Towards Microorganism-Based Biofuel Cells: The Viability of Saccharomyces cerevisiae Modified by Multiwalled Carbon Nanotubes †
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strain and Chemicals
2.2. Preparation of MW-CNT Suspension
2.3. Preparation of Solutions for Biofuel Cell
2.4. Graphite Electrode Preparation
2.5. Characterization of Nanoparticles
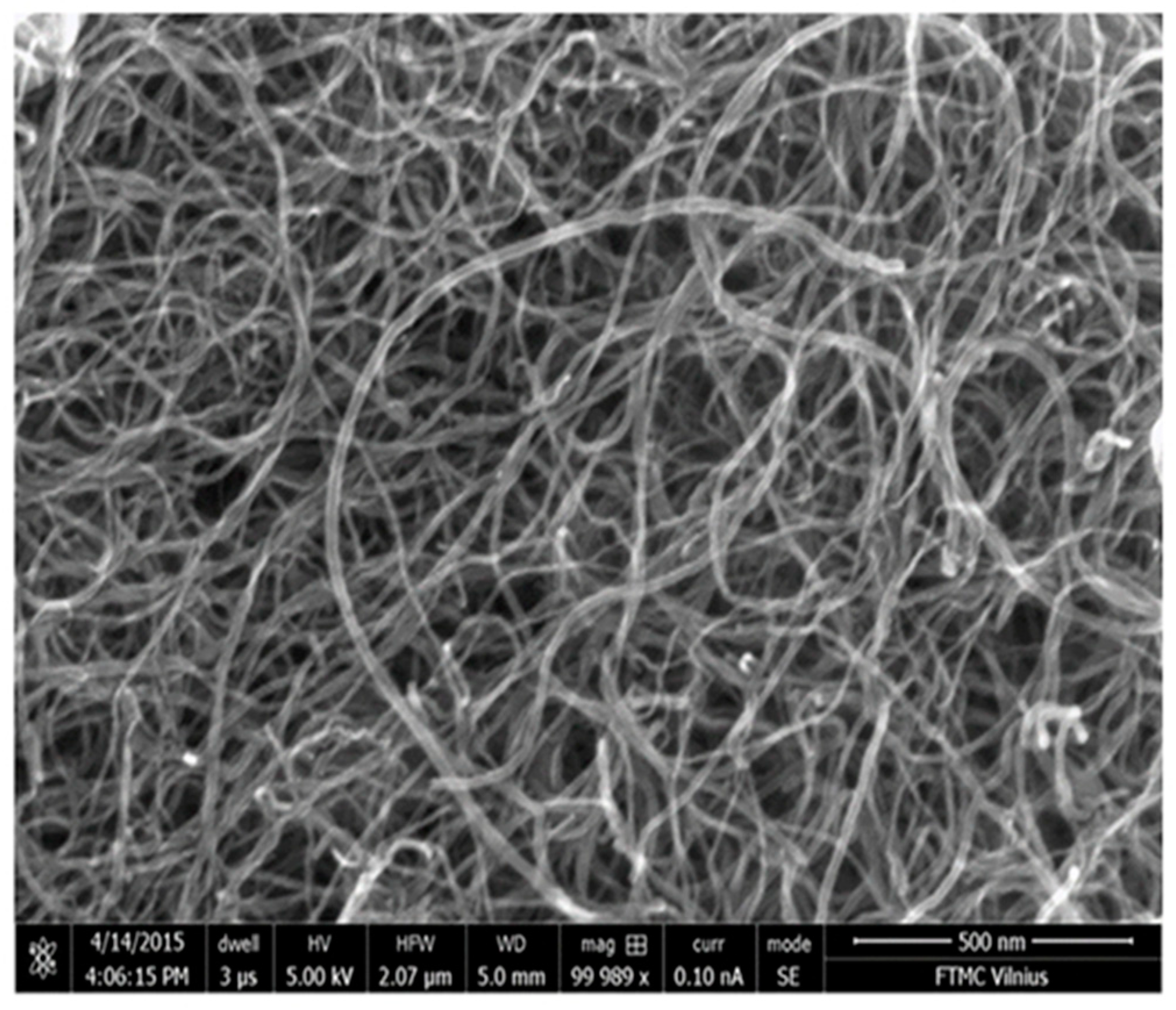
2.5.1. Scanning Electron Microscopy (SEM)
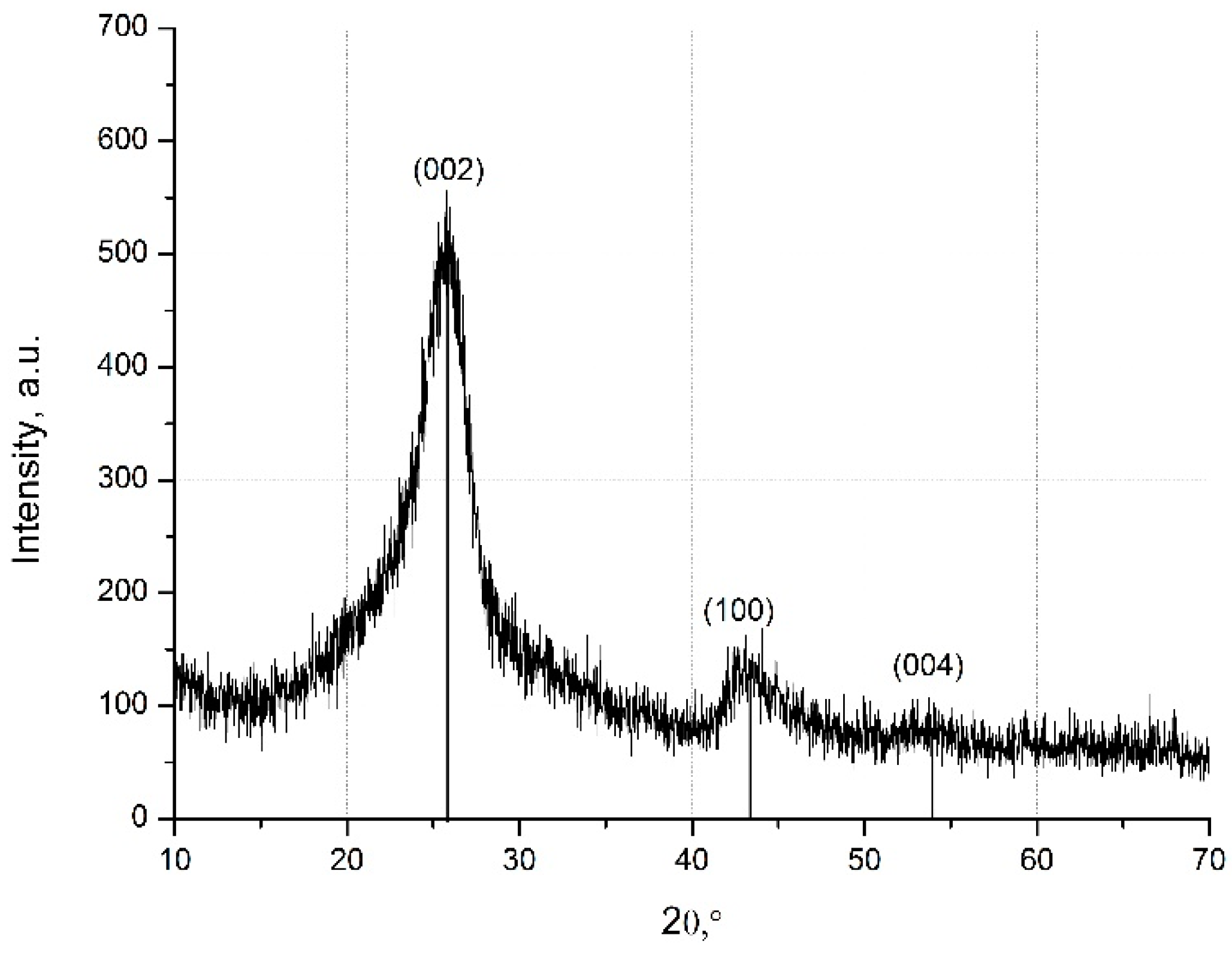
2.5.2. X-ray Diffraction Analysis (XRD)
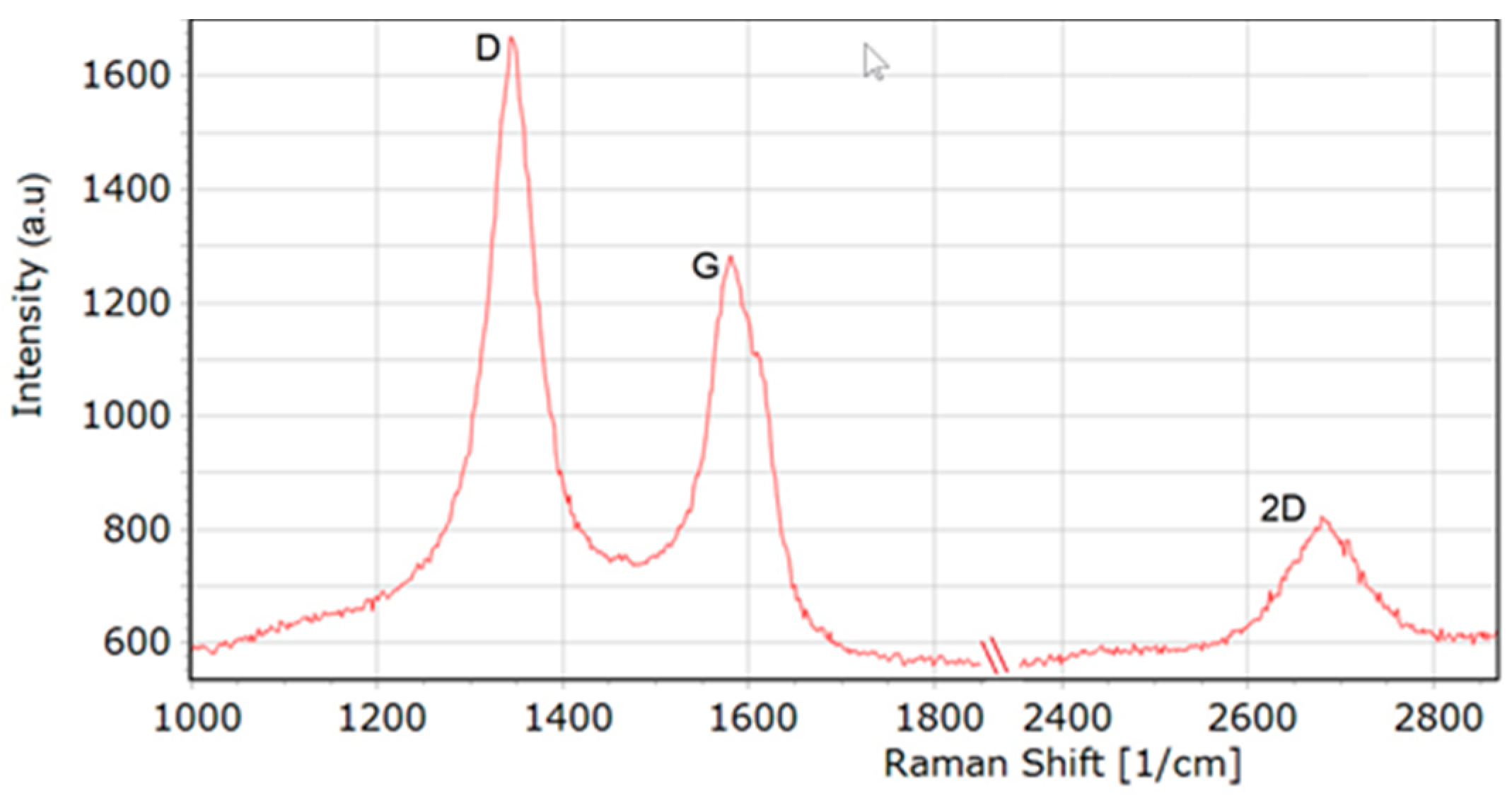
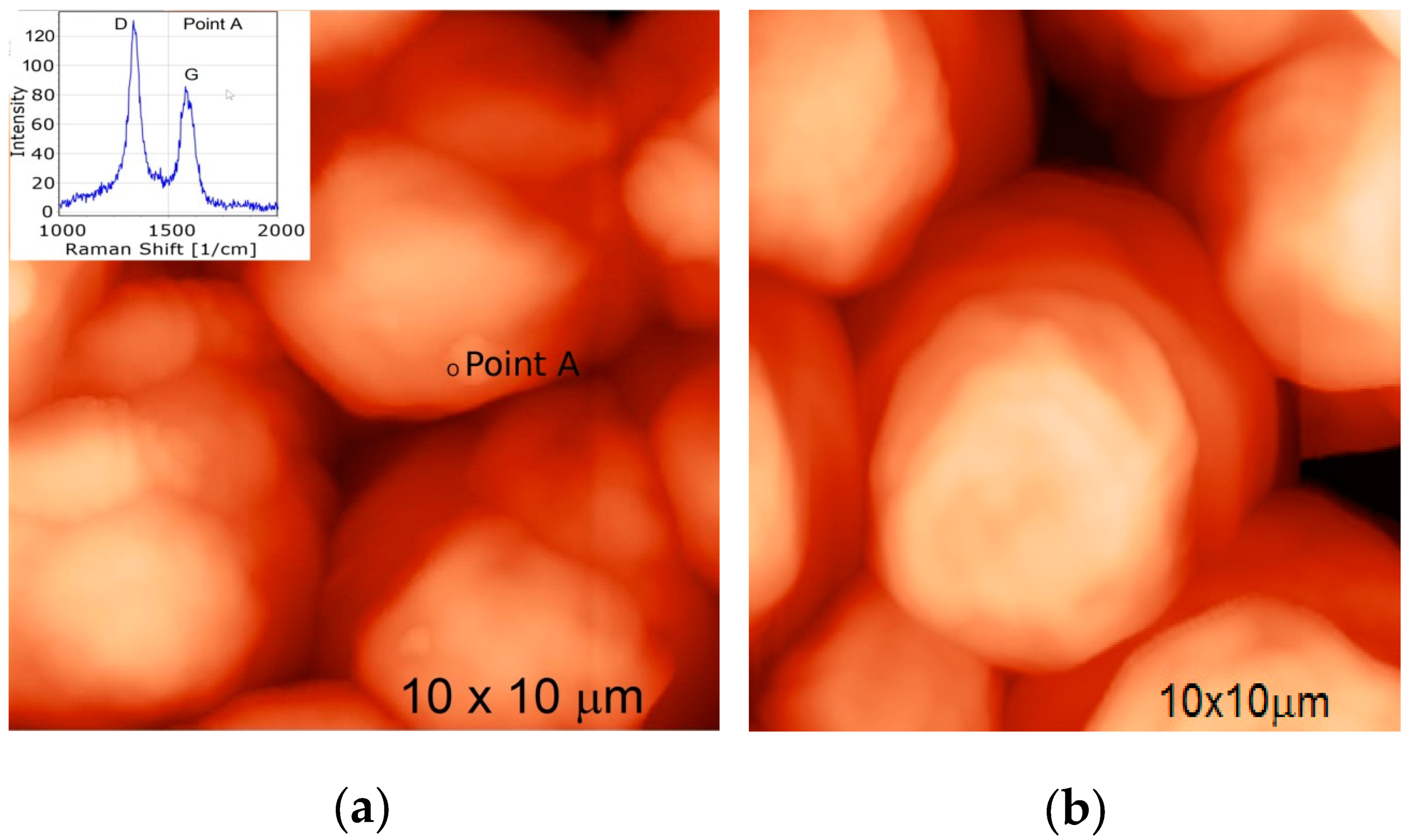
2.5.3. Raman Spectroscopy Measurements
2.6. Exposure of Yeast Saccharomyces Cerevisiae Cells to MW-CNTs
2.7. Yeast Cell Viability Assays
Assessment of Temporal Change of Yeast Population Size
2.8. Electrochemical Measurements
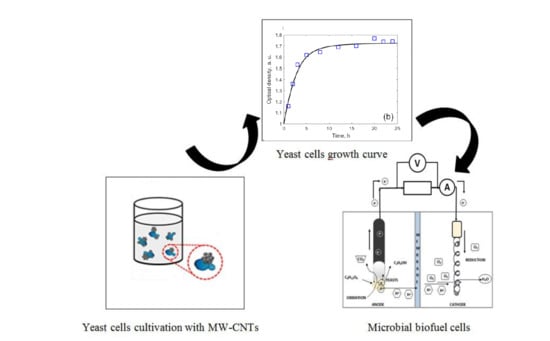
3. Results
3.1. Analysis of the Structure of MW-CNTs
3.2. Evaluation of Toxicity of MW-CNTs on Yeast Cells
3.3. Electrochemical Analysis and Biofuel Cell Development
4. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramanavicius, A.; Ramanaviciene, A. Hemoproteins in Design of Biofuel Cells. Fuel Cells 2009, 9, 25–36. [Google Scholar] [CrossRef]
- Kisieliute, A.; Popov, A.; Apetrei, R.-M.; Cârâc, G.; Morkvenaite-Vilkonciene, I.; Ramanaviciene, A.; Ramanavicius, A. Towards microbial biofuel cells: Improvement of charge transfer by self-modification of microoganisms with conducting polymer–Polypyrrole. Chem. Eng. J. 2019, 356, 1014–1021. [Google Scholar] [CrossRef]
- Davis, F.; Higson, S.P.J. Biofuel cells-Recent advances and applications. Biosens. Bioelectron. 2007. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Cheng, Y.; Jiang, S.P. Effect of Carbon Nanotubes on Direct Electron Transfer and Electrocatalytic Activity of Immobilized Glucose Oxidase. ACS Omega 2018. [Google Scholar] [CrossRef]
- Apetrei, R.M.; Carac, G.; Bahrim, G.; Ramanaviciene, A.; Ramanavicius, A. Modification of Aspergillus niger by conducting polymer, Polypyrrole, and the evaluation of electrochemical properties of modified cells. Bioelectrochemistry 2018, 121, 46–55. [Google Scholar] [CrossRef]
- Apetrei, R.M.; Carac, G.; Ramanaviciene, A.; Bahrim, G.; Tanase, C.; Ramanavicius, A. Cell-assisted synthesis of conducting polymer–polypyrrole–for the improvement of electric charge transfer through fungal cell wall. Colloids Surf. B Biointerfaces 2019, 175, 671–679. [Google Scholar] [CrossRef]
- Stirke, A.; Apetrei, R.M.; Kirsnyte, M.; Dedelaite, L.; Bondarenka, V.; Jasulaitiene, V.; Pucetaite, M.; Selskis, A.; Carac, G.; Bahrim, G.; et al. Synthesis of polypyrrole microspheres by Streptomyces spp. Polymer (Guildford) 2016, 84, 99–106. [Google Scholar] [CrossRef]
- Andriukonis, E.; Stirke, A.; Garbaras, A.; Mikoliunaite, L.; Ramanaviciene, A.; Remeikis, V.; Thornton, B.; Ramanavicius, A. Yeast-assisted synthesis of polypyrrole: Quantification and influence on the mechanical properties of the cell wall. Colloids Surf. B Biointerfaces 2018, 164, 224–231. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Andriukonis, E.; Stirke, A.; Mikoliunaite, L.; Balevicius, Z.; Ramanaviciene, A. Synthesis of polypyrrole within the cell wall of yeast by redox-cycling of [Fe(CN)6]3−/[Fe(CN)6]4−. Enzyme Microb. Technol. 2016, 83, 40–47. [Google Scholar] [CrossRef]
- Atalay, P.B.; Asci, O.; Kaya, F.O.; Tuna, B.G. Hydrogen peroxide prolongs mitotic arrest in a dose dependent manner and independently of the spindle assembly checkpoint activity in saccharomyces cerevisiae. Acta Biol. Hung. 2017, 68, 477–489. [Google Scholar] [CrossRef]
- Pagano, L.; Caldara, M.; Villani, M.; Zappettini, A.; Marmiroli, N.; Marmiroli, M. In Vivo-In Vitro Comparative Toxicology of Cadmium Sulphide Quantum Dots in the Model Organism Saccharomyces cerevisiae. Nanomaterials 2019, 9, 512. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, X.; Haldolaarachchige, N.; Wang, Q.; Luo, Z.; Ryu, J.; Young, D.P.; Wei, S.; Guo, Z. Polypyrrole metacomposites with different carbon nanostructures. J. Mater. Chem. 2012, 22, 4996–5005. [Google Scholar] [CrossRef]
- Le, T.H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers (Basel) 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, P. Electro-Optic and Optical Devices; Springer International Publishing: Cham, Switzerland, 2018; ISBN 9783319693767. [Google Scholar]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H.; Baumann, T.F. Synthesis of graphene aerogel with high electrical conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef]
- Liu, S.; Chevali, V.S.; Xu, Z.; Hui, D.; Wang, H. A review of extending performance of epoxy resins using carbon nanomaterials. Compos. Part B Eng. 2018, 136, 197–214. [Google Scholar] [CrossRef]
- Cheng, C.; Müller, K.H.; Koziol, K.K.K.; Skepper, J.N.; Midgley, P.A.; Welland, M.E.; Porter, A.E. Toxicity and imaging of multi-walled carbon nanotubes in human macrophage cells. Biomaterials 2009, 30, 4152–4160. [Google Scholar] [CrossRef]
- Handy, R.D.; Von Der Kammer, F.; Lead, J.R.; Hassellöv, M.; Owen, R.; Crane, M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 2008, 17, 287–314. [Google Scholar] [CrossRef]
- Kisin, E.R.; Murray, A.R.; Keane, M.J.; Shi, X.C.; Schwegler-Berry, D.; Gorelik, O.; Arepalli, S.; Castranova, V.; Wallace, W.E.; Kagan, V.E.; et al. Single-walled carbon nanotubes: Geno- and cytotoxic effects in lung fibroblast V79 cells. J. Toxicol. Environ. Health Part A Curr. Issues 2007, 70, 2071–2079. [Google Scholar] [CrossRef]
- Ghafari, P.; St-Denis, C.H.; Power, M.E.; Jin, X.; Tsou, V.; Mandal, H.S.; Bols, N.C.; Tang, X. Impact of carbon nanotubes on the ingestion and digestion of bacteria by ciliated protozoa. Nat. Nanotechnol. 2008, 3, 347–351. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef]
- Francis, A.P.; Devasena, T. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon nanotubes: Evaluation of toxicity at biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.L.; Yah, C.S.; Iyuke, S.E.; Rumbold, K.; Pillay, V. The cellular response of Saccharomyces cerevisiae to multi-walled carbon nanotubes (MWCNTs). J. Saudi Chem. Soc. 2015, 19, 147–154. [Google Scholar] [CrossRef]
- Safarova, K.; Drovak, A.; Kubinek, R. Usage of AFM, SEM and TEM for the research of carbon nanotubes. Mod. Res. Educ. Top. Microsc. 2007, 513–519. [Google Scholar]
- Saleh, T.A.; Gondal, M.A.; Drmosh, Q.A.; Yamani, Z.H.; AL-yamani, A. Enhancement in photocatalytic activity for acetaldehyde removal by embedding ZnO nano particles on multiwall carbon nanotubes. Chem. Eng. J. 2011, 166, 407–412. [Google Scholar] [CrossRef]
- Zamaleeva, A.I.; Sharipova, I.R.; Porfireva, A.V.; Evtugyn, G.A.; Fakhrullin, R.F. Polyelectrolyte-Mediated Assembly of Multiwalled Carbon Nanotubes on Living Yeast Cells. Langmuir 2010, 26, 2671–2679. [Google Scholar] [CrossRef]
- Juška, A. Minimal models of growth and decline of microbial populations. J. Theor. Biol. 2011, 269, 195–200. [Google Scholar] [CrossRef]
- Zhu, Z.Z.; Wang, Z.; Li, H.L. Functional multi-walled carbon nanotube/polyaniline composite films as supports of platinum for formic acid electrooxidation. Appl. Surf. Sci. 2008, 254, 2934–2940. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, B.; Huang, A.; Hu, Y.; Wang, G.; Ling, F. Toxicological effects of multi-walled carbon nanotubes on Saccharomyces cerevisiae: The uptake kinetics and mechanisms and the toxic responses. J. Hazard. Mater. 2016, 318, 650–662. [Google Scholar] [CrossRef]
- Morkvenaite-Vilkonciene, I.; Ramanaviciene, A.; Ramanavicius, A. 9,10-Phenanthrenequinone as a redox mediator for the imaging of yeast cells by scanning electrochemical microscopy. Sensors Actuators, B Chem. 2016, 228, 200–206. [Google Scholar] [CrossRef]

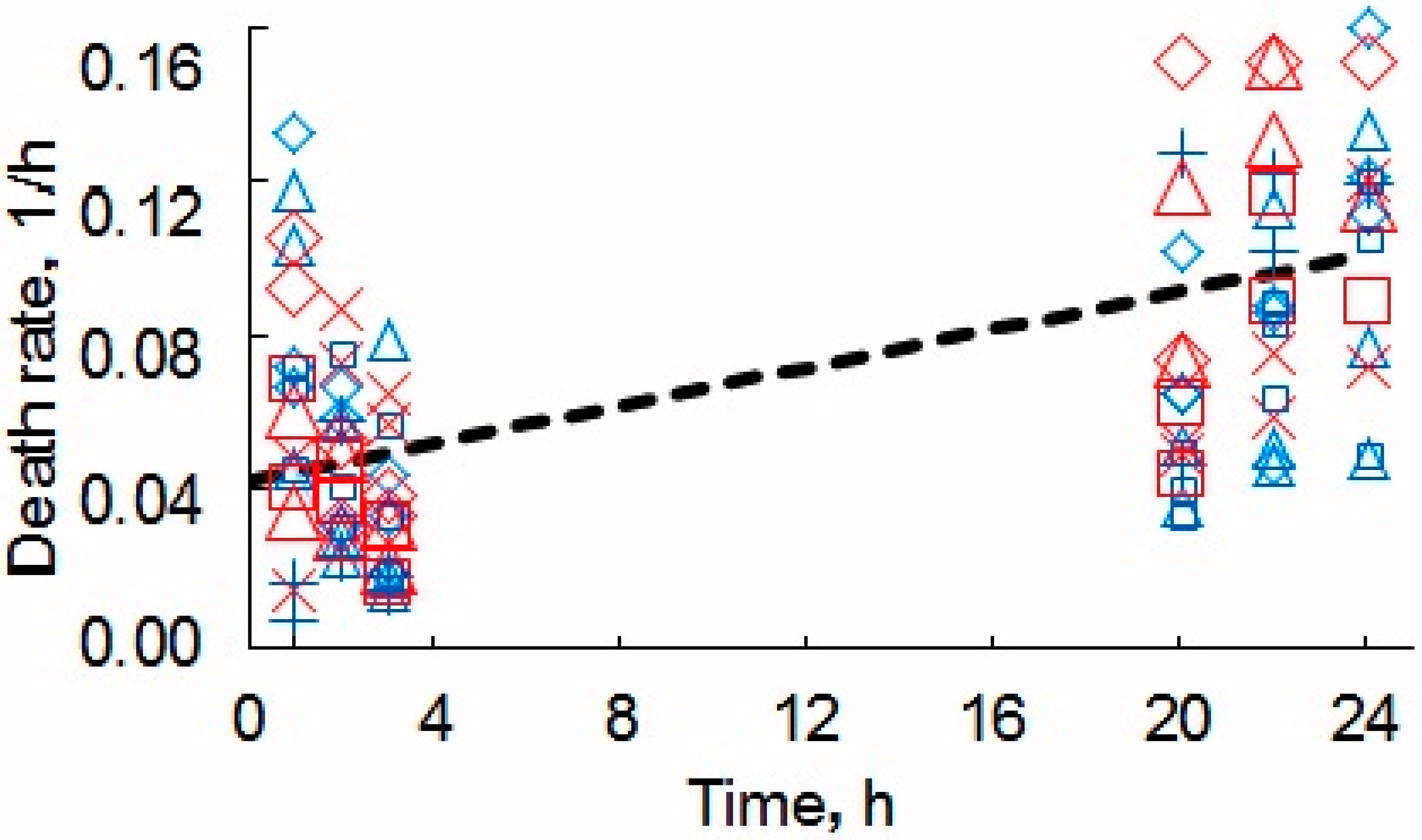
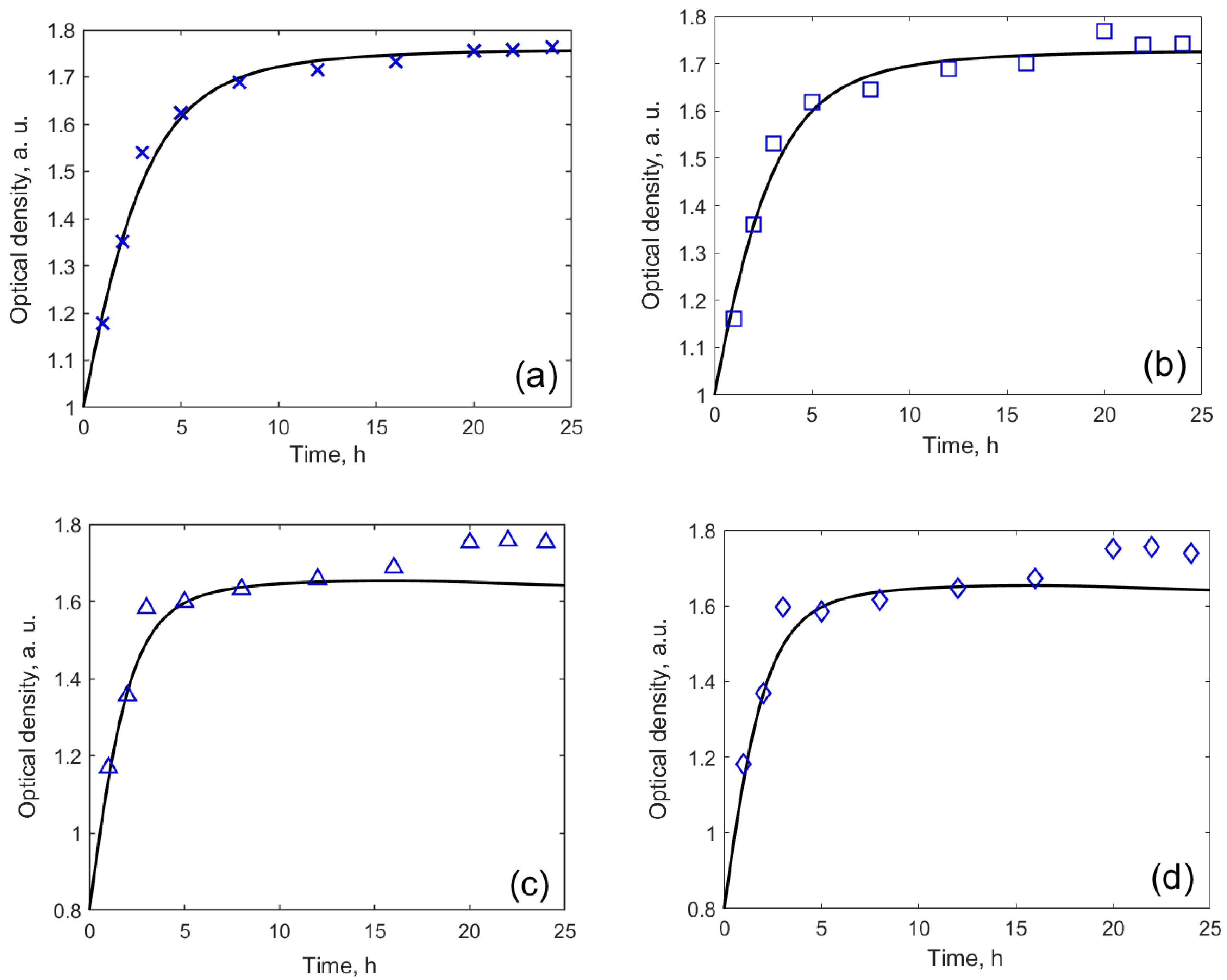
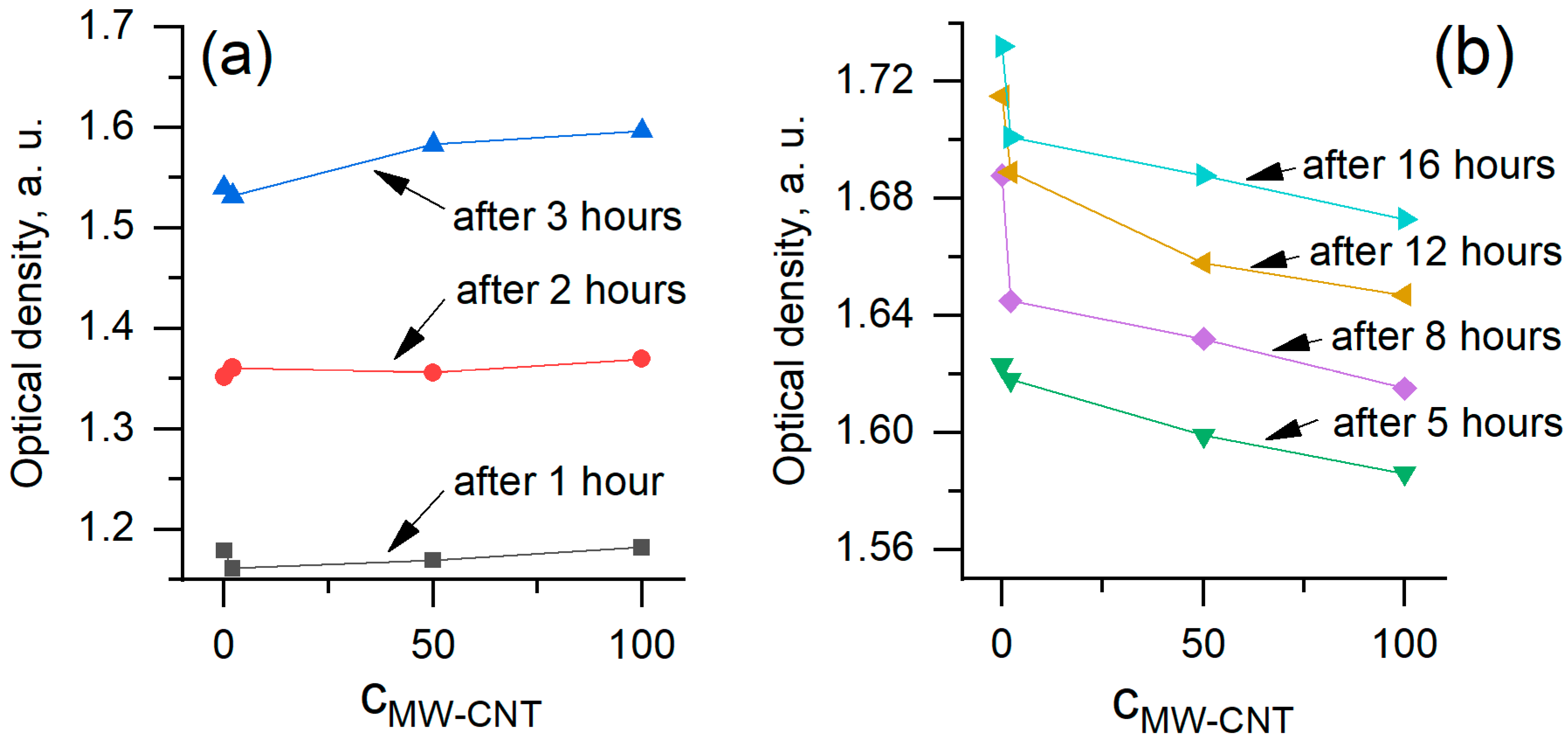

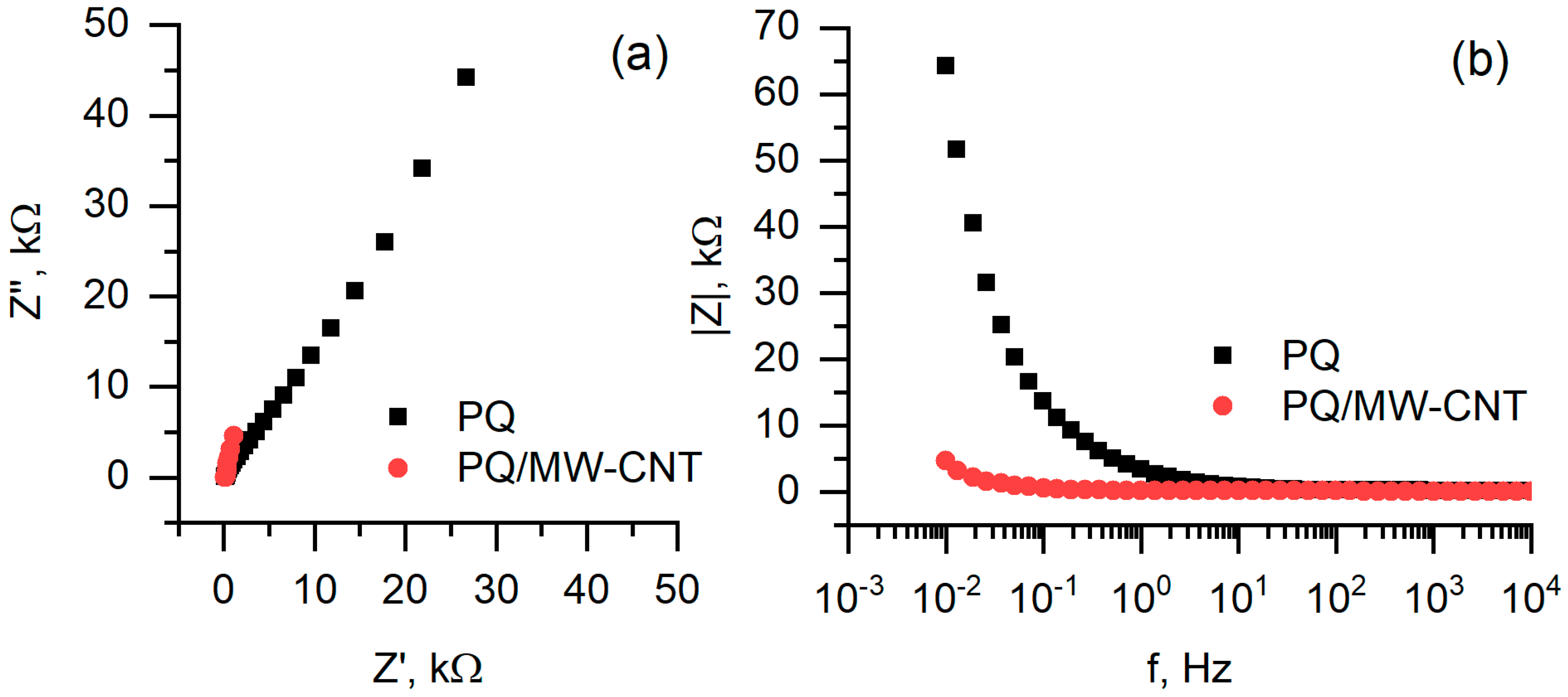
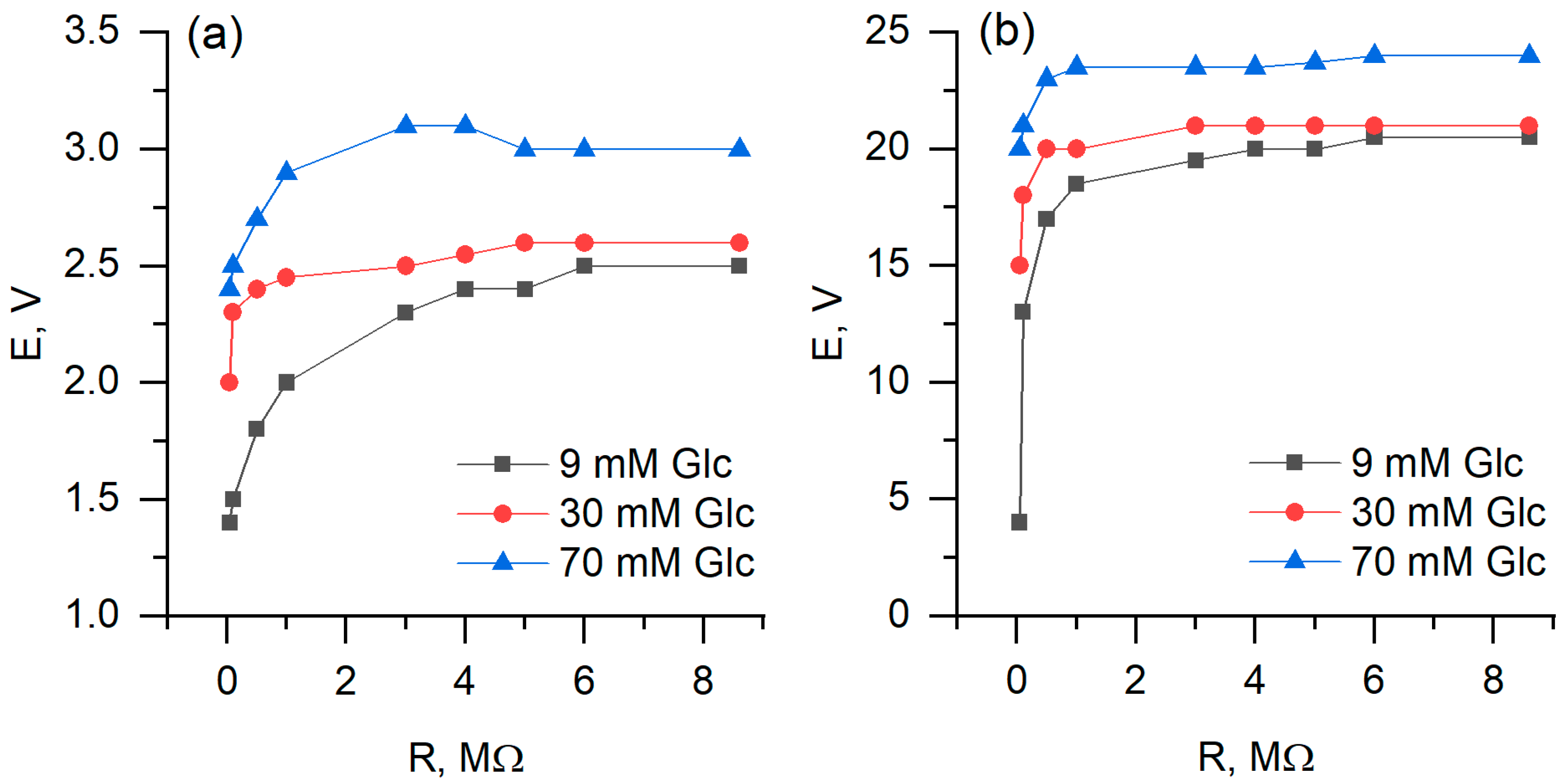
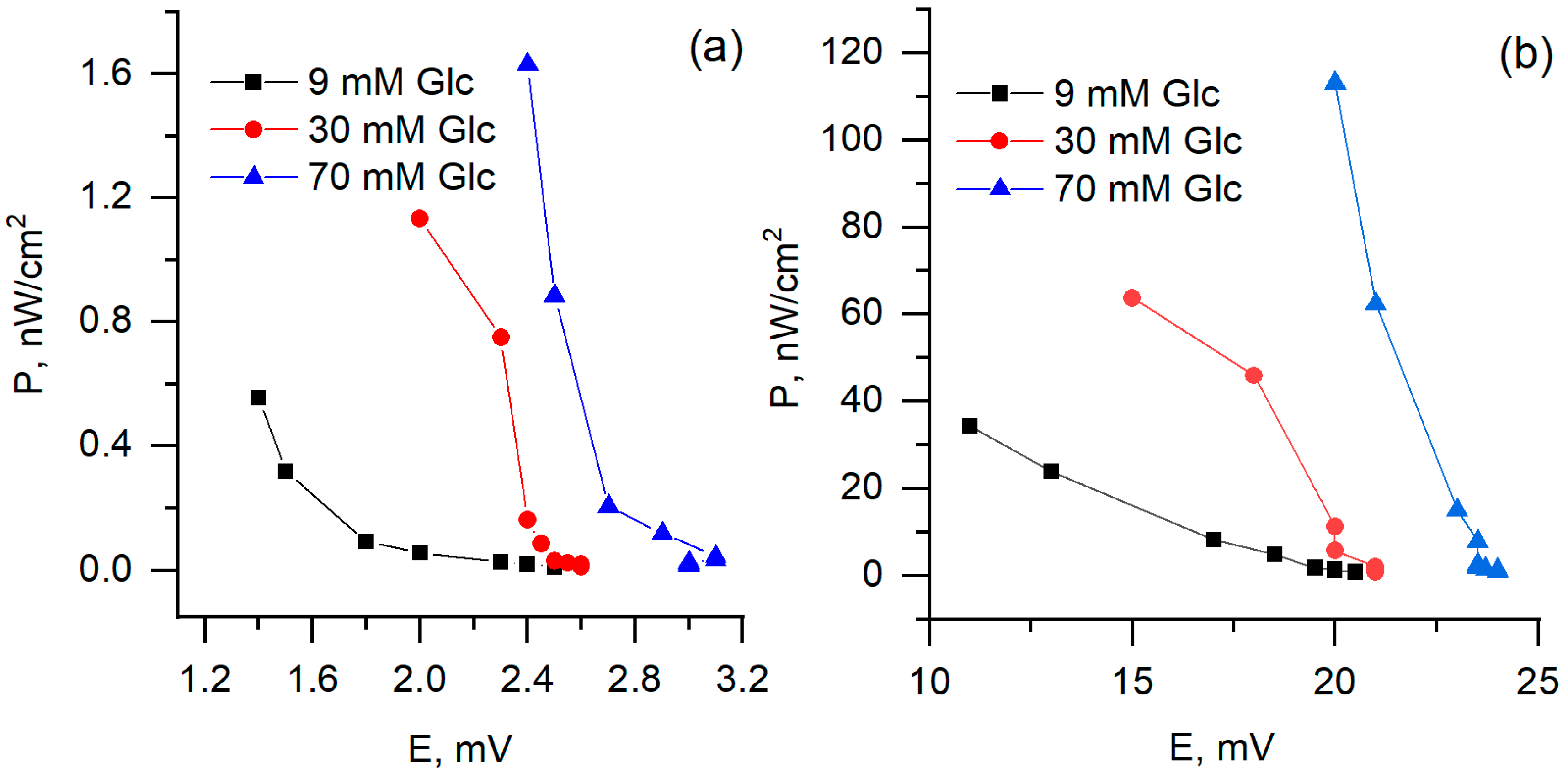
| Relative Growth Rate (α), h−1 | Relative Death Rate (µ), h−1 | Consumption of Non-Saccharide Resources (ζ) | Initial Population Size x(0) * | |
|---|---|---|---|---|
| Unaffected cells | 0.215 | 0.0207 | 5 | 1 |
| 2 µg/mL MW-CNTs | 0.219 | 0.022 | 5 | 1 |
| 50 µg/mL MW-CNTs | 0.46 | 0.041 | 4.8 | 0.8 |
| 100 µg/mL MW-CNTs | 0.46 | 0.041 | 4.8 | 0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruzaite, I.; Rozene, J.; Morkvenaite-Vilkonciene, I.; Ramanavicius, A. Towards Microorganism-Based Biofuel Cells: The Viability of Saccharomyces cerevisiae Modified by Multiwalled Carbon Nanotubes. Nanomaterials 2020, 10, 954. https://doi.org/10.3390/nano10050954
Bruzaite I, Rozene J, Morkvenaite-Vilkonciene I, Ramanavicius A. Towards Microorganism-Based Biofuel Cells: The Viability of Saccharomyces cerevisiae Modified by Multiwalled Carbon Nanotubes. Nanomaterials. 2020; 10(5):954. https://doi.org/10.3390/nano10050954
Chicago/Turabian StyleBruzaite, Ingrida, Juste Rozene, Inga Morkvenaite-Vilkonciene, and Arunas Ramanavicius. 2020. "Towards Microorganism-Based Biofuel Cells: The Viability of Saccharomyces cerevisiae Modified by Multiwalled Carbon Nanotubes" Nanomaterials 10, no. 5: 954. https://doi.org/10.3390/nano10050954
APA StyleBruzaite, I., Rozene, J., Morkvenaite-Vilkonciene, I., & Ramanavicius, A. (2020). Towards Microorganism-Based Biofuel Cells: The Viability of Saccharomyces cerevisiae Modified by Multiwalled Carbon Nanotubes. Nanomaterials, 10(5), 954. https://doi.org/10.3390/nano10050954