Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects
Abstract
:1. Introduction
2. Synthesis of Zero-Valent Iron Nanoparticles
2.1. Top-Down Synthesis
2.2. Bottom-Up Synthesis
2.2.1. Solution Based Processes
2.2.2. High-Temperature Thermal Processes
3. Application of nZVI in Catalysis
4. Removal of Toxic Pollutants from Water, Wastewater, Ground Water, or Soil/Sediments
4.1. Removal of Organic Compounds from Water and Soil
4.1.1. Removal of Organic Compounds from Water
4.1.2. Removal of Organic Compounds from Spiked Soil
4.2. Organic Dye Discolouration/Decomposition in Water
| nZVI Cap/Support | Dye | pH, Reagent | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|---|
| bare | Methylene blue | 7.5 | n.a. | [78] |
| STSPF 2 | Methylene blue | 5.0‒9.0 | 140.80 | [33] |
| Cellulose | Methylene blue | 5.96 | n.a. | [83] |
| Cellulose | Methyl blue | 5.96 | n.a. | [83] |
| STSPF 2 | Malachite green | 5.0‒9.0 | 92.59 | [33] |
| STSPF 2 | Methyl violet 2B | 5.0‒9.0 | 92.59 | [33] |
| Montmorillonite | Rhodamine B | Air | n.a. | [79] |
| Fe2O3 shell | Orange II | 3, air | n.a. | [80] |
| Cellulose | Orange II | 5.96 | n.a. | [83] |
| Bare | Disperse Red 1 | 3, H2O2 | n.a. | [18] |
| rGO/attapulgite | Acid Red 18 | 2–8 | 400 | [81] |
| Cellulose | Methyl orange | neutral | n.a. | [82] |
| Cellulose | Methyl orange | 5.96 | n.a. | [83] |
| Kaolin | Rosso Zetanyl B-NG | 4.8 | n.a. | [84] |
| Bentonite | Rosso Zetanyl B-NG | 4.8 | n.a. | [84] |
| Native clay | Rosso Zetanyl B-NG | 4.8 | n.a. | [84] |
4.3. Degradation of Halogenated Organic Compounds
4.3.1. Removal of Halogenated Compounds from Water and Wastewater
| nZVI Cap/Support | Pollutant | pH, Reagent | Degradation Product | Ref. |
|---|---|---|---|---|
| Mg-aminoclay | Perfluorooctanoic acid | 3 | Not detected | [86] |
| Mg-aminoclay | Perfluorononanoic acid | 3 | Not detected | [86] |
| Mg-aminoclay | Perfluorodecanoic acid | 3 | Not detected | [86] |
| Mg-aminoclay | Perfluorooctane sulfonate | 3 | Not detected | [86] |
| CTAB | Perfluorooctanoic acid | 0.5 | n.a. | [87] |
| Graphene | Trichloronitromethane | 6.5 | Methylamine | [88] |
| Fe2O3 | Chlorinated hydrocarbons 2 | Neutral | n.a. | [12] |
| MEG | Chlorinated hydrocarbons 3 | Neutral | n.a. | [13] |
| Carbon | Trichloroethene | 7 | Ethene | [15] |
| Sulfite hydrate | Trichloroethylene | Neutral | n.a. | [24] |
| FeO | Lindane | 5–9 | Benzene | [85] |
| Bare | Chloramphenicol | 6.8 | C11H16N2O3 | [89] |
| Sulfide | Florfenicol | 7 | C12H17NO4S 4 | [90] |
| Bare | Diazepam | 2.2 | n.a. | [66] |
| FeOOH/protein | Dichlorvos | Neutral, H2O2 | PO43−, Cl− | [44] |
| Fe3O4 | Decabromodiphenyl ether | 7.1, H2O2 | CO2, H2O 5 | [91] |
| Sepiolite | Bromamine acid | 3–11 | n.a. | [93] |
| Fe3O4 | Iopromide | 7.2–7.9 | n.a. | [94] |
| PAA/Fe3O4 | Iopromide | 7.2–7.9 | n.a. | [94] |
| FeO/Fe3O4 | Iopromide | 7.2–7.9 | n.a. | [94] |
4.3.2. Removal of Halogenated Compounds from Groundwater and Soil
4.4. Heavy Metal and Metalloid Ion Removal
- Reduction (examples: Cr, As, Cu, U, Pb, Ni, Se, Co, Pd, Pt, Hg, and Ag ions),
- Adsorption (examples: Cr, As, U, Pb, Ni, Se, Co, Cd, Zn, and Ba ions),
- Oxidation/reoxidation (examples: As, U, Se, and Pb ions),
- Coprecipitation (examples: Cr, As, Ni, and Se ions),
- Precipitation (examples: Cu, Pb, Cd, Co, and Zn ions).
4.4.1. Removal of Heavy Metals and Metalloids from Water and Wastewater
| nZVI Cap/Support | Pollutant | pH | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|---|
| Graphene-silica | As(III) | 6.0–8.0 | 45.57 | [101] |
| Graphene-silica | As(V) | 4.0 | 45.12 | [101] |
| Montmorillonite | As(III) | 7.0 | 59.9 | [103] |
| Montmorillonite | As(V) | 7.0 | 45.5 | [103] |
| Bare | As(III) | 7 | 102 | [104] |
| Bare | As(V) | 7 | 118 | [104] |
| Bare | As(V) | 7 | 26.36 | [106] |
| Zeolite | As(V) | 7 | 47.30 | [106] |
| Fe-oxide | As(V) | 6–8 | 245 | [130] |
| FeOOH | Au(III) | neutral | 25 | [107] |
| Biochar | Cd(II) | 6 | 22.37 | [108] |
| rGO | Cd(II) | 5 | 425.72 | [49] |
| kaolinite | Co(II) | n.a. | 25 | [110] |
| rGO | Co(II) | 4–9 | 131.58 | [111] |
| kaolinite | Cu(II) | n.a. | 140 | [110] |
| Bare | Cu(II) | n.a. | 250 | [116] |
| MWCNT-PAA/PVA | Cu(II) | 4.5–5 | 107.8 | [118] |
| alumina | Cu(II) | 3–11 | 95.3 | [117] |
| Fe-oxide | Cu(II) | 6–8 | 226 | [130] |
| Biochar | Cr(VI) | 5 | 26.63 | [112] |
| Lignin/Al-bentonite | Cr(VI) | 5.6 | 46.2 | [113] |
| CMC | Cr(VI) | 7 | 3.33 | [114] |
| MWCNT | Cr(VI) | 7 | 2.71 | [114] |
| pumice | Cr(VI) | n.a. | 23.6 | [27] |
| Carbon nanofiber | Cr(VI) | 4 | n.a. | [115] |
| pumice | Hg(II) | n.a. | 25.6 | [27] |
| FeOOH | Ni(II) | neutral | 130 | [120] |
| Bare | Pb(II) | 6 | 807.23 | [22] |
| Bare | Pb(II) | neutral | 1718.4 | [121] |
| Mg(OH)2 | Pb(II) | neutral | 1986.6 | [121] |
| zeolite | Pb(II) | 4 | 806 | [122] |
| CMC | Se(IV/VI) | 7 | 2.26 | [114] |
| MWCNT | Se(IV/VI) | 7 | 2.52 | [114] |
| Fe-oxide/PVDF/PAA | Se(IV/VI) | 4.5 | n.a. | [124] |
| Bare | Se(IV) | neutral | n.a. | [9] |
| Bare | U(VI) | 5 | 8173 | [126] |
| rGO | U(VI) | 5 | 4174 | [126] |
| Na-bentonite | U(VI) | <7 | 120 | [127] |
| Bare | Zn(II) | 5–7 | n.a. | [32] |
| bentonite | Zn(II)/Cu(II) | 3.9 | n.a. | [128] |
4.4.2. Removal of Heavy Metal Contaminants from Groundwater and Soil
4.5. Removal of Ammonium, Bromate, Nitrate, and Perchlorate Ions from Water
5. Toxicity of nZVI
6. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Machado, S.; Pacheco, J.G. Nanoremediation with zero-valent iron nanoparticles. In From Soil Remediation, 1st ed.; Albergaria, J.T., Nouws, H.P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 108–120. [Google Scholar]
- Liu, P.; Hong, Y. Magnetic nanomaterials for water remediation. In Magnetic Nanomaterials, 1st ed.; Hou, Y., Sellmyer, D.J., Eds.; Wiley-VCH Verlag GmbH & Co.: New York, NY, USA, 2017; pp. 515–546. [Google Scholar]
- Phenrat, T.; Lowry, G.V. (Eds.) Nanoscale Zerovalent Iron Particles for Environmental Restoration, Fundamental Science to Field Scale Engineering Applications; Springer International Publishing AG: Cham, Switzerland, 2019. [Google Scholar]
- Yan, W.; Lien, H.-L.; Koel, B.E.; Zhang, W.-X. Iron nanoparticles for environmental clean-up: Recent developments and future outlook. Environ. Sci. Proc. Impacts. 2013, 15, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.C.; Braun, J.; Bruns, J.; Černík, M.; Rissing, P.; Rickerby, D.; Nowack, B. Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environ. Sci. Pollut. Res. 2012, 19, 550–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, N.C.; Nowack, B. Nanoparticles for remediation: Solving big problems with little particles. Elements 2010, 6, 395–400. [Google Scholar] [CrossRef]
- Phenrat, T.; Lowry, G.V.; Babakhani, P. Nanoscale Zerovalent Iron (NZVI) for Environmental Decontamination: A Brief History of 20 Years of Research and Field-Scale Application. In Nanoscale Zerovalent Iron Particles for Environmental Restoration, Fundamental Science to Field Scale Engineering Applications; Phenrat, T., Lowry, G.V., Eds.; Springer International Publishing AG: Cham, Switzerland, 2019; pp. 1–43. [Google Scholar]
- Adusei-Gyamfi, J.; Acha, V. Carriers for nano zerovalent iron (nZVI): Synthesis, application and efficiency. RSC Adv. 2016, 6, 91025–91044. [Google Scholar] [CrossRef]
- Ling, L.; Pan, B.; Zhang, W.-X. Removal of selenium from water with nanoscale zero-valent iron: Mechanisms of intraparticle reduction of Se(IV). Water Res. 2015, 71, 274–281. [Google Scholar] [CrossRef]
- Liu, A.; Liu, J.; Pan, B.; Zhang, W.-X. Formation of lepidocrocite (g-FeOOH) from oxidation of nanoscale zero-valent iron (nZVI) in oxygenated water. RSC Adv. 2014, 4, 57377–57382. [Google Scholar] [CrossRef]
- Mackenzie, K.; Georgi, A. NZVI Synthesis and Characterization. In Nanoscale Zerovalent Iron Particles for Environmental Restoration, Fundamental Science to Field Scale Engineering Applications; Phenrat, T., Lowry, G.V., Eds.; Springer International Publishing AG: Cham, Switzerland, 2019; pp. 45–95. [Google Scholar]
- Li, S.; Yan, W.; Zhang, W.-X. Solvent-free production of nanoscale zero-valent iron (nZVI) with precision milling. Green Chem. 2009, 11, 1618–1626. [Google Scholar] [CrossRef]
- Ribas, D.; Peskova, K.; Jubany, I.; Parma, P.; Cernik, M.; Benito, J.A.; Marti, V. High reactive nano zero-valent iron produced via wet milling through abrasion by alumina. Chem. Eng. J. 2019, 366, 235–245. [Google Scholar] [CrossRef]
- Köber, R.; Hollert, H.; Hornbruch, G.; Jekel, M.; Kamptner, A.; Klaas, N.; Maes, H.; Mangold, K.M.; Martac, E.; Matheis, A.; et al. Nanoscale zero-valent iron flakes for groundwater treatment. Environ. Earth Sci. 2014, 72, 3339–3352. [Google Scholar] [CrossRef]
- Gao, J.; Wang, A.; Rondinon, A.J.; He, F.; Liang, L. Degradation of Trichloroethene with a Novel Ball Milled Fe–C Nanocomposite. J. Hazard. Mater. 2015, 300, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, L.T.; Bojesen, A.; Timmermann, L.; Nielsen, M.M.; Mørup, S. Structural and magnetic properties of core–shell iron–iron oxide nanoparticles. J. Phys. Condens. Matter 2002, 14, 13551–13567. [Google Scholar] [CrossRef]
- Okazoe, S.; Yasaka, Y.; Kudo, M.; Maeno, H.; Murakami, Y.; Kimura, Y. Synthesis of zero-valent iron nanoparticles via laser ablation in a formate ionic liquid under atmospheric conditions. Chem. Commun. 2018, 54, 7834–7837. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Rodrigues, M.; Silveira, J.; Zazo, J.A.; Rodriguez, J.J. Synthesis, characterization and application of nanoscale zero-valent iron in the degradation of the azo dye Disperse Red 1. J. Environ. Chem. Eng. 2017, 5, 628–634. [Google Scholar] [CrossRef]
- Han, Y.; Yang, M.D.Y.; Zhang, W.; Yan, W. Optimizing synthesis conditions of nanoscale zero-valent iron (nZVI) through aqueous reactivity assessment. Front. Environ. Sci. Eng. 2015, 9, 813–822. [Google Scholar] [CrossRef]
- Jamei, M.R.; Khosravi, M.R.; Anvaripour, B. A novel ultrasound assisted method in synthesis of NZVI particles. Ultrason. Sonochem. 2014, 21, 226–233. [Google Scholar] [CrossRef]
- Kamali, M.; Costa, M.E.V.; Otero-Irurueta, G.; Capela, I.; Capela, I. Ultrasonic irradiation as a green production route for coupling crystallinity and high specific surface area in iron nanomaterials. J. Clean. Prod. 2019, 211, 185–197. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, W.; Chang, G.; Shuai, L.; Jiao, W.; Liu, Y. Removal of heavy metal lead(II) using nanoscale zero-valent iron with different preservation methods. Adv. Powder Technol. 2019, 30, 581–589. [Google Scholar]
- Parimala, L.; Santhanalakshmi, J. Studies on the Iron Nanoparticles Catalyzed Reduction of Substituted Aromatic Ketones to Alcohols. J. Nanoparticles 2014, 2014, 156868. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Feitz, A.J.; Guan, J.; Waite, T.D. Comparison of the reactivity of nanosized zero valent iron (nZVI) particles produced by borohydride and dithionite reduction of iron salts. Nano: Brief. Rep. Rev. 2008, 3, 341–349. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Wang, Y.; Wang, Z.L. Preparation of Monodispersed Fe-Mo Nanoparticles as the Catalyst for CVD Synthesis of Carbon Nanotubes. Chem. Mater. 2001, 13, 1008–1014. [Google Scholar] [CrossRef]
- Hu, Y.-B.; Li, X.-Y. Influence of a thin aluminum hydroxide coating layer on the suspension stability and reductive reactivity of nanoscale zero-valent iron. Appl. Cat. B: Environ. 2018, 226, 554–564. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Z.L.; Yan, X.; Zhang, B. Removal of mercury (II) and chromium (VI) from wastewater using a new and effective composite: Pumice-supported nanoscale zero-valent iron. Chem. Eng. J. 2014, 245, 34–40. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, Y.C.; Mines, P.D.; Suk Huh, Y.; Andersen, H.R. Nanoscale zerovalent iron (nZVI) synthesis in a Mg-aminoclay solution exhibits increased stability and reactivity for reductive decontamination. Appl. Catal. B Environ. 2014, 147, 748–755. [Google Scholar] [CrossRef]
- Sombra dos Santos, F.; Lago, F.R.; Yokoyama, L.; Fonseca, F.V. Synthesis and characterization of zero-valent iron nanoparticles supported on SBA-15. J. Mater. Res. Technol. 2017, 6, 178–183. [Google Scholar] [CrossRef]
- Khuntia, B.K.; Anwar, M.F.; Alam, T.; Samim, M.; Kumari, M.; Arora, I. Synthesis and Characterization of Zero-Valent Iron Nanoparticles, and the Study of Their Effect against the Degradation of DDT in Soil and Assessment of Their Toxicity against Collembola and Ostracods. ACS Omega 2019, 4, 18502–18509. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Peng, Z.; Huang, D.; Zeng, G.; Wan, J.; Xu, R.; Cheng, M.; Zhang, C.; Jiang, D.; Hu, Z. Nanoremediation of cadmium contaminated river sediments: Microbial response and organic carbon changes. J. Hazard. Mater. 2018, 359, 290–299. [Google Scholar] [CrossRef]
- Kržišnik, N.; Mladenovič, A.; Škapin, A.S.; Škrlep, L.; Ščančar, J.; Milačič, R. Nanoscale zero-valent iron for the removal of Zn2+, Zn(II)–EDTA and Zn(II)–citrate from aqueous solutions. Sci. Tot. Environ. 2014, 476–477, 20–28. [Google Scholar] [CrossRef]
- Parlayici, S.; Pehlivan, E. Fast decolorization of cationic dyes by nano-scale zero valent iron immobilized in sycamore tree seed pod fibers: Kinetics and modelling study. Int. J. Phytoremed. 2019, 21, 1130–1144. [Google Scholar] [CrossRef]
- Vilardi, G.; Stoller, M.; Di Palma, L.; Boodhoo, K.; Verdone, N. Metallic iron nanoparticles intensified production by spinning disk reactor: Optimization and fluid dynamics modelling. Chem. Eng. Process Intensif. 2019, 146, 107683. [Google Scholar] [CrossRef] [Green Version]
- Vilardi, G.; Stoller, M.; Verdone, N.; Di Palma, L. Production of nano Zero Valent Iron particles by Means of a Spinning Disk Reactor. Chem. Eng. Trans. 2017, 57, 751–756. [Google Scholar]
- Vilardi, G.; Verdone, N. Production of metallic iron nanoparticles in a baffled stirred tank reactor: Optimization via computational fluid dynamics simulation. Particuology 2020, in press. [Google Scholar] [CrossRef]
- Jiao, W.; Qin, Y.; Luo, S.; Feng, Z.; Liu, Y. Continuous preparation of nanoscale zero-valent iron using impinging stream-rotating packed bed reactor and their application in reduction of nitrobenzene. J. Nanopart. Res. 2017, 19, 52. [Google Scholar] [CrossRef]
- Fan, H.; Ren, H.; Ma, X.; Zhou, S.; Huang, J.; Jiao, W.; Qi, G.; Liu, Y. High-gravity continuous preparation of chitosan-stabilized nanoscale zero-valent iron towards Cr(VI) removal. Chem. Eng. J. 2020, 390, 124639. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Wang, D.; Zhang, L.-L.; Wang, J.-X. Efficient preparation of nanoscale zero-valent iron by high gravity technology for enhanced Cr(VI) removal. Can. J. Chem. Eng. 2019, 97, 1451–1458. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [Green Version]
- Machado, S.; Pacheco, J.G.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Green zero-valent iron nanoparticles for the degradation of amoxicillin. Int. J. Environ. Sci. Technol. 2017, 14, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Machado, S.; Pacheco, J.G.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci. Tot. Environ. 2015, 533, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Machado, S.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Utilization of food industry wastes for the production of zero-valent iron nanoparticles. Sci. Tot. Environ. 2014, 496, 233–240. [Google Scholar] [CrossRef]
- Mehrotra, N.; Tripathi, R.M.; Zafar, F.; Singh, M.P. Catalytic Degradation of Dichlorvos Using Biosynthesized Zero Valent Iron Nanoparticles. IEEE Trans. Nanobiosci. 2017, 16, 280–286. [Google Scholar] [CrossRef]
- Xia, Q.; Jiang, Z.; Wang, J.; Yao, Z. A facile preparation of hierarchical dendritic zero-valent iron for Fenton-like degradation of phenol. Catal. Commun. 2017, 100, 57–61. [Google Scholar] [CrossRef]
- Chen, S.-S.; Hsu, H.-D.; Li, C.-W. A new method to produce nanoscale iron for nitrate removal. J. Nanopart. Res. 2004, 6, 639–647. [Google Scholar] [CrossRef]
- Goswami, A.; Kadam, R.G.; Tucek, J.; Sofer, Z.; Bousa, D.; Varma, R.S.; Gawande, M.B..; Zboril, R. Fe(0)-embedded thermally reduced graphene oxide as efficient nanocatalyst for reduction of nitro compounds to amines. Chem. Eng. J. 2020, 382, 122469. [Google Scholar] [CrossRef]
- Tucek, J.; Sofer, Z.; Bousa, D.; Pumera, M.; Hola, K.; Mala, A.; Polakova, K.; Havrdova, M.; Cepe, K.; Tomanec, O.; et al. Air-stable superparamagnetic metal nanoparticles entrapped in graphene oxide matrix. Nature Comm. 2016, 7, 12879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chen, C.; Zhu, K.; Wang, X. Nanoscale zero-valent iron particles modified on reduced graphene oxides using a plasma technique for Cd(II) removal. J. Taiwan Inst. Chem. Eng. 2016, 59, 389–394. [Google Scholar] [CrossRef]
- He, D.; Niu, H.; He, S.; Mao, L.; Cai, Y.; Liang, Y. Strengthened Fenton degradation of phenol catalyzed by core/shell Fe-Pd@C nanocomposites derived from mechanochemically synthesized Fe-Metal organic frameworks. Water Res. 2019, 162, 151–160. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M.; Kótai, L.; Sajó, I.E.; Homonnay, Z.; Kuzmann, E.; Kiss, L.F.; Váczi, T.; Kovács, I. Nanofurry magnetic carbon microspheres for separation processes and catalysis: Synthesis, phase composition, and properties. J. Mater. Sci. 2015, 50, 7353–7363. [Google Scholar] [CrossRef]
- Kong, Q.; Guo, C.; Wang, B.; Ji, Q.; Xia, Y. A facile preparation of carbon-supported nanoscale zero-valent iron fibers. Mater. Sci. Forum 2011, 688, 349–352. [Google Scholar] [CrossRef]
- Nistico, R.; Carlos, L. High yield of nano zero-valent iron (nZVI) from carbothermal synthesis using lignin-derived substances from municipal biowaste. J. Anal. Appl. Pyrol. 2019, 140, 239–244. [Google Scholar] [CrossRef]
- Uegami, M.; Kawano, J.; Okita, T.; Fujii, Y.; Okinaka, K.; Kakuyua, K. Iron Particles for Purifying Contaminated Soil or Ground Water. Process for Producing the Iron Particles, Purifying Agent Comprising the Iron Particles, Process for Producing the Purifying Agent and Method of Purifying Contaminated Soil or Ground Water; Toda Kogyo Corp. US Patent Application 2003/0217974 A1, 2003. [Google Scholar]
- Choi, C.J.; Dong, X.L.; Kim, B.K. Characterization of Fe and Co nanoparticles synthesized by chemical vapor condensation. Scripta Mater. 2001, 44, 2225–2229. [Google Scholar] [CrossRef]
- Visentin, C.; da Silva Trentin, A.W.; Braun, A.B.; Thome, A. Lifecycle assessment of environmental and economic impacts of nano-iron synthesis process for application in contaminated site remediation. J. Clean. Prod. 2019, 231, 307–319. [Google Scholar] [CrossRef]
- Bae, S.; Gim, S.; Kim, H.; Hanna, K. Effect of NaBH4 on properties of nanoscale zero-valent iron and its catalytic activity for reduction of pnitrophenol. Appl. Cat. B: Environ. 2016, 182, 541–549. [Google Scholar] [CrossRef]
- Walling, C. Fenton’s reagent revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Karim, S.; Bae, S.; Greenwood, D.; Hanna, K.; Singhal, N. Degradation of 17a-ethinylestradiol by nano zero valent iron under different pH and dissolved oxygen levels. Water Res. 2017, 125, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Sharma, V.K. Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ. Chem. Lett. 2018, 16, 947–967. [Google Scholar] [CrossRef] [Green Version]
- Nidheesh, P.V. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015, 5, 40552–40577. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Rezaei, F.; Vione, D. Effect of pH on zero valent iron performance in heterogeneous fenton and fenton-like processes: A review. Molecules 2018, 23, 3127. [Google Scholar] [CrossRef] [Green Version]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar]
- Zhou, Y.; Wang, T.; Zhi, D.; Guo, B.; Zhou, Y.; Nie, J.; Huang, A.; Yang, Y.; Huang, H.; Luo, L. Applications of nanoscale zero-valent iron and its composites to the removal of antibiotics: A review. J. Mater. Sci. 2019, 54, 12171–12188. [Google Scholar] [CrossRef]
- Bautitz, I.R.; Velosa, A.C.; Pupo Nogueira, R.F. Zero valent iron mediated degradation of the pharmaceutical diazepam. Chemosphere 2012, 88, 688–692. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Clemente, A.; Torres-Palma, R.A.; Peñuela, G.A. Removal of polycyclic aromatic hydrocarbons in aqueous environment by chemical treatments: A review. Sci. Tot. Environ. 2014, 478, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Q.; Liu, Y.; Lei, M. Recyclable nanoscale zero-valent iron-based magnetic polydopamine coated nanomaterials for the adsorption and removal of phenanthrene and anthracene. Sci. Technol. Adv. Mater. 2017, 18, 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, Z.-H.; Xu, X.-R.; Jiang, D.; Kong, L.-J.; Sun, Y.-X.; Hu, Y.-X.; Hao, Q.-W.; Chen, H. Bentonite-supported nanoscale zero-valent iron/persulfate system for the simultaneous removal of Cr(VI) and phenol from aqueous solutions. Chem. Eng. J. 2016, 302, 213–222. [Google Scholar] [CrossRef]
- Ma, L.; He, H.; Zhu, R.; Zhu, J.; Mackinnon, I.D.R.; Xi, Y. Bisphenol A degradation by a new acidic nano zero-valent iron diatomite composite. Cat. Sci. Technol. 2016, 6, 6066–6075. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Qiu, X.; Fang, Z.; Yang, M.; Pokeung, T.; Gu, F.; Cheng, W.; Lan, B. Removal mechanism of antibiotic metronidazole from aquatic solutions by using nanoscale zero-valent iron particles. Chem. Eng. J. 2012, 181, 113–119. [Google Scholar] [CrossRef]
- Ghauch, A.; Tuqan, A.; Assi, H.A. Antibiotic removal from water: Elimination of amoxicillin and ampicillin by microscale and nanoscale iron particles. Environ. Pollut. 2009, 157, 1626–1635. [Google Scholar] [CrossRef]
- Diao, Z.-H.; Qian, W.; Lei, Z.-X.; Kong, L.-J.; Dua, J.-J.; Liu, H.; Yang, J.-W.; Pu, S.Y. Insights on the nitrate reduction and norfloxacin oxidation over a novel nanoscale zero valent iron particle: Reactivity, products, and mechanism. Sci. Tot. Environ. 2019, 660, 541–549. [Google Scholar] [CrossRef]
- Chen, H.; Luo, H.; Lan, Y.; Dong, T.; Hu, B.; Wang, Y. Removal of tetracycline from aqueous solutions using polyvinylpyrrolidone (PVP-K30) modified nanoscale zero valent iron. J. Hazard. Mater. 2011, 192, 44–53. [Google Scholar] [CrossRef]
- Daneshkhah, M.; Hossaini, H.; Malakootian, M. Removal of metoprolol from water by sepiolite-supported nanoscale zero-valent iron. J. Environ. Chem. Eng. 2017, 5, 3490–3499. [Google Scholar] [CrossRef]
- Singhal, R.K.; Gangadhar, B.; Basu, H.; Manisha, V.; Naidu, G.R.K.; Reddy, A.V.R. Remediation of malathion contaminated soil using zero valent iron nano-particles. Amer. J. Anal. Chem. 2012, 3, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Kurokawa, T.; Suzuki, M.; Takagi, M.; Kawase, Y. Removal of cationic dye methylene blue by zero-valent iron: Effects of pH and dissolved oxygen on removal mechanisms. J. Environ. Sci. Health. A 2015, 50, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Kamali, M.; Capela, I.; Costa, M.E. Ultrasonic synthesis of zero valent iron nanoparticles for the efficient discoloration of aqueous solutions containing methylene blue dye. In Nanomaterials in the Wet Processing of Textiles, 1st ed.; Islam, S.-U., Butola, B.S., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2018; p. 261284. [Google Scholar]
- Son, Y.-H.; Lee, J.-K.; Soong, Y.; Martello, D.; Chyu, M.K. Heterostructured zero valent iron-montmorillonite nanohybrid and their catalytic efficacy. Appl. Clay Sci. 2012, 62, 21–26. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Zhou, J.; Ma, J.; Komarneni, S. Degradation of orange II by Fe@Fe2O3 core shell nanomaterials assisted by NaHSO3. Chemosphere 2020, 244, 125588. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tian, W.; Zhang, Y.; Tang, J.; Zhao, Z.; Chen, Y. Reduced Graphene Oxide/Attapulgite-Supported Nanoscale Zero-Valent Iron Removal of Acid Red 18 from Aqueous Solution. Water Air Soil Pollut. 2018, 229, 388. [Google Scholar] [CrossRef]
- Bossa, N.; Carpenter, A.W.; Kumar, N.; de Lannoy, C.-F.; Wiesner, M. Cellulose nanocrystal zero-valent iron nanocomposites for groundwater remediation. Environ. Sci. Nano 2017, 4, 1294–1303. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P.; Ma, J.; Liu, H.; Ning, P. Synthesis, characterization, and reactivity of cellulose modified nano zero-valent iron for dye discoloration. Appl. Surf. Sci. 2015, 345, 57–66. [Google Scholar] [CrossRef]
- Kerkez, D.V.; Tomasevic, D.D.; Kozma, G.; Becelic-Tomin, M.R.; Prica, M.D.; Roncevic, S.D.; Kukovecz, A.; Dalmacija, B.D.; Konya, Z. Three different clay-supported nanoscale zero-valent iron materials for industrial azo dye degradation: A comparative study. J. Taiwan Inst. Chem. Eng. 2014, 45, 2451–2461. [Google Scholar] [CrossRef]
- Elliott, D.W.; Lien, H.-L.; Zhang, W.-X. Degradation of lindane by zero-valent iron nanoparticles. J. Environ. Eng. 2009, 135, 317–324. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Hwang, Y.; Andersen, H.R.; Stasinakis, A.S.; Thomaidis, N.S.; Aloupi, M. Reductive degradation of perfluorinated compounds in water using Mg-aminoclay coated nanoscale zero valent iron. Chem. Eng. J. 2015, 262, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Weinholtz, L.; Zheng, L.; Peiravi, M.; Wu, Y.; Chen, D. Removal of PFOA in groundwater by Fe0 and MnO2 nanoparticles under visible light. J. Environ. Sci. Health, Part A 2017, 52, 1048–1054. [Google Scholar] [CrossRef]
- Chen, H.F.; Cao, Y.; Wei, E.; Gong, T.T.; Xian, Q.M. Facile synthesis of graphene nano zero-valent iron composites and their efficient removal of trichloronitromethane from drinking water. Chemosphere 2016, 146, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Gu, Z.; Zhang, Z.; Zhang, J.; Hermanowicz, S.W. Removal of chloramphenicol from aqueous solution by nanoscale zero-valent iron particles. Chem. Eng. J. 2014, 257, 98–104. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, X.; Xu, J.; Zhang, J.; Yang, Y.; Zhou, J.; Xu, X.; Lowry, G.V. Removal of Antibiotic Florfenicol by Sulfide-Modified Nanoscale Zero-Valent Iron. Environ. Sci. Technol. 2017, 51, 11269–11277. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Lu, S.; Fang, Z.; Cheng, W.; Tsang, E.P. Enhanced reductive debromination and subsequent oxidative ring-opening of decabromodiphenyl ether by integrated catalyst of nZVI supported on magnetic Fe3O4 nanoparticles. Appl. Cat. B: Environm. 2017, 200, 200–210. [Google Scholar] [CrossRef]
- Tan, L.; Liang, B.; Cheng, W.; Fang, Z.; Tsang, E.P. Effect of solvent on debromination of decabromodiphenyl ether by Ni/Fe nanoparticles and nano zero-valent iron particles. Environ. Sci. Pollut. Res. 2016, 23, 22172–22182. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Cao, L.; Zhou, L.; Gu, Y.; Wang, X. Degradation of bromamine acid by nanoscale zero-valent iron (nZVI) supported on sepiolite. Water Sci. Technol. 2012, 66, 2539–2545. [Google Scholar] [CrossRef]
- Schmid, D.; Micic, V.; Laumann, S.; Hofmann, T. Measuring the reactivity of commercially available zero-valent iron nanoparticles used for environmental remediation with iopromide. J. Contam. Hydrol. 2015, 181, 36–45. [Google Scholar] [CrossRef]
- Wei, Y.-T.; Wu, S.-C.; Chou, C.-M.; Che, C.-H.; Tsai, S.-M.; Lien, H.-L. Influence of nanoscale zero-valent iron on geochemical properties of groundwater and vinyl chloride degradation: A field case study. Water Res. 2010, 44, 131–140. [Google Scholar] [CrossRef]
- Bennett, P.; He, F.; Zhao, D.; Aiken, B.; Feldman, L. In situ testing of metallic iron nanoparticle mobility and reactivity in a shallow granular aquifer. J. Cont. Hydrol. 2010, 116, 35–46. [Google Scholar] [CrossRef]
- Quinn, J.; Geiger, C.; Clausen, C.; Brooks, K.; Coon, C.; O’Hara, S.; Krug, T.; Major, D.; Yoon, W.S.; Gavaskar, A.; et al. Field demonstration of DNAPL dehalogenation using emulsified zero-valent iron. Environ. Sci. Technol. 2005, 39, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, S.; Krug, T.; Quinn, J.; Clausen, C.; Geiger, C. Field and laboratory evaluation of the treatment of DNAPL source zones using emulsified zero-valent iron. Remediation 2006, 16, 35–56. [Google Scholar]
- Jordan, M.; Shetty, N.; Zenker, M.J.; Brownfield, C. Remediation of a former dry cleaner using nanoscale zero valent iron. Remediation 2013, 24, 31–48. [Google Scholar] [CrossRef]
- Filip, J.; Kolarik, J.; Petala, E.; Petr, M.; Sracek, O.; Zboril, L. Nanoscale Zerovalent Iron Particles for Treatment of Metalloids. In Nanoscale Zerovalent Iron Particles for Environmental Restoration, Fundamental Science to Field Scale Engineering Applications; Phenrat, T., Lowry, G.V., Eds.; Springer International Publishing AG: Cham, Switzerland, 2019; pp. 157–199. [Google Scholar]
- Liu, P.; Liang, Q.; Luo, H.; Fang, W.; Geng, J. Synthesis of nano-scale zero-valent iron-reduced graphene oxide-silica nano-composites for the efficient removal of arsenic from aqueous solutions. Environ. Sci. Pollut. Res. 2019, 26, 33507–33516. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tu, J.; Liu, W.; Zhang, J.; Chu, S.; Lu, G.; Lin, Z.; Dang, Z. The double influence mechanism of pH on arsenic removal by nano zero valent iron: Electrostatic interactions and the corrosion of Fe0. Environ. Sci. Nano 2017, 4, 1544–1552. [Google Scholar] [CrossRef]
- Bhowmick, S.; Chakraborty, S.; Mondal, P.; Van Renterghem, W.; Van den Berghe, S.; Roman-Ross, G.; Chatterjee, D.; Iglesias, M. Montmorillonite-supported nanoscale zero-valent iron for removal of arsenic from aqueous solution: Kinetics and mechanism. Chem. Eng. J. 2014, 243, 14–23. [Google Scholar] [CrossRef]
- Tanboonchuy, V.; Hsu, J.-C.; Grisdanurak, N.; Liao, C.-H. Impact of selected solution factors on arsenate and arsenite removal by nanoiron particles. Environ. Sci. Pollut. Res. 2011, 18, 857–864. [Google Scholar] [CrossRef]
- Yan, W.; Ramos, M.A.V.; Koel, B.E.; Zhang, W.-X. Multi-tiered distributions of arsenic in iron nanoparticles: Observation of dual redox functionality enabled by a core-shell structure. Chem. Commun. 2010, 46, 6995–6997. [Google Scholar] [CrossRef]
- Suazo-Hernandez, J.; Sepulveda, P.; Manquian-Cerda, K.; Ramirez-Tagle, R.; Rubio, M.A.; Bolan, N.; Sarkar, B.; Arancibia-Miranda, N. Synthesis and characterization of zeolite-based composites functionalized with nanoscale zero-valent iron for removing arsenic in the presence of selenium from water. J. Hazard. Mater. 2019, 373, 810–819. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Wang, W.; Zhang, W.-X. Recovery of gold from wastewater using nanoscale zero-valent iron. Environ. Sci. Nano 2019, 6, 519–527. [Google Scholar] [CrossRef]
- Zhu, L.; Tong, L.; Zhao, N.; Li, J.; Lv, Y. Coupling interaction between porous biochar and nano zero valent iron/nano a-hydroxyl iron oxide improves the remediation efficiency of cadmium in aqueous solution. Chemosphere 2019, 219, 493–503. [Google Scholar] [CrossRef]
- Saffari, M. Response surface methodological approach for optimizing the removal of cadmium from aqueous solutions using pistachio residues biochar supported/non-supported by nanoscale zero-valent iron. Main Group Metal Chem. 2018, 41, 167–181. [Google Scholar] [CrossRef]
- Üzüm, C.; Shahwan, T.; Eroğlu, A.E.; Hallam, K.R.; Scott, T.B.; Lieberwirth, I. Synthesis and characterization of kaolinite-supported zero-valent iron nanoparticles and their application for the removal of aqueous Cu2+ and Co2+ ions. Appl. Clay Sci. 2009, 43, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.; Xu, L.J.; Wang, J.L. Mechanism of Co(II) adsorption by zero valent iron/graphene nanocomposite. J. Hazard. Mater. 2016, 301, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Ji, B.; Cui, B.; Shi, Y.; Wang, J.; Hu, M.; Luo, S.; Guo, D. Almond Shell-Derived, Biochar-Supported, Nano-Zero-Valent Iron Composite for Aqueous Hexavalent Chromium Removal: Performance and Mechanisms. Nanomaterials 2020, 10, 198. [Google Scholar] [CrossRef] [Green Version]
- Chi, Z.; Hao, L.; Dong, H.; Yu, H.; Liu, H.; Yu, H. The innovative application of organosolv lignin for nanomaterial modification to boost its heavy metal detoxification performance in the aquatic environment. Chem. Eng. J. 2020, 382, 122789. [Google Scholar] [CrossRef]
- Vilardi, G.; Mpouras, T.; Dermatas, D.; Verdone, N.; Polydera, A.; Di Palma, L. Nanomaterials application for heavy metals recovery from polluted water: The combination of nano zerovalent iron and carbon nanotubes. Competitive adsorption non-linear modelling. Chemosphere 2018, 201, 716–729. [Google Scholar] [CrossRef]
- Mucha, N.R.; Ravella, R.; Reddy, M.R.; Zhang, L. Electrospun Carbon Nanofiber Supported Zero Valent Iron Nanoparticles (nZVI@ECNFs) for Cr (VI) Remediation in Ground and Waste Water. MRS Adv. 2016, 1, 3593–3599. [Google Scholar] [CrossRef]
- Karabelli, D.; Üzüm, C.; Shahwan, T.; Eroglu, A.E.; Scott, T.B.; Hallam, K.R.; Lieberwirth, I. Batch Removal of Aqueous Cu2+ Ions Using Nanoparticles of Zero-Valent Iron: A Study of the Capacity and Mechanism of Uptake. Ind. Eng. Chem. Res. 2008, 47, 4758–4764. [Google Scholar] [CrossRef] [Green Version]
- Karabellia, D.; Ünal, S.; Shahwan, T.; Eroglu, A.E. Preparation and characterization of alumina-supported iron nanoparticles and its application for the removal of aqueous Cu2+ ions. Chem. Eng. J. 2011, 168, 979–984. [Google Scholar] [CrossRef]
- Xiao, S.; Ma, H.; Shen, M.; Wang, S.; Huang, Q.; Shi, X. Excellent copper(II) removal using zero-valent iron nanoparticle-immobilized hybrid electrospun polymer nanofibrous mats. Coll. Surf. A: Physicochem. Eng. Aspects 2011, 381, 48–54. [Google Scholar] [CrossRef]
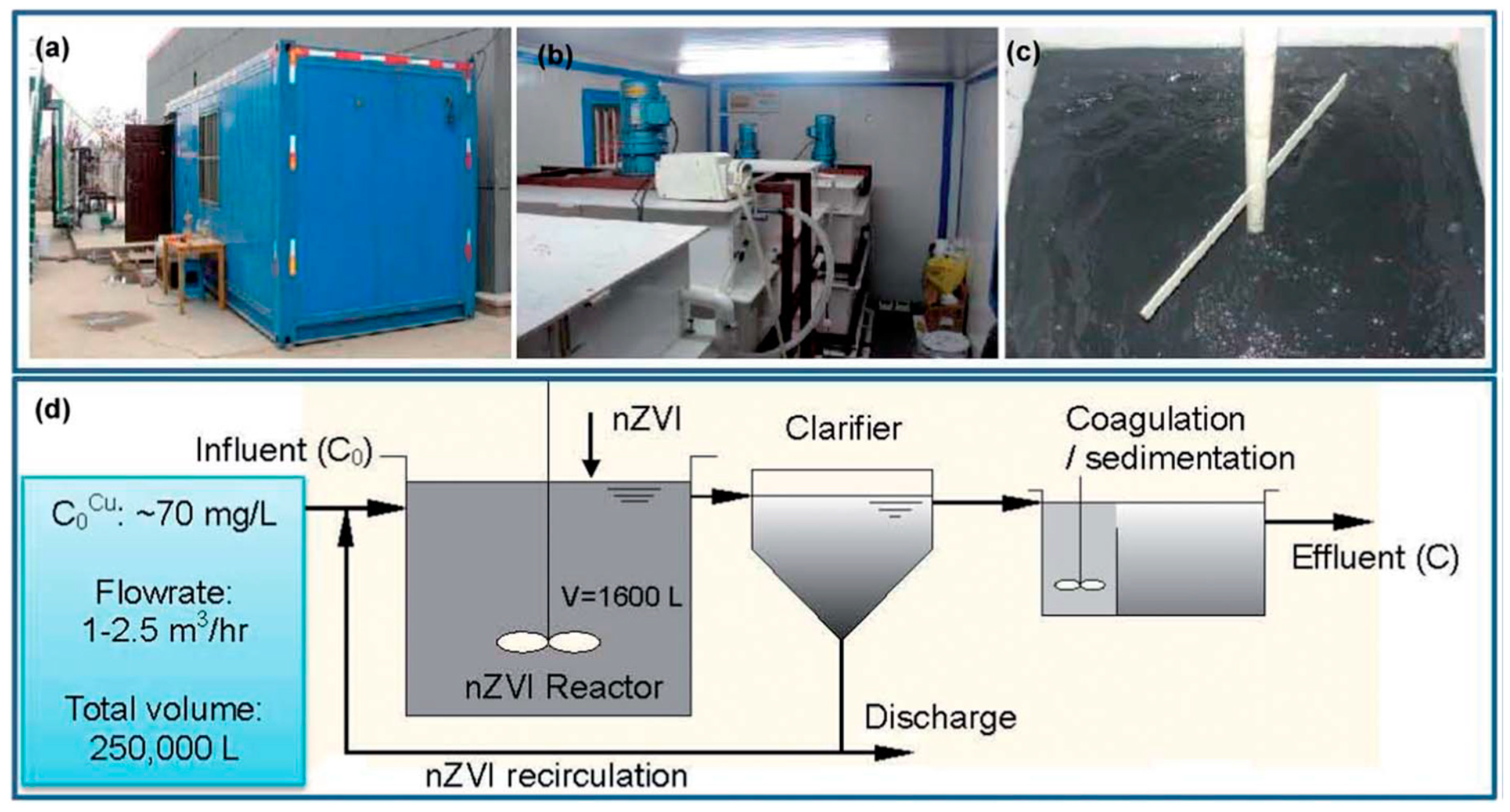
- Li, S.; Wang, W.; Yan, W.; Zhang, W.-x. Nanoscale zero-valent iron (nZVI) for the treatment of concentrated Cu(II) wastewater: A field demonstration. Environ. Sci. Processes Impacts 2014, 16, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Q.; Zhang, W.-X. Iron Nanoparticles: The Core-Shell Structure and Unique Properties for Ni(II) Sequestration. Langmuir 2006, 22, 4638–4642. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Chen, L.; Zhang, Y.; Lin, Z. Mg(OH)2 Supported Nanoscale Zero Valent Iron Enhancing the Removal of Pb(II) from Aqueous Solution. ACS Appl. Mater. Interf. 2015, 7, 7961–7969. [Google Scholar] [CrossRef]
- Kim, S.A.; Kamala-Kannan, S.; Lee, K.-J.; Park, Y.-J.; Shea, P.J.; Lee, W.-H.; Kim, H.-M.; Oh, B.-T. Removal of Pb(II) from aqueous solution by a zeolite–nanoscale zero-valent iron composite. Chem. Eng. J. 2013, 217, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Yang, W.; Guan, X.; Li, J.; Xu, Z.; Wu, J.; Huang, Y.; Zhang, X. Kinetics and mechanisms of pH-dependent selenite removal by zero valent iron. Water Res. 2013, 47, 5846–5855. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.; Papp, J.K.; Colburn, A.S.; Bhattacharyya, D.; Meeks, N.D.; Weaver, B.; Wilf, I. Engineered Iron/Iron Oxide Functionalized Membranes for Selenium and Other Toxic Metal Removal from Power Plant Scrubber Water. J. Membrane Sci. 2015, 488, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Shang, C.; Shao, J.; Zhang, J.; Huang, H. The application of iron-based technologies in uranium remediation: A review. Sci. Tot. Environ. 2017, 575, 1291–1306. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Wang, L.; Yuan, L.Y.; Xiao, C.L.; Mei, L.; Zheng, L.R.; Zhang, J.; Yang, J.H.; Zhao, Y.L.; Zhu, Z.T.; et al. Efficient removal of uranium from aqueous solution by zero-valent iron nanoparticle and its graphene composite. J. Hazard. Mater. 2015, 290, 26–33. [Google Scholar] [CrossRef]
- Sheng, G.; Shao, X.; Li, Y.; Li, J.; Dong, H.; Cheng, W.; Gao, X.; Huang, Y. Enhanced Removal of Uranium(VI) by Nanoscale Zerovalent Iron Supported on Na−Bentonite and an Investigation of Mechanism. J. Phys. Chem. A 2014, 118, 2952–2958. [Google Scholar] [CrossRef]
- Shi, L.-N.; Zhou, Y.; Chen, Z.; Megharaj, M.; Naidu, R. Simultaneous adsorption and degradation of Zn2+ and Cu2+ from wastewaters using nanoscale zero-valent iron impregnated with clays. Environ. Sci. Pollut. Res. 2013, 20, 3639–3648. [Google Scholar] [CrossRef]
- Wang, W.; Hua, Y.; Li, S.; Yan, W.; Zhang, W.-X. Removal of Pb(II) and Zn(II) using lime and nanoscale zero-valent iron (nZVI): A comparative study. Chem. Eng. J. 2016, 304, 79–88. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.-X.; Wang, W.; Liang, F. Heavy metal removal using nanoscale zero-valent iron (nZVI): Theory and application. J. Hazard. Mater. 2017, 322, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Baragano, D.; Alonso, J.; Gallego, J.R.; Lobo, M.C.; Gil-Diaz, M. Zero valent iron and goethite nanoparticles as new promising remediation techniques for As-polluted soils. Chemosphere 2020, 238, 124624. [Google Scholar] [CrossRef] [PubMed]
- Tomašević Filipović, D.; Kerkez, D.; Dalmacija, B.; Slijepčević, N.; Krčmar, D.; Rađenovic, D.; Bečelić-Tomin, M. Remediation of Toxic Metal Contaminated Sediment Using Three Types of nZVI Supported Materials. Bull. Environ. Contam. Toxicol. 2018, 101, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Nemecek, J.; Pokorny, P.; Lacinova, L.; Cernik, M.; Masopustova, Z.; Lhotsky, O.; Filipova, A.; Cajthaml, T. Combined abiotic and biotic in-situ reduction of hexavalent chromium in groundwater using nZVI and whey: A remedial pilot test. J. Hazard. Mater. 2015, 300, 670–679. [Google Scholar] [CrossRef]
- Němeček, J.; Lhotský, O.; Cajthaml, T. Nanoscale zero-valent iron application for in situ reduction of hexavalent chromium and its effects on indigenous microorganism populations. Sci. Tot. Environ. 2014, 485, 739–747. [Google Scholar] [CrossRef]
- Němeček, J.; Pokorný, P.; Lhotský, O.; Knytl, V.; Najmanová, P.; Steinová, J.; Černík, M.; Filipová, A.; Filip, J.; Cajthaml, T. Combined nano-biotechnology for in situ remediation of mixed contamination of groundwater by hexavalent chromium and chlorinated solvents. Sci. Tot. Environ. 2016, 563–564, 822–834. [Google Scholar] [CrossRef]
- Bao, Z.; Hu, Q.; Qi, W.; Tang, Y.; Wang, W.; Wan, P.; Chao, J.; Yang, X.J. Nitrate reduction in water by aluminum alloys particles. J. Environ. Manag. 2017, 196, 666–673. [Google Scholar] [CrossRef]
- Ryu, A.; Jeong, S.-W.; Jang, A.; Choi, H. Reduction of highly concentrated nitrate using nanoscale zero-valent iron: Effects of aggregation and catalyst on reactivity. Appl. Cat. B: Environ. 2011, 105, 128–135. [Google Scholar] [CrossRef]
- Vilardi, G.; De Caprariis, B.; Stoller, M.; Di Palma, L.; Verdone, N. Intensified water denitrification by means of a spinning disk reactor and stirred tank in series: Kinetic modelling and computational fluid dynamics. J. Water Proc. Eng. 2020, 34, 101147. [Google Scholar] [CrossRef]
- Liu, M.; Xi, B.-D.; Hou, L.-A.; Yu, S. Magnetic multi-functional nano-fly ash-derived zeolite composites for environmental applications. J. Mater. Chem. A 2013, 1, 12617–12626. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhao, D.; Pan, G. Rapid and complete destruction of perchlorate in water and ion-exchange brine using stabilized zero-valent iron nanoparticles. Water Res. 2007, 41, 3497–3505. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Elliott, D.; Zhang, W. Perchlorate reduction by nanoscale iron particles. J. Nanopart. Res. 2005, 7, 499–506. [Google Scholar] [CrossRef]
- Wang, Q.; Snyder, S.; Kim, J.; Choi, H. Aqueous Ethanol modified Nanoscale Zerovalent Iron in Bromate Reduction: Synthesis, Characterization, and Reactivity. Environ. Sci. Technol. 2009, 43, 3292–3299. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.F.; Nielsen, K.N.; Knudsen, N.; Grieger, K.D.; Baun, A. Operationalization and application of “early warning signs” to screen nanomaterials for harmful properties. Environ. Sci.: Processes Impacts 2013, 15, 190–203. [Google Scholar] [CrossRef] [Green Version]
- Pettibone, J.M.; Louie, S.M. Research highlights: Improved understanding of ecological impacts resulting from nanomaterial-based in situ remediation. Environ. Sci. Nano 2016, 3, 236–239. [Google Scholar] [CrossRef]
- Sun, X.; Yan, Y.; Wang, M.; Han, Z. Effect of nanoscale zero-valent iron confined in mesostructure on Escherichia coli. Environ. Sci. Pollut. Res. 2017, 24, 24038–24045. [Google Scholar] [CrossRef]
- Semerád, J.; Moeder, M.; Filip, J.; Pivokonský, M.; Filipová, A.; Cajthaml, T. Oxidative stress in microbes after exposure to iron nanoparticles: Analysis of aldehydes as oxidative damage products of lipids and proteins. Environ. Sci. Poll. Res. 2019, 26, 33670–33682. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Stevenson, L.M.; Su, Y.; Nisbet, R.M.; Zhang, Y.; Keller, A.A. Influence of phytoplankton on fate and effects of modified zero-valent iron nanoparticles. Environ. Sci. Technol. 2016, 50, 5597–5605. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, D.; Seo, S.M.; Kim, D. Physiological effects of zero-valent iron nanoparticles in rhizosphere on edible crop, Medicago sativa (Alfalfa), grown in soil. Ecotoxicology 2019, 28, 869–877. [Google Scholar] [CrossRef]
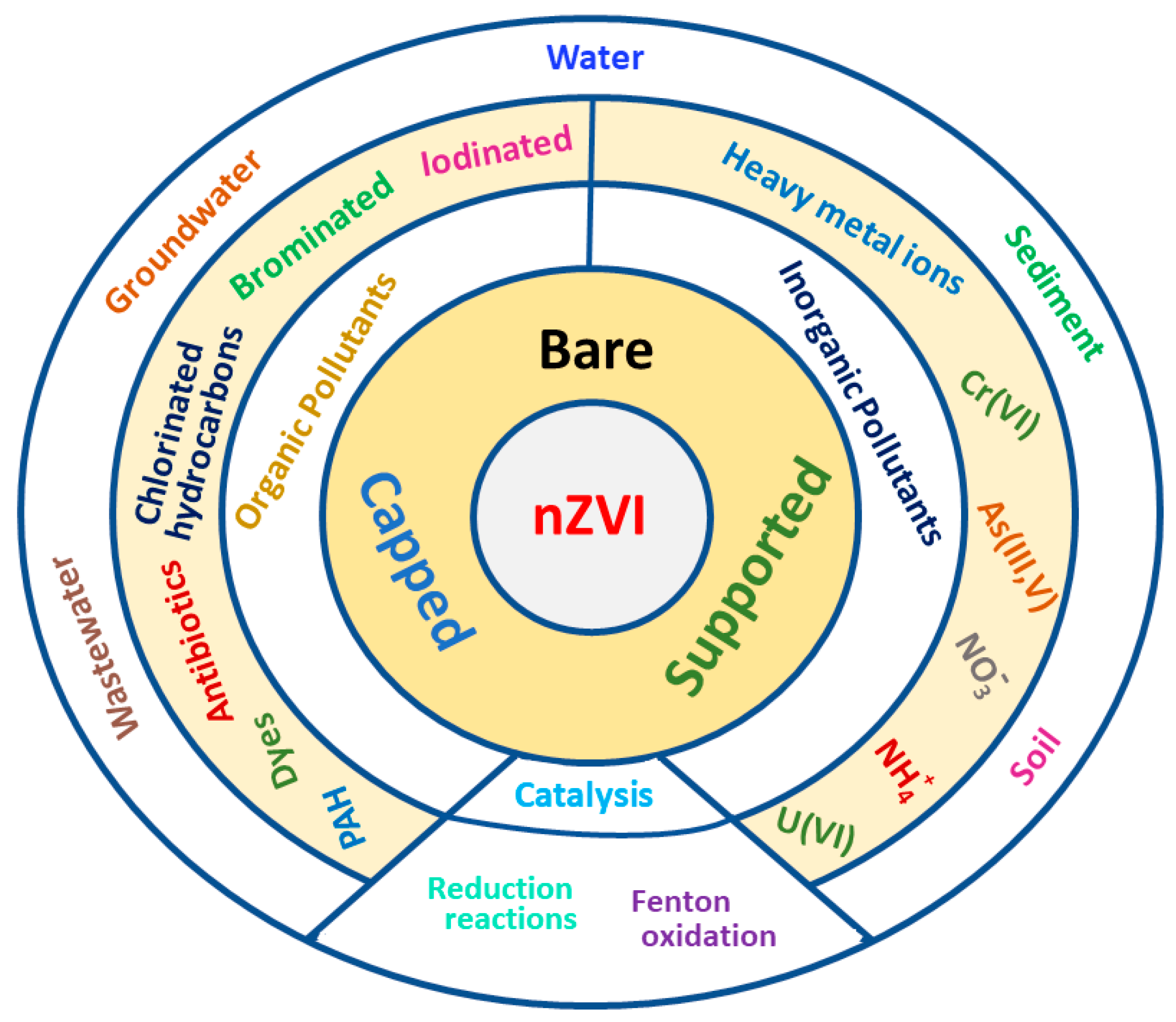

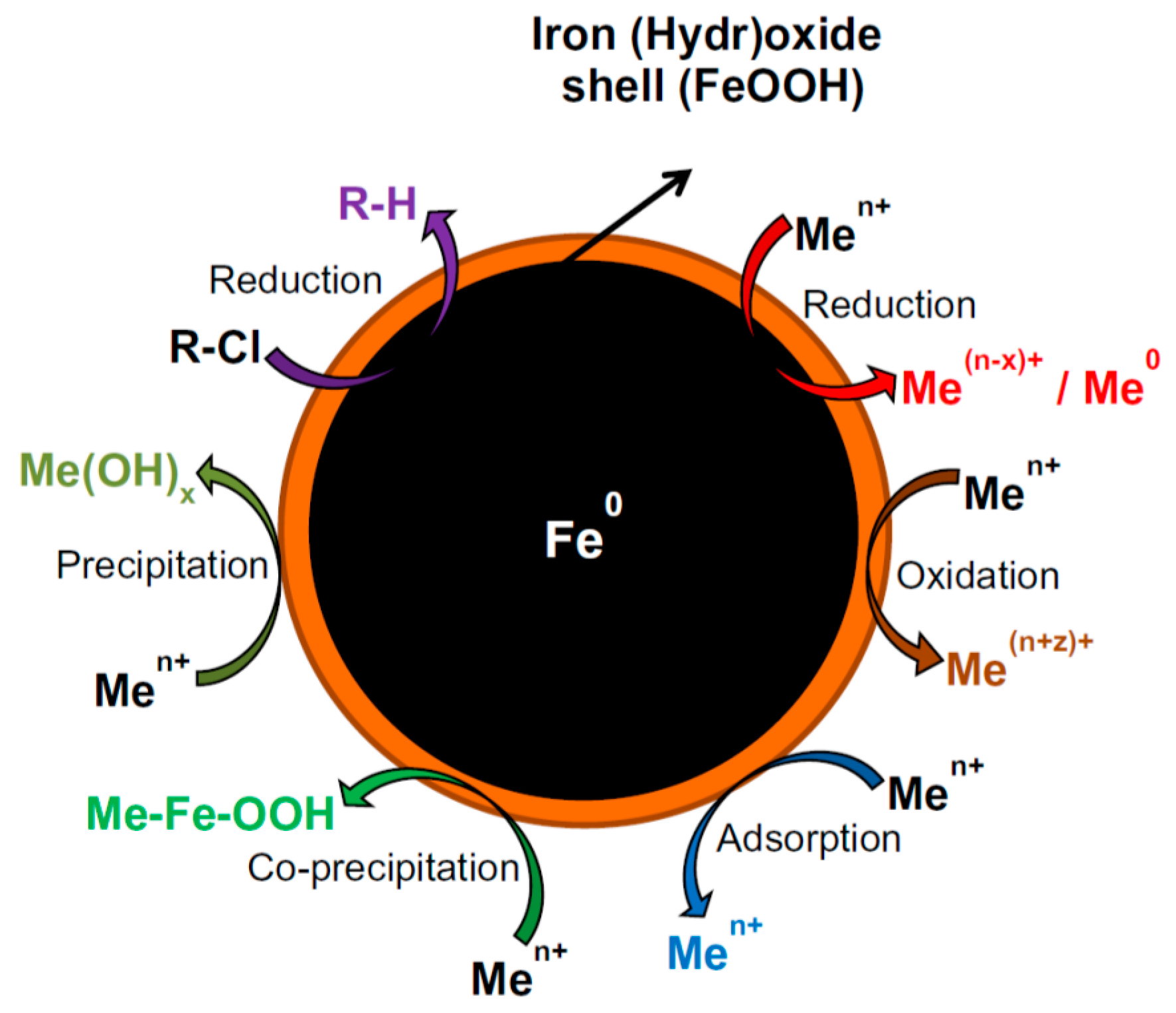

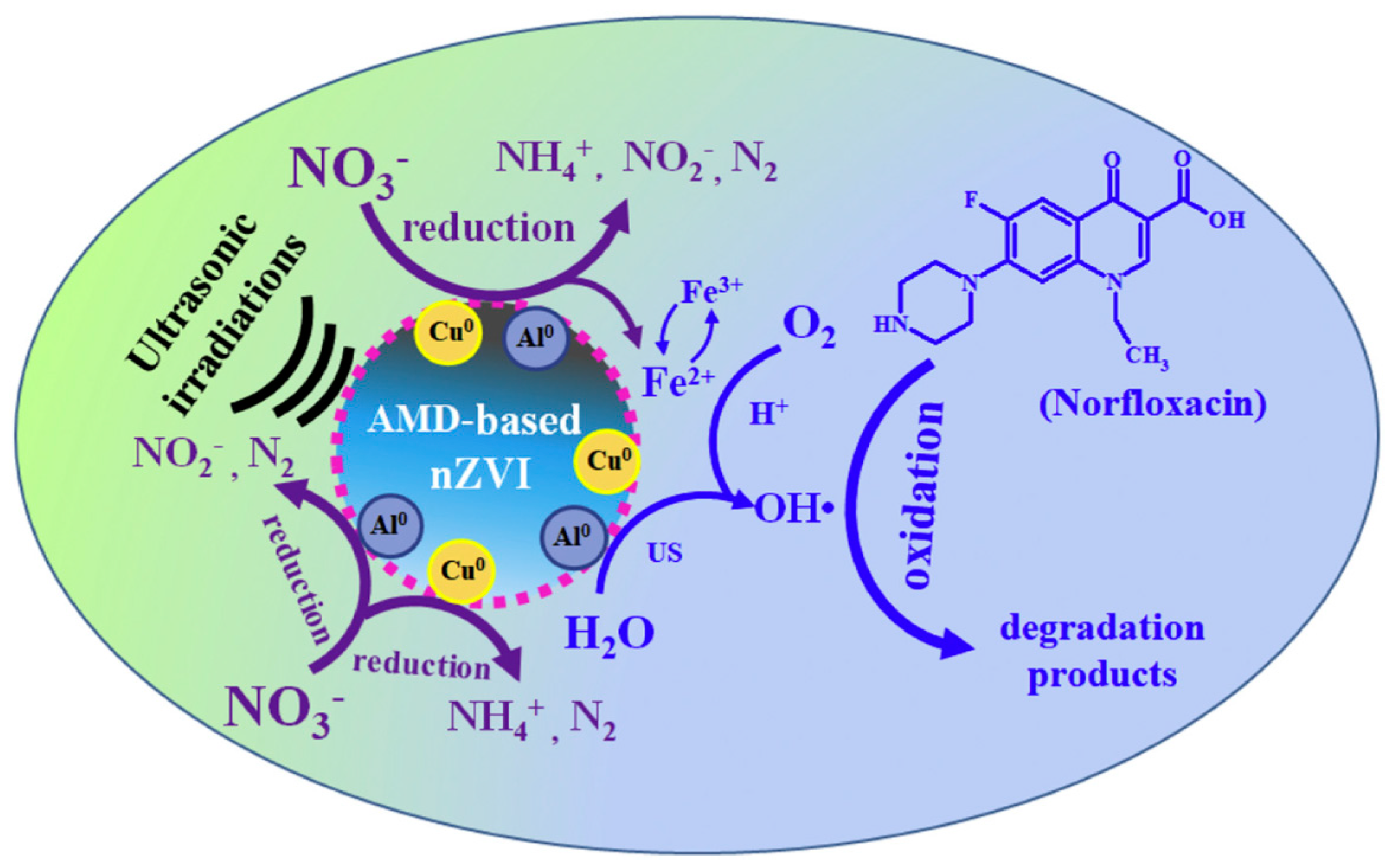
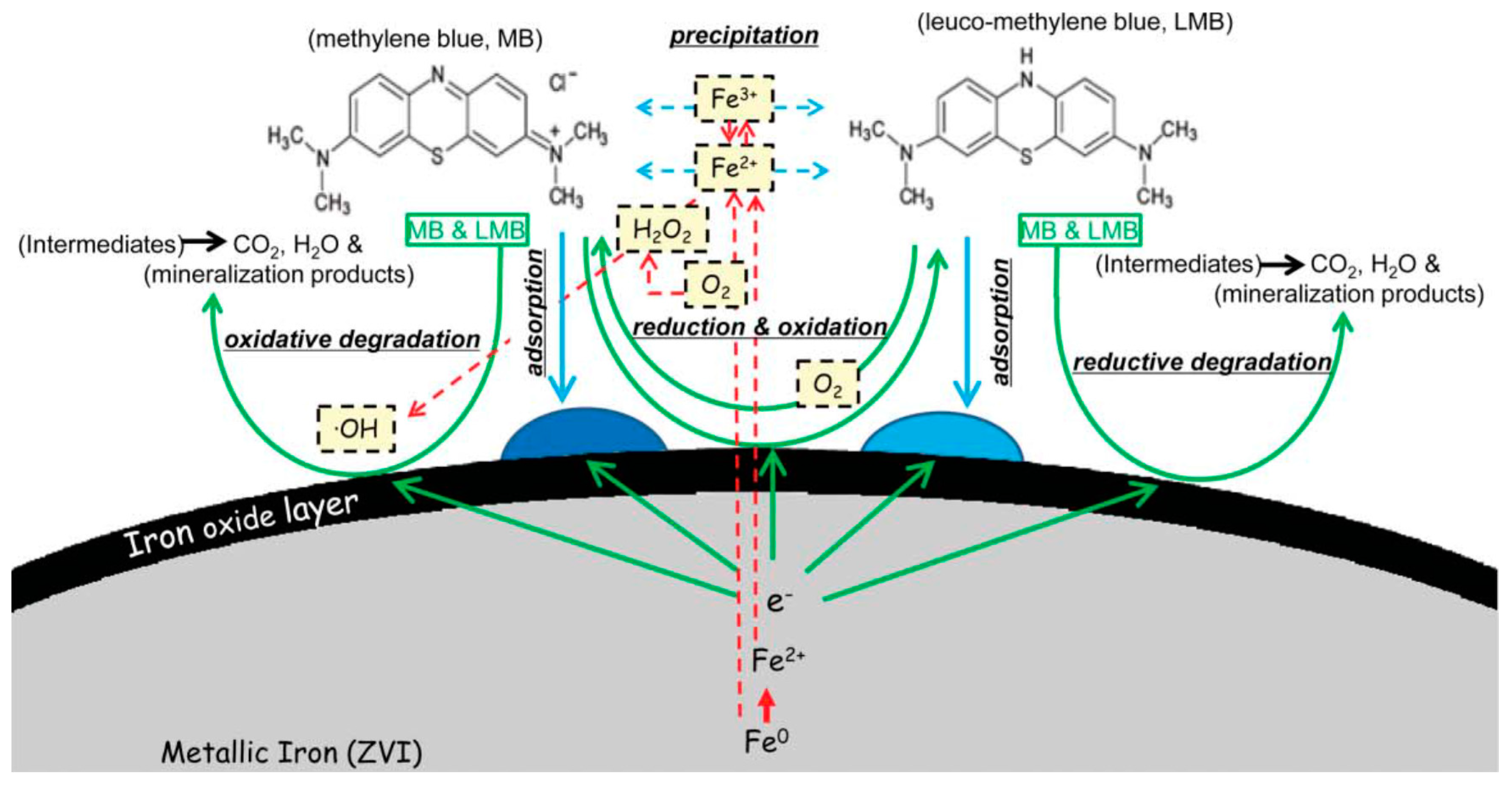
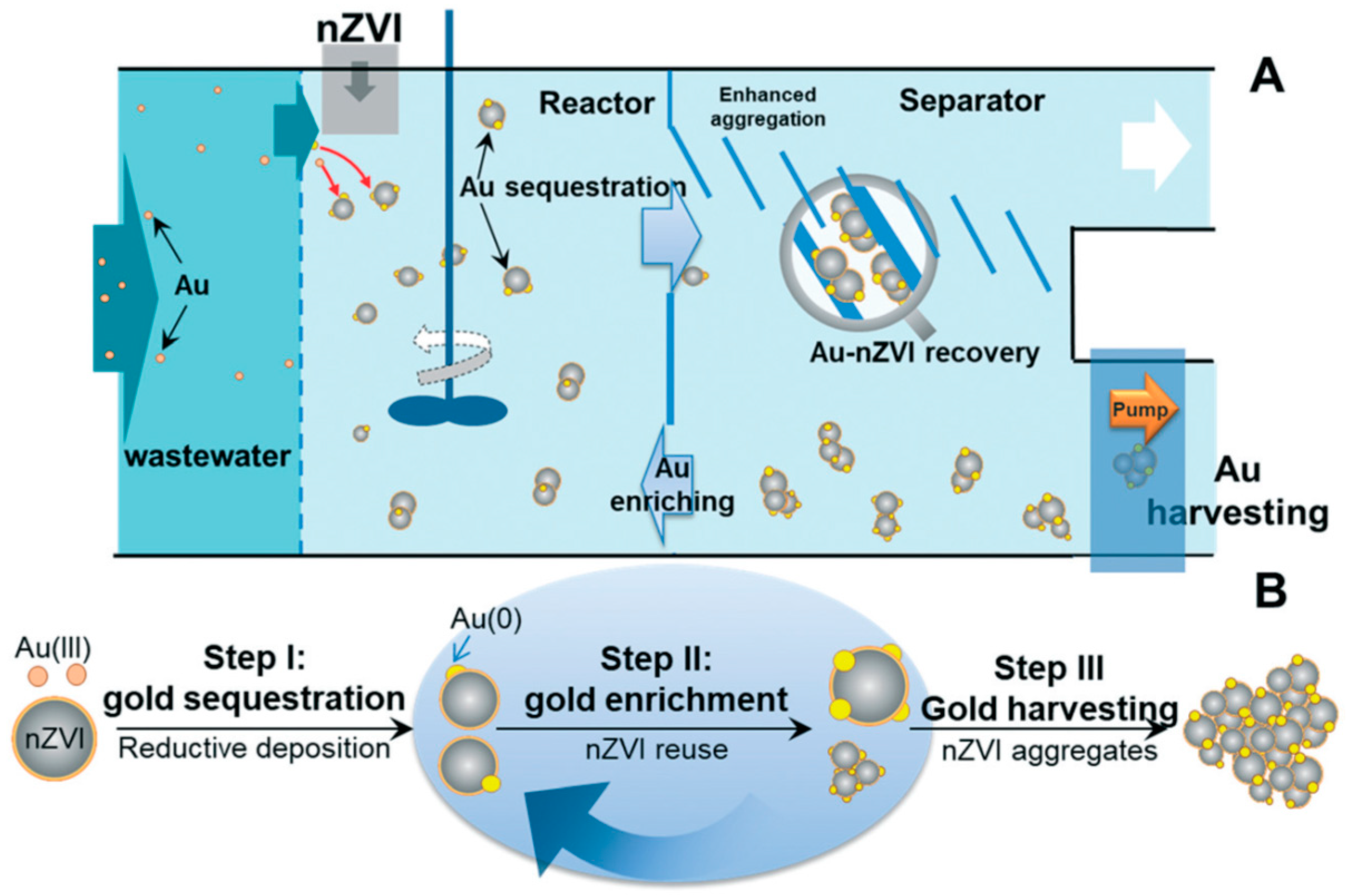

| Support/Cap | Precursor | Reagent | Product | Reference |
|---|---|---|---|---|
| rGO | Ar-NO2 2 | N2H4 | Ar-NH2 | [47] |
| no support | p-nitrophenol | NaBH4 | p-aminophenol | [57] |
| PEG, CMC, or PVP 3 | Ketones 4 | NaNH4 | alcohols | [23] |
| Octanoic acid, bis-2-ethylhexylamine | CH4 | — | SWCNT | [25] |
| nZVI Cap/Support | Pollutant | pH, Reagent | Adsorption Capacity (mg/g) or dec. Product | Ref. |
|---|---|---|---|---|
| From water | ||||
| Silica/PDA 2 | Anthracene | 3–11 | 0.367 | [68] |
| Silica/PDA | Phenanthrene | 3–11 | 0.185 | [68] |
| Al(OH)3 | 4-nitrophenol | 7.3 | 4-aminophenol | [26] |
| Carbon | Phenol | 4–5, H2O2 | n.a. | [50] |
| Bare | Phenol | 4, H2O2 | CO2, H2O | [45] |
| Bentonite | Phenol, Cr(VI) | 5, S2O82− | Formic acid | [69] |
| Diatomite | Bisphenol A | 5.75, H2O2 | CO2, H2O | [70] |
| Bare | 17α-ethinylestradiol | 3, 5, 7, O2 | C20H28O2 | [59] |
| polyphenols | Amoxicillin | 3, H2O2 | CO2, H2O | [41] |
| PVP | Metronidazole | 5.6 | C6H11N3O | [71] |
| PEG | Amoxicillin | 6.6 | AMX penicilloic acid | [72] |
| PEG | Ampicillin | 6.6 | AMP penicilloic acid | [72] |
| Bare | Norfloxacin | 4, air | CO2, H2O | [73] |
| PVP | Tetracycline | 6.5 | C19H26O | [74] |
| Sepiolite | Metoprolol | 3, H2O2 | n.a. | [75] |
| From spiked soil | ||||
| Polyphenols | Amoxicillin | 2.6–3.4, H2O2 | CO2, H2O | [41] |
| Bare | Malathion | 7.6 | ODP | [76] |
| nZVI Cap/Support | Pollutant | Soil | pH, Product | Ref. |
|---|---|---|---|---|
| Bare | Vinyl chloride | Ground water | 6–7, methane, ethene | [95] |
| CMC | PCE and TCE | Sediment | Ethene | [96] |
| Emulsion | TCE | Soil | Ethene 2 | [97] |
| Emulsion | TCE | Soil | Ethene 2 | [98] |
| Pectin | DDT | Spiked soil | 5.9 | [30] |
| MEG | PCE | Sand layers | Ethene | [14] |
| Bare | PCE | Soil | Ethene 2 | [99] |
| nZVI Cap/Support | Pollutant | Soil | pH | Ref. |
|---|---|---|---|---|
| Bare | As(V) | Sandy loam | n.a. | [131] |
| Rhamnolipid | Cd(II) | River sediment | 7.71 | [31] |
| CMC | Ni(II), Pb(II), Zn(II) | River sediment | n.a. | [132] |
| Bentonite | Ni(II), Pb(II), Zn(II) | River sediment | n.a. | [132] |
| Caolinite | Ni(II), Pb(II), Zn(II) | River sediment | n.a. | [132] |
| Bare | Cr(VI) | Groundwater | 5.4 | [133] |
| Bare | Cr(VI) | Groundwater | 5.4 | [134] |
| Bare | Cr(VI) 2 | Groundwater | n.a. | [135] |
| nZVI Cap/Support | Pollutant | pH, Reagent | Adsorption Capacity (mg/g) or Product | Ref. |
|---|---|---|---|---|
| CPC | NO3− | 4–7 | NH4+, N2 | [46] |
| Mg-aminoclay | NO3− | 8.8 | NH4+ | [28] |
| Bare | NO3− | n.a. | NH4+, NO2− | [137] |
| Bare | NO3− | 4 | NH4+ | [73] |
| CMC | NO3− | n.a. | n.a. | [138] |
| Zeolite | NH4+ | 8 | 62.82 | [139] |
| Starch | ClO4− | 7–7.4 | Cl− | [140] |
| CMC | ClO4− | 7–7.4 | Cl− | [140] |
| Bare | ClO4− | 6 | Cl− | [141] |
| Bare | ClO3− | 6 | Cl− | [141] |
| Bare | ClO2− | 6 | Cl− | [141] |
| Bare | ClO− | 6 | Cl− | [141] |
| Fe3O4/γ-Fe2O3 | BrO3− | 3‒7 | Br− | [142] |
| nZVI Cap/Support | Organism | Effect | Cause of Toxicity | Ref. |
|---|---|---|---|---|
| Bare | Vibrio fischeri | No | — | [134] |
| Bare | G+ bacteria | Positive | — | [134] |
| Rhamnolipid | Proteobacteria and Firmicutes | Positive | — | [31] |
| Pectin | Collembola (Folsomia candida) | Negative | n.a. | [30] |
| Pectin | Ostracods (H. incongruens) | Negative | Anoxia | [30] |
| Bare | Escherichia coli | Negative | Reduction | [145] |
| SBA-15 2 | Escherichia coli | Minimal | — | [145] |
| Bare | Bacillus cereus | Negative | Oxidative stress | [146] |
| Bare | Serratia marcescens | Negative | Oxidative stress | [146] |
| Bare | Saccharomyces cerevisiae | Negative | Oxidative stress | [146] |
| Bare | Desmodesmus subspicatus | Negative | Oxidative stress | [146] |
| Sulfide/silica | Chlamydomonas reinhardtii | Lag | — | [147] |
| Bare | Arabidopsis thaliana | No toxic | — | [87] |
| Bare | Medicago sativa | No toxic | — | [148] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasinszki, T.; Krebsz, M. Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects. Nanomaterials 2020, 10, 917. https://doi.org/10.3390/nano10050917
Pasinszki T, Krebsz M. Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects. Nanomaterials. 2020; 10(5):917. https://doi.org/10.3390/nano10050917
Chicago/Turabian StylePasinszki, Tibor, and Melinda Krebsz. 2020. "Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects" Nanomaterials 10, no. 5: 917. https://doi.org/10.3390/nano10050917






