Remarkable Physical and Thermal Properties of Hydrothermal Carbonized Nanoscale Cellulose Observed from Citric Acid Catalysis and Acetone Rinsing
Abstract
:1. Introduction
2. Methods
3. Analysis
4. Results and Discussion
4.1. Yield and Compositions
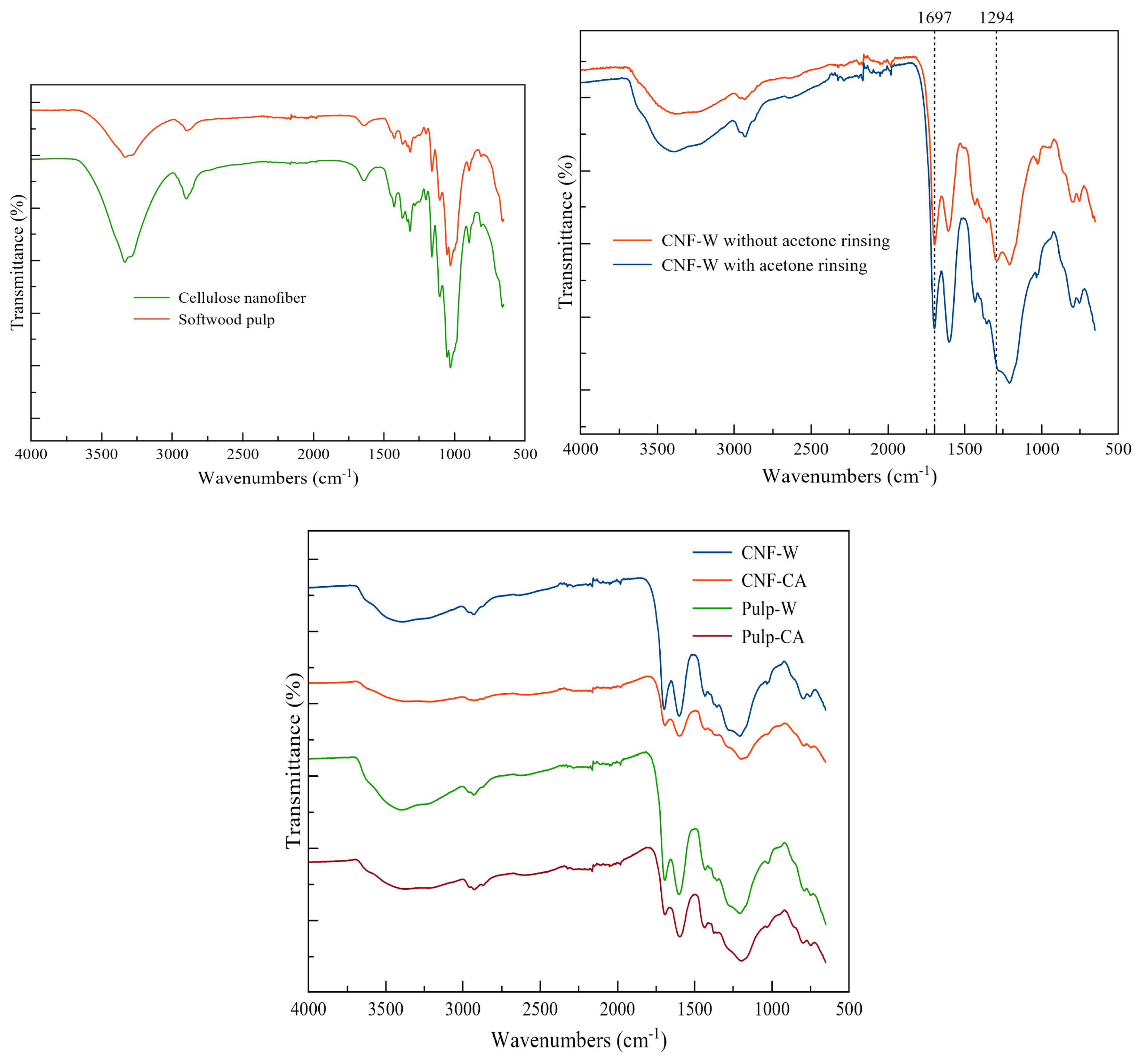
4.2. ATR-FTIR Analysis
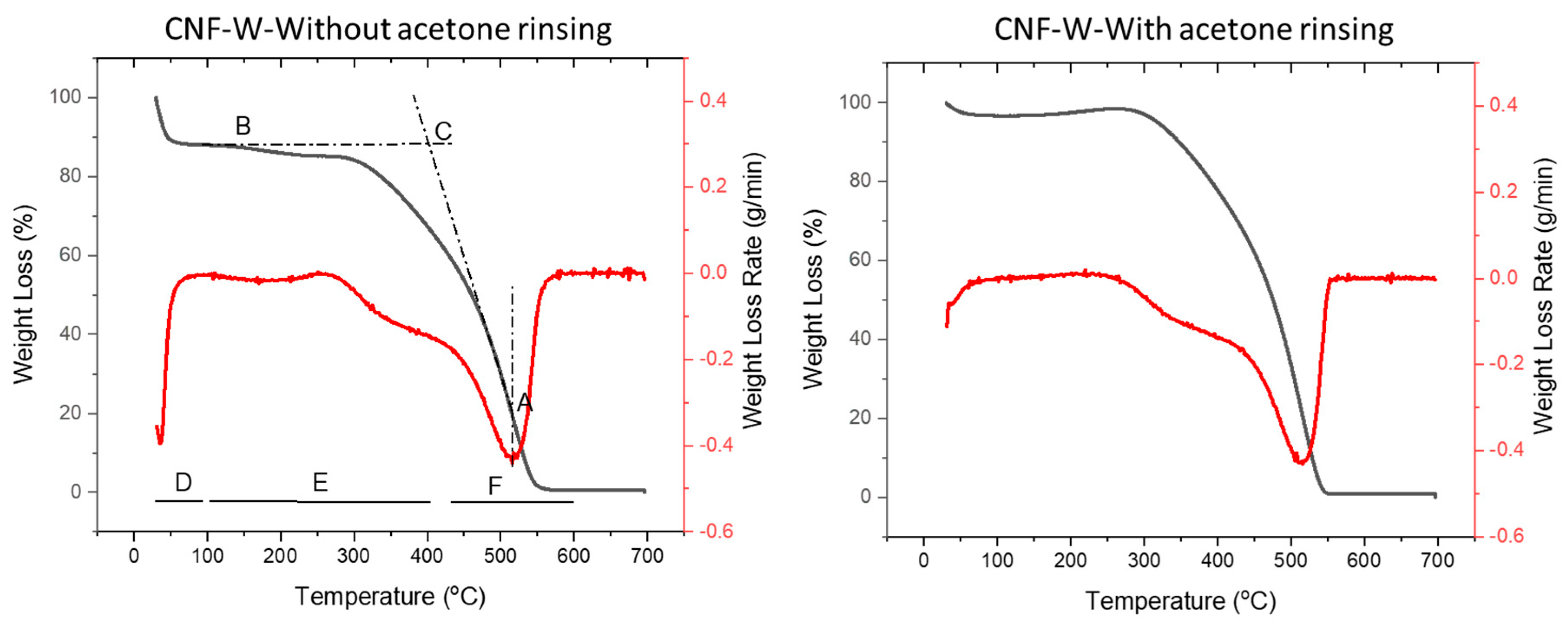
4.3. Combustion and Thermal Properties
4.4. Surface Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sevilla, M.; Maciá-Agulló, J.A.; Fuertes, A.B. Hydrothermal Carbonization of Biomass as a Route for the Sequestration of CO2: Chemical and Structural Properties of the Carbonized Products. Biomass Bioenergy 2011, 35, 3152–3159. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Loganathan, V.A.; Gupta, R.B.; Barnett, M.O. An Assessment of U(VI) Removal from Groundwater Using Biochar Produced from Hydrothermal Carbonization. J. Environ. Manag. 2011, 92, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Parshetti, G.K.; Liu, Z.; Jain, A.; Srinivasan, M.P.; Balasubramanian, R. Hydrothermal Carbonization of Sewage Sludge for Energy Production with Coal. Fuel 2013, 111, 201–210. [Google Scholar] [CrossRef]
- Gai, C.; Zhang, F.; Lang, Q.; Liu, T.; Peng, N.; Liu, Z. Facile One-Pot Synthesis of Iron Nanoparticles Immobilized into the Porous Hydrochar for Catalytic Decomposition of Phenol. Appl. Catal. B Environ. 2017, 204, 566–576. [Google Scholar] [CrossRef]
- Mau, V.; Gross, A. Energy Conversion and Gas Emissions from Production and Combustion of Poultry-Litter-Derived Hydrochar and Biochar. Appl. Energy 2018, 213, 510–519. [Google Scholar]
- Darmawan, A.; Budianto, D.; Aziz, M.; Tokimatsu, K. Retrofitting Existing Coal Power Plants through Cofiring with Hydrothermally Treated Empty Fruit Bunch and a Novel Integrated System. Appl. Energy 2017, 204, 1138–1147. [Google Scholar]
- Zhao, L.; Bacsik, Z.; Hedin, N.; Wei, W.; Sun, Y.; Antonietti, M.; Titirici, M. Carbon Dioxide Capture on Amine-Rich Carbonaceous Materials Derived from Glucose. ChemSusChem 2010, 3, 840–845. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, L.; Liu, X.; Zhang, J.; Diao, H.; Ma, X. Preparation of Monodispersed Carbon Spheres via Hydrothermal Carbonization of Ascorbic Acid and Their Application in Lithium Ion Batteries. Chem. Res. Chin. Univ. 2018, 34, 628–634. [Google Scholar]
- Zhang, Y.; Jiang, Q.; Xie, W.; Wang, Y.; Kang, J. Effects of Temperature, Time and Acidity of Hydrothermal Carbonization on the Hydrochar Properties and Nitrogen Recovery from Corn Stover. Biomass Bioenergy 2019, 122, 175–182. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J. Hydrothermal Carbonization of Biomass Residuals: A Comparative Review of the Chemistry, Processes and Applications of Wet and Dry Pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Shen, Y.; Ge, S.; Chen, Z.; Yoshikawa, K. Clean Solid Biofuel Production from High Moisture Content Waste Biomass Employing Hydrothermal Treatment. Appl. Energy 2014, 131, 345–367. [Google Scholar] [CrossRef] [Green Version]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An Overview of Effect of Process Parameters on Hydrothermal Carbonization of Biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Li, R.; Shahbazi, A. A Review of Hydrothermal Carbonization of Carbohydrates for Carbon Spheres Preparation. Trends Renew. Energy 2015, 1, 43–56. [Google Scholar] [CrossRef]
- Johnson, S.; Faradilla, R.H.F.; Venditti, R.A.; Lucia, L.; Hakovirta, M. Hydrothermal Carbonization of Nanofibrillated Cellulose: A Pioneering Model Study Demonstrating the Effect of Size on Final Material Qualities. ACS Sustain. Chem. Eng. 2020, 8, 1823–1830. [Google Scholar]
- Van Putten, R.-J.; Van Der Waal, J.C.; De Jong, E.D.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Reiche, S.; Kowalew, N.; Schlögl, R. Influence of Synthesis PH and Oxidative Strength of the Catalyzing Acid on the Morphology and Chemical Structure of Hydrothermal Carbon. ChemPhysChem 2015, 16, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Susanti, R.F.; Arie, A.A.; Kristianto, H.; Erico, M.; Kevin, G.; Devianto, H. Activated Carbon from Citric Acid Catalyzed Hydrothermal Carbonization and Chemical Activation of Salacca Peel as Potential Electrode for Lithium Ion Capacitor’s Cathode. Ionics (Kiel) 2019, 25, 3915–3925. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Zhao, X.; Wang, D.P.; Liu, S.X. Carbon Spheres Obtained via Citric Acid Catalysed Hydrothermal Carbonisation of Cellulose. Mater. Res. Innov. 2013, 17, 546–551. [Google Scholar] [CrossRef]
- Simsir, H.; Eltugral, N.; Karagoz, S. Hydrothermal Carbonization for the Preparation of Hydrochars from Glucose, Cellulose, Chitin, Chitosan and Wood Chips via Low-Temperature and Their Characterization. Bioresour. Technol. 2017, 246, 82–87. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.-Y. Conversion of Sewage Sludge to Clean Solid Fuel Using Hydrothermal Carbonization: Hydrochar Fuel Characteristics and Combustion Behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Ryu, J.; Suh, Y.-W.; Suh, D.J.; Ahn, D.J. Hydrothermal Preparation of Carbon Microspheres from Mono-Saccharides and Phenolic Compounds. Carbon 2010, 48, 1990–1998. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Pires, E.; Fraile, J.M. Parametric Study of the Hydrothermal Carbonization of Cellulose and Effect of Acidic Conditions. Carbon 2017, 123, 421–432. [Google Scholar] [CrossRef]
- Kumar, M.; Oyedun, A.O.; Kumar, A. A Review on the Current Status of Various Hydrothermal Technologies on Biomass Feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Latham, K.G.; Jambu, G.; Joseph, S.D.; Donne, S.W. Nitrogen Doping of Hydrochars Produced Hydrothermal Treatment of Sucrose in H2O, H2SO4, and NaOH. ACS Sustain. Chem. Eng. 2014, 2, 755–764. [Google Scholar] [CrossRef]
- Mumme, J.; Eckervogt, L.; Pielert, J.; Diakité, M.; Rupp, F.; Kern, J. Hydrothermal Carbonization of Anaerobically Digested Maize Silage. Bioresour. Technol. 2011, 102, 9255–9260. [Google Scholar] [CrossRef] [PubMed]
- Lynam, J.G.; Coronella, C.J.; Yan, W.; Reza, M.T.; Vasquez, V.R. Acetic Acid and Lithium Chloride Effects on Hydrothermal Carbonization of Lignocellulosic Biomass. Bioresour. Technol. 2011, 102, 6192–6199. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, Y.; Zhu, Y.; Li, Y.; Qu, Y.; Rong, C.; Ma, X.; Wang, Z. A New Route for Preparation of Hydrochars from Rice Husk. Bioresour. Technol. 2010, 101, 9807–9810. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of Hydrochars Produced by Hydrothermal Carbonization of Lignin, Cellulose, D-Xylose, and Wood Meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Jatzwauck, M.; Schumpe, A. Kinetics of Hydrothermal Carbonization (HTC) of Soft Rush. Biomass Bioenergy 2015, 75, 94–100. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Xiao, H.; Guo, Y.; Liang, X.; Qi, C. One-Step Synthesis of Novel Biacidic Carbon via Hydrothermal Carbonization. J. Solid State Chem. 2010, 183, 1721–1725. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Y.; Balasubramanian, R.; Hoekman, S.K. Mechanical Stability and Combustion Characteristics of Hydrochar/Lignite Blend Pellets. Fuel 2016, 164, 59–65. [Google Scholar] [CrossRef]
- Karatepe, N.; Küçükbayrak, S. Proximate Analysis of Some Turkish Lignites by Thermogravimetry. Thermochim. Acta 1993, 213, 147–150. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Qian, F.; Lei, Z.; Zhang, Z.; Zhang, S.; Chen, J.; Ren, Z.J. Demethanation Trend of Hydrochar Induced by Organic Solvent Washing and Its Influence on Hydrochar Activation. Environ. Sci. Technol. 2017, 51, 10756–10764. [Google Scholar] [CrossRef]
- Cao, X.; Ro, K.S.; Chappell, M.; Li, Y.; Mao, J. Chemical Structures of Swine-Manure Chars Produced under Different Carbonization Conditions Investigated by Advanced Solid-State 13C Nuclear Magnetic Resonance (NMR) Spectroscopy. Energy Fuels 2010, 25, 388–397. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S.; Wu, J. Characterization and Application of Chars Produced from Pinewood Pyrolysis and Hydrothermal Treatment. Fuel 2010, 89, 510–514. [Google Scholar] [CrossRef]
- Yang, G.; Song, S.; Li, J.; Tang, Z.; Ye, J.; Yang, J. Preparation and CO2 Adsorption Properties of Porous Carbon by Hydrothermal Carbonization of Tree Leaves. J. Mater. Sci. Technol. 2019, 35, 875–884. [Google Scholar] [CrossRef]
- Xu, M.; Sheng, C. Influences of the Heat-Treatment Temperature and Inorganic Matter on Combustion Characteristics of Cornstalk Biochars. Energy Fuels 2011, 26, 209–218. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, R.; Parshetti, G.K.; Liu, Z.; Balasubramanian, R. Enzyme-Assisted Hydrothermal Treatment of Food Waste for Co-Production of Hydrochar and Bio-Oil. Bioresour. Technol. 2014, 168, 267–274. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Y.; Zou, B.; Rong, C.; Ma, X.; Qu, Y.; Li, Y.; Wang, Z. High Surface Area Porous Carbons Prepared from Hydrochars by Phosphoric Acid Activation. Bioresour. Technol. 2011, 102, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
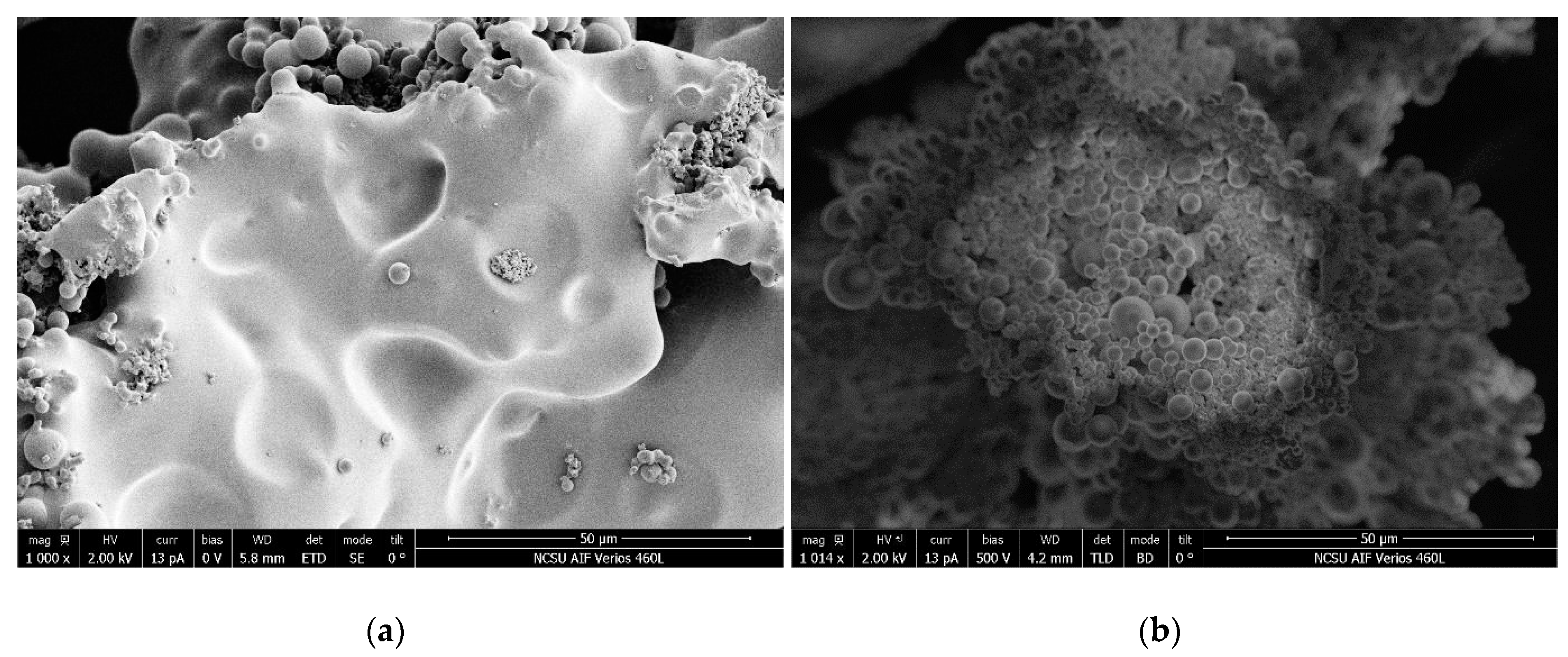
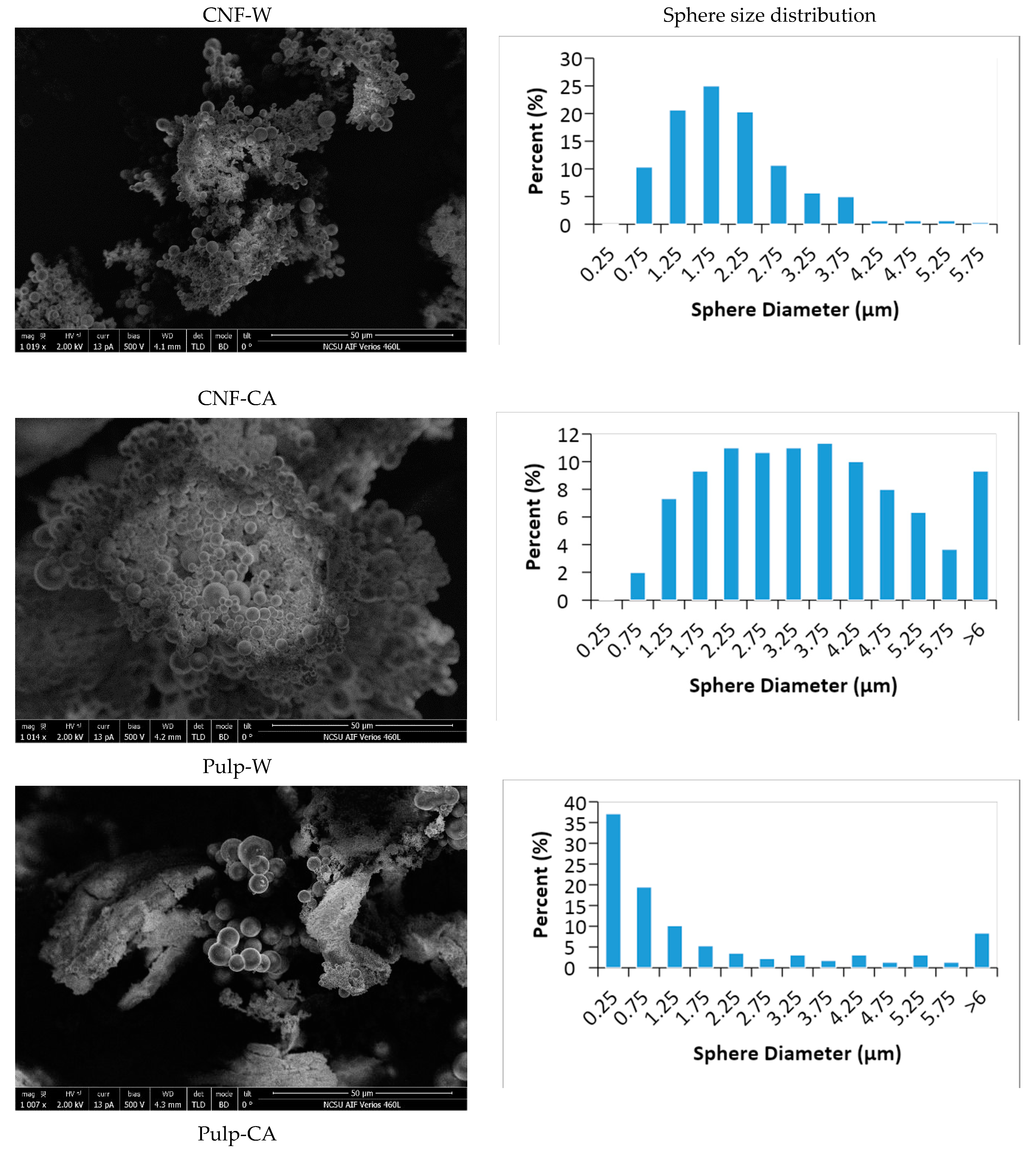
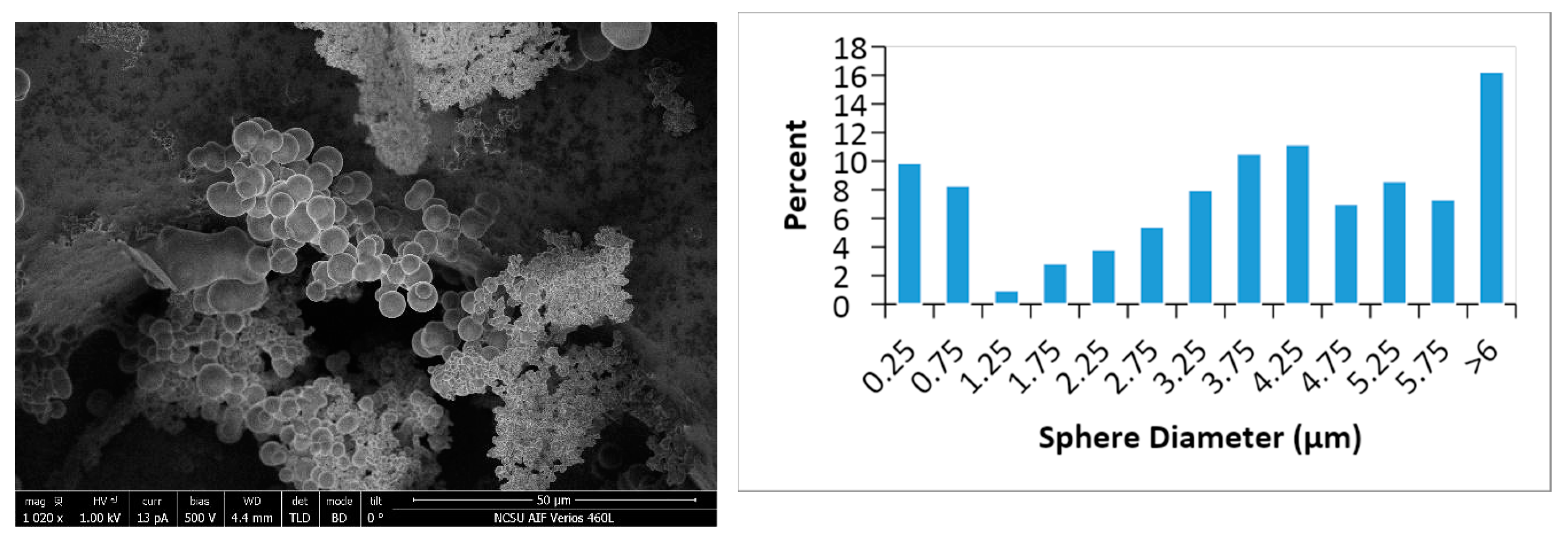
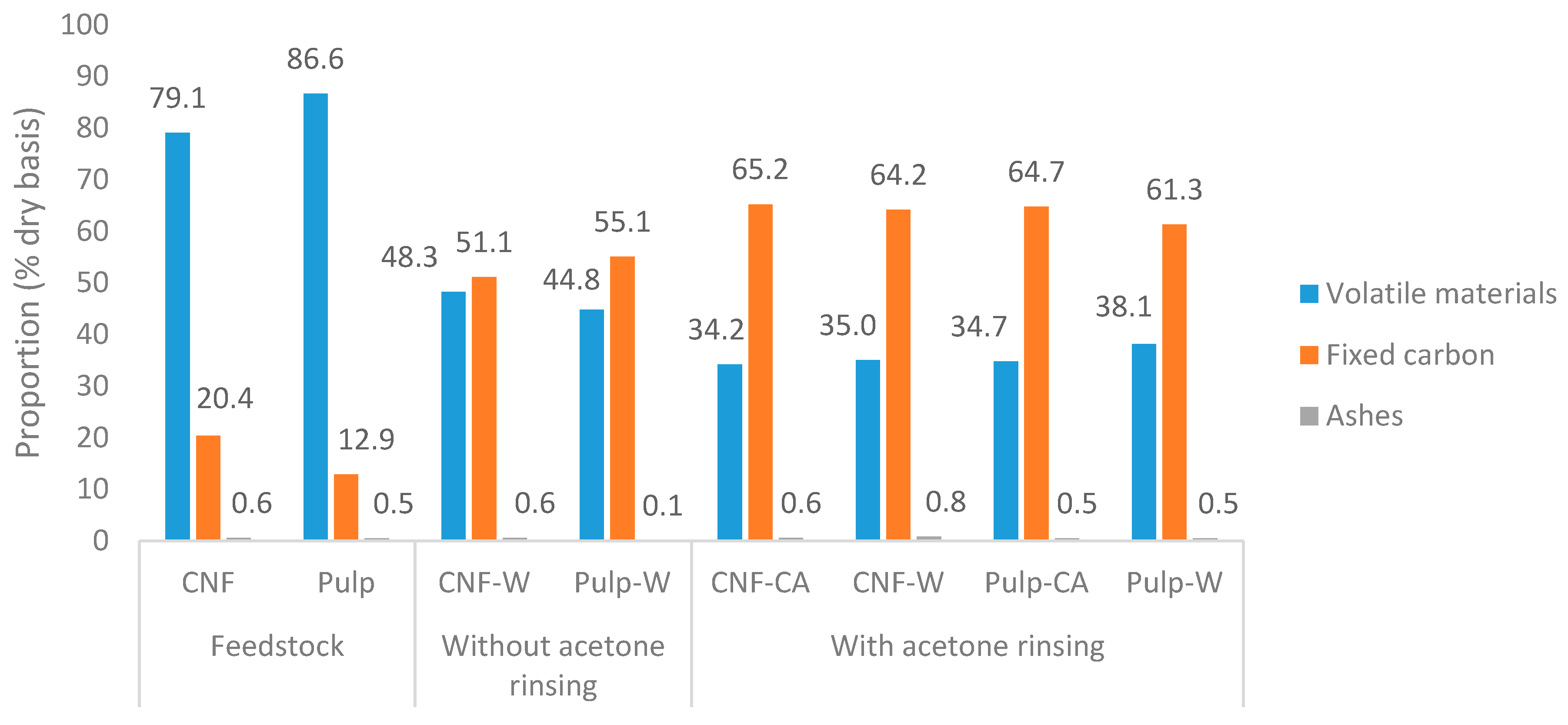

| Sample Codes | Yield (%) | Hydrochar | Tar | Liquid | Initial pH | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Acetone Rinsing | After Acetone Rinsing | ||||||||||||||||||
| C (%) | H (%) | N (%) | O (%) | C (%) | H (%) | N (%) | O (%) | H/C | O/C | HHV (MJ kg−1) | C (%) | H (%) | N (%) | TC (mg L−1) | TOC (mg L−1) | pH | |||
| CNF-W | 42.8 | 62.90 | 3.88 | 0.10 | 33.12 | 66.42 | 4.09 | 0.10 | 29.39 | 0.06 | 0.44 | 23.0 | 70.14 | 5.54 | 0.08 | 5558 | 5537 | 2.94 | 3.59 |
| CNF-CA | 44.5 | 65.10 | 4.29 | 0.06 | 30.55 | 0.07 | 0.47 | 22.7 | 15,010 | 14,929 | 2.95 | 1.87 | |||||||
| Pulp-W | 32.2 | 70.73 | 4.39 | 0.13 | 24.75 | 66.95 | 4.20 | 0.06 | 28.79 | 0.06 | 0.43 | 23.5 | 70.08 | 5.76 | 0.09 | 7938 | 7917 | 2.78 | 6.34 |
| Pulp-CA | 50.5 | 68.11 | 4.29 | 0.10 | 27.50 | 0.06 | 0.40 | 24.2 | 22,820 | 22,352 | 2.70 | 1.68 | |||||||
| Samples | Dehydration Stage (°C) | Devolatilization and Combustion Stage (°C) | Char Combustion Stage (°C) | Characteristic Temperature (°C) | ||
|---|---|---|---|---|---|---|
| Ti | Tm | Tb | ||||
| Feedstocks | ||||||
| CNF | 178 | 220–300 | 520–580 | 518.5 | 558.8 | 573.0 |
| 330–450 | ||||||
| Pulp | 125 | 290–450 | 490–570 | 515.9 | 543.7 | 555.6 |
| Without acetone rinsing | ||||||
| CNF-W | 75 | 95–265 | 440–580 | 401.1 | 515.9 | 558.2 |
| 265–440 | ||||||
| Pulp-W | 93 | 105–260 | 430–620 | 403.7 | 510.8 | 583.9 |
| 260–430 | ||||||
| With acetone rinsing | ||||||
| CNF-W | 78 | 240–430 | 430–560 | 415.0 | 513.4 | 553.7 |
| CNF-CA | 108 | 260–470 | 470–630 | 440.4 | 540.0 | 624.8 |
| Pulp-W | 92 | 240–430 | 430–590 | 404.2 | 509.6 | 577.6 |
| Pulp-CA | 85 | 260–443 | 443–593 | 450.8 | 532.5 | 587.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faradilla, R.F.; Lucia, L.; Hakovirta, M. Remarkable Physical and Thermal Properties of Hydrothermal Carbonized Nanoscale Cellulose Observed from Citric Acid Catalysis and Acetone Rinsing. Nanomaterials 2020, 10, 1049. https://doi.org/10.3390/nano10061049
Faradilla RF, Lucia L, Hakovirta M. Remarkable Physical and Thermal Properties of Hydrothermal Carbonized Nanoscale Cellulose Observed from Citric Acid Catalysis and Acetone Rinsing. Nanomaterials. 2020; 10(6):1049. https://doi.org/10.3390/nano10061049
Chicago/Turabian StyleFaradilla, RH Fitri, Lucian Lucia, and Marko Hakovirta. 2020. "Remarkable Physical and Thermal Properties of Hydrothermal Carbonized Nanoscale Cellulose Observed from Citric Acid Catalysis and Acetone Rinsing" Nanomaterials 10, no. 6: 1049. https://doi.org/10.3390/nano10061049
APA StyleFaradilla, R. F., Lucia, L., & Hakovirta, M. (2020). Remarkable Physical and Thermal Properties of Hydrothermal Carbonized Nanoscale Cellulose Observed from Citric Acid Catalysis and Acetone Rinsing. Nanomaterials, 10(6), 1049. https://doi.org/10.3390/nano10061049







