Bi-Magnetic Core-Shell CoFe2O4@MnFe2O4 Nanoparticles for In Vivo Theranostics
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of the Nanoparticles
- Synthesis of single-core nanoparticles.
- Synthesis of core-shell bi-magnetic nanoparticles.
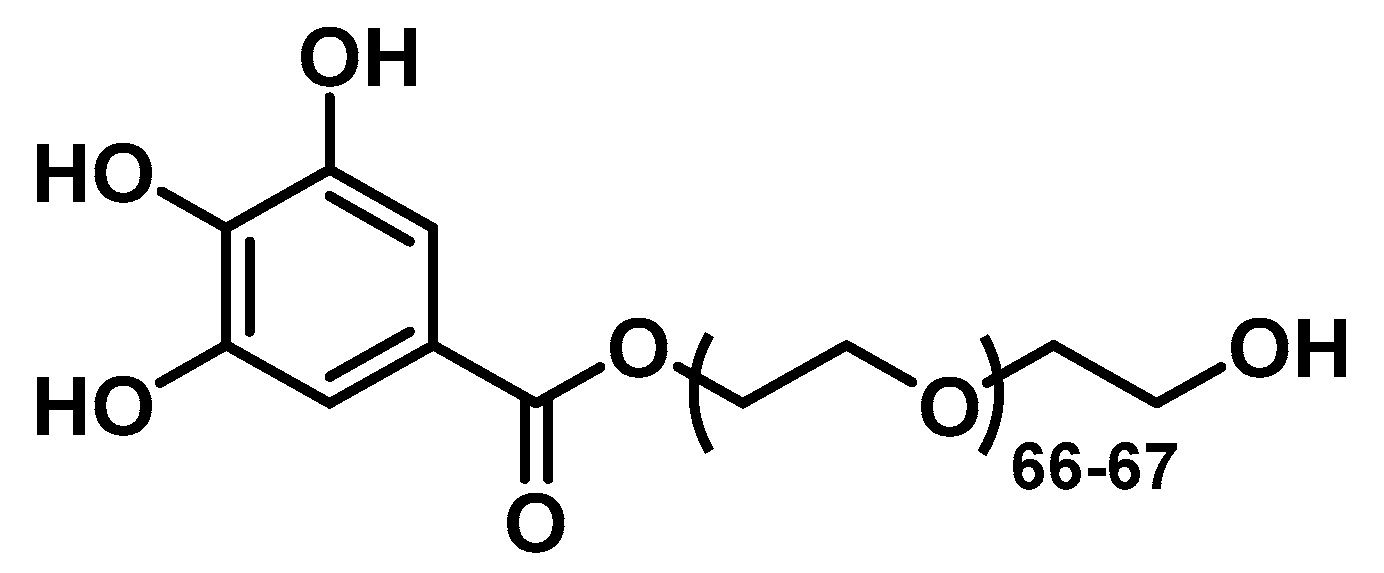
2.3. Synthesis of the PEGylated Ligand
2.4. Functionalization of Bi-Magnetic NPs
2.5. Characterization Methods
- Transmission Electron Microscopy.
- X-ray diffraction patterns (XRD).
- Vibrating Sample Magnetometer (VSM).
- Specific Absorption Rate (SAR).
- Dynamic Light Scattering (DLS).
- Nuclear Magnetic Resonance Spectroscopy (NMR).
- Inductively Coupled Plasma High Resolution Mass Spectroscopy (ICP-HRMS).
- In vitro longitudinal and transversal relaxivities (r1 and r2).
- Cell Culture.
- Cytotoxicity assays.
- In vivo Magnetic Resonance Imaging.
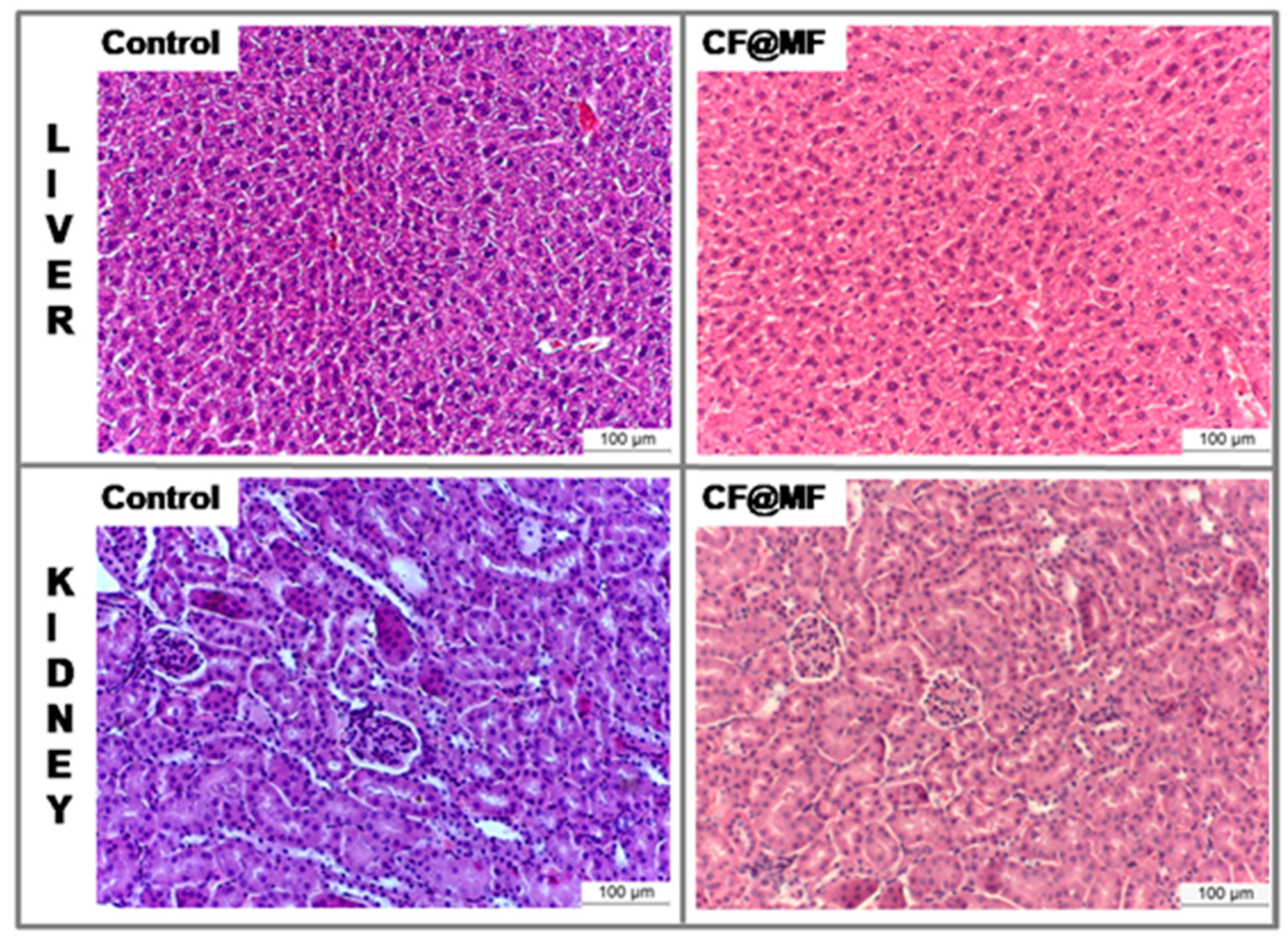
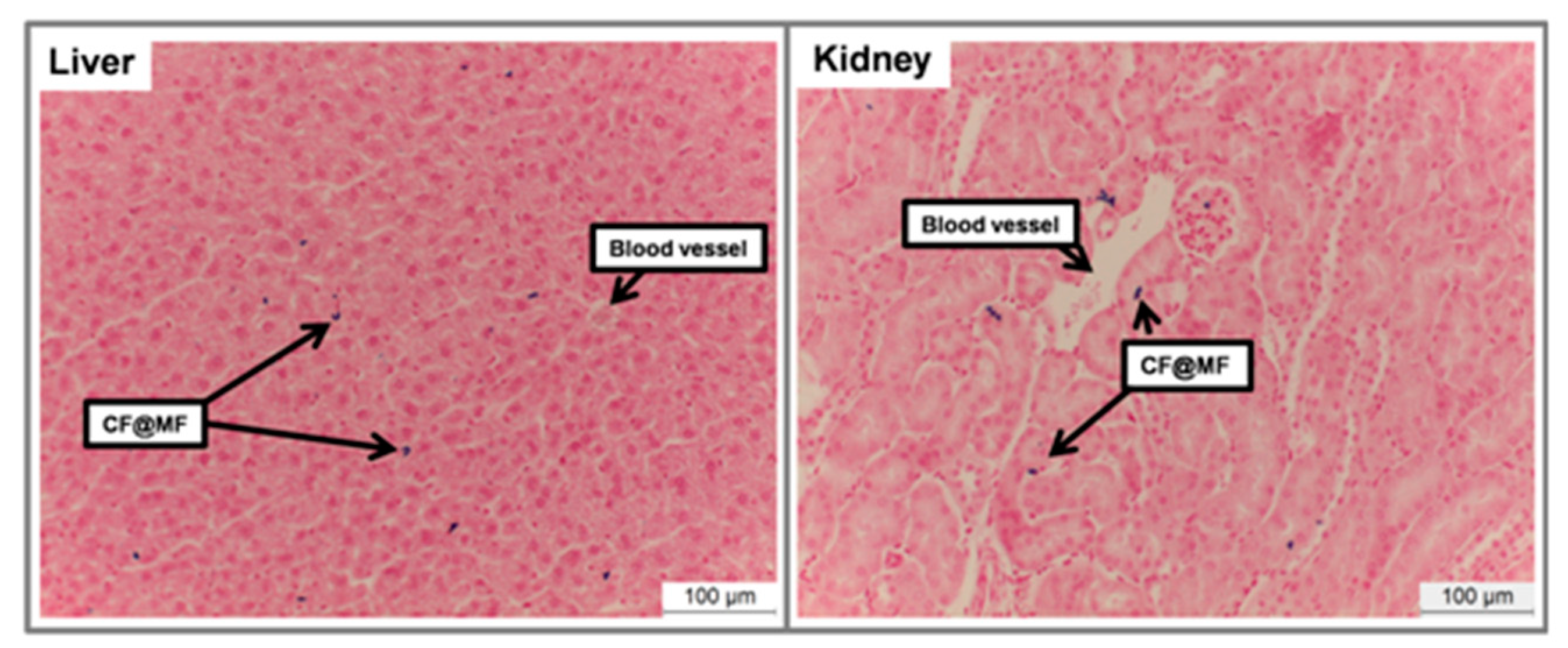
- Histology.
2.6. Statistical Analysis
3. Results and Discussion
3.1. X-ray Diffraction Analysis
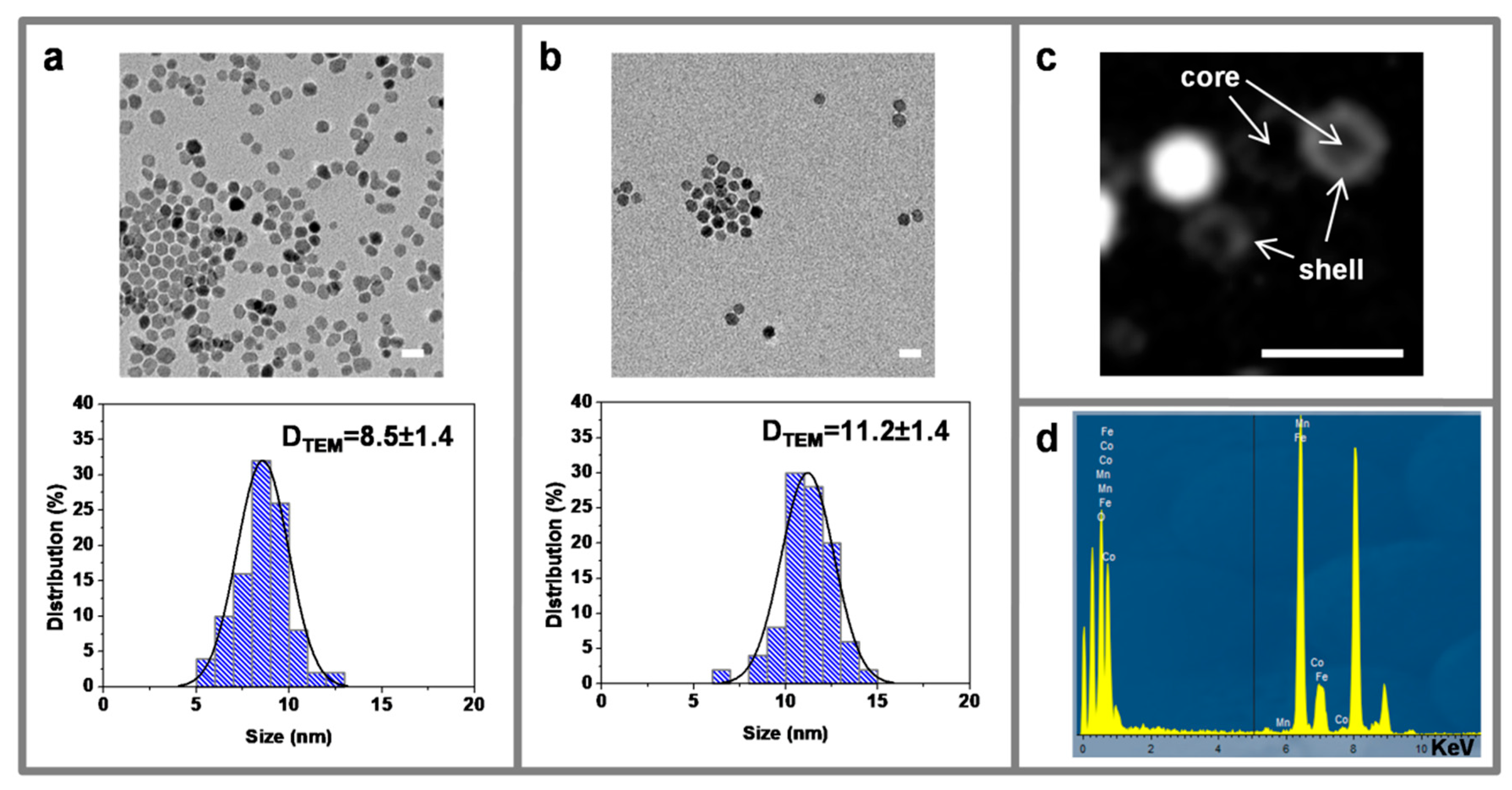
3.2. Transmission Electron Microscopy
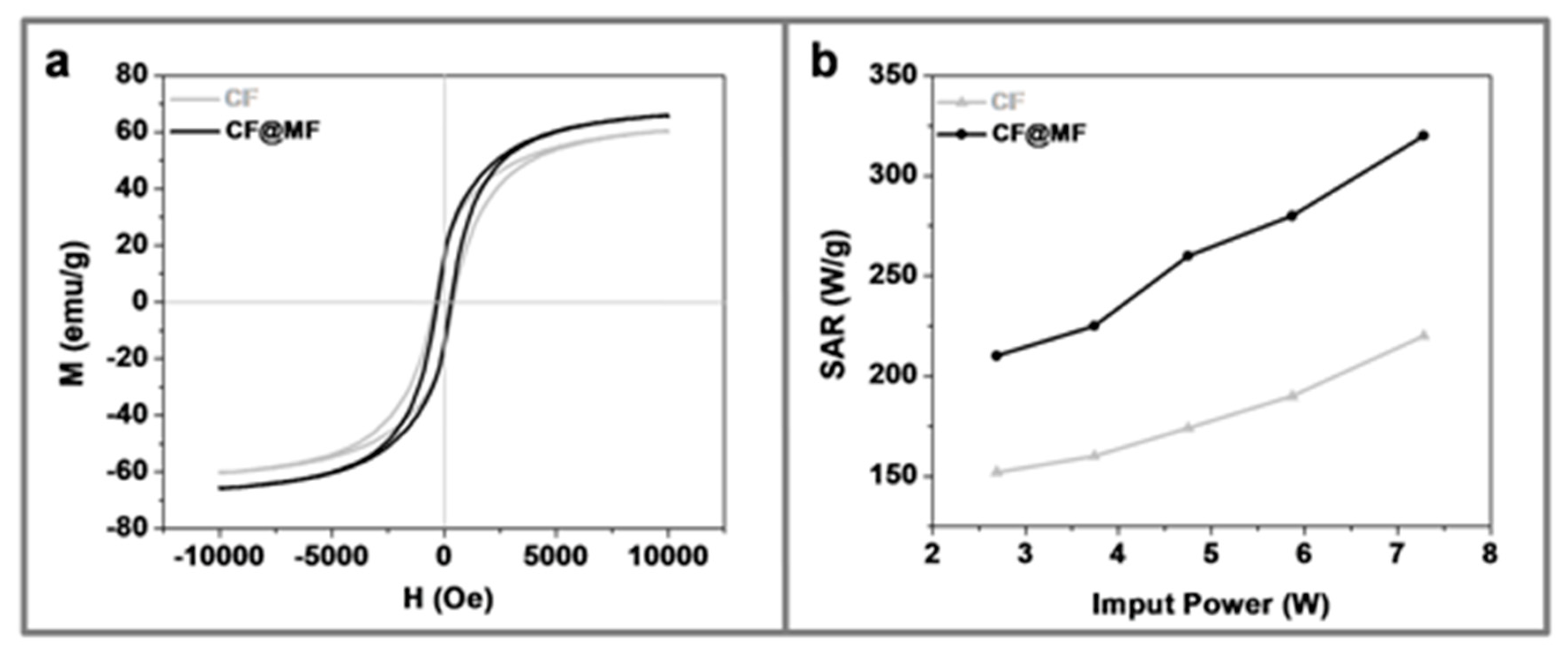
3.3. Magnetic Characterization
3.4. Fourier-Transform Infrared Spectroscopy(FTIR)
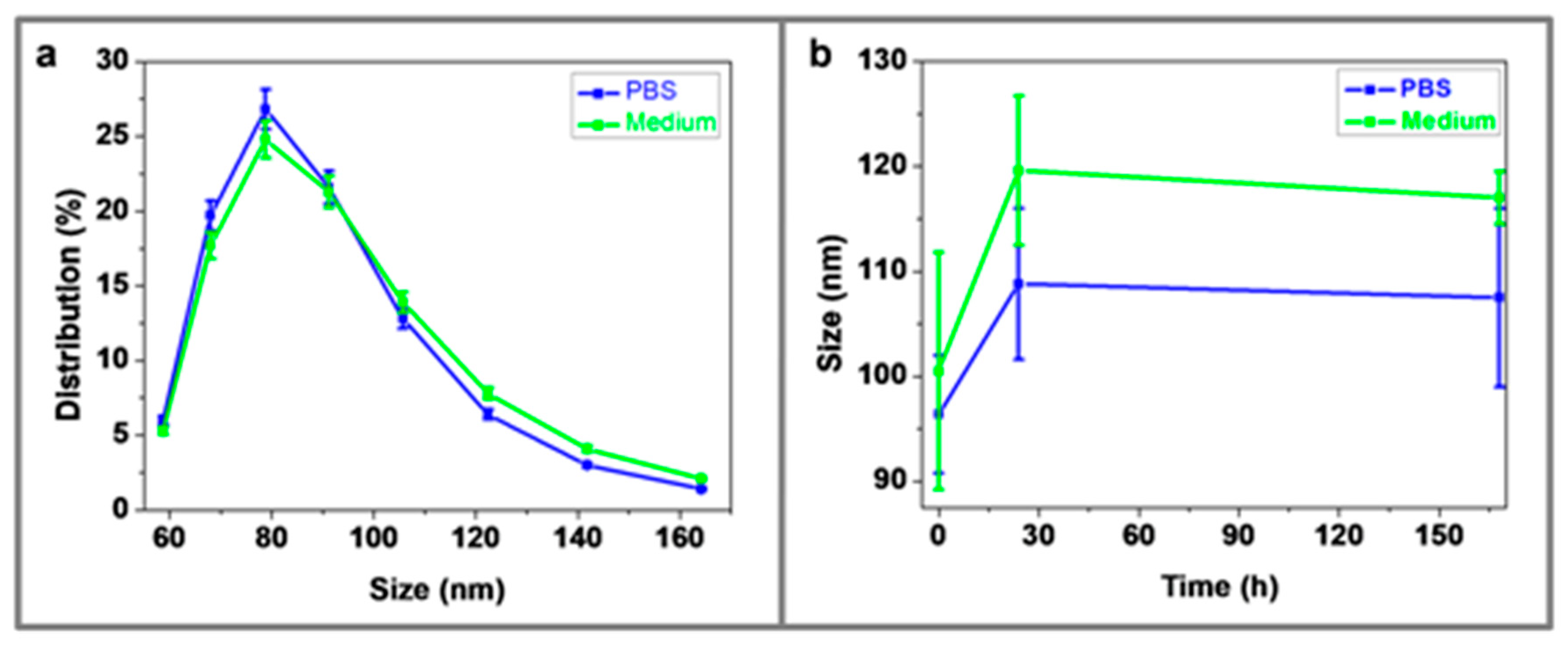
3.5. Dynamic Light Scattering (DLS)
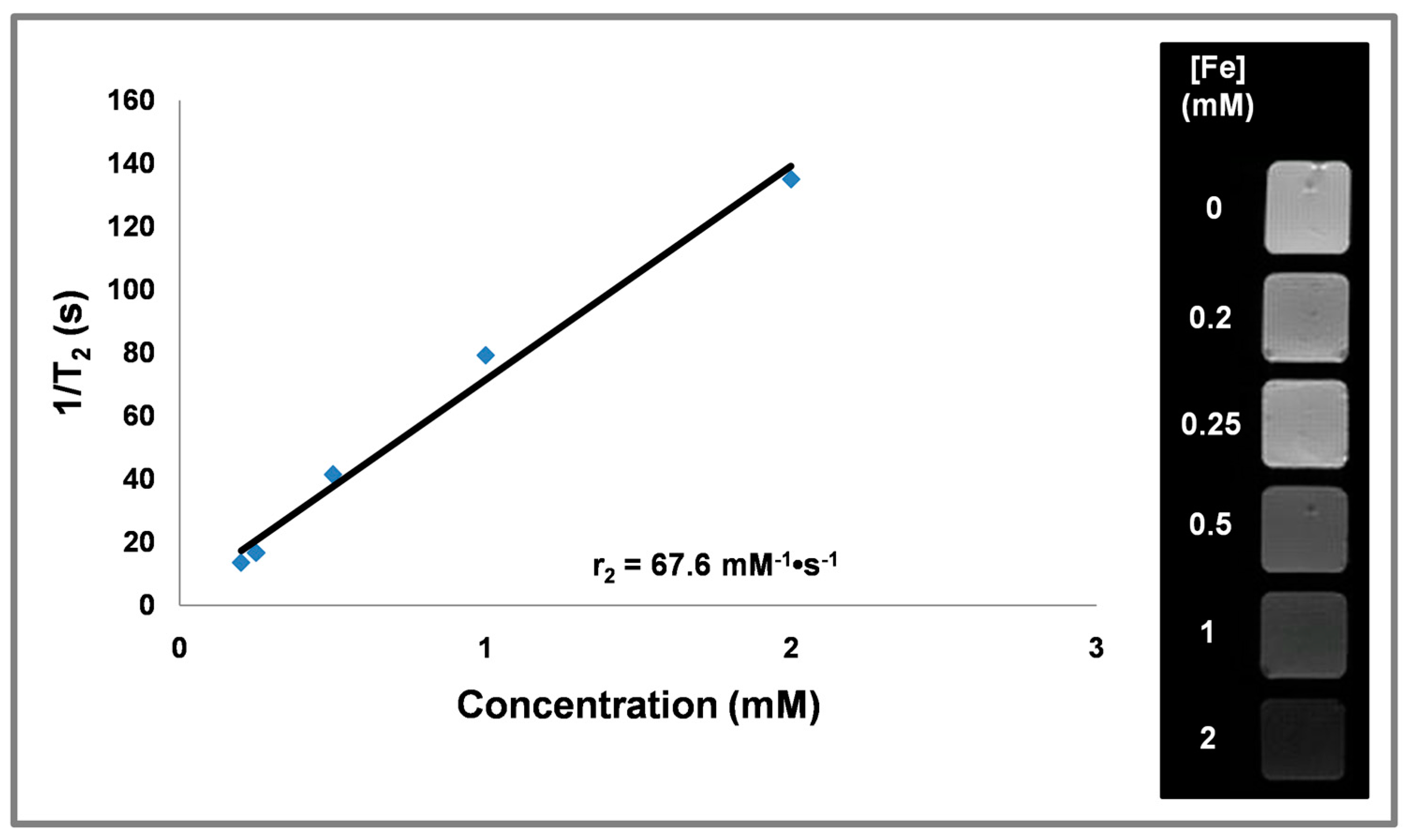
3.6. Magnetic Relaxivity
3.7. Cytotoxicity Assays
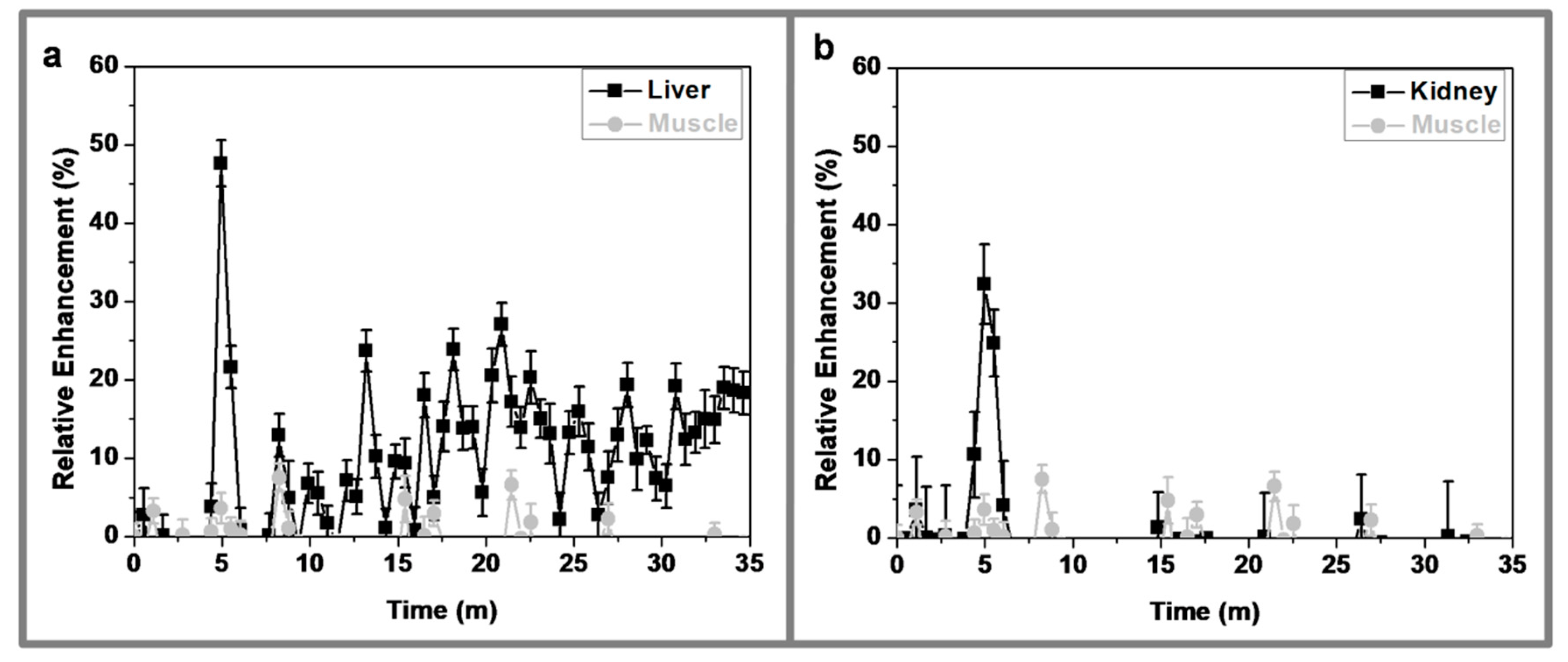
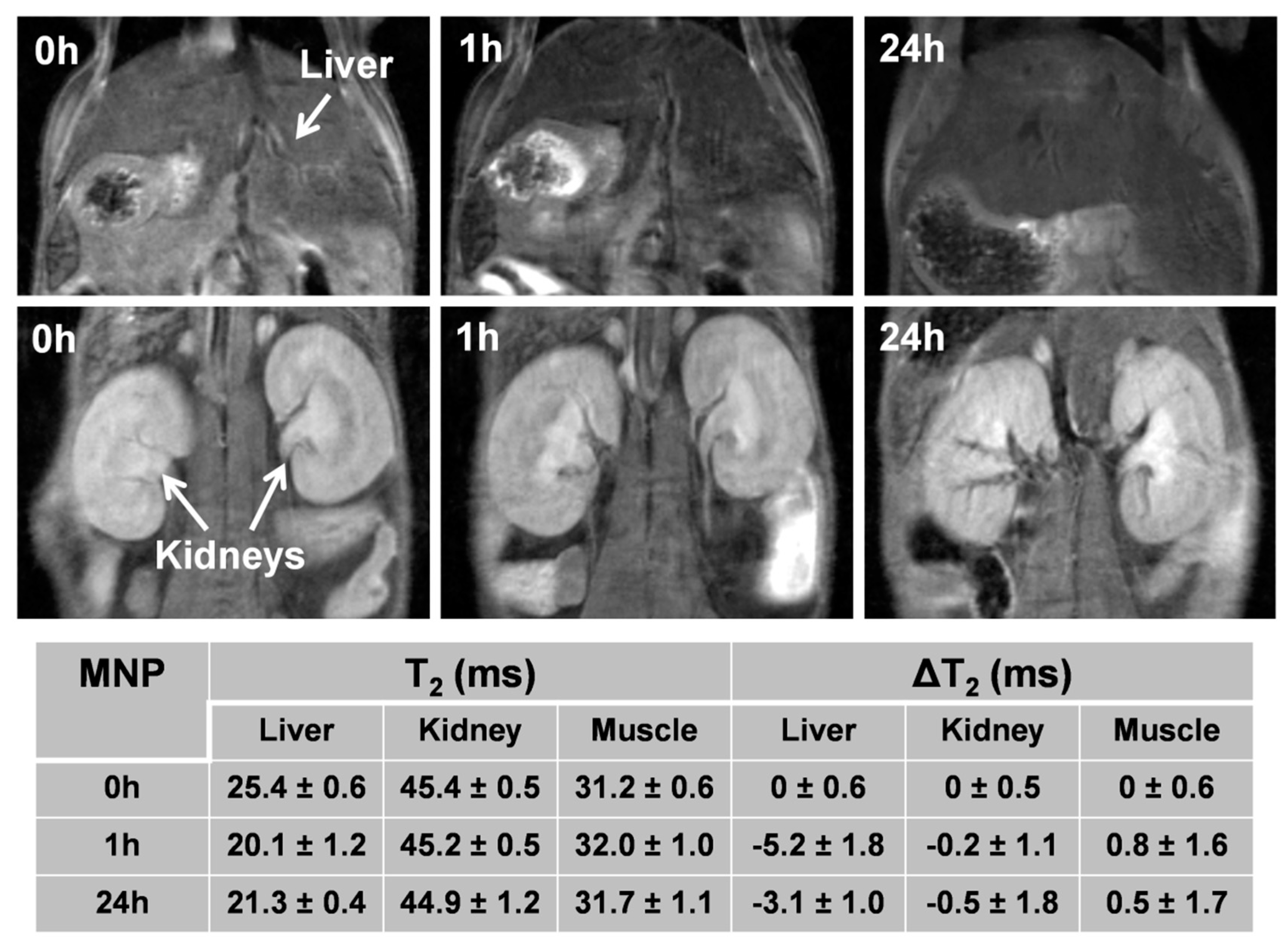
3.8. In Vivo MRI Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Brawley, O.W.; Wender, R.C. Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2017, 67, 100–121. [Google Scholar] [CrossRef]
- Weissleder, R.; Mahmood, U. Molecular imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef]
- Caro, C.; Garcia-Martin, M.L.; Pernia Leal, M. Manganese-Based Nanogels as pH Switches for Magnetic Resonance Imaging. Biomacromolecules 2017, 18, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.-P.; Robinson, L.; Hogg, P. Magnetic resonance imaging contrast agents: Overview and perspectives. Radiography 2007, 13, e5–e19. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Murray, D.L.; Thielen, K.R.; Williamson, E.E.; Eckel, L.J. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015, 275, 772–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Caro, C.; Pozo, D. Polysaccharide Colloids as Smart Vehicles in Cancer Therapy. Curr. Pharm. Des. 2015, 21, 4822–4836. [Google Scholar] [CrossRef]
- Pernia Leal, M.; Munoz-Hernandez, C.; Berry, C.C.; Garcia-Martin, M. In vivo pharmacokinetics of T2 contrast agents based on iron oxide nanoparticles: Optimization of blood circulation times. RSC Adv. 2015, 5, 76883–76891. [Google Scholar] [CrossRef] [Green Version]
- Pernia Leal, M.; Rivera-Fernandez, S.; Franco, J.M.; Pozo, D.; de la Fuente, J.M.; Garcia-Martin, M.L. Long-circulating PEGylated manganese ferrite nanoparticles for MRI-based molecular imaging. Nanoscale 2015, 7, 2050–2059. [Google Scholar] [CrossRef]
- Pernia Leal, M.; Caro, C.; García-Martín, M.L. Shedding light on zwitterionic magnetic nanoparticles: Limitations for in vivo applications. Nanoscale 2017, 9, 8176–8184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, Y.; Shen, J.; Wei, J.; Yu, C.; Kong, B.; Liu, W.; Yang, H.; Yang, S.; Wang, W. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials 2014, 35, 7470–7478. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, K.; Cheng, L.; Zhu, J.; Ma, X.; Xu, H.; Li, Y.; Guo, L.; Gu, H.; Liu, Z. PEGylated FePt@Fe2O3 core-shell magnetic nanoparticles: Potential theranostic applications and in vivo toxicity studies. Nanomedicine 2013, 9, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.A.; Urquiza, E.S.; Presa, P.D.L.; Tobón, S.H.; Pal, U.; Fraijo, P.H.; Yacaman, M.J.; Lozada Ramírez, J.D.; Ivkov, R.; Angulo-Molina, A.; et al. Enhanced magnetic properties and MRI performance of bi-magnetic core–shell nanoparticles. RSC Adv. 2016, 6, 77558–77568. [Google Scholar] [CrossRef]
- Yoon, T.-J.; Lee, H.; Shao, H.; Weissleder, R. Highly Magnetic Core–Shell Nanoparticles with a Unique Magnetization Mechanism. Angew. Chem. Int. Ed. 2011, 50, 4663–4666. [Google Scholar] [CrossRef] [Green Version]
- Aadinath, W.; Ghosh, T.; Anandharamakrishnan, C. Multimodal magnetic nano-carriers for cancer treatment: Challenges and advancements. J. Magn. Magn. Mater. 2016, 401, 1159–1172. [Google Scholar] [CrossRef]
- Hammad, M.; Nica, V.; Hempelmann, R. Synthesis and Characterization of Bi-Magnetic Core/Shell Nanoparticles for Hyperthermia Applications. IEEE Trans. Magn. 2017, 53, 1–6. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Schirra, H.; Thomas, S.; Schmidt, H.; Felix, R. Endocytosis of dextran and silan-coated magnetite nanoparticles and the effect of intracellular hyperthermia on human mammary carcinoma cells in vitro. J. Magn. Magn. Mater. 1999, 194, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Moroz, P.; Jones, S.K.; Gray, B.N. Magnetically mediated hyperthermia: Current status and future directions. Int. J. Hyperth. 2002, 18, 267–284. [Google Scholar] [CrossRef]
- Beola, L.; Asín, L.; Fratila, R.M.; Herrero, V.; de la Fuente, J.M.; Grazú, V.; Gutiérrez, L. Dual Role of Magnetic Nanoparticles as Intracellular Hotspots and Extracellular Matrix Disruptors Triggered by Magnetic Hyperthermia in 3D Cell Culture Models. ACS Appl. Mater. Interfaces 2018, 10, 44301–44313. [Google Scholar] [CrossRef]
- Moros, M.; Idiago-Lopez, J.; Asin, L.; Moreno-Antolin, E.; Beola, L.; Grazu, V.; Fratila, R.M.; Gutierrez, L.; de la Fuente, J.M. Triggering antitumoural drug release and gene expression by magnetic hyperthermia. Adv. Drug Deliv. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Fratila, R.M.; de la Fuente, J.M. Introduction to Hyperthermia. In Nanomaterials for Magnetic and Optical Hyperthermia Applications; Fratila, R.M., De La Fuente, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–10. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, J.T.; Choi, J.S.; Moon, S.H.; Noh, S.H.; Kim, J.W.; Kim, J.G.; Kim, I.S.; Park, K.I.; Cheon, J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol. 2011, 6, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the magnetic properties of nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Mendoza-Garcia, A.; Ning, B.; Sun, S. Cobalt-substituted magnetite nanoparticles and their assembly into ferrimagnetic nanoparticle arrays. Adv. Mater. 2013, 25, 3090–3094. [Google Scholar] [CrossRef]
- Khurshid, H.; Alonso, J.; Nemati, Z.; Phan, M.H.; Mukherjee, P.; Fdez-Gubieda, M.L.; Barandiarán, J.M.; Srikanth, H. Anisotropy effects in magnetic hyperthermia: A comparison between spherical and cubic exchange-coupled FeO/Fe3O4 nanoparticles. J. Appl. Phys. 2015, 117, 17A337. [Google Scholar] [CrossRef]
- Phadatare, M.R.; Meshram, J.V.; Gurav, K.V.; Kim, J.H.; Pawar, S.H. Enhancement of specific absorption rate by exchange coupling of the core–shell structure of magnetic nanoparticles for magnetic hyperthermia. J. Phys. D Appl. Phys. 2016, 49, 095004. [Google Scholar] [CrossRef]
- Dippong, T.; Toloman, D.; Levei, E.-A.; Cadar, O.; Mesaros, A. A possible formation mechanism and photocatalytic properties of CoFe2O4/PVA-SiO2 nanocomposites. Thermochim. Acta 2018, 666, 103–115. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O.; Deac, I.G.; Diamandescu, L.; Barbu-Tudoran, L. Effect of nickel content on structural, morphological and magnetic properties of NixCo1-xFe2O4/SiO2 nanocomposites. J. Alloys Compd. 2019, 786, 330–340. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Carta, D.; Casula, M.F.; Floris, P.; Falqui, A.; Mountjoy, G.; Boni, A.; Sangregorio, C.; Corrias, A. Synthesis and microstructure of manganese ferrite colloidal nanocrystals. Phys. Chem. Chem. Phys. 2010, 12, 5074–5083. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, Z.J. Controlled Synthesis and Magnetic Properties of Bimagnetic Spinel Ferrite CoFe2O4 and MnFe2O4 Nanocrystals with Core–Shell Architecture. J.Am. Chem. Soc. 2012, 134, 10182–10190. [Google Scholar] [CrossRef]
- Moon, S.H.; Noh, S.-H.; Lee, J.-H.; Shin, T.-H.; Lim, Y.; Cheon, J. Ultrathin Interface Regime of Core–Shell Magnetic Nanoparticles for Effective Magnetism Tailoring. Nano Lett. 2017, 17, 800–804. [Google Scholar] [CrossRef] [PubMed]
- López-Ortega, A.; Estrader, M.; Salazar-Alvarez, G.; Roca, A.G.; Nogués, J. Applications of exchange coupled bi-magnetic hard/soft and soft/hard magnetic core/shell nanoparticles. Phys. Rep. 2015, 553, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Nikam, D.S.; Jadhav, S.V.; Khot, V.M.; Ningthoujam, R.S.; Hong, C.K.; Mali, S.S.; Pawar, S.H. Colloidal stability of polyethylene glycol functionalized Co0.5Zn0.5Fe2O4 nanoparticles: Effect of pH, sample and salt concentration for hyperthermia application. RSC Adv. 2014, 4, 12662–12671. [Google Scholar] [CrossRef]
- Caro, C.; Dalmases, M.; Figuerola, A.; Garcia-Martin, M.L.; Leal, M.P. Highly water-stable rare ternary Ag-Au-Se nanocomposites as long blood circulation time X-ray computed tomography contrast agents. Nanoscale 2017, 9, 7242–7251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedinger, A.; Pernia Leal, M.; Deka, S.R.; George, C.; Franchini, I.R.; Falqui, A.; Cingolani, R.; Pellegrino, T. “nanohybrids” based on pH-responsive hydrogels and inorganic nanoparticles for drug delivery and sensor applications. Nano Lett. 2011, 11, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Pichon, B.P.; Gerber, O.; Lefevre, C.; Florea, I.; Fleutot, S.; Baaziz, W.; Pauly, M.; Ohlmann, M.; Ulhaq, C.; Ersen, O.; et al. Microstructural and Magnetic Investigations of Wüstite-Spinel Core-Shell Cubic-Shaped Nanoparticles. Chem. Mater. 2011, 23, 2886–2900. [Google Scholar] [CrossRef]
- Ungár, T. Characterization of nanocrystalline materials by X-ray line profile analysis. J. Mater. Sci. 2007, 42, 1584–1593. [Google Scholar] [CrossRef]
- Knappett, B.R.; Abdulkin, P.; Ringe, E.; Jefferson, D.A.; Lozano-Perez, S.; Rojas, T.C.; Fernández, A.; Wheatley, A.E.H. Characterisation of Co@Fe3O4 core@shell nanoparticles using advanced electron microscopy. Nanoscale 2013, 5, 5765–5772. [Google Scholar] [CrossRef] [Green Version]
- Hammad, M.; Nica, V.; Hempelmann, R. On-command controlled drug release by diels-Alder reaction using Bi-magnetic core/shell nano-carriers. Colloids Surf. B Biointerfaces 2017, 150, 15–22. [Google Scholar] [CrossRef]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Nica, V.; Hempelmann, R. On–off switch-controlled doxorubicin release from thermo- and pH-responsive coated bimagnetic nanocarriers. J. Nanopart. Res. 2016, 18, 234. [Google Scholar] [CrossRef]
- Hammad, M.; Hempelmann, R. Enhanced specific absorption rate of bi-magnetic nanoparticles for heating applications. Mater. Chem. Phys. 2017, 188, 30–38. [Google Scholar] [CrossRef]
- Peter, A.I.; Naidu, E.C.; Akang, E.; Ogedengbe, O.O.; Offor, U.; Rambharose, S.; Kalhapure, R.; Chuturgoon, A.; Govender, T.; Azu, O.O. Investigating Organ Toxicity Profile of Tenofovir and Tenofovir Nanoparticle on the Liver and Kidney: Experimental Animal Study. Toxicol. Res. 2018, 34, 221–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
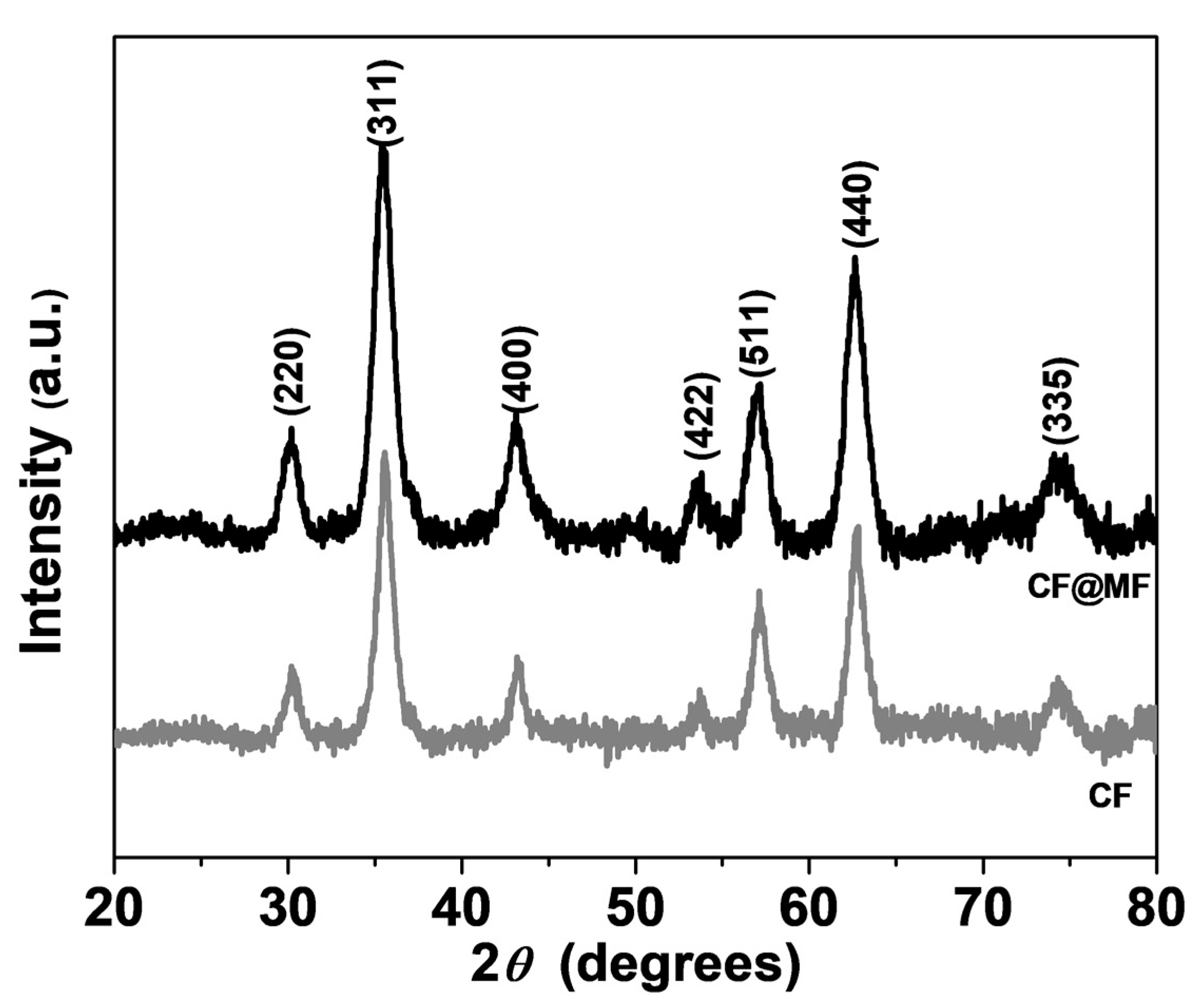
| Sample | <d> (nm) | a (Å) | ε |
|---|---|---|---|
| CF | 9.8(7) | 8.3832(4) | 6.55 × 10−5 |
| CF@MF | 10.4(8) | 8.3961(2) | 7.16 × 10−4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nica, V.; Caro, C.; Páez-Muñoz, J.M.; Leal, M.P.; Garcia-Martin, M.L. Bi-Magnetic Core-Shell CoFe2O4@MnFe2O4 Nanoparticles for In Vivo Theranostics. Nanomaterials 2020, 10, 907. https://doi.org/10.3390/nano10050907
Nica V, Caro C, Páez-Muñoz JM, Leal MP, Garcia-Martin ML. Bi-Magnetic Core-Shell CoFe2O4@MnFe2O4 Nanoparticles for In Vivo Theranostics. Nanomaterials. 2020; 10(5):907. https://doi.org/10.3390/nano10050907
Chicago/Turabian StyleNica, Valentin, Carlos Caro, Jose Maria Páez-Muñoz, Manuel Pernia Leal, and Maria Luisa Garcia-Martin. 2020. "Bi-Magnetic Core-Shell CoFe2O4@MnFe2O4 Nanoparticles for In Vivo Theranostics" Nanomaterials 10, no. 5: 907. https://doi.org/10.3390/nano10050907
APA StyleNica, V., Caro, C., Páez-Muñoz, J. M., Leal, M. P., & Garcia-Martin, M. L. (2020). Bi-Magnetic Core-Shell CoFe2O4@MnFe2O4 Nanoparticles for In Vivo Theranostics. Nanomaterials, 10(5), 907. https://doi.org/10.3390/nano10050907







