Hydroxyapatite Surfaces Functionalized with a Self-Assembling Peptide: XPS, RAIRS and NEXAFS Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Samples Investigation
2.2.1. X-ray Photoelectron Spectroscopy (XPS)
2.2.2. Reflection–Absorption Infrared Spectroscopy (RAIRS)
2.2.3. Fourier Transform Spectroscopic Imaging (μFTIR)
2.2.4. Near Edge X-ray Absorption Fine Structure (NEXAFS) Spectroscopy
3. Results
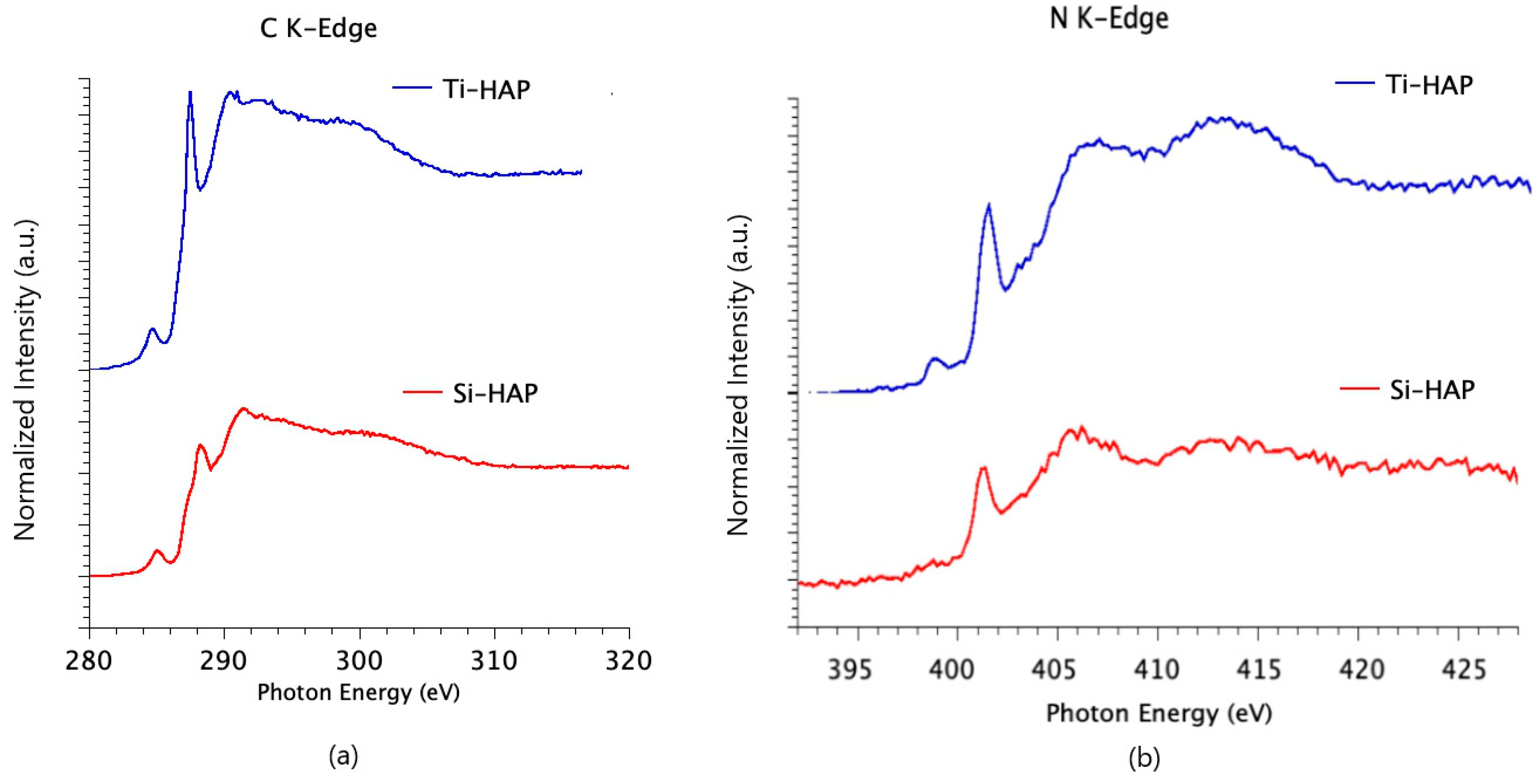
3.1. Analysis of Pristine Samples
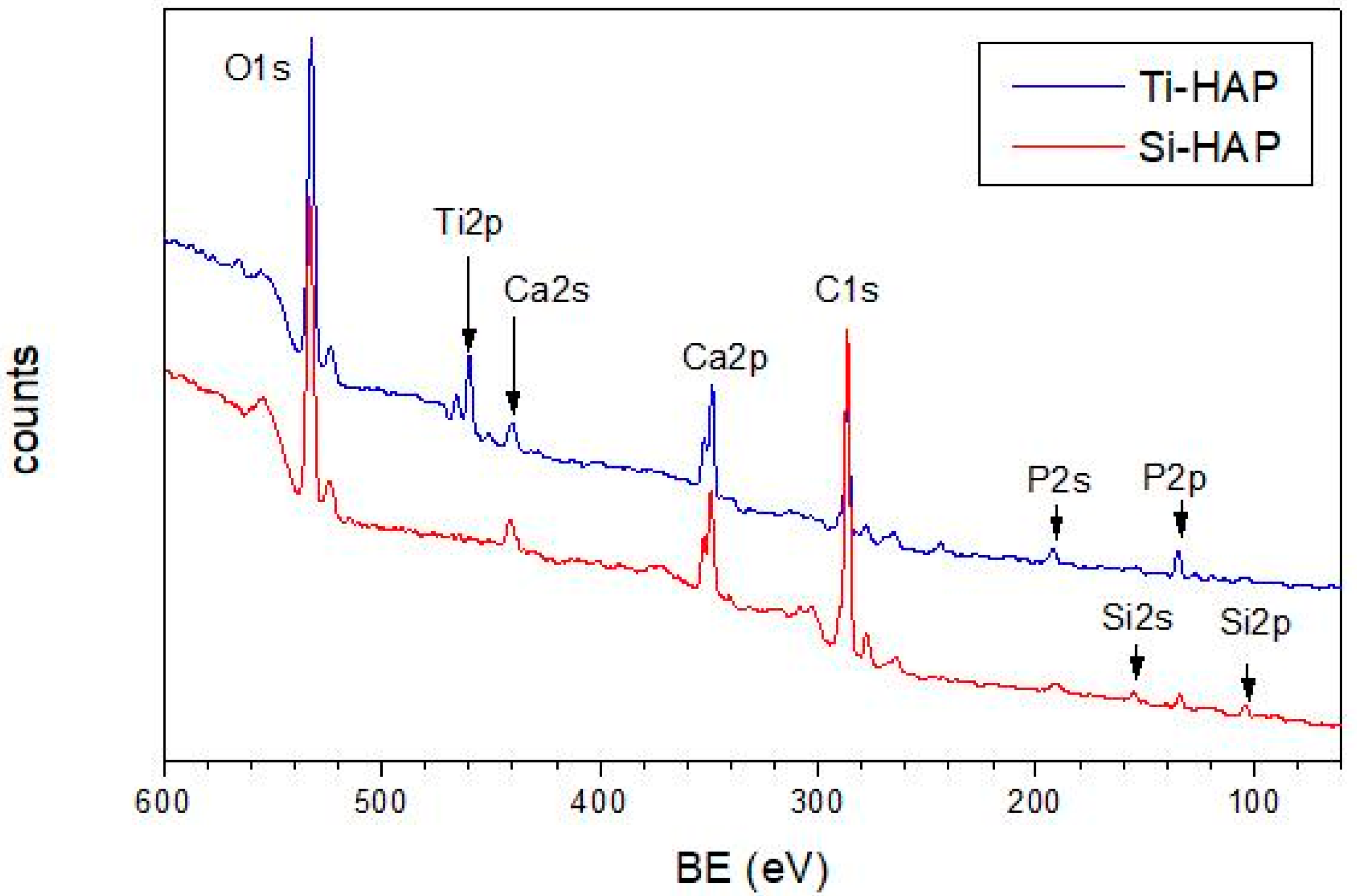
3.1.1. XPS Investigations
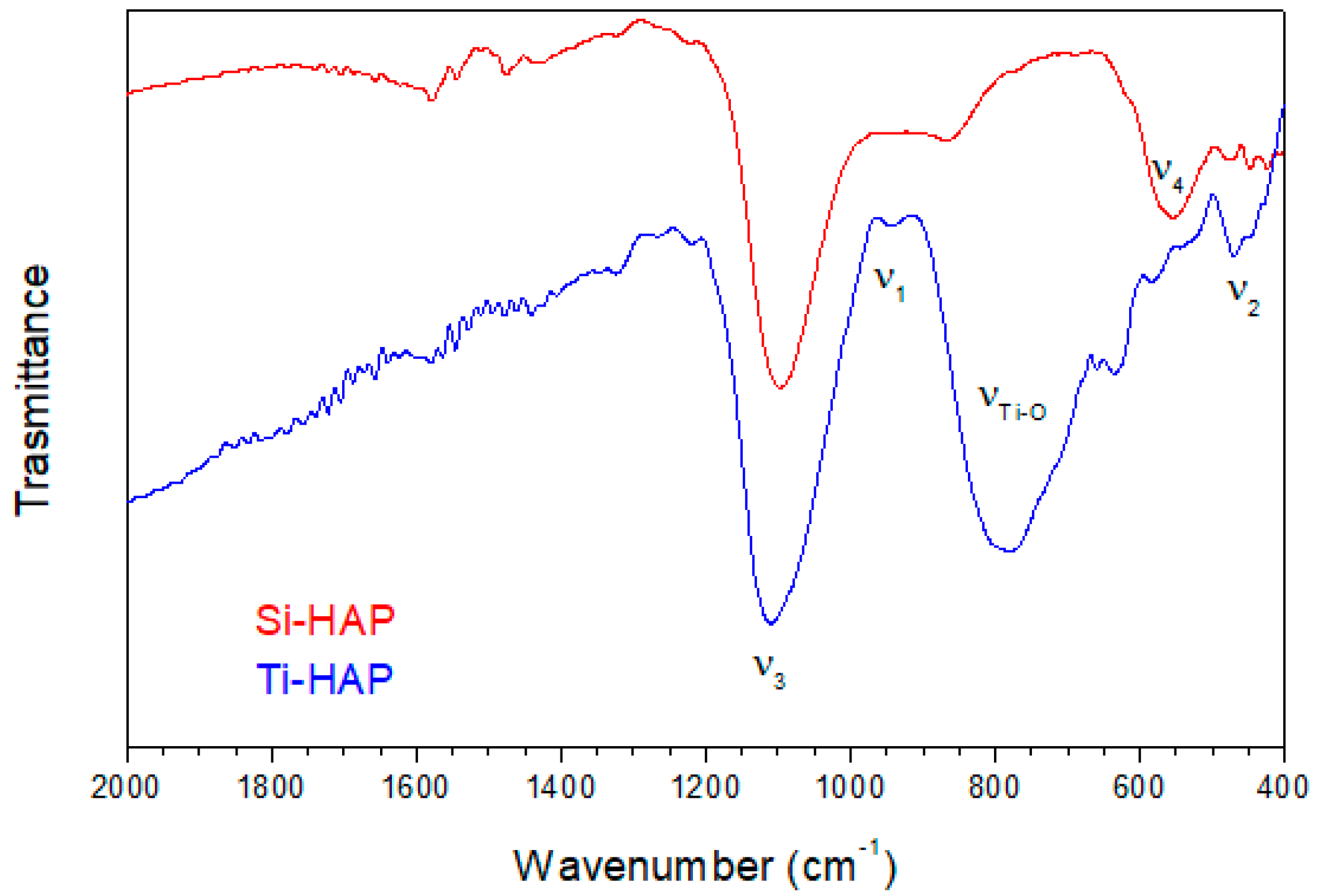
3.1.2. FTIR Investigations
3.2. SAP Adsorption on the HAP Surface
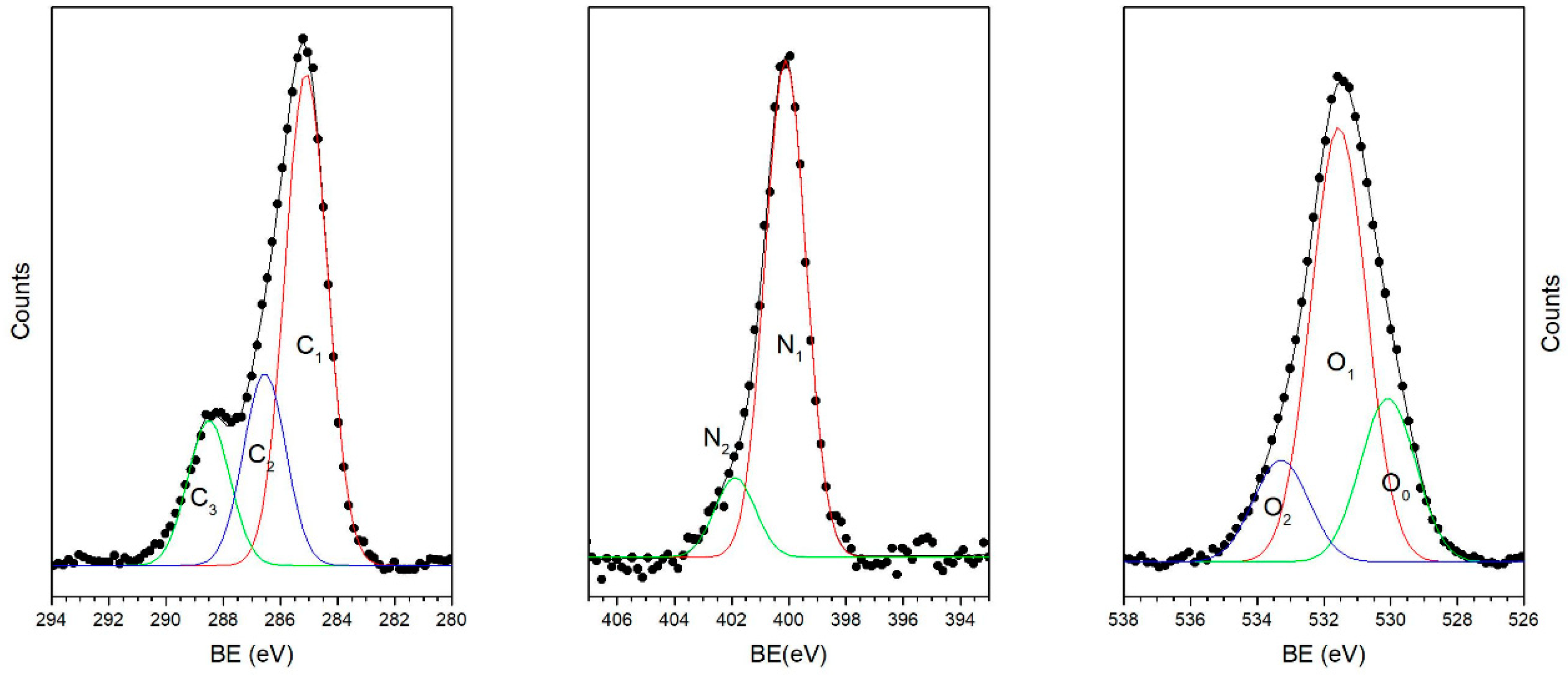
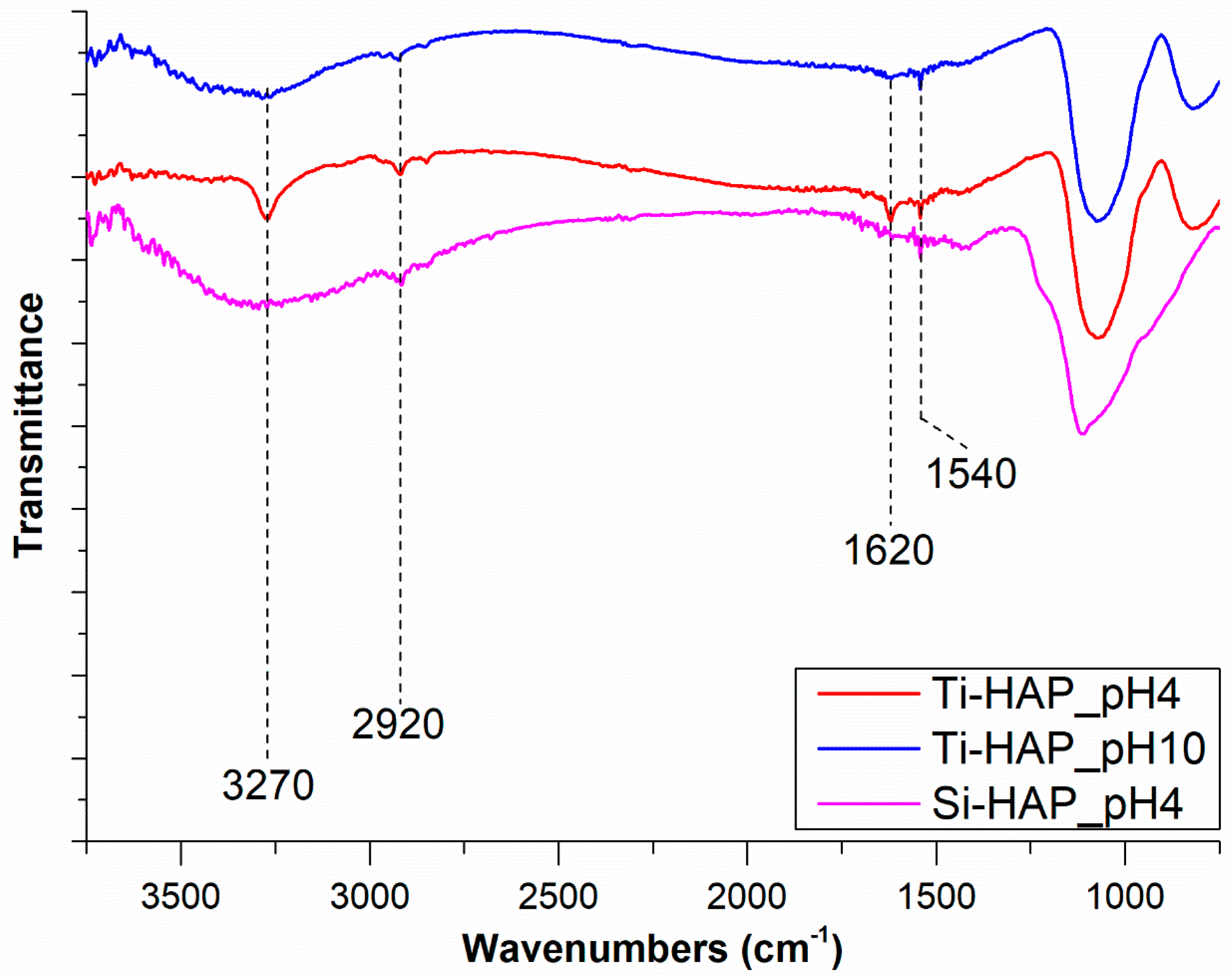
3.2.1. FTIR and XPS Results
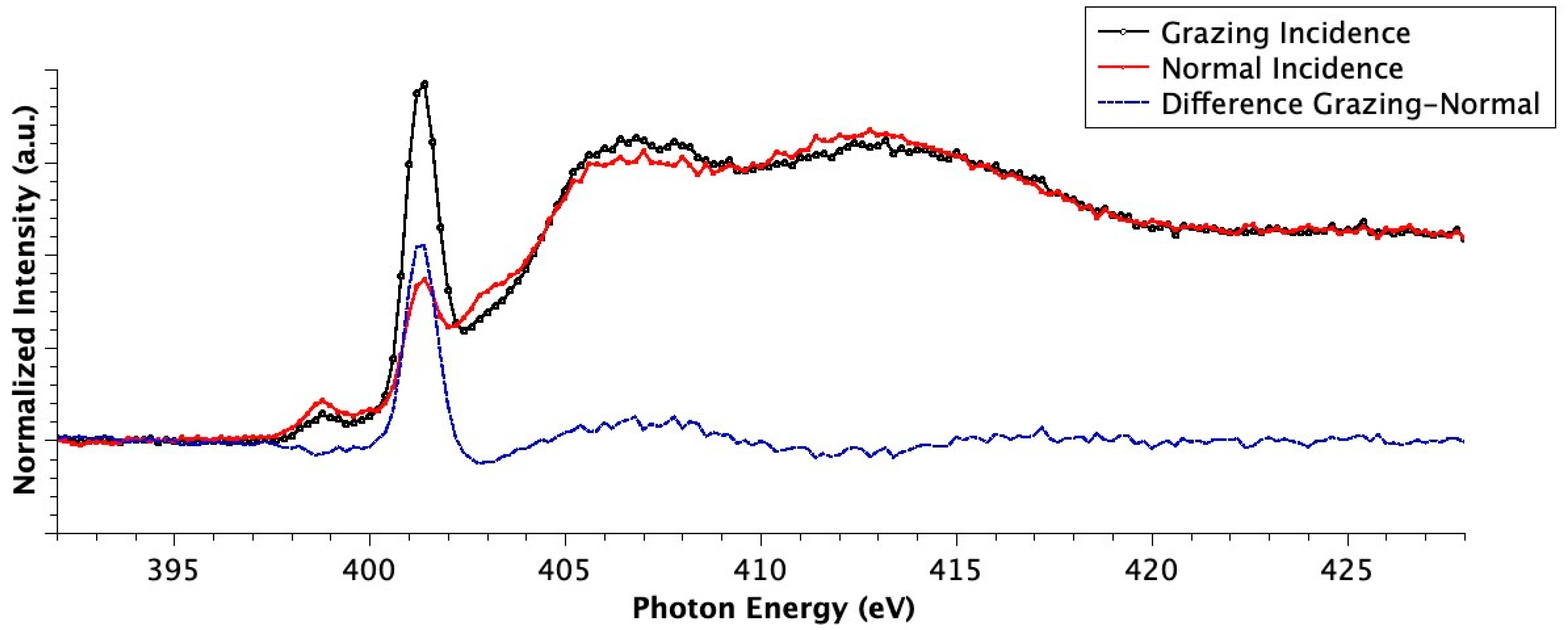
3.2.2. NEXAFS Spectra
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faria, R.C.S.; Rodrigues, R.C.S.; Rosa, A.L.; Ribeiro, R.F. Experimental titanium alloys for dental applications. J. Prosthet. Dent. 2014, 112, 1448–1460. [Google Scholar] [CrossRef]
- Textor, M.; Sittig, C.; Frauchiger, V.; Tosatti, S.; Brunette, D.M. Properties and biological significance of natural oxide films on titanium and its alloys. In Titanium in Medicine; Springer: Berlin/Heidelberg, Germany, 2001; pp. 171–230. [Google Scholar]
- Shah, F.A.; Trobos, M.; Thomsen, P.; Palmquist, A. Commercially pure titanium (cp-Ti) versus titanium alloy (Ti6Al4V) materials as bone anchored implants—Is one truly better than the other? Mater. Sci. Eng. C 2016, 62, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Dettin, M.; Zamuner, A.; Iucci, G.; Messina, M.L.; Battocchio, C.; Picariello, G.; Gallina, G.; Marletta, G.; Castagliuolo, I.; Brun, P. Driving h-osteoblast adhesion and proliferation on titania: Peptide hydrogels decorated with growth factors and adhesive conjugate. J. Pept. Sci. 2014, 20, 585–594. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Sharkeev, Y.P.; Komarova, E.G.; Khlusov, I.A.; Chebodaeva, V.V. Structure and properties of the wollastonite calcium phosphate coatings deposited on titanium and titanium niobium alloy using microarc oxidation method. Surf. Coat. Technol. 2016, 307, 1274–1283. [Google Scholar] [CrossRef]
- Pardun, K.; Treccani, L.; Volkmann, E.; Streckbein, P.; Heiss, C.; Destri, G.L.; Marletta, G.; Rezwan, K. Mixed zirconia calcium phosphate coatings for dental implants: Tailoring coating stability and bioactivity potential. Mater. Sci. Eng. C 2015, 48, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Tanaka, Y.; Ide-Ektessabi, A. Fabrication of hydroxyapatite thin films for biomedical applications using RF magnetron sputtering. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2006, 249, 723–725. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. C 2015, 55, 272–326. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Monsees, T.K.; Ak Azem, F.; Cotrut, C.M.; Braic, M.; Abdulgader, R.; Pana, I.; Birlik, I.; Kiss, A.; Booysen, R.; Vladescu, A. Biodegradable ceramics consisting of hydroxyapatite for orthopaedic implants. Coatings 2017, 7, 184. [Google Scholar] [CrossRef]
- Vladescu, A.; Cotrut, C.M.; Ak Azem, F.; Bramowicz, M.; Pana, I.; Braic, V.; Birlik, I.; Kiss, A.; Braic, M.; Abdulgader, R.; et al. Sputtered Si and Mg doped hydroxyapatite for biomedical applications. Biomed. Mater. 2018, 13, 025011. [Google Scholar] [CrossRef]
- Boyd, A.R.; Rutledge, L.; Randolph, L.D.; Meenan, B.J. Strontium-substituted hydroxyapatite coatings deposited via a co-deposition sputter technique. Mater. Sci. Eng. C 2015, 46, 290–300. [Google Scholar] [CrossRef]
- Revilla-López, G.; Bertran, O.; Casanovas, J.; Turon, P.; Puiggalí, J.; Alemán, C. Effects of hydroxyapatite (0001) Ca2+/Mg2+ substitution on adsorbed D-ribose ring puckering. RSC Adv. 2016, 6, 69634–69640. [Google Scholar] [CrossRef]
- Vladescu, A.; Padmanabhan, S.C.; Ak Azem, F.; Braic, M.; Titorencu, I.; Birlik, I.; Morris, M.A.; Braic, V. Mechanical properties and biocompatibility of the sputtered Ti doped hydroxyapatite. J. Mech. Behav. Biomed. Mater. 2016, 63, 314–325. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate-based biocomposites and hybrid biomaterials. J. Mater. Sci. 2009, 44, 2343–2387. [Google Scholar] [CrossRef]
- Nayak, S.; Dey, T.; Naskar, D.; Kundu, S.C. The promotion of osseointegration of titanium surfaces by coating with silk protein sericin. Biomaterials 2013, 34, 2855–2864. [Google Scholar] [CrossRef]
- Capriotti, L.A.; Beebe, T.P., Jr.; Schneider, J.P. Hydroxyapatite surface-induced peptide folding. J. Am. Chem. Soc. 2007, 129, 5281–5287. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Ren, T.; Gu, S.; Tan, Q.; Zhang, L.; Lv, K.; Pan, K.; Jiang, X. Bone marrow stromal cells cultured on poly (lactide-co-glycolide)/ nano-hydroxyapatite composites with chemical immobilization of Arg-Gly-Asp peptide and preliminary bone regeneration of mandibular defect thereof. J. Biomed. Mater. Res. A 2010, 95, 993–1003. [Google Scholar] [CrossRef]
- Durrieu, M.C.; Pallu, S.; Guillemot, F.; Barreille, R.; Amédée, J.; Baquey, C.H.; Labrugère, C.; Dard, M. Grafting RGD containing peptides onto hydroxyapatite to promote osteoblastic cell adhesion. J. Mat. Sci. Mat. Med. 2004, 15, 779–786. [Google Scholar] [CrossRef]
- Rodriguez, G.M.; Bowen, J.; Grossin, D.; Ben-Nissan, B.; Stamboulis, A. Functionalisation of Ti6Al4V and hydroxyapatite surfaces with combined peptides based on KKLPDA and EEEEEEEE peptides. Colloids Surf. B Biointerfaces 2017, 160, 154–160. [Google Scholar] [CrossRef]
- Battocchio, C.; Iucci, G.; Dettin, M.; Carravetta, V.; Monti, S.; Polzonetti, G. Self-assembling behaviour of self-complementary oligopeptides on biocompatible substrates. Mat. Sci. Eng. C 2010, 169, 36–42. [Google Scholar] [CrossRef]
- Franchi, S.; Secchi, V.; Santi, M.; Dettin, M.; Zamuner, A.; Battocchio, C.; Iucci, G. Biofunctionalization of TiO2 surfaces with self assembling oligopeptides in different pH and ionic strength conditions: Charge effects and molecular organization. Mat. Sci. Eng. C 2018, 90, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Zamuner, A.; Peretti, A.; Conti, J.; Messina, G.M.L.; Marletta, G.; Dettin, M. 3D Synthetic Peptide-based Architectures for the Engineering of the Enteric Nervous System. Sci Rep 2019, 9, 5583. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Kaciulis, S.; Mattogno, G.; Mezzi, A.; Ambrosio, L.; Branda, F. XPS characterization of biocompatible hydroxyapatite-polymer coatings. Surf. Interf. Anal. 2013, 34, 45–49. [Google Scholar] [CrossRef]
- Lebugle, A.; Rovira, A.; Rabaud, M.; Rey, C. XPS study of elastin-solubilized peptides binding onto apatite in orthopaedic biomaterials. J. Mat. Sci. Mat. Med. 1996, 7, 223–226. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. C1s peak of adventitious carbon aligns to the vacuum level: Dire consequences for material’s bonding assignment by photoelectron spectroscopy. ChemPhysChem 2017, 18, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Greczynski, G.; Hultman, L. Compromising science by ignorant instrument calibration—Need to revisit half a century of published XPS data. Angew. Chem. Ed. 2020, 59, 5002–5006. [Google Scholar] [CrossRef] [PubMed]
- Maachou, H.; Genet, M.J.; Aliouche, D.; Dupont-Gillain, C.C.; Rouxhet, P.G. XPS analysis of chitosan-hydroxyapatite biomaterials: From elements to compounds. Surf. Interf. Anal. 2013, 45, 1088–1095. [Google Scholar] [CrossRef]
- NIST. X-ray Photoelectron Spectroscopy Database; Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Polzonetti, G.; Battocchio, C.; Iucci, G.; Dettin, M.; Gambaretto, R.; Di Bello, C.; Carravetta, V. Thin films of a self-assembling peptide on TiO2 and Au studied by NEXAFS, XPS and IR spectroscopies. Mat. Sci. Eng. C 2006, 26, 929–934. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Division, Perkin-Elmer Corp: Norwalk, CT, USA, 1995. [Google Scholar]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-raman and FTIR studies of synthetic and natural apatite. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR Spectroscopy. J. Mat. Sci. Mat. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, D.C.L.; Costa, V.C.; Nunes, E.H.M.; Sabioni, A.C.S.; Gasparon, M.; Vasconcelos, W.L. Infrared spectroscopy of titania sol-gel coatings on 316L stainless steel. Mater. Sci. Appl. 2011, 2, 1375–1382. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, W.; Ye, J. FTIR study on the polymorphic structure of tricalcium silicate. Cem. Concr. Res. 2017, 99, 129–136. [Google Scholar] [CrossRef]
- Franchi, S.; Secchi, V.; Santi, M.; Vladescu, A.; Braic, M.; Skála, T.; Lavkova, J.; Dettin, M.; Zamuner, A.; Iucci, G.; et al. Biocompatible materials based on self-assembling peptides on Ti25Nb10Zr alloy: Molecular structure and organization. Nanomaterials 2018, 8, 148. [Google Scholar] [CrossRef]
- Seah, M.; Dench, W.A. Quantitative electron spectroscopy of surfaces: A standard data base for electron inelastic mean free paths in solids. Surf. Interf. Anal. 1979, 1, 2–11. [Google Scholar] [CrossRef]
- Haris, P.I.; Chapman, D. The conformational analysis of peptides using Fourier Transform IR spectroscopy. Biopolymers 1995, 37, 251–263. [Google Scholar] [CrossRef]
- Castano, S.; Desbat, B.; Laguerre, M.; Dufourcq, J. Structure, orientation and affinity for interfaces and lipids of ideally amphipathic lytic LiKj(i = 2j) peptides. Biochim. Biophys. Acta 1999, 1416, 176–194. [Google Scholar] [CrossRef]
- Stohr, J. NEXAFS Spectroscopy Springer Series in Surface Sciences; Gomer, C., Ed.; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
| Sample | RF Powers Fed (W) | |||
|---|---|---|---|---|
| HAP Cathode | SiC Cathode | TiO2 Cathode | MgO Cathode | |
| Ti-HAP | 50 | - | 25 | 50 |
| Si-HAP | 50 | 15 | - | 50 |
| Sample | BE (eV) | ||||||
|---|---|---|---|---|---|---|---|
| Ca2p3/2 | P2p | Si2p | Ti2p3/2 | Mg2p | O1s(0) | O1s (1) | |
| Ti-HAP | 347.5 | 133.6 | 458.8 | 50.3 | 530.4 | 531.6 | |
| Si-HAP | 347.5 | 132.4 | 102.6 | 50.9 | 531.9 | ||
| Sample | P/Ca | Si/Ca | Ti/Ca | Mg/Ca | O/Ca | C/Ca | O0/O1 |
|---|---|---|---|---|---|---|---|
| Ti-HAP | 0.57 | 0.39 | 0.34 | 3.8 | 3.2 | 0.54 | |
| Si-HAP | 0.40 | 0.54 | 0.15 | 2.48 | 3.6 |
| Sample | pH | C/Ca | N/Ca | O/Ca | P/Ca | Ti/Ca | C2/C1 | C3/C1 | N2/N1 | O0/O1 | O2/O1 | Å |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti-HAP | 4 | 35 | 4.3 | 17 | 2.1 | 0. | 0.5 | 0.3 | 0.14 | 0.16 | 0.16 | 59 |
| 10 | 15 | 1.1 | 13 | 1.2 | 1.5 | 0.6 | 0.5 | 0.18 | 0.4 | 0.3 | 43 | |
| pH | C/Ca | N/Ca | O/Ca | P/Ca | Si/Ca | C2/C1 | C3/C1 | O2/O1 | Å | |||
| Si-HAP | 4 | 10 | 0.23 | 5.0 | 0.9 | 0.2 | 0.4 | 0.25 | 0.18 | 9 | ||
| 10 | 8 | 0.26 | 6.6 | 0.7 | 0.9 | 0.3 | 0.2 | 0.1 | 0.11 | 17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secchi, V.; Franchi, S.; Dettin, M.; Zamuner, A.; Beranová, K.; Vladescu, A.; Battocchio, C.; Graziani, V.; Tortora, L.; Iucci, G. Hydroxyapatite Surfaces Functionalized with a Self-Assembling Peptide: XPS, RAIRS and NEXAFS Study. Nanomaterials 2020, 10, 1151. https://doi.org/10.3390/nano10061151
Secchi V, Franchi S, Dettin M, Zamuner A, Beranová K, Vladescu A, Battocchio C, Graziani V, Tortora L, Iucci G. Hydroxyapatite Surfaces Functionalized with a Self-Assembling Peptide: XPS, RAIRS and NEXAFS Study. Nanomaterials. 2020; 10(6):1151. https://doi.org/10.3390/nano10061151
Chicago/Turabian StyleSecchi, Valeria, Stefano Franchi, Monica Dettin, Annj Zamuner, Klára Beranová, Alina Vladescu, Chiara Battocchio, Valerio Graziani, Luca Tortora, and Giovanna Iucci. 2020. "Hydroxyapatite Surfaces Functionalized with a Self-Assembling Peptide: XPS, RAIRS and NEXAFS Study" Nanomaterials 10, no. 6: 1151. https://doi.org/10.3390/nano10061151
APA StyleSecchi, V., Franchi, S., Dettin, M., Zamuner, A., Beranová, K., Vladescu, A., Battocchio, C., Graziani, V., Tortora, L., & Iucci, G. (2020). Hydroxyapatite Surfaces Functionalized with a Self-Assembling Peptide: XPS, RAIRS and NEXAFS Study. Nanomaterials, 10(6), 1151. https://doi.org/10.3390/nano10061151






.jpg)



