Reagent-Free Colorimetric Assay for Galactose Using Agarose Gel Entrapping Nanoceria and Galactose Oxidase
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Agarose Gel Composites Entrapping Nanoceria Only (Agarose_Nanoceria) and Both Gal Ox and Nanoceria (Agarose_Nanoceria + Gal Ox)
2.3. Determination of H2O2 Using Agarose_Nanoceria
2.4. Determination of Galactose Using Agarose_Nanoceria + Gal Ox
3. Results and Discussion
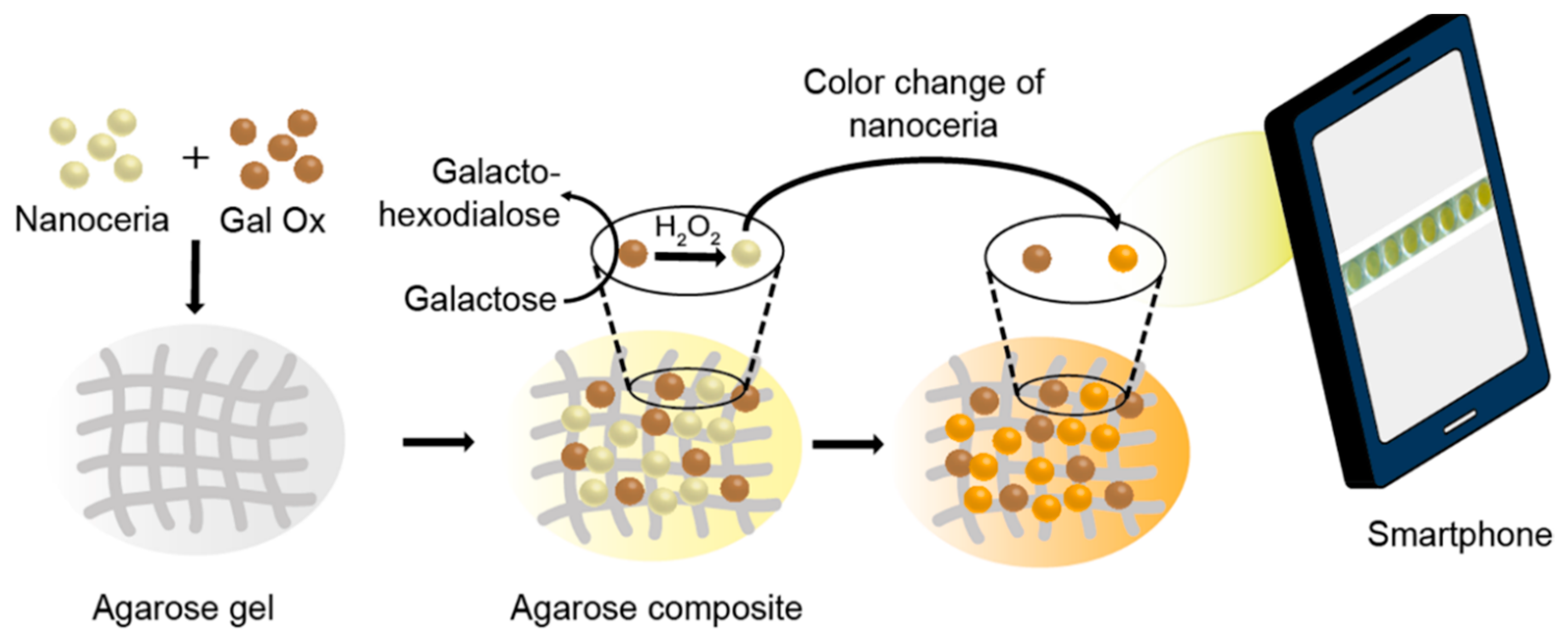
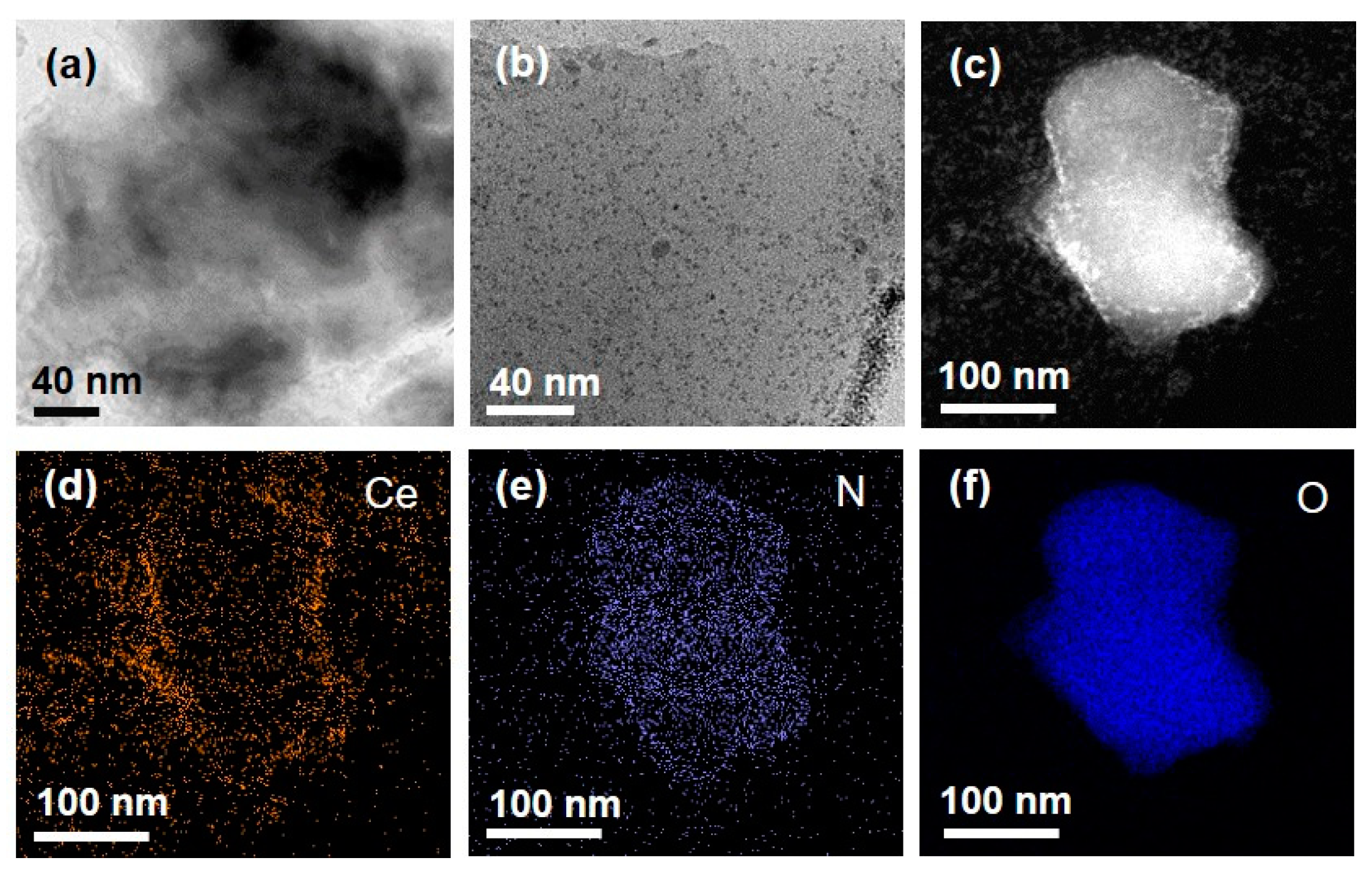
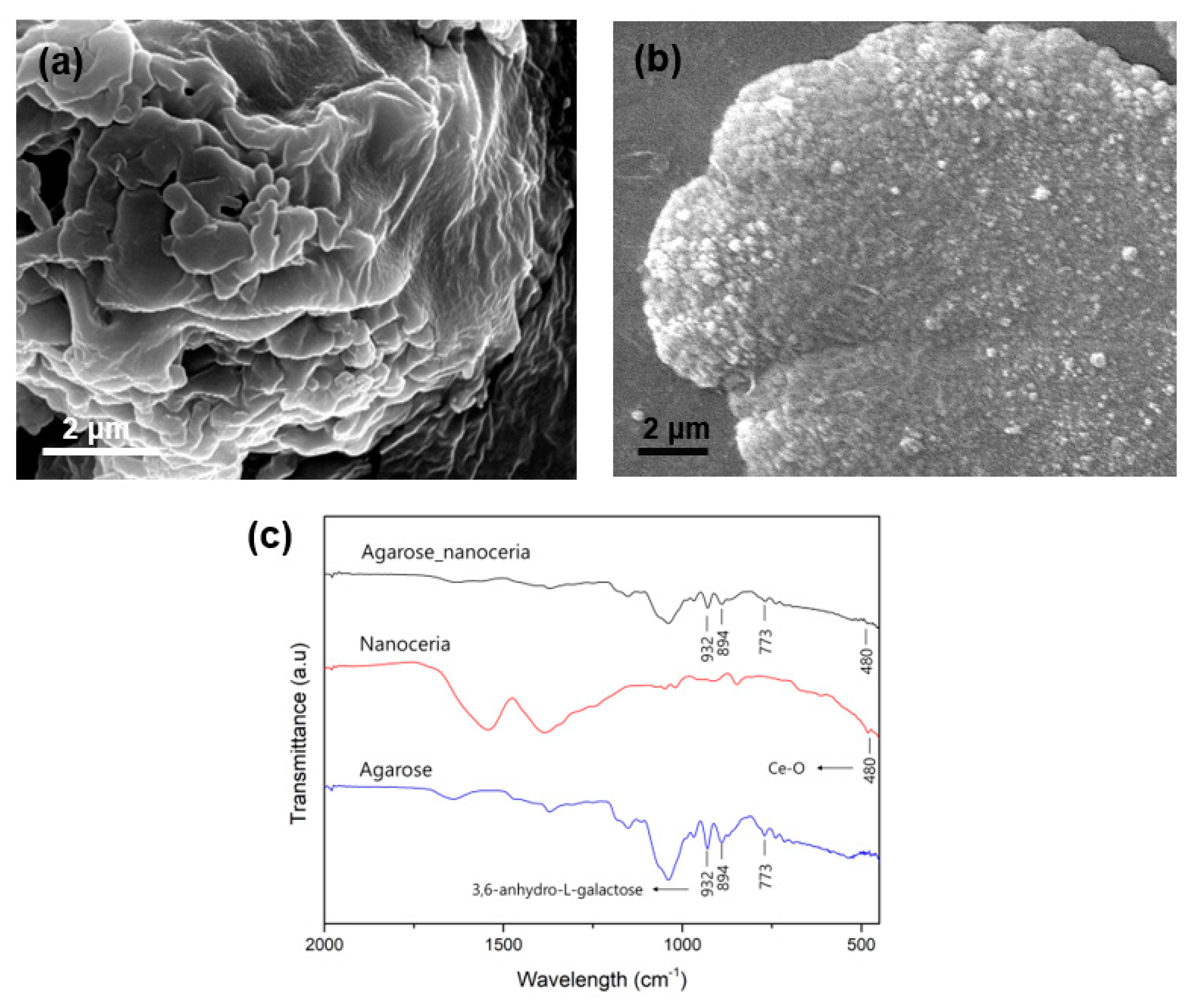
3.1. Construction of Agarose Composite for Colorimetric Determination of Galactose
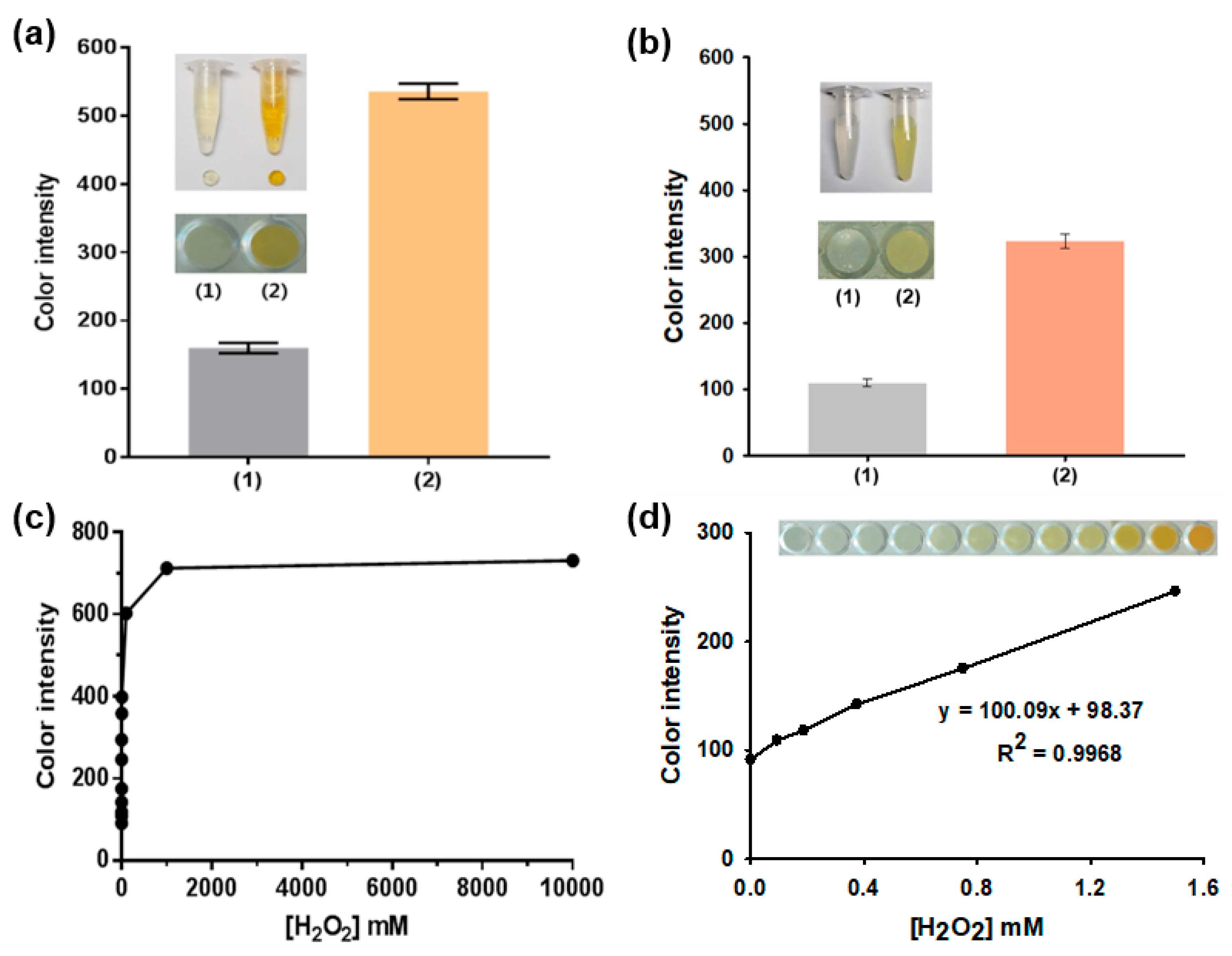
3.2. Vivid Colorimetric Responses of Agarose_Nanoceria toward H2O2
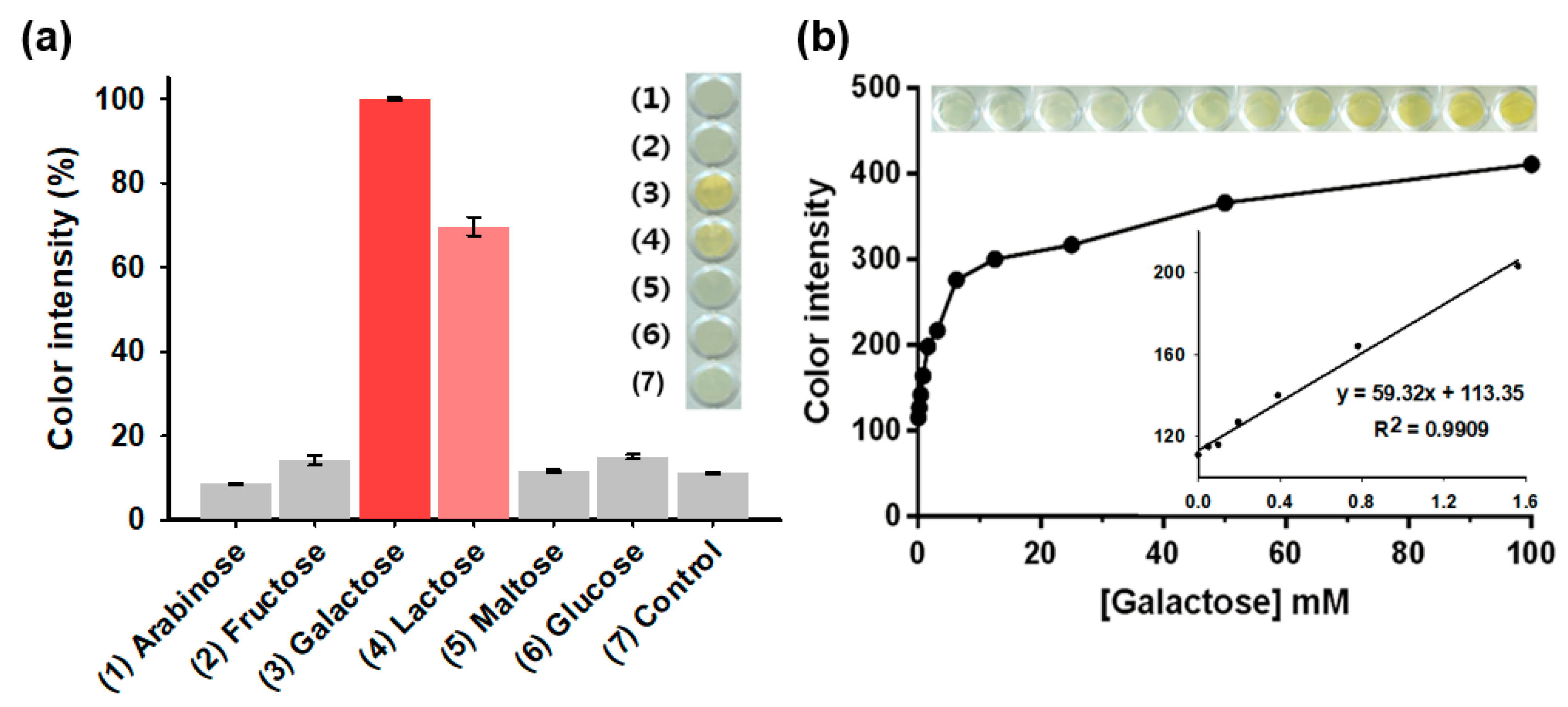
3.3. Analytical Capabilities of the Agarose Composite: Specificity, Linearity, Sensitivity, and Precision for Galactose Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Weese, S.J.; Gosnell, K.; West, P.; Gropper, S.S. Galactose content of baby food meats: Considerations for infants with galactosemia. J. Am. Diet. Assoc. 2003, 103, 373–375. [Google Scholar] [PubMed]
- Holton, J. Galactose disorders: An overview. J. Inherit. Metab. Dis. 1990, 13, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Holden, H.M.; Rayment, I.; Thoden, J.B. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J. Biol. Chem. 2003, 278, 43885–43888. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-S.; Kwon, H.-J.; Yoon, H.-R.; Lee, Y.-M.; Choi, T.-Y.; Hong, S.-P. A pulsed amperometric detection method of galactose 1-phosphate for galactosemia diagnosis. Anal. Biochem. 2008, 376, 200–205. [Google Scholar] [CrossRef]
- Bosch, A.M. Classical galactosaemia revisited. J. Inherit. Metab. Dis. 2006, 29, 516–525. [Google Scholar] [CrossRef]
- Bennett, M.J. Galactosemia diagnosis gets an upgrade. Clin. Chem. 2010, 56, 690–692. [Google Scholar] [CrossRef][Green Version]
- Paigen, K.; Pacholec, F.; Levy, H.L. A new method of screening for inherited disorders of galactose metabolism. J. Lab. Clin. Med. 1982, 99, 895–907. [Google Scholar]
- Woo, M.-A.; Kim, M.I.; Cho, D.; Park, H.G. Cell-based galactosemia diagnosis system based on a galactose assay using a bioluminescent Escherichia coli array. Anal. Chem. 2013, 85, 11083–11089. [Google Scholar] [CrossRef]
- Hu, O.Y.-P.; Hu, T.-M.; Tang, H.-S. Determination of galactose in human blood by high-performance liquid chromatography: Comparison with an enzymatic method and application to the pharmacokinetic study of galactose in patients with liver dysfunction. J. Pharm. Sci. 1995, 84, 231–235. [Google Scholar]
- Nishimura, Y.; Tajima, G.; Bahagia, A.D.; Sakamoto, A.; Ono, H.; Sakura, N.; Naito, K.; Hamakawa, M.; Yoshii, C.; Kubota, M. Differential diagnosis of neonatal mild hypergalactosaemia detected by mass screening: Clinical significance of portal vein imaging. J. Inherit. Metab. Dis. 2004, 27, 11–18. [Google Scholar] [CrossRef]
- Ning, C.; Segal, S. Plasma galactose and galactitol concentration in patients with galactose-1-phosphate uridyltransferase deficiency galactosemia: Determination by gas chromatography/mass spectrometry. Metabolism 2000, 49, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Diepenbrock, F.; Heckler, R.; Schickling, H.; Engelhard, T.; Bock, D.; Sander, J. Colorimetric determination of galactose and galactose-1-phosphate from dried blood. Clin. Biochem. 1992, 25, 37–39. [Google Scholar] [CrossRef]
- Kim, M.I.; Shim, J.; Li, T.; Woo, M.-A.; Cho, D.; Lee, J.; Park, H.G. Colorimetric quantification of galactose using a nanostructured multi-catalyst system entrapping galactose oxidase and magnetic nanoparticles as peroxidase mimetics. Analyst 2012, 137, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Kanyong, P.; Krampa, F.D.; Aniweh, Y.; Awandare, G.A. Enzyme-based amperometric galactose biosensors: A review. Microchim. Acta 2017, 184, 3663–3671. [Google Scholar] [CrossRef]
- Kanyong, P.; Pemberton, R.M.; Jackson, S.K.; Hart, J.P. Amperometric screen-printed galactose biosensor for cell toxicity applications. Anal. Lett. 2016, 49, 236–244. [Google Scholar] [CrossRef]
- Sharma, S.K.; Singhal, R.; Malhotra, B.D.; Sehgal, N.; Kumar, A. Langmuir-Blodgett film based biosensors for estimation of galactose in milk. Electrochim. Acta 2004, 49, 2479–2485. [Google Scholar] [CrossRef]
- Kim, D.H.; Hur, J.; Park, H.G.; Kim, M.I. Reagentless colorimetric biosensing platform based on nanoceria within an agarose gel matrix. Biosens. Bioelectron. 2017, 93, 226–233. [Google Scholar] [CrossRef]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef]
- Scholes, F.H.; Soste, C.; Hughes, A.E.; Hardin, S.G.; Curtis, P.R. The role of hydrogen peroxide in the deposition of cerium-based conversion coatings. Appl. Surf. Sci. 2006, 253, 1770–1780. [Google Scholar] [CrossRef]
- Feng, X.; Sayle, D.C.; Wang, Z.L.; Paras, M.S.; Santora, B.; Sutorik, A.C.; Sayle, T.X.; Yang, Y.; Ding, Y.; Wang, X.; et al. Converting ceria polyhedral nanoparticles into single-crystal nanospheres. Science 2006, 312, 1504–1508. [Google Scholar] [CrossRef]
- Prasad, K.; Mehta, G.; Meena, R.; Siddhanta, A.K. Hydrogel-forming agar-graft-PVP and κ-carrageenan-graft-PVP blends: Rapid synthesis and characterization. J. Appl. Polym. Sci. 2006, 102, 3654–3663. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Rao, K.S.; Kumar, A. Facile preparation of agarose–chitosan hybrid materials and nanocomposite ionogels using an ionic liquid via dissolution, regeneration and sol–gel transition. Green Chem. 2014, 16, 320–330. [Google Scholar] [CrossRef]
- Das, J.; Han, J.W.; Choi, Y.-J.; Song, H.; Cho, S.-G.; Park, C.; Seo, H.G.; Kim, J.-H. Cationic lipid-nanoceria hybrids, a novel nonviral vector-mediated gene delivery into mammalian cells: Investigation of the cellular uptake mechanism. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- McDevitt, N.T.; Baun, W.L. Infrared absorption study of metal oxides in the low frequency region (700-240 cm−1). Spectrochim. Acta 1964, 20, 799–808. [Google Scholar] [CrossRef]
- Ford, J.D.; Haworth, J.C. The estimation of galactose in plasma using galactose oxidase. Clin. Chem. 1964, 10, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.U.; Song, S.; Kim, S.; Sim, S.J. A shape-code nanoplasmonic biosensor for multiplex detection of Alzheimer’s disease biomarkers. Biosens. Bioelectron. 2018, 101, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Cho, H.J.; Lee, D.H.; Song, J.H. Galactosemia detected by meonatal screening test. J. Korean Pediatr. Soc. 2003, 46, 440–446. [Google Scholar]
- Jensen, U.G.; Brandt, N.J.; Christensen, E.; Skovby, F.; Nørgaard-Pedersen, B.; Simonsen, H. Neonatal screening for galactosemia by quantitative analysis of hexose monophosphates using tandem mass spectrometry: A retrospective study. Clin. Chem. 2001, 47, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
| Original Amount (mM) | Added Galactose Concentration (mM) | Expected Galactose Concentration (mM) | Measured a Galactose Concentration (mM) | SD b | CV c (%) | Recovery d (%) | |
|---|---|---|---|---|---|---|---|
| Normal | 0.060 | 0.139 | 0.199 | 0.188 | 0.012 | 6.38 | 94.47 |
| Boundary | 0.589 | 0.649 | 0.687 | 0.033 | 4.80 | 105.86 | |
| High | 1.333 | 1.393 | 1.377 | 0.095 | 6.90 | 98.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, P.T.; Ahn, H.T.; Kim, M.I. Reagent-Free Colorimetric Assay for Galactose Using Agarose Gel Entrapping Nanoceria and Galactose Oxidase. Nanomaterials 2020, 10, 895. https://doi.org/10.3390/nano10050895
Nguyen PT, Ahn HT, Kim MI. Reagent-Free Colorimetric Assay for Galactose Using Agarose Gel Entrapping Nanoceria and Galactose Oxidase. Nanomaterials. 2020; 10(5):895. https://doi.org/10.3390/nano10050895
Chicago/Turabian StyleNguyen, Phuong Thy, Hee Tae Ahn, and Moon Il Kim. 2020. "Reagent-Free Colorimetric Assay for Galactose Using Agarose Gel Entrapping Nanoceria and Galactose Oxidase" Nanomaterials 10, no. 5: 895. https://doi.org/10.3390/nano10050895
APA StyleNguyen, P. T., Ahn, H. T., & Kim, M. I. (2020). Reagent-Free Colorimetric Assay for Galactose Using Agarose Gel Entrapping Nanoceria and Galactose Oxidase. Nanomaterials, 10(5), 895. https://doi.org/10.3390/nano10050895







