Black-Si as a Photoelectrode
Abstract
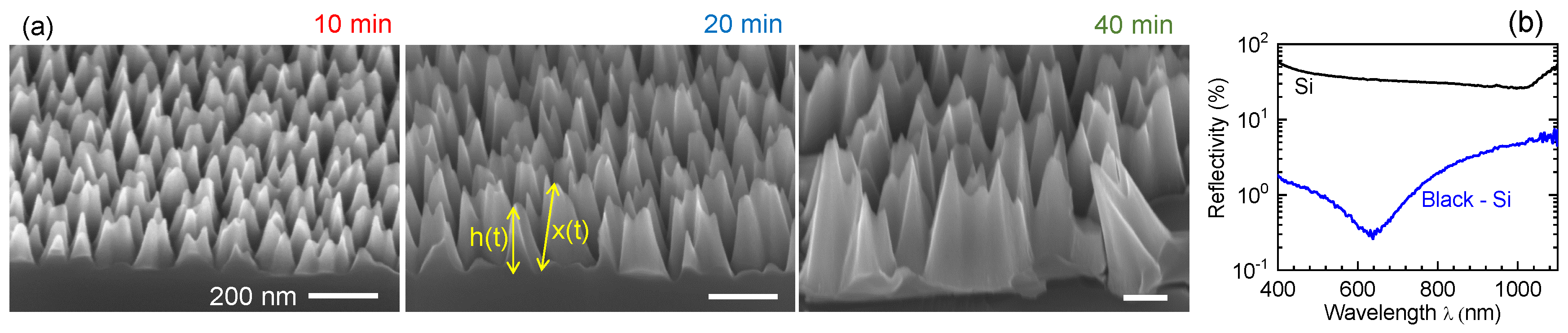
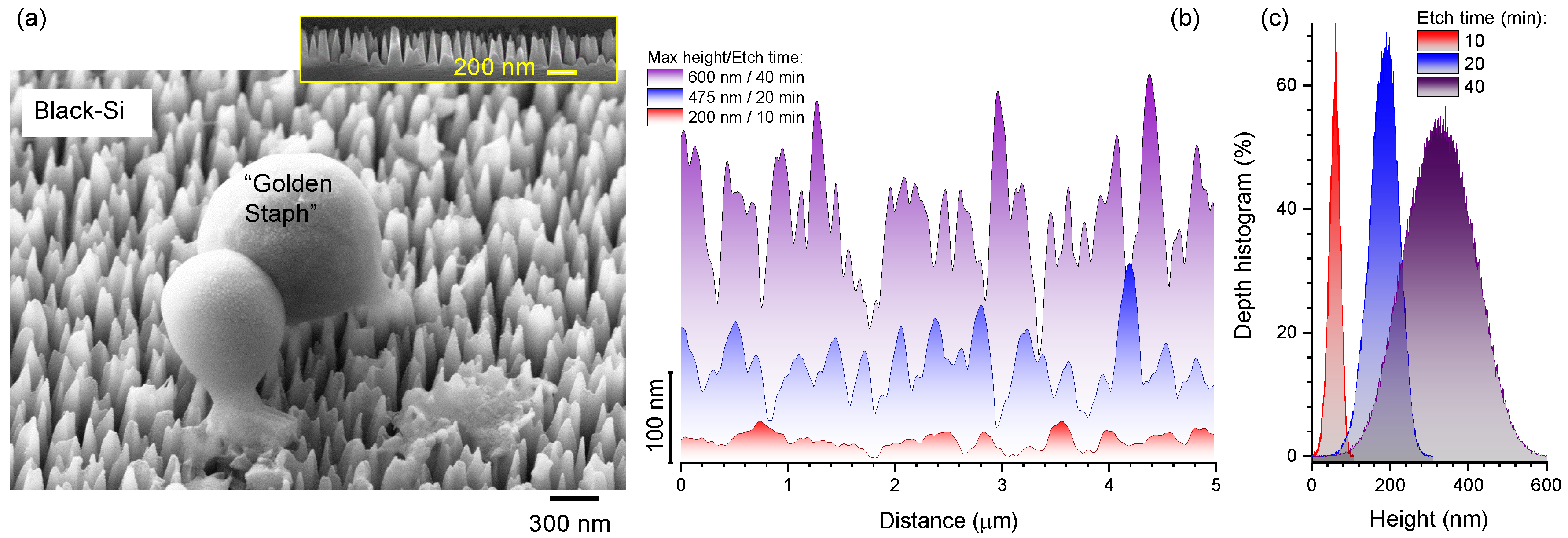
1. Introduction
2. Experimental
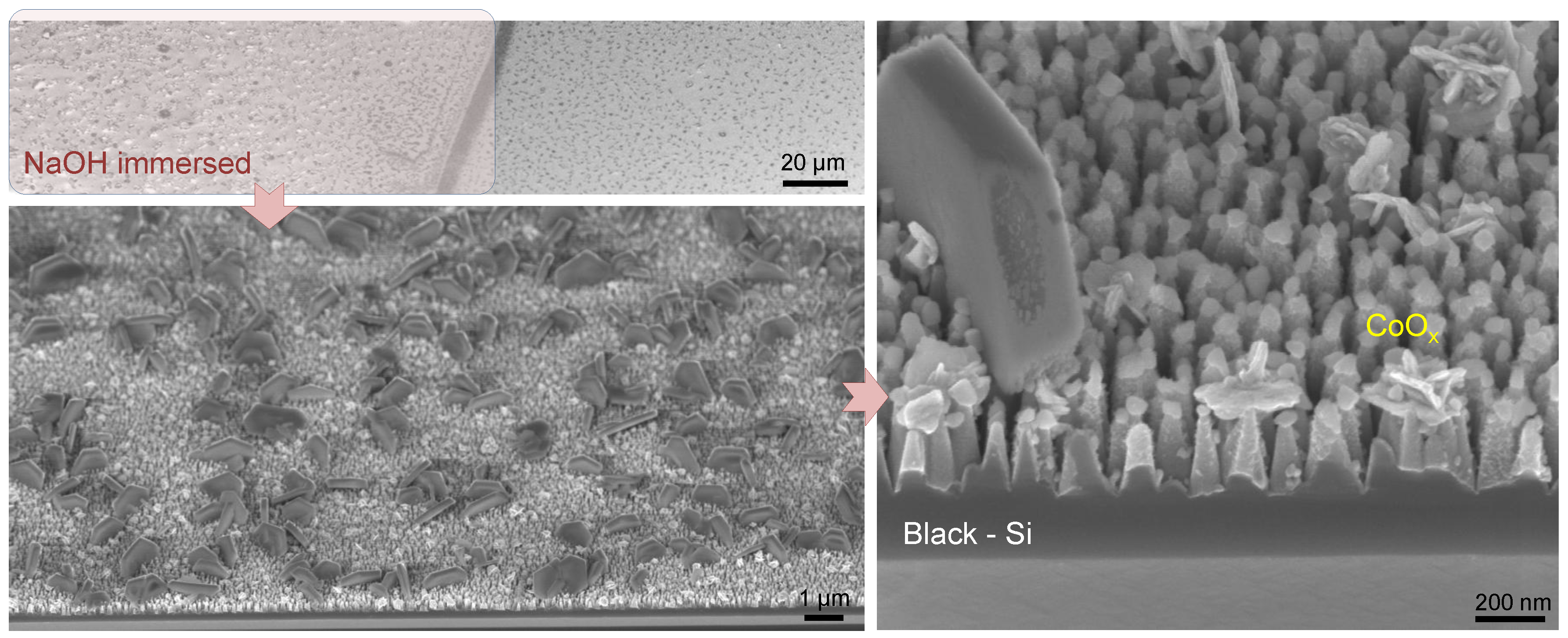
2.1. Fabrication of Photoanodes
2.2. TiO2 and CoO Cocatalyst Deposition
2.3. PEC Measurements
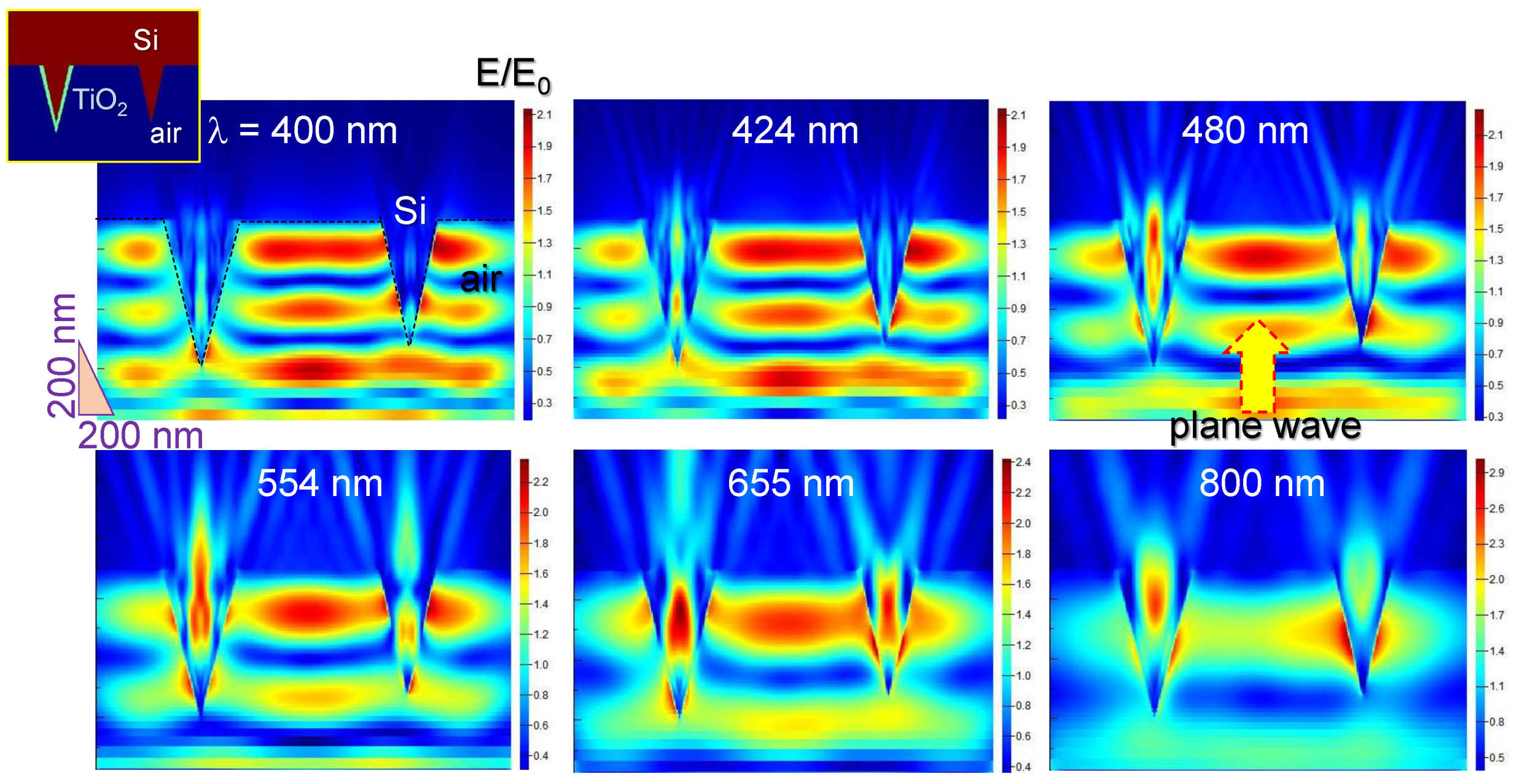
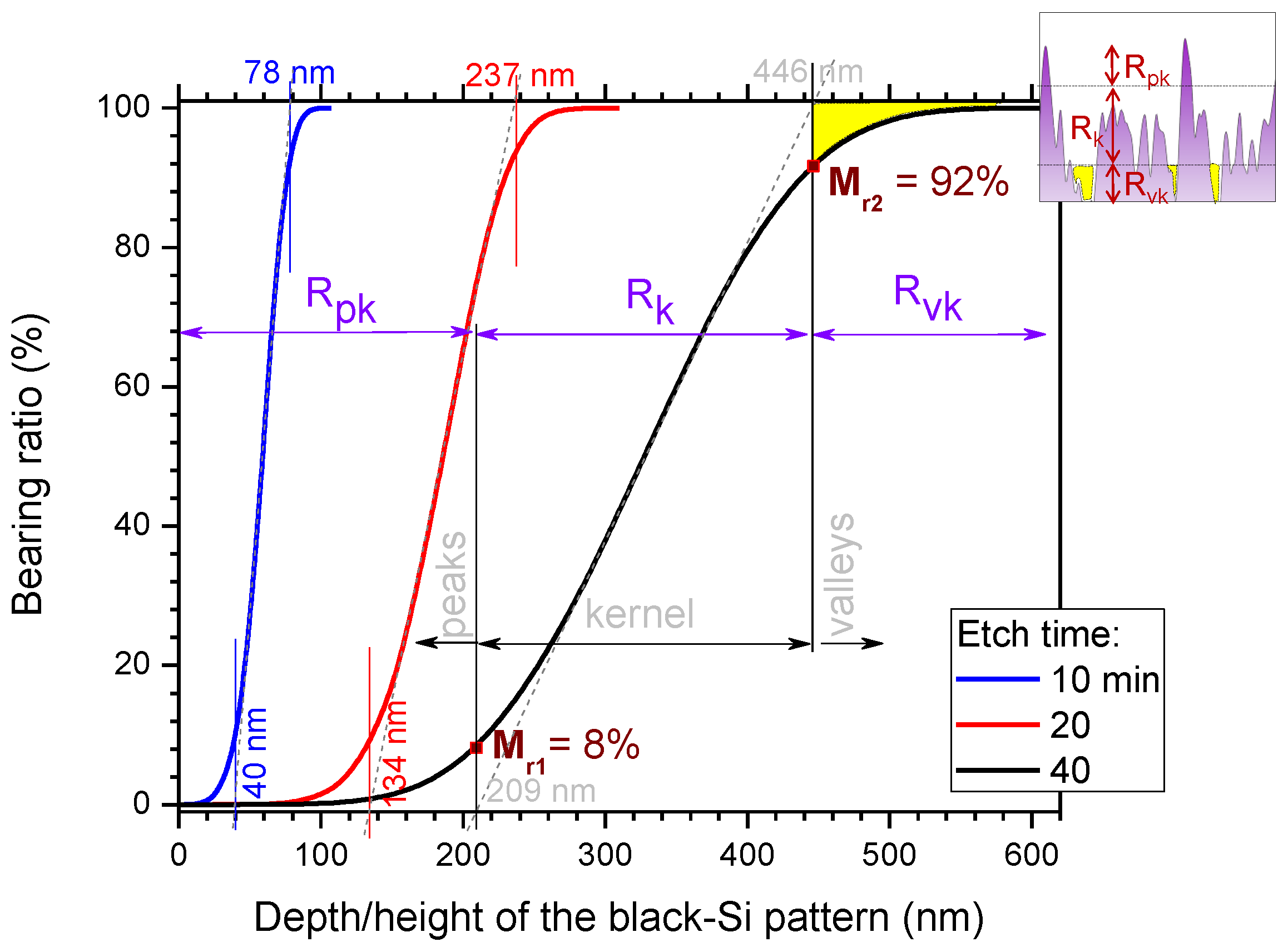
3. Results and Discussion
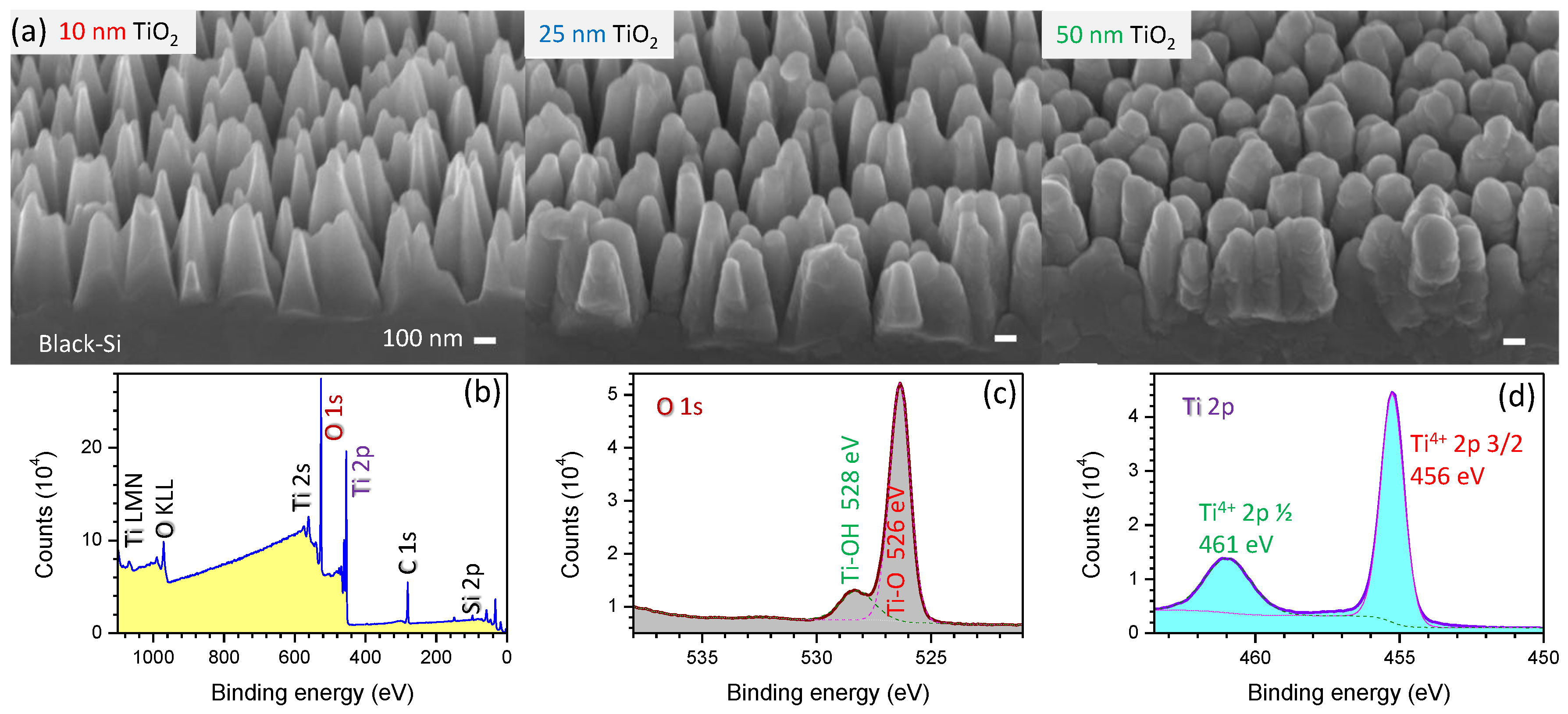
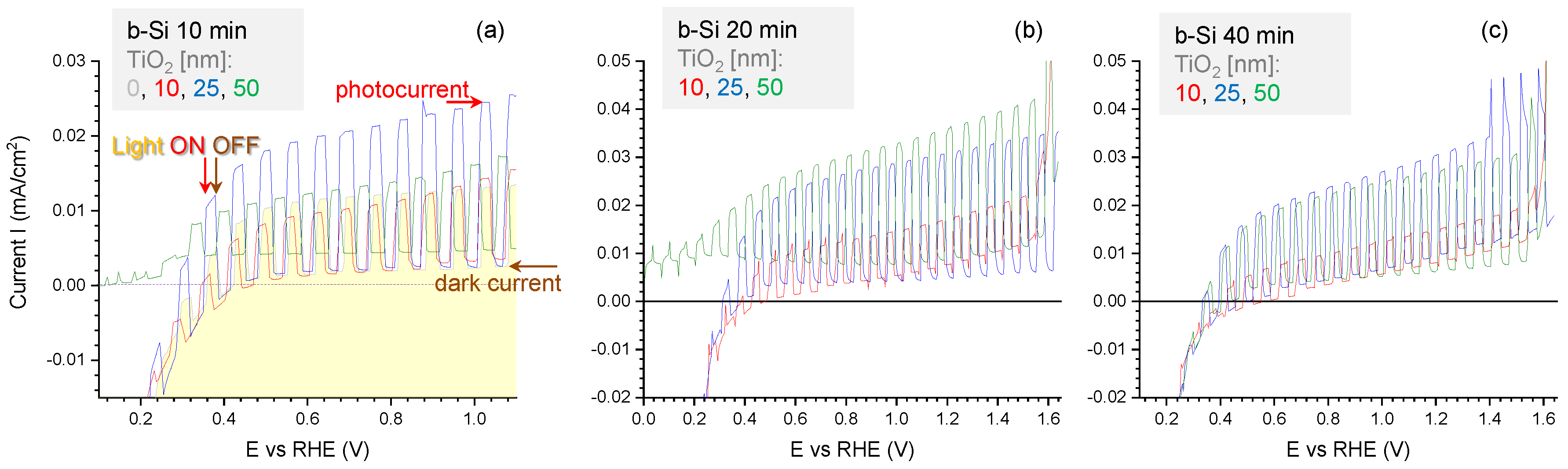
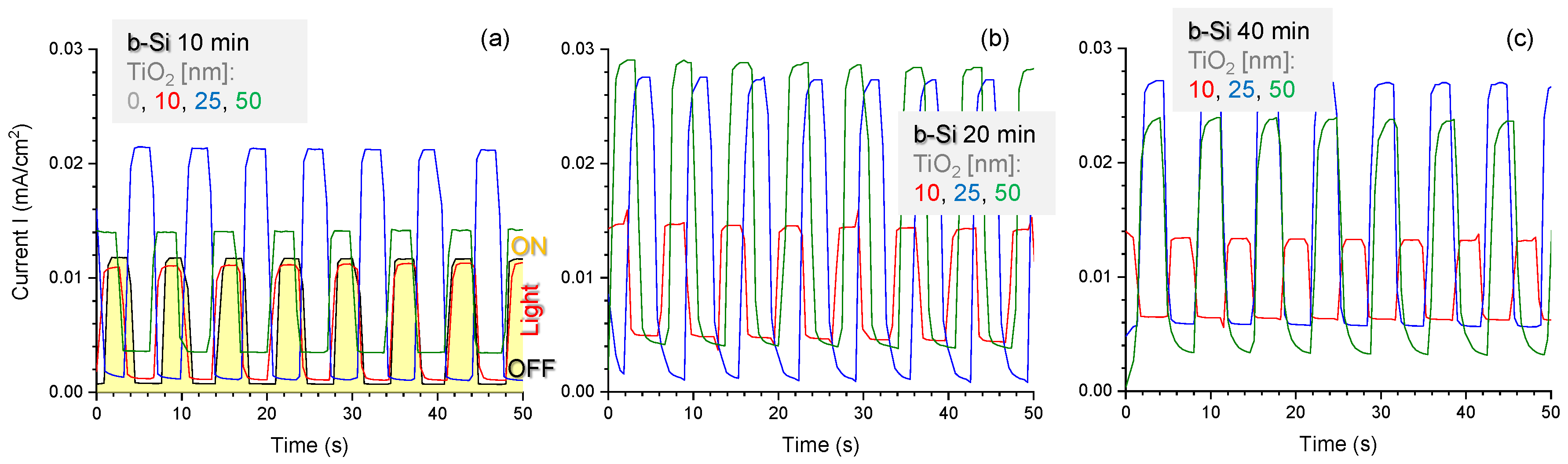
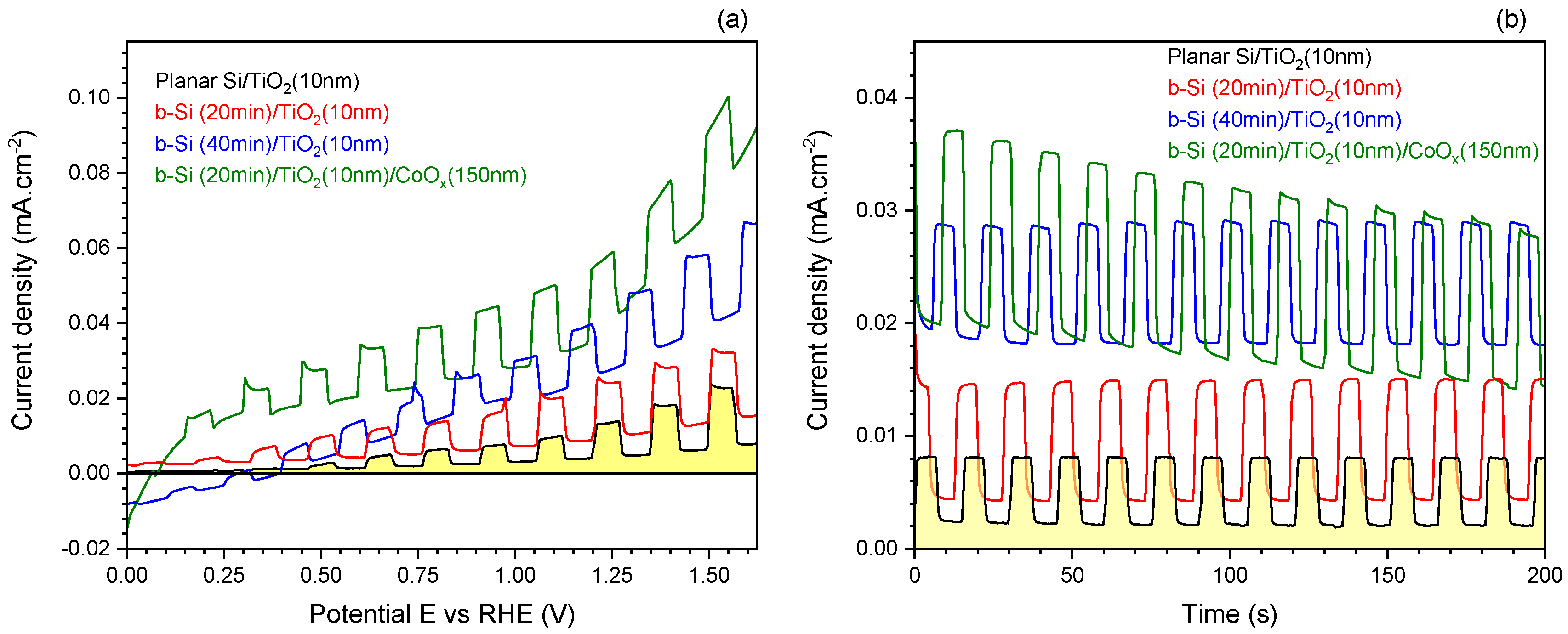
3.1. Black-Si with TiO2: Potentiostatic and Potentiodynamic Scans
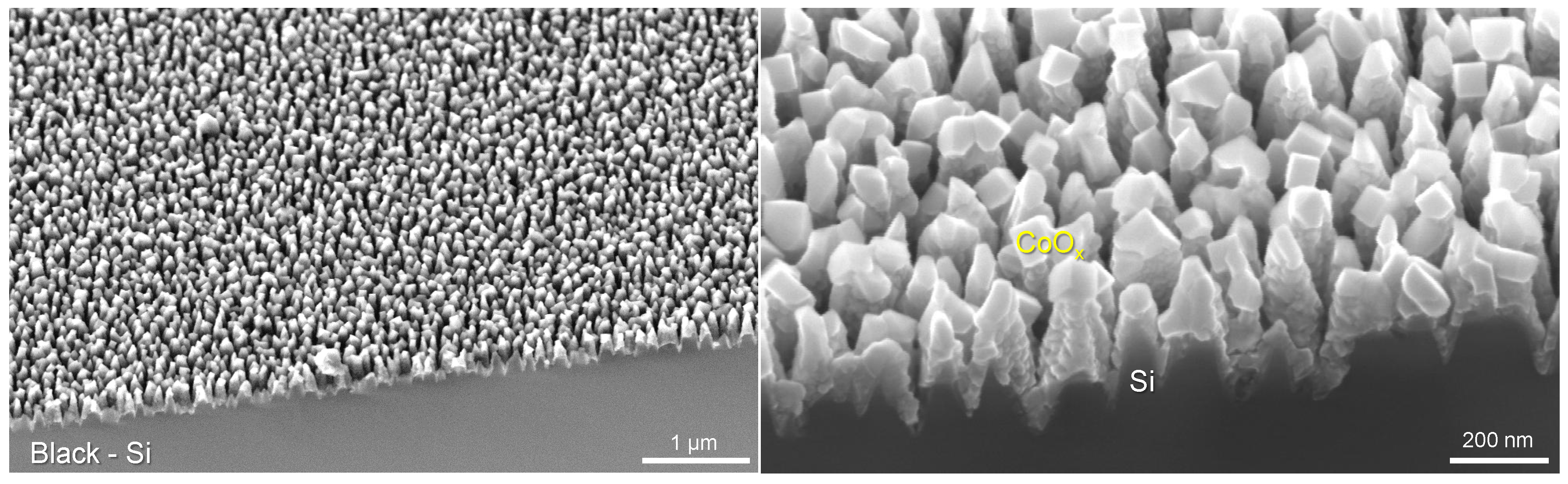
3.2. Black-Si with TiO2 and CoO: Potentiostatic and Potentiodynamic Scans
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Jansen, H.; de Boer, M.; Legtenberg, R.; Elwenspoek, M. The black silicon method: A universal method for determining the parameter setting of a fluorine-based reactive ion etcher in deep silicon trench etching with profile control. J. Micromech. Microeng. 1995, 5, 115. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.; Watson, G.S.; et al. Bactericidal activity of nanostructured black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.H.; Truong, V.K.; Mainwaring, D.; Guo, Y.; Baulin, V.; Kobaisi, M.A.; Gervinskas, G.; Juodkazis, S.; Zeng, W.R.; Doran, P.; et al. Nanotopography as a trigger for the microscale, autogenous and passive lysis of erythrocytes. J. Mater. Chem. B 2014, 2, 2819–2826. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.; Prommesberger, C.; Ławrowski, R.; Schreiner, R.; Serbun, P.; Müller, G.; Düsberg, F.; Hofmann, M.; Bachmann, M.; Pahlke, A. Field emission properties of p-type black silicon on pillar structures. J. Vac. Sci. Technol. B 2020, 34, 02G107. [Google Scholar] [CrossRef]
- Seniutinas, G.; Gervinskas, G.; Verma, R.; Gupta, B.D.; Lapierre, F.; Stoddart, P.R.; Clark, F.; McArthur, S.L.; Juodkazis, S. Versatile SERS sensing based on black silicon. Opt. Express 2015, 23, 6763–6772. [Google Scholar] [CrossRef]
- Ščajev, P.; Malinauskas, T.; Seniutinas, G.; Arnold, M.D.; Gentle, A.; Aharonovich, I.; Gervinskas, G.; Michaux, P.; Hartley, J.S.; Mayes, E.L.H.; et al. Light-induced reflectivity transients in black-Si nanoneedles. Sol. Energy Mater. Sol. Cells 2016, 144, 221–227. [Google Scholar] [CrossRef]
- Nishijima, Y.; Komatsu, R.; Ota, S.; Seniutinas, G.; Balčytis, A.; Juodkazis, S. Anti-reflective surfaces: Cascading nano/microstructuring. Appl. Phys. Lett. Photonics 2016, 1, 076104. [Google Scholar] [CrossRef]
- Shoji, T.; Mototsuji, A.; Balcytis, A.; Linklater, D.; Juodkazis, S.; Tsuboi, Y. Optical tweezing and binding at high irradiation powers on black-Si. Sci. Rep. 2017, 7, 12298. [Google Scholar] [CrossRef]
- Pekarek, R.T.; Christensen, S.T.; Liu, J.; Neale, N.R. Energetic Effects of Hybrid Organic/Inorganic Interfacial Architecture on Nanoporous Black Silicon Photoelectrodes. Sustain. Energy Fuels 2019, 3, 1600–1667. [Google Scholar] [CrossRef]
- Yang, F.; Aguiar, J.A.; Fairchild, M.; Vakki, W.; Younan, S.; Zhou, Y.; Zhuo, L.; Gu, J. Dual Protection Layer Strategy to Increase Photoelectrode—Catalyst Interfacial Stability: A Case Study on Black Silicon Photoelectrodes. Adv. Mater. Interfaces 2019, 6, 1802085. [Google Scholar] [CrossRef]
- Oh, K.; Joanny, L.; Gouttefangeas, F.; Fabre, B.; Dorcet, V.; Lassalle-Kaiser, B.; Vacher, A.; Meriadec, C.; Ababou-Girard, S.; Loget, G. Black Silicon Photoanodes Entirely Prepared with Abundant Materials by Low-Cost Wet Methods. ACS Appl. Energy Mater. 2019, 2, 1006–1010. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.; Yin, X.; Kvit, A.; Liao, Q.; Kang, Z.; Yan, X.; Zhang, Y.; Wang, X. Enhanced photoelectrochemical efficiency and stability using a conformal TiO2 film on a black silicon photoanode. Nat. Energy 2017, 2, 17045. [Google Scholar] [CrossRef]
- Yao, X.; Schneider, C.W.; Lippert, T.; Wokaun, A. Manipulation of ion energies in pulsed laser deposition to improve film growth. Appl. Phys. A 2019, 125, 344. [Google Scholar] [CrossRef]
- Gervinskas, G.; Seniutinas, G.; Hartley, J.S.; Kandasamy, S.; Stoddart, P.R.; Fahim, N.F.; Juodkazis, S. Surface-enhanced Raman scattering sensing on black silicon. Ann. Der Phys. 2013, 525, 907–914. [Google Scholar] [CrossRef]
- Balčytis, A.; Ryu, M.; Seniutinas, G.; Nishijima, Y.; Hikima, Y.; Zamengo, M. Si-based infrared optical filters. Opt. Eng. 2015, 54, 127103. [Google Scholar] [CrossRef]
- Buividas, R.; Fahim, N.; Juodkazytė, J.; Juodkazis, S. Novel method to determine the actual surface area of a laser-nanotextured sensor. Appl. Phys. A 2013, 14, 169–175. [Google Scholar] [CrossRef]
- Juodkazis, K.; Juodkazytė, J.; Šebeka, B.; Savickaja, I.; Juodkazis, S. Photoelectrochemistry of silicon in HF solution. J. Solid State Electrochem. 2013, 17, 2269–2276. [Google Scholar] [CrossRef]
- Brack, P.; Dann, S.E.; Wijayantha, K.; Adcock, P.; Foster, S. An assessment of the viability of hydrogen generation from the reaction of silicon powder and sodium hydroxide solution for portable applications. Int. J. Energy Res. 2017, 41, 220–228. [Google Scholar] [CrossRef]
- Mizeikis, V.; Kowalska, E.; Juodkazis, S. Resonant localization, enhancement, and polarization. In Nanoplasmonics: Advanced Device Applications; Chon, J.W.M., Iniewski, K., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 126–134. [Google Scholar]
- Si, W.; Pergolesi, D.; Haydous, F.; Fluri, A.; Wokaun, A.; Lippert, T. Investigating the behavior of various cocatalysts on LaTaON2 photoanode for visible light water splitting. Phys. Chem. Chem. Phys. 2017, 19, 656–662. [Google Scholar] [CrossRef]
- Deng, X.; Tüysüz, H. Cobalt-Oxide-Based Materials as Water Oxidation Catalyst: Recent Progress and Challenges. ACS Catal. 2014, 4, 3701–3714. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Martens, W.N.; Frost, R.L. Synthesis and Characterization of Cobalt Hydroxide, Cobalt Oxyhydroxide, and Cobalt Oxide Nanodiscs. J. Phys. Chem. C 2010, 114, 111–119. [Google Scholar] [CrossRef]
- Stevens, M.; Kast, M.; Trotochaud, L.; Smith, A.; Boettcher, S. Cobalt–Iron (Oxy)hydroxide Oxygen Evolution Electrocatalysts: The Role of Structure and Composition on Activity, Stability, and Mechanism. J. Am. Chem. Soc. 2015, 137, 10–1021. [Google Scholar]
- Weidler, N.; Paulus, S.; Schuch, J.; Klett, J.; Hoch, S.; Stenner, P.; Maljusch, A.; Brötz, J.; Wittich, C.; Kaisera, B.; et al. CoOx thin film deposited by CVD as efficient water oxidation catalyst: Change of oxidation state in XPS and its correlation to electrochemical activity. Phys. Chem. Chem. Phys. 2016, 18, 10708–10718. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, R.; Sun, K.; Papadantonakis, K.M.; Brunschwig, B.S.; Lewis, N.S. 570 mV photovoltage, stabilized n-Si/CoOx heterojunction photoanodes fabricated using atomic layer deposition. Energy Environ. Sci. 2016, 9, 892–897. [Google Scholar] [CrossRef]
- Zhang, M.; de Respinis, M.; Frei, H. Time-resolved observations of water oxidation intermediates on a cobalt oxide nanoparticle catalyst. Nat. Chem 2014, 6, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Nellist, M.R.; Laskowski, F.A.L.; Lin, F.; Mills, T.J.; Boettcher, S.W. Semiconductor–Electrocatalyst Interfaces: Theory, Experiment, and Applications in Photoelectrochemical Water Splitting. Acc. Chem. Res. 2016, 49, 733–740. [Google Scholar] [CrossRef]
- Juodkazis, K.; Juodkazytė, J.; Kalinauskas, P.; Jelmakas, E.; Juodkazis, S. Photoelectrolysis of Water: Solar Hydrogen—Achievements and Perspectives. Opt. Express Energy Express 2010, 18, A147–A160. [Google Scholar] [CrossRef]
- Juodkazytė, J.; Seniutinas, G.; Šebeka, B.; Savickaja, I.; Malinauskas, T.; Badokas, K.; Juodkazis, K.; Juodkazis, S. Solar water splitting: Efficiency discussion. Int. J. Hydrogen Energy 2016, 41, 11941–11948. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, H.X.; Chen, Y.P.; Meng, X.Y.; Ghanbaja, J.; Horwat, D.; Pierson, J.F. Wurtzite CoO: A direct band gap oxide suitable for a photovoltaic absorber. Chem. Commun. 2018, 54, 13949–13952. [Google Scholar] [CrossRef]
- Berenschot, E.; Jansen, H.V.; Tas, N.R. Fabrication of 3D fractal structures using nanoscale anisotropic etching of single crystalline silicon. J. Micromech. Microeng. 2013, 23, 055024. [Google Scholar] [CrossRef]
- Majumdar, A.; Bhushan, B. Role of Fractal Geometry in Roughness Characterization and Contact Mechanics of Surfaces. J. Tribol. 1990, 112, 205–216. [Google Scholar] [CrossRef]
- Ţalu, Ş.; Bramowicz, M.; Kulesza, S.; Pignatelli, F.; Salerno, M. Surface Morphology Analysis of Composite Thin Films based on Titanium-Dioxide Nanoparticles. Acta Phys. Pol. 2017, 131, 1529–1533. [Google Scholar] [CrossRef]
- Balcytis, A.; Ryu, M.; Seniutinas, G.; Stoddart, P.R.; Mamun, M.; Morikawa, J.; Juodkazis, S. Nano-rescaling of gold films on polystyrene: Thermal management for SERS. Nanoscale 2017, 9, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Nonomura, Y.; Seino, E.; Abe, S.; Mayama, H. Preparation and Characterization of Fractal Elastomer Surfaces. J. Oleo Sci. 2013, 62, 587–590. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linklater, D.P.; Haydous, F.; Xi, C.; Pergolesi, D.; Hu, J.; Ivanova, E.P.; Juodkazis, S.; Lippert, T.; Juodkazytė, J. Black-Si as a Photoelectrode. Nanomaterials 2020, 10, 873. https://doi.org/10.3390/nano10050873
Linklater DP, Haydous F, Xi C, Pergolesi D, Hu J, Ivanova EP, Juodkazis S, Lippert T, Juodkazytė J. Black-Si as a Photoelectrode. Nanomaterials. 2020; 10(5):873. https://doi.org/10.3390/nano10050873
Chicago/Turabian StyleLinklater, Denver P., Fatima Haydous, Cheng Xi, Daniele Pergolesi, Jingwen Hu, Elena P. Ivanova, Saulius Juodkazis, Thomas Lippert, and Jurga Juodkazytė. 2020. "Black-Si as a Photoelectrode" Nanomaterials 10, no. 5: 873. https://doi.org/10.3390/nano10050873
APA StyleLinklater, D. P., Haydous, F., Xi, C., Pergolesi, D., Hu, J., Ivanova, E. P., Juodkazis, S., Lippert, T., & Juodkazytė, J. (2020). Black-Si as a Photoelectrode. Nanomaterials, 10(5), 873. https://doi.org/10.3390/nano10050873






