high-temperature synthesis: The case of CdSe QDs. Angew. Chem. Int. Ed. 2005, 44, 5447–5451. [Google Scholar] [CrossRef]
- Wang, H.Z.; Nakamura, H.; Uehara, M.; Yamaguchi, Y.; Miyazaki, M.; Maeda, H. Highly luminescent CdSe/ZnS nanocrystals synthesized using a single-molecular ZnS source in a microfluidic reactor. Adv. Funct. Mater. 2005, 15, 603–608. [Google Scholar] [CrossRef]
- Naughton, M.S.; Kumar, V.; Bonita, Y.; Deshpande, K.; Kenis, P.J.A. High temperature continuous flow synthesis of CdSe/CdS/ZnS, CdS/ZnS, and CdSeS/ZnS nanocrystals. Nanoscale 2015, 7, 15895–15903. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, A.M.; de Mello, J.C. Controlled synthesis of III-V QDs in microfluidic reactors. Chemphyschem 2009, 10, 2612–2614. [Google Scholar] [CrossRef]
- Baek, J.; Allen, P.M.; Bawendi, M.G.; Jensen, K.F. Investigation of indium phosphide nanocrystal synthesis using a high-temperature and high-pressure continuous flow microreactor. Angew. Chem. Int. Ed. 2011, 50, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Ippen, C.; Schneider, B.; Pries, C.; Kroepke, S.; Greco, T.; Hollaender, A. Large-scale synthesis of high quality InP QDs in a continuous flow-reactor under supercritical conditions. Nanotechnology 2015, 26, 085604. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jeong, S.; Woo, J.Y.; Han, C.-S. Successive and large-scale synthesis of InP/ZnS QDs in a hybrid reactor and their application to white LEDs. Nanotechnology 2012, 23, 065602. [Google Scholar] [CrossRef]
- Baek, J.; Shen, Y.; Lignos, I.; Bawendi, M.G.; Jensen, K.F. Multistage microfluidic platform for the continuous synthesis of III-V core/shell QDs. Angew. Chem. Int. Ed. 2018, 130, 11081–11084. [Google Scholar] [CrossRef]
- Pan, J.; El-Ballouli, A.a.O.; Rollny, L.; Voznyy, O.; Burlakov, V.M.; Goriely, A.; Sargent, E.H.; Bakr, O.M. Automated synthesis of photovoltaic-quality colloidal QDs using separate nucleation and growth stages. ACS Nano 2013, 7, 10158–10166. [Google Scholar] [CrossRef]
- Lignos, I.; Protesescu, L.; Stavrakis, S.; Piveteau, L.; Speirs, M.J.; Loi, M.A.; Kovalenko, M.V.; de Mello, A.J. Facile droplet-based microfluidic synthesis of monodisperse IV-VI semiconductor nanocrystals with coupled In-line NIR fluorescence detection. Chem. Mater. 2014, 26, 2975–2982. [Google Scholar] [CrossRef]
- Yamamoto, D.; Maki, T.; Watanabe, S.; Tanaka, H.; Miyahara, M.T.; Mae, K. Synthesis and adsorption properties of ZIF-8 nanoparticles using a micromixer. Chem. Eng. 2013, 227, 145–150. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Xu, Z.; Closson, A.B.; Usherwood, T.; Zhang, J.X.J. Microfluidic continuous flow synthesis of functional hollow spherical silica with hierarchical sponge-like large porous shell. Chem. Eng. J. 2019, 366, 433–438. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Zhang, J.X.J. Biomimetic hierarchical walnut kernel-like and erythrocyte-like mesoporous silica nanomaterials: Controllable synthesis and versatile applications. Microporous Mesoporous Mater. 2018, 261, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Chen, X.; Jeon, S.; Yan, M. Carbohydrate-conjugated hollow oblate mesoporous silica nanoparticles as nanoantibiotics to target mycobacteria. Adv. Healthc. Mater. 2015, 4, 2797–2801. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Hedrick, J.L.; Ong, Z.Y.; Yang, C.; Ee, P.L.R.; Hammond, P.T.; Yang, Y.Y. The effects of polymeric nanostructure shape on drug delivery. Adv. Drug Del. Rev. 2011, 63, 1228–1246. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Li, L.; Tang, F. Facile preparation of ellipsoid-like MCM-41 with parallel channels along the short axis for drug delivery and assembly of Ag nanoparticles for catalysis. J. Mater. Chem. 2014, 2, 11565–11568. [Google Scholar] [CrossRef]
- Geng, Y.; Dalhaimer, P.; Cai, S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef]
- Hao, N.; Yang, H.; Li, L.; Li, L.; Tang, F. The shape effect of mesoporous silica nanoparticles on intracellular reactive oxygen species in A375 cells. New J. Chem. 2014, 38, 4258–4266. [Google Scholar] [CrossRef]
- Carroll, N.J.; Rathod, S.B.; Derbins, E.; Mendez, S.; Weitz, D.A.; Petsev, D.N. Droplet-based microfluidics for emulsion and solvent evaporation synthesis of monodisperse mesoporous silica microspheres. Langmuir 2008, 24, 658–661. [Google Scholar] [CrossRef]
- Fang, A.; Gaillard, C.; Douliez, J.-P. Template-free formation of monodisperse doughnut-shaped silica microparticles by droplet-based microfluidics. Chem. Mater. 2011, 23, 4660–4662. [Google Scholar] [CrossRef]
- Zhao, C.X.; Middelberg, A.P.J. Microfluidic synthesis of monodisperse hierarchical silica particles with raspberry-like morphology. RSC Adv. 2013, 3, 21227–21230. [Google Scholar] [CrossRef]
- Ju, M.; Ji, X.; Wang, C.; Shen, R.; Zhang, L. Preparation of solid, hollow, hole-shell and asymmetric silica microspheres by microfluidic-assisted solvent extraction process. Chem. Eng. J. 2014, 250, 112–118. [Google Scholar] [CrossRef]
- Yan, H.; Kim, C. Formation of monodisperse silica microparticles with various shapes and surface morphologies using double emulsion templates. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 88–95. [Google Scholar] [CrossRef]
- Shang, L.; Shangguan, F.; Cheng, Y.; Lu, J.; Xie, Z.; Zhao, Y.; Gu, Z. Microfluidic generation of magnetoresponsive Janus photonic crystal particles. Nanoscale 2013, 5, 9553–9557. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, W.; Shen, P.; Li, Y.; Li, Y.; Deng, Y.; Zheng, Q.; Liu, Y.; Ding, Z.; Li, J. Microfluidic fabrication of photonic encoding magnetized silica microspheres for aptamer-based enrichment of Ochratoxin A. Microchimica Acta 2017, 184, 3755–3763. [Google Scholar] [CrossRef]
- Lan, W.; Li, S.; Xu, J.; Luo, G. Synthesis of titania-silica core-shell microspheres via a controlled interface reaction in a microfluidic device. Langmuir 2011, 27, 13242–13247. [Google Scholar] [CrossRef]
- Abou-Hassan, A.; Bazzi, R.; Cabuil, V. Multistep continuous-flow microsynthesis of magnetic and fluorescent γ-Fe2O3 @ SiO2 core/shell nanoparticles. Angew. Chem. Int. Ed. 2009, 121, 7316–7319. [Google Scholar] [CrossRef]
- Hassan, N.; Cabuil, V.; Abou-Hassan, A. Continuous multistep microfluidic assisted assembly of fluorescent, plasmonic, and magnetic nanostructures. Angew. Chem. Int. Ed. 2013, 52, 1841. [Google Scholar] [CrossRef]
- Gomez, L.; Arruebo, M.; Sebastian, V.; Gutierrez, L.; Santamaria, J. Facile synthesis of SiO2-Au nanoshells in a three-stage microfluidic system. J. Mater. Chem. 2012, 22, 21420. [Google Scholar] [CrossRef]
- Watanabe, S.; Hiratsuka, T.; Asahi, Y.; Tanaka, A.; Mae, K.; Miyahara, M.T. Flow synthesis of plasmonic gold nanoshells via a microreactor. Part. Part. Syst. Charact. 2015, 32, 234–242. [Google Scholar] [CrossRef]
- Shiba, K.; Sugiyama, T.; Takei, T.; Yoshikawa, G. Controlled growth of silica-titania hybrid functional nanoparticles through a multistep microfluidic approach. Chem. Commun. 2015, 51, 15854–15857. [Google Scholar] [CrossRef]
- Lee, S.-K.; Liu, X.; Cabeza, V.S.; Jensen, K.F. Synthesis, assembly and reaction of a nanocatalyst in microfluidic systems: A general platform. Lab Chip 2012, 12, 4080–4084. [Google Scholar] [CrossRef] [PubMed]
- Strass, A.; Maier, R.; Guettel, R. Continuous synthesis of nanostructured Co3O4@SiO2 core-shell particles in a laminar-flow reactor. Chem. Ing. Tech. 2017, 89, 963–967. [Google Scholar] [CrossRef]
- Hao, N.; Nie, Y.; Tadimety, A.; Shen, T.; Zhang, J.X.J. Microfluidics-enabled rapid manufacturing of hierarchical silica-magnetic microflower toward enhanced circulating tumor cell screening. Biomater. Sci. 2018, 6, 3121–3125. [Google Scholar] [CrossRef] [PubMed]
- Ettema, T.J.G.; Huynen, M.A.; Vos, W.M.D.; Oost, J.V.D. TRASH: A novel metal-binding domain predicted to be involved in heavy-metal sensing, trafficking and resistance. Trends Biochem. Sci. 2003, 28, 170–173. [Google Scholar] [CrossRef]
- Cabrera, F.C.; Melo, A.F.A.A.; de Souza, J.C.P.; Job, A.E.; Crespilho, F.N. A flexible lab-on-a-chip for the synthesis and magnetic separation of magnetite decorated with gold nanoparticles. Lab Chip 2015, 15, 1835–1841. [Google Scholar] [CrossRef]
- Maier, S.A.; Kik, P.G.; Atwater, H.A.; Meltzer, S.; Harel, E.; Koel, B.E.; Requicha, A.A.G. Local detection of electromagnetic energy transport below the diffraction limit in metal nanoparticle plasmon waveguides. Nat. Mater. 2003, 2, 229–232. [Google Scholar] [CrossRef]
- Schwarz, J.A.; Contescu, C.; Contescu, A. Methods for preparation of catalytic materials. Chem. Rev. 1995, 95, 477–510. [Google Scholar] [CrossRef]
- Hartman, R.L.; McMullen, J.P.; Jensen, K.F. Deciding whether to go with the flow: Evaluating the merits of flow reactors for synthesis. Angew. Chem. Int. Ed. 2011, 50, 7502–7519. [Google Scholar] [CrossRef]
- Boutonnet, M.; Kizling, J.; Stenius, P.; Maire, G. The preparation of monodisperse colloidal metal particles from microemulsions. Colloids Surf. 1982, 5, 209–225. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Cobley, C.; Chen, J.; Cho, E.; Wang, L.; Xia, Y. Gold nanostructures: A class of multifunctional materials for biomedical applications. Chem. Soc. Rev. 2011, 40, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Chen, C. Fate and toxicity of metallic and metal-containing nanoparticles for biomedical applications. Small 2011, 7, 2965–2980. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Ji, C.; Shi, J.; Pridgen, E.M.; Frieder, J.; Wu, J.; Farokhzad, O.C. DNA self-assembly of targeted near-infrared-responsive gold nanoparticles for cancer thermo-chemotherapy. Angew. Chem. Int. Ed. 2012, 124, 12023–12027. [Google Scholar] [CrossRef]
- Ren, F.; Bhana, S.; Norman, D.D.; Johnson, J.; Xu, L.; Baker, D.L.; Parrill, A.L.; Huang, X. Gold nanorods carrying paclitaxel for photothermal-chemotherapy of cancer. Bioconj. Chem. 2013, 24, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, L.; Jing, W.; Jiang, X.; Li, X.; Hu, Z.; Ji, Y.; Wu, X.; Chen, C. Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv. Mater. 2012, 24, 1418–1423. [Google Scholar] [CrossRef]
- Melancon, M.P.; Elliott, A.M.; Shetty, A.; Huang, Q.; Stafford, R.J.; Li, C. Near-infrared light modulated photothermal effect increases vascular perfusion and enhances polymeric drug delivery. J. Control. Release 2011, 156, 265–272. [Google Scholar] [CrossRef]
- Khan, A.K.; Rashid, R.; Murtaza, G.; Zahra, A. Gold nanoparticles: Synthesis and applications in drug delivery. Trop. J. Pharm. Res. 2014, 13, 1169–1177. [Google Scholar] [CrossRef]
- Sau, T.K.; Pal, A.; Jana, N.R.; Wang, Z.L.; Pal, T. Size controlled synthesis of gold nanoparticles using photochemically prepared seed particles. J. Nanopart. Res. 2001, 3, 257–261. [Google Scholar] [CrossRef]
- Lu, M.; Yang, S.; Ho, Y.P.; Grigsby, C.L.; Leong, K.W.; Huang, T.J. Shape-controlled synthesis of hybrid nanomaterials three-dimensional hydrodynamic focusing. ACS Nano 2014, 8, 10026–10034. [Google Scholar] [CrossRef]
- Osawa, T.; Harada, T.; Takayasu, O. Asymmetrically modified nickel catalyst for the enantio-differentiating hydrogenation of prochiral ketones. Curr. Org. Chem. 2006, 10, 1513–1531. [Google Scholar] [CrossRef]
- Guillou, N.; Gao, Q.; Forster, P.M.; Chang, J.S.; Nogues, M.; Park, S.E.; Ferey, G.; Cheetham, A.K. Nickel(II) phosphate VSB-5: A magnetic nanoporous hydrogenation catalyst with 24-ring tunnels. Curr. Org. Chem. 2010, 32, 2831–2834. [Google Scholar]
- Lugaresi, O.; Perales-Rondón, J.V.; Minguzzi, A.; Solla-Gullón, J.; Sánchez-Sánchez, C.M. Rapid screening of silver nanoparticles for the catalytic degradation of chlorinated pollutants in water. Appl. Catal. B 2015, 163, 554–563. [Google Scholar] [CrossRef]
- Wang, J.; Blau, W.J. Inorganic and hybrid nanostructures for optical limiting. J. Opt. A Pure Appl. Opt. 2009, 11, 024001. [Google Scholar] [CrossRef]
- Ong, C.; Lim, J.Z.Z.; Ng, C.T.; Li, J.J.; Yung, L.Y.L.; Bay, B.H. Silver nanoparticles in cancer: Therapeutic efficacy and toxicity. Curr. Med. Chem. 2013, 20, 772–781. [Google Scholar] [PubMed]
- Mishra, A.; Mehdi, S.J.; Irshad, M.; Ali, A.; Sardar, M.; Rizvi, M.M.A. Effect of biologically synthesized silver nanoparticles on human cancer cells. Sci. Adv. Mater. 2012, 4, 1200–1206. [Google Scholar] [CrossRef]
- Zhu, N.; Huang, W.; Hu, X.; Liu, Y.; Fang, Z.; Guo, K. Chemoselective polymerization platform for flow synthesis of functional polymers and nanoparticles. Chem. Eng. J. 2018, 333, 43–48. [Google Scholar] [CrossRef]
- Leonard, B.M.; Nsp, B.; Schaak, R.E. Low-temperature polyol synthesis of AuCuSn2 and AuNiSn2: Using solution chemistry to access ternary intermetallic compounds as nanocrystals. J. Am. Chem. Soc. 2010, 36, 7326–7327. [Google Scholar] [CrossRef]
- Mcdonald, J.; Rodriguez, A.; Jones, E.D.; Adams, P. Rare-earth transition-metal intermetallic compounds produced via self-propagating, high-temperature synthesis. J. Mater. Res. 2010, 25, 718–727. [Google Scholar] [CrossRef]
- Lazarus, L.L.; Riche, C.T.; Marin, B.C.; Gupta, M.; Malmstadt, N.; Brutchey, R.L. Two-phase microfluidic droplet flows of ionic liquids for the synthesis of gold and silver nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 3077–3083. [Google Scholar] [CrossRef]
- Yashina, A.; Lignos, I.; Stavrakis, S.; Choo, J.; de Mello, A.J. Scalable production of CuInS2/ZnS QDs in a two-step droplet-based microfluidic platform. J. Mater. Chem. 2016, 4, 6401–6408. [Google Scholar]
- Wang, C.; Thompson, R.; Baltrus, J.; Matranga, J. Visible light photoreduction of CO2 using CdSe/Pt/TiO2 heterostructured catalysts. J. Phys. Chem. Lett. 2010, 1, 48–53. [Google Scholar] [CrossRef]
- Marti, A.; Lopez, N.; Antolin, E.; Canovas, E.; Luque, A.; Stanley, C.F.; Farmer, C.D.; Diaz, P. Emitter degradation in QD intermediate band solar cells. Appl. Phys. Lett. 2007, 90, 233510. [Google Scholar] [CrossRef]
- Schramm, A.; Tukiainen, A.; Pessa, M.; Konetzni, C.; Hansen, W. Neutron-irradiated Schottky diodes with self-assembled InAs QDs: Optical and electrical properties. J. Appl. Phys. 2009, 105, 104308. [Google Scholar] [CrossRef]
- Upadhyay, S.; Mandal, A.; Ghadi, H.; Pal, D.; Basu, A.; Agarwal, A.; Subrahmanyam, N.B.V.; Singh, P.; Chakrabarti, S. Effects of high energy proton implantation on the optical and electrical properties of In(Ga)as/GaAs QD heterostructures with variations in the capping layer. J. Lumin. 2015, 161, 129–134. [Google Scholar] [CrossRef]
- Rodarte, A.; Cisneros, F.; Hein, J.; Ghosh, S.; Hirst, L. Quantum dot/liquid crystal nanocomposites in photonic devices. Photonics 2015, 2, 855–864. [Google Scholar] [CrossRef]
- Vikram, A.; Kumar, V.; Ramesh, U.; Balakrishnan, K.; Oh, N.; Deshpande, K.; Ewers, T.; Trefonas, P.; Shim, M.; Kenis, P.J.A. A millifluidic reactor system for multistep continuous synthesis of InP/ZnSeS nanoparticles. ChemNanoMat 2018, 4, 943–953. [Google Scholar] [CrossRef]
- Kwon, B.-H.; Lee, K.G.; Park, T.J.; Kim, H.; Lee, T.J.; Lee, S.J.; Jeon, D.Y. Continuous In situ synthesis of ZnSe/ZnS core/shell QDs in a microfluidic reaction system and its application for light-emitting diodes. Small 2012, 8, 3257–3262. [Google Scholar] [CrossRef]
- Chung, W.; Yu, H.J.; Park, S.H.; Chun, B.H.; Kim, S.H. YAG and CdSe/ZnSe nanoparticles hybrid phosphor for white LED with high color rendering index. Mater. Chem. Phys. 2011, 126, 162–166. [Google Scholar] [CrossRef]
- Wang, H.Z.; Li, X.Y.; Uehara, M.; Yamaguchi, Y.; Nakamura, H.; Miyazaki, M.P.; Shimizu, H.; Maeda, H. Continuous synthesis of CdSe-ZnS composite nanoparticles in a microfluidic reactor. Chem. Commun. 2004, 48–49. [Google Scholar] [CrossRef]
- Lu, Z.S.; Li, C.M. Quantum dot-based nanocomposites for biomedical applications. Curr. Med. Chem. 2011, 18, 3516–3528. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, K.; Tang, A.; Xie, Y.; Qian, L.; Cao, W.; Yang, Y.; Chen, Y.; Teng, F. Solution-processed high-efficiency cadmium-free Cu-Zn-In-S-based quantum-dot light-emitting diodes with low turn-on voltage. Org. Electron. 2016, 36, 97–102. [Google Scholar] [CrossRef]
- Gygi, D.; Bloch, E.D.; Mason, J.A.; Hudson, M.R.; Gonzalez, M.I.; Siegelman, R.L.; Darwish, T.A.; Queen, W.L.; Brown, C.M.; Long, J.R. Hydrogen storage in the expanded pore metal-organic frameworks M-2(dobpdc) (M = Mg, Mn, Fe, Co, Ni, Zn). Chem. Mater. 2016, 28, 1128–1138. [Google Scholar] [CrossRef]
- Banerjee, D.; Simon, C.M.; Plonka, A.M.; Motkuri, R.K.; Liu, J.; Chen, X.; Smit, B.; Parise, J.B.; Haranczyk, M.; Thallapally, P.K. Metal-organic framework with optimally selective xenon adsorption and separation. Nat. Commun. 2016, 7, 11831. [Google Scholar] [CrossRef] [PubMed]
- Bag, P.P.; Wang, D.; Chen, Z.; Cao, R. Outstanding drug loading capacity by water stable microporous MOF: A potential drug carrier. Chem. Commun. 2016, 52, 3669–3672. [Google Scholar] [CrossRef]
- Chowdhuri, A.R.; Bhattacharya, D.; Sahu, S.K. Magnetic nanoscale metal organic frameworks for potential targeted anticancer drug delivery, imaging and MRI contrast agent. Dalton Trans. 2015, 45, 2963–2973. [Google Scholar] [CrossRef]
- Lian, X.; Yan, B. A postsynthetic modified MOF hybrid as heterogeneous photocatalyst for alpha-phenethyl alcohol and reusable fluorescence sensor. Inorg. Chem. 2016, 55, 11831–11838. [Google Scholar] [CrossRef]
- Genna, D.T.; Pfund, L.Y.; Samblanet, D.C.; Wong-Foy, A.G.; Matzger, A.J.; Sanford, M.S. Rhodium hydrogenation catalysts supported in metal organic frameworks: Influence of the framework on catalytic activity and selectivity. ACS Catal. 2016, 6, 3569–3574. [Google Scholar] [CrossRef]
- Faustini, M.; Kim, J.; Jeong, G.Y.; Jin, Y.K.; Moon, H.R.; Ahn, W.S.; Kim, D.P. Microfluidic approach toward continuous and ultrafast synthesis of metal-organic framework crystals and hetero structures in confined microdroplets. J. Am. Chem. Soc. 2013, 135, 14619–14626. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, G.; Guo, J.; Liu, Y.; Ren, J.; Kong, T.; Zhao, Y. Vitamin metal–organic framework-laden microfibers from microfluidics for wound healing. Mater. Horiz. 2018, 5, 1137–1142. [Google Scholar] [CrossRef]
- Hu, C.; Bai, Y.; Hou, M.; Wang, Y.; Wang, L.; Cao, X.; Chan, C.-W.; Sun, H.; Li, W.; Ge, J.; et al. Defect-induced activity enhancement of enzyme-encapsulated metal-organic frameworks revealed in microfluidic gradient mixing synthesis. Sci. Adv. 2020, 6, eaax5785. [Google Scholar] [CrossRef]
- Ottino, J.M.; Wiggins, S. Introduction: Mixing in microfluidics. Philos. Trans. R. Soc. Lond. A 2004, 362, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Bains, A.; Cao, Y.; Moffitt, M.G. Multiscale control of hierarchical structure in crystalline block copolymer nanoparticles using microfluidics. Macromol. Rapid Commun. 2015, 36, 2000–2005. [Google Scholar] [CrossRef]
- Karbalaei, A.; Kumar, R.; Cho, H.J. Thermocapillarity in microfluidics-a review. Micromachines 2016, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.; Cheng, G.; Ma, X.; Pang, X.; Qiang, Z. Surface modification of zinc oxide nanoparticle by PMAA and its dispersion in aqueous system. Appl. Surf. Sci. 2006, 252, 5227–5232. [Google Scholar] [CrossRef]
- TYu, D.G.; Lin, W.C.; Yang, M.C. Surface modification of poly(l-lactic acid) membrane via layer-by-layer assembly of silver nanoparticle-embedded polyelectrolyte multilayer. Bioconj. Chem. 2007, 18, 1521–1529. [Google Scholar]
- Bohannon, J. ‘Smart coatings’ research shows the virtues of superficiality. Science 2005, 309, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, K.; Wu, C.; Zhao, J.; Xie, Y. Surface chemical-modification for engineering the intrinsic physical properties of inorganic two-dimensional nanomaterials. Chem. Soc. Rev. 2015, 44, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Petosa, A.R.; Jaisi, D.P.; Quevedo, I.R.; Elimelech, M.; Tufenkji, N. Aggregation and deposition of engineered nanomaterials in aquatic environments: Role of physicochemical interactions. Environ. Sci. Technol. 2010, 44, 6532–6549. [Google Scholar] [CrossRef]
- Haider, S.; Maparu, A.K.; Ashok, K.; Sri, S.; Shiv, S.S. Generic delivery of payload of nanoparticles intracellularly via hybrid polymer capsules for bioimaging applications. PLoS ONE 2012, 7, e36195. [Google Scholar]
- Valencia, P.M.; Basto, P.A.; Zhang, L.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Single-step assembly of homogenous lipid—polymeric and lipid-QD nanoparticles enabled by microfluidic rapid mixing. ACS Nano 2010, 4, 1671–1679. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Tam, Y.Y.C.; Chen, S.; Tam, Y.K.; Zaifman, J.; Cullis, P.R.; Biswas, S. Rapid synthesis of lipid nanoparticles containing hydrophobic inorganic nanoparticles. Nanoscale 2017, 9, 13600–13609. [Google Scholar] [CrossRef] [PubMed]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Bahlakeh, G.; Majedi, F.S.; Keshvari, H.; Dersarl, J.J.V.; Bertsch, A.; Panahifar, A.; Renaud, P.; Tayebi, L.; et al. On-chip synthesis of fine-tuned bone-seeking hybrid nanoparticles. Nanomedicine 2015, 10, 3431–3449. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Cai, B.; Bu, L.-L.; Liao, Q.-Q.; Guo, S.-S.; Zhao, X.-Z.; Dong, W.-F.; Liu, W. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano 2017, 11, 3496–3505. [Google Scholar] [CrossRef]
- Liu, T.; Wan, Q.; Luo, Y.; Chen, M.; Zou, C.; Ma, M.; Liu, X.; Chen, H. On-demand detaching nanosystem for the spatiotemporal control of cancer theranostics. ACS Appl. Mater. Interfaces 2019, 11, 16285–16295. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Ma, M.; Zhang, H.; Zhang, Y.; Wan, G.; Shen, J.; Chen, H.; Wu, R. Mesopore-induced aggregation of cobalt protoporphyrin for photoacoustic imaging and antioxidant protection of stem cells. Adv. Funct. Mater. 2018, 28, 1804497. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, N.; Wang, Z.; Wu, M.; Chen, Y.; Ma, M.; Chen, H.; Shi, J. Endogenous catalytic heneration of O2 bubbles for in-situ ultrasound-guided high intensity focused ultrasound ablation. ACS Nano 2017, 11, 9093–9102. [Google Scholar] [CrossRef]
- Ma, M.; Chen, H.; Chen, Y.; Wang, X.; Chen, F.; Cui, X.; Shi, J. Au capped magnetic core/mesoporous silica shell nanoparticles for combined photothermo-/chemo-therapy and multimodal imaging. Biomaterials 2012, 33, 989–998. [Google Scholar] [CrossRef]
- Wang, T.; Chai, F.; Fu, Q.; Zhang, L.; Liu, H.; Li, L.; Liao, Y.; Su, Z.; Wang, C.; Duan, B. Uniform hollow mesoporous silica nanocages for drug delivery in vitro and in vivo for liver cancer therapy. J. Mater. Chem. 2011, 21, 5299–5306. [Google Scholar] [CrossRef]
- Shen, S.; Tang, H.; Zhang, X.; Ren, J.; Pang, Z.; Wang, D.; Gao, H.; Qian, Y.; Jiang, X.; Yang, W. Targeting mesoporous silica-encapsulated gold nanorods for chemo-photothermal therapy with near-infrared radiation. Biomaterials 2013, 34, 3150–3158. [Google Scholar] [CrossRef]
- Li, Z.Z.; Wen, L.X.; Shao, L.; Chen, J.F. Fabrication of porous hollow silica nanoparticles and their applications in drug release control. J. Control. Release 2004, 98, 245–254. [Google Scholar] [CrossRef]
- Li, T.; Zhang, A.; Shao, G.; Wei, M.; Guo, B.; Zhang, G.; Li, L.; Wang, W. Janus Microdimer Surface Walkers Propelled by Oscillating Magnetic Fields. Adv. Funct. Mater. 2018, 28, 1706066. [Google Scholar] [CrossRef]
- Uhlmann, P.; Ionov, L.; Houbenov, N.; Nitschke, M.; Grundke, K.; Motornov, M.; Minko, S.; Stamm, M. Surface functionalization by smart coatings: Stimuli-responsive binary polymer brushes. Prog. Org. Coat. 2006, 55, 168–174. [Google Scholar] [CrossRef]
- Chilkoti, A. Creating smart surfaces using stimuli responsive polymers. Adv. Mater. 2010, 14, 1243–1247. [Google Scholar]
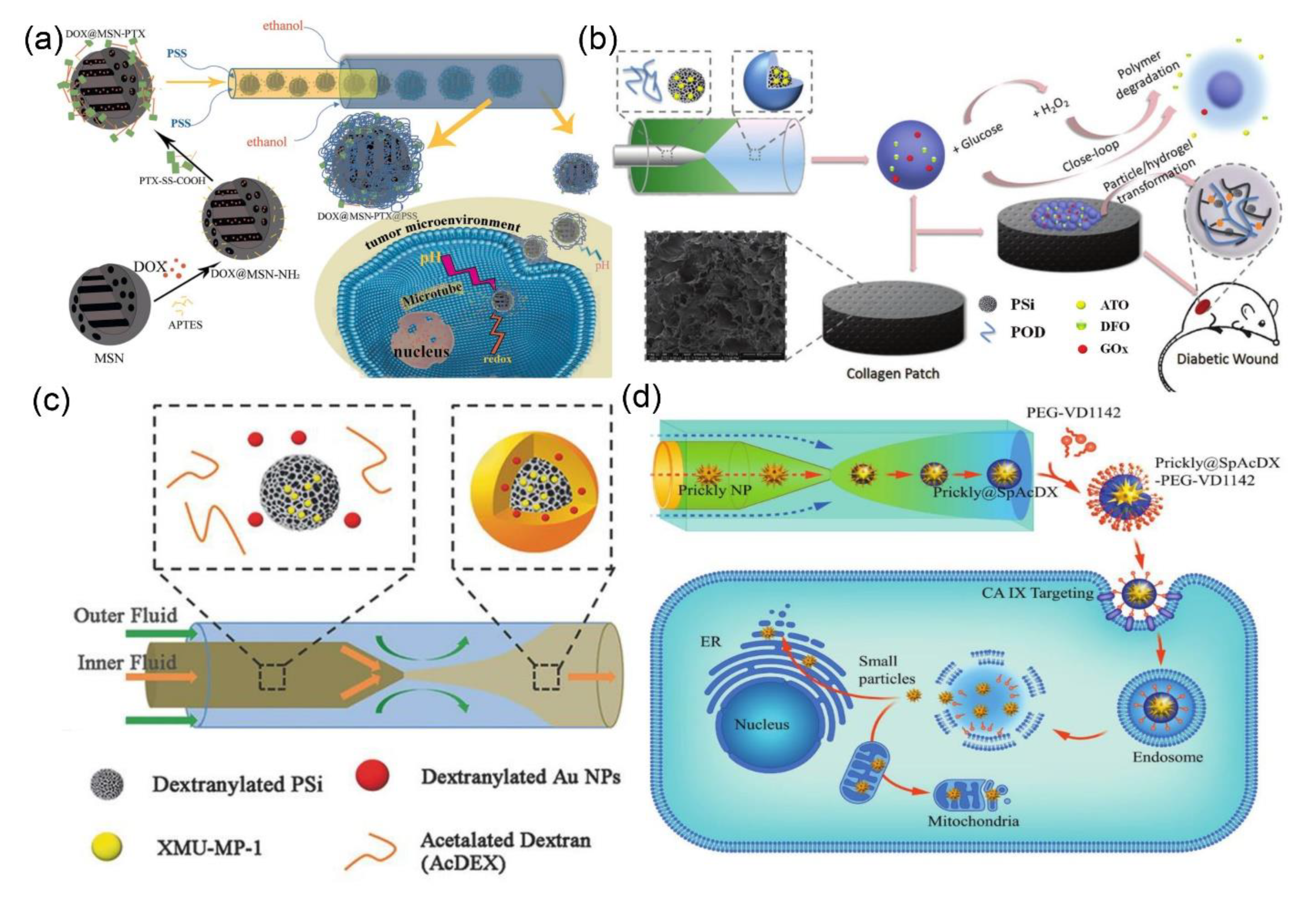
- Yan, J.; Xu, X.; Zhou, J.; Liu, C.; Zhang, L.; Wang, D.; Yang, F.; Zhang, H. Fabrication of a pH/redox-triggered mesoporous silica-based nanoparticle with microfluidics for anticancer drugs doxorubicin and paclitaxel codelivery. ACS Appl. Bio. Mater. 2020, 3, 1216–1225. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Li, W.; Lian, W.; Kemell, M.; Hietala, S.; Figueiredo, P.; Li, L.; Makila, E.; Ma, M.; et al. Close-loop dynamic nanohybrids on collagen-ark with in situ gelling transformation capability for biomimetic stage-specific diabetic wound healing. Mater. Horiz. 2019, 6, 385–393. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Li, W.; Xiao, C.; Liu, D.; Dong, C.; Zhang, M.; Makila, E.; Kemell, M.; Salonen, J.; et al. Multifunctional nanohybrid based on porous silicon nanoparticles, gold nanoparticles, and acetalated dextran for liver regeneration and acute liver failure theranostics. Adv. Mater. 2018, 30, 1703393. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, D.; Wang, L.; Liu, Z.; Wu, R.; Janoniene, A.; Ma, M.; Pan, G.; Baranauskiene, L.; Zhang, L.; et al. Microfluidic encapsulation of prickly zinc-doped copperoxide nanoparticles with VD1142 modified spermine acetalated dextran for efficient cancer therapy. Adv. Healthc. Mater. 2017, 6, 1601406. [Google Scholar] [CrossRef]



| Material Type | Microfluidic Systems | Size | Shape | Yield | Applications | References |
|---|---|---|---|---|---|---|
| SiO2 | single-phase flow | 164–321 nm | solid sphere | [23] | ||
| SiO2 | single-phase flow | 50–300 nm | solid sphere | [24] | ||
| SiO2 | single-phase flow | 53–176 nm | solid sphere | 62 ± 6% | [25] | |
| SiO2 | single-phase flow | 200–400 nm | mesoporous (~2 nm) sphere | [27] | ||
| SiO2 | single-phase flow | 50–650 nm | hollow; mesoporous sphere | [28] | ||
| SiO2; SiO2-QDs/Fe3O4 | single-phase flow | 804 nm | hollow sphere | cell imaging, dye adsorption and drug delivery. | [26] | |
| SiO2 | single-phase flow | 100 nm/ 15 μm | hollow; mesoporous sheet | organics adsorption, protein immobilization, and drug encapsulation | [29] | |
| SiO2; SiO2-Ag/Fe3O4 | single-phase flow | ~130 /1500 nm | mesoporous (~3 nm) fiber | gram-scale | doxorubicin (DOX) loading; 4-nitrophenol reduction | [30] |
| SiO2 | single-phase flow | 80 × 150 nm | hollow; mesoporous ellipsoid | DOX loading | [31] | |
| Fe2O3@SiO2 | single-phase flow | 50–350 nm | solid sphere; cube; rod; belt | circulating tumor cell screening | [32] | |
| SiO2@TiO2 | multiphase flow | ~250 nm | solid sphere | [33] | ||
| SiO2@Au | multiphase flow | 177–260 nm | solid sphere | [34] | ||
| SiO2–FITC | multiphase flow | 50–350 nm | solid sphere | [35] | ||
| SiO2–HDDA | multiphase flow | ~500 nm | mesoporous patchy | [36] | ||
| SiO2@Au | multiphase flow | 230 nm | solid sphere | [37] | ||
| SiO2–dextran | multiphase flow | 150–400 nm | mesoporous sphere | PTX, SFN, and MTX drug loading | [38] | |
| SiO2@Fe2O3 | multiphase flow | ~100 nm | solid sphere | [39] | ||
| SiO2@Au | multiphase flow | ~175 nm | solid sphere | [40] | ||
| SiO2–PEGDA | multiphase flow | 100–150 nm (sectional diameter) | macroporous fiber | [41] | ||
| SiO2–FITC | multiphase flow | 10–65 nm | solid sphere | [18] | ||
| Au | single-phase flow | 15–24 nm | solid sphere | labelling | [42] | |
| Au | single-phase flow | 1–2.5 nm | nanoplates | glucose oxidation | [43] | |
| Au | single-phase flow | 3–50 nm | nanorods | [44] | ||
| Ni | multiphase flow | 60–114 nm | solid sphere | 11.5 g h−1 | catalytic hydrogenation of p-nitrophenol to p-aminophenol | [45] |
| Ni; Ni/SiO2 | multiphase flow | 8.8–15.4 nm | solid sphere | >27 g d−1 | catalytic hydrodeoxygenation of guaiacol. | [46] |
| Ag | single-phase flow | ~8 nm | solid sphere | [47] | ||
| Ag | single-phase flow | edge length: 27–60 nm thickness: 11 nm | solid triangle | LSPR sensing | [48] | |
| Ag | single-phase flow | 5–10 nm | solid sphere | 4-nitrophenol reduction | [49] | |
| Pd | multiphase flow | 2.3 ± 0.3 nm | solid sphere | ~10 L d−1 | [50] | |
| Pd | multiphase flow | ~4 nm | nanorods | 96.5% | catalytic hydrogenation of styrene | [51] |
| Rh | multiphase flow | 3–8 nm | multipods; cuboctahedra | vapor-phase cyclohexene hydrogenation | [52] | |
| Pt1Bi1; Pt1Bi2 | single-phase flow | ~17 nm; ~33.5 nm | V-shaped nanorods; solid sphere | electrocatalysis | [53] | |
| AuPd | single-phase flow | 1–3 nm | nanoclusters | catalytic aerobic oxidation of benzyl alcohol | [54] | |
| AuPd | single-phase flow | 1–2 nm | solid sphere | CO oxidation | [55] | |
| Pt–Ni | multiphase flow | 6–12 nm | octahedra | 20–160 mg h−1 | oxygen reduction reaction catalysts | [56] |
| Pd@Pt | multiphase flow | 12–20 nm | core-shell; icosahedra | oxygen reduction reaction catalysts | [57] | |
| CdSe | single-phase flow | 2–4.5 nm | solid sphere | [58] | ||
| CdSe | single-phase flow | 3.6–5.4 nm | solid sphere | [59] | ||
| CdSe | multiphase flow | narrow size distribution. | solid sphere | 40–70% | [60] | |
| CdSe/ZnS | single-phase flow | 2.8–4.9 nm | core-shell; solid sphere | [61] | ||
| CdSe/CdS/ZnS; CdS/ZnS; CdSeS/ZnS | single-phase flow | ~1–5 nm | core-shell; solid sphere | [62] | ||
| InP | single-phase flow | ~5 nm | solid sphere | [63] | ||
| InP | single-phase flow | 4 nm | solid sphere | [64] | ||
| InP | single-phase flow | 2.7 nm | solid sphere | 63.1 g d−1 | [65] | |
| InP/ZnS | single-phase flow | 2.8–3.9 nm | core-shell; solid sphere | white-light-emitting diode | [66] | |
| InP/ ZnS; InP/ ZnSe; InP/ CdS; InAs/ InP | single-phase flow | 4.1–4.9 nm | core-shell; solid sphere | [67] | ||
| PbS | multiphase flow | 2–5 nm | solid sphere | 2.4–2.5 g h−1 | photovoltaic device | [68] |
| PbS; PbSe | multiphase flow | 3.8–4.5 nm | solid sphere | fabrication of Schottky solar cells | [69] | |
| ZIF-8 | single-phase flow | 150–465 nm | larger particles with a polygonal shape; smaller particles with roughly spherical shape | [70] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Shafiq, M.; Ma, M.; Chen, H. Synthesis and Surface Engineering of Inorganic Nanomaterials Based on Microfluidic Technology. Nanomaterials 2020, 10, 1177. https://doi.org/10.3390/nano10061177
Shen J, Shafiq M, Ma M, Chen H. Synthesis and Surface Engineering of Inorganic Nanomaterials Based on Microfluidic Technology. Nanomaterials. 2020; 10(6):1177. https://doi.org/10.3390/nano10061177
Chicago/Turabian StyleShen, Jie, Muhammad Shafiq, Ming Ma, and Hangrong Chen. 2020. "Synthesis and Surface Engineering of Inorganic Nanomaterials Based on Microfluidic Technology" Nanomaterials 10, no. 6: 1177. https://doi.org/10.3390/nano10061177
APA StyleShen, J., Shafiq, M., Ma, M., & Chen, H. (2020). Synthesis and Surface Engineering of Inorganic Nanomaterials Based on Microfluidic Technology. Nanomaterials, 10(6), 1177. https://doi.org/10.3390/nano10061177