Polyelectrolyte Multilayer Films Modification with Ag and rGO Influences Platelets Activation and Aggregate Formation under In Vitro Blood Flow
Abstract
1. Introduction
2. Materials and Methods
2.1. Polyelectrolyte Multilayer Film Build-Up
2.2. PEMs Chemical Cross-Linking
2.3. Nanoparticles Immobilization within PEMs
2.3.1. Graphene Oxide Nanoparticles Immobilization
2.3.2. In Situ Synthesis of Ag Nanoparticles
2.4. Coating Properties
2.5. Dynamic Blood-Material Interaction Assay
2.6. Hemocompatibility Analysis
2.6.1. Blood Quality Analysis
2.6.2. PEMs Surface Quality Evaluation
2.7. Statistical Investigation
3. Results
3.1. Coating Properties
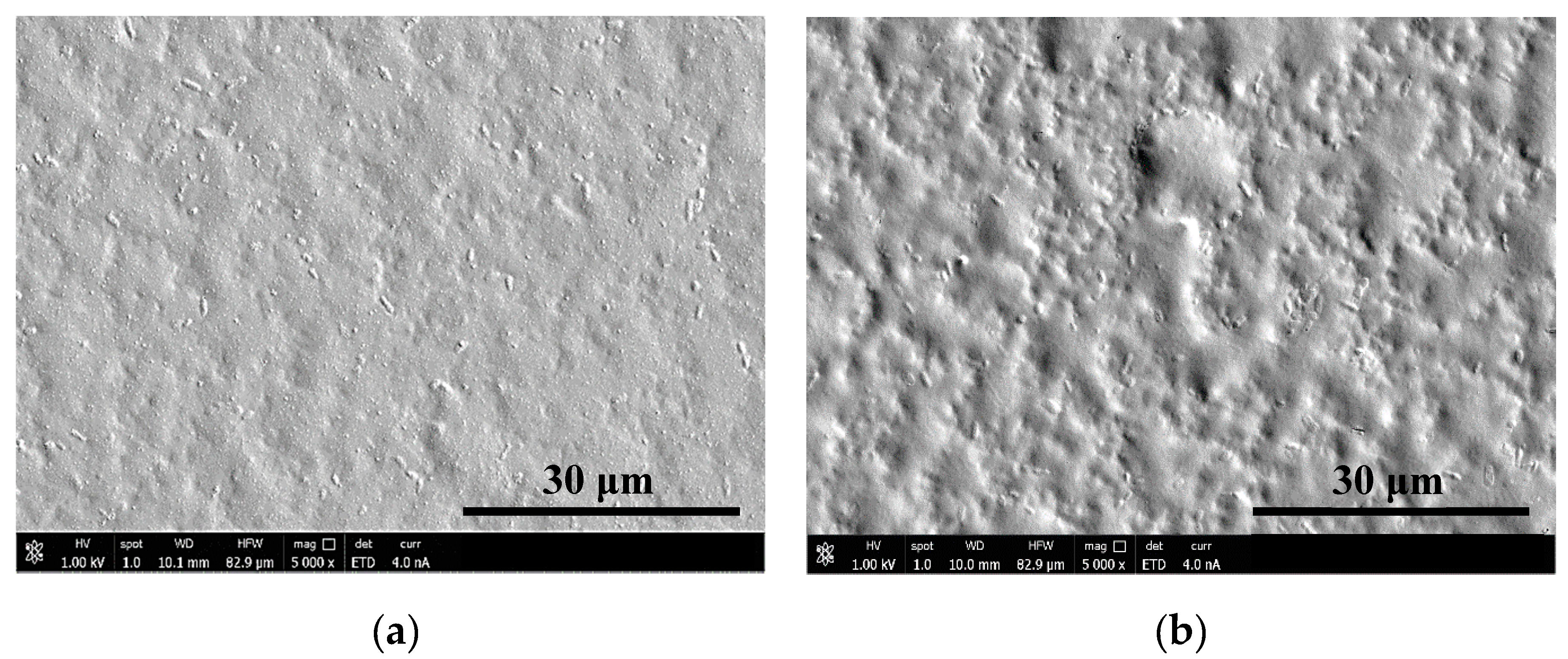
3.1.1. Surface Morphology
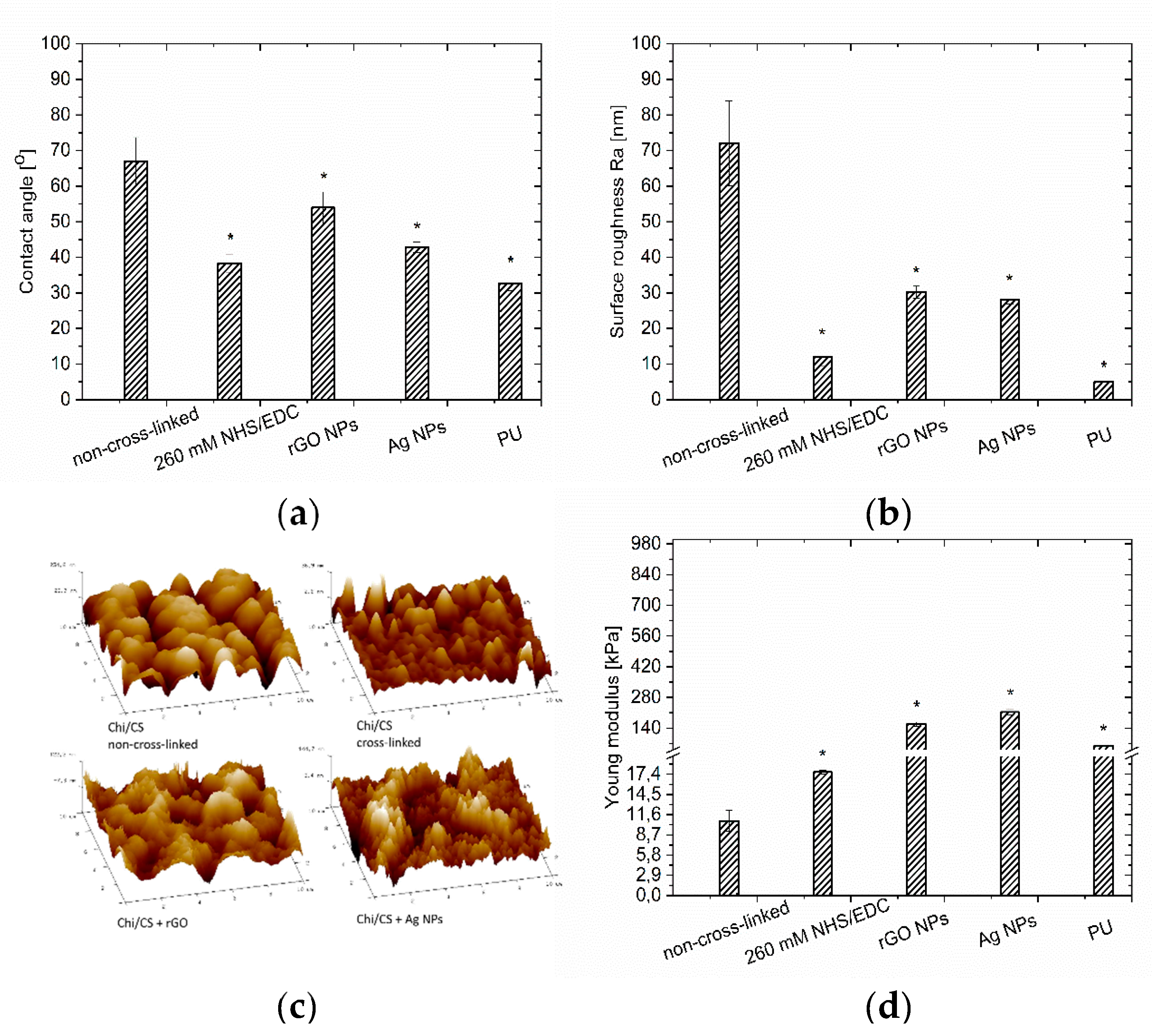
3.1.2. Wettability
3.1.3. Coating Stiffness
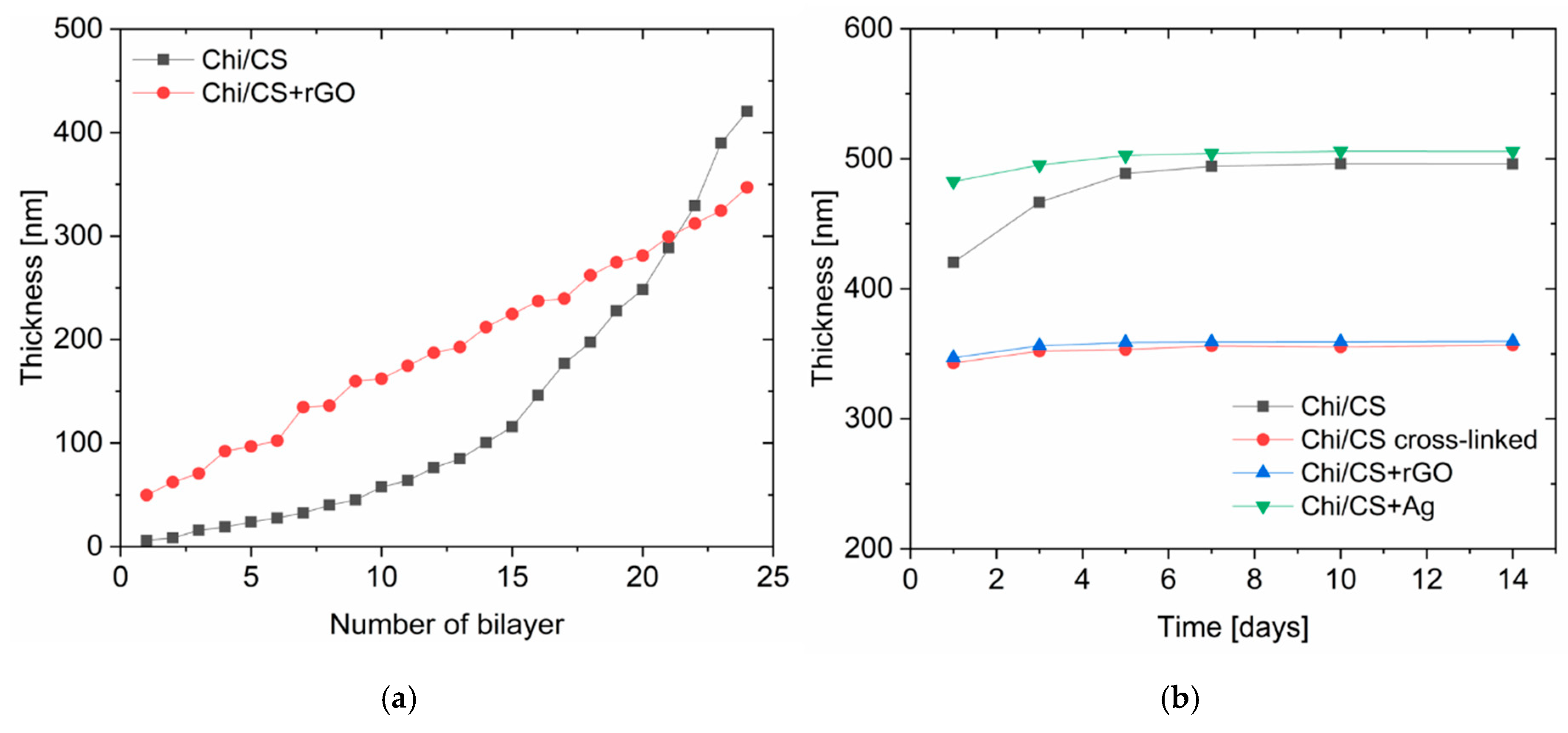
3.1.4. Coating Thickness and Stability
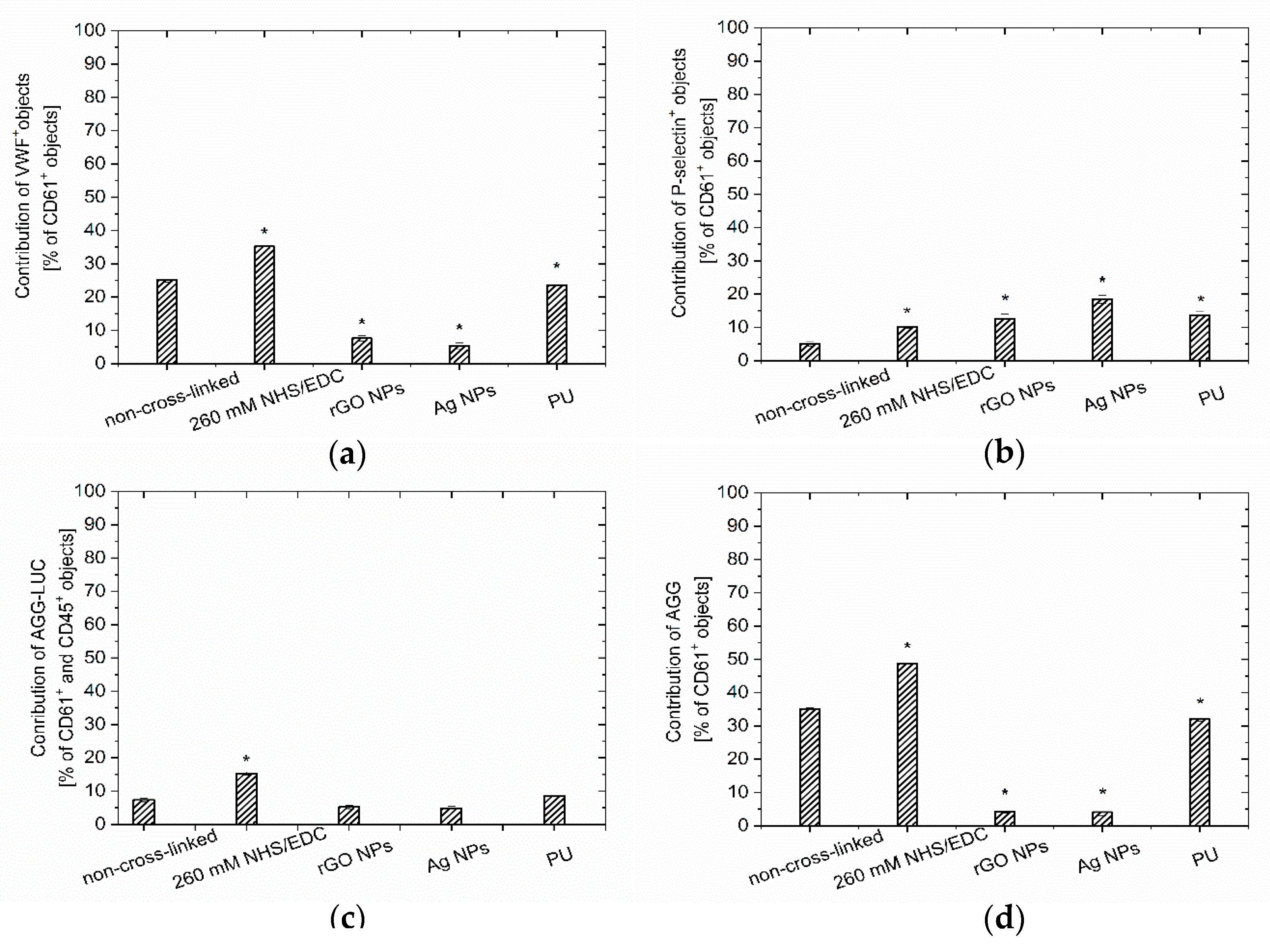
3.2. Hemocompatibility Analysis
3.2.1. Blood Quality Analysis
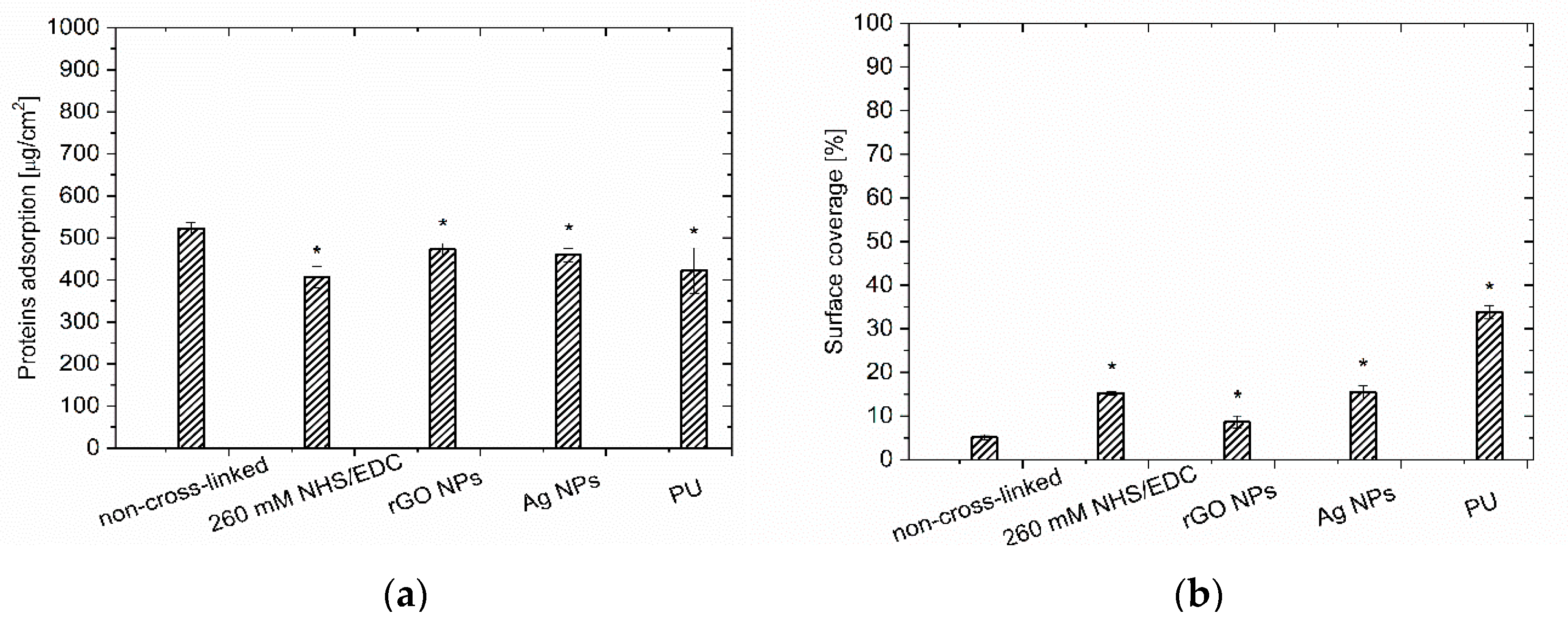
3.2.2. PEMs Surface Quality Evaluation
3.3. Correlation of PEMs Physicochemical Properties with Hemocompatibility
4. Discussion
4.1. Simultaneous Multiparameter Changes in the Physicochemical Properties of PEMs
4.2. Correlation of the PEMs Physicochemical Properties with Hemocompatibility
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brass, L.M.; Prohovnik, I.; Pavlakis, S.G.; DeVivo, D.C.; Piomelli, S.; Mohr, J.P. Middle cerebral artery blood velocity and cerebral blood flow in sickle cell disease. Stroke 1991, 22, 27–30. [Google Scholar] [CrossRef]
- Ratner, B.D. The catastrophe revisited: Blood compatibility in the 21st Century. Biomaterials 2007, 28, 5144–5147. [Google Scholar] [CrossRef] [PubMed]
- Braune, S.; Grunze, M.; Straub, A.; Jung, F. Are there sufficient standards for the in vitro hemocompatibility testing of biomaterials? Biointerphases 2013, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D. Blood compatibility—A perspective. J. Biomater. Sci. Polym. Ed. 2000, 11, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Gribova, V.; Auzely-Velty, R.; Picart, C. Polyelectrolyte Multilayer Assemblies on Materials Surfaces: From Cell Adhesion to Tissue Engineering. Chem. Mater. 2011, 24, 854–869. [Google Scholar] [CrossRef]
- Ren, K.-F.; Hu, M.; Zhang, H.; Li, B.-C.; Lei, W.-X.; Chen, J.-Y.; Chang, H.; Wang, L.-M.; Ji, J. Layer-by-layer assembly as a robust method to construct extracellular matrix mimic surfaces to modulate cell behavior. Prog. Polym. Sci. 2019, 92, 1–34. [Google Scholar] [CrossRef]
- Sashiwa, H.; Aiba, S.-I. Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
- Séon, L.; LaValle, P.; Schaaf, P.; Boulmedais, F. Polyelectrolyte Multilayers: A Versatile Tool for Preparing Antimicrobial Coatings. Langmuir 2015, 31, 12856–12872. [Google Scholar] [CrossRef]
- Perreault, F.; Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Richert, L.; LaValle, P.; Payan, E.; Shu, X.Z.; Prestwich, G.D.; Stoltz, J.-F.; Schaaf, P.; Voegel, J.-C.; Picart, C. Layer by Layer Buildup of Polysaccharide Films: Physical Chemistry and Cellular Adhesion Aspects. Langmuir 2004, 20, 448–458. [Google Scholar] [CrossRef]
- Schneider, A.; Picart, C.; Senger, B.; Schaaf, P.; Voegel, J.-C.; Frisch, B. Layer-by-Layer Films from Hyaluronan and Amine-Modified Hyaluronan. Langmuir 2007, 23, 2655–2662. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Jang, E.; Son, K.J.; Koh, W.-G. Metal-enhanced fluorescence using silver nanoparticles-embedded polyelectrolyte multilayer films for microarray-based immunoassays. Colloid Polym. Sci. 2014, 292, 1355–1364. [Google Scholar] [CrossRef]
- Vogt, B.D.; Lin, E.K.; Wu, W.-L.; White, C.C. Effect of Film Thickness on the Validity of the Sauerbrey Equation for Hydrated Polyelectrolyte Films. J. Phys. Chem. B 2004, 108, 12685–12690. [Google Scholar] [CrossRef]
- Sanak, M.; Jakieła, B.; Węgrzyn, W. Assessment of hemocompatibility of materials with arterial blood flow by platelet functional tests. Bull. Pol. Acad. Sci. Tech. Sci. 2010, 58, 317–322. [Google Scholar] [CrossRef][Green Version]
- Mzyk, A.; Lackner, J.M.; Wilczek, P.; Lipińska, L.; Niemiec-Cyganek, A.; Samotus, A.; Morenc, M. Polyelectrolyte multilayer film modification for chemo-mechano-regulation of endothelial cell response. RSC Adv. 2016, 6, 8811–8828. [Google Scholar] [CrossRef]
- Fu, J.; Ji, J.; Yuan, W.; Shen, J. Construction of anti-adhesive and antibacterial multilayer films via layer-by-layer assembly of heparin and chitosan. Biomater. 2005, 26, 6684–6692. [Google Scholar] [CrossRef]
- Crouzier, T.; Boudou, T.; Picart, C. Polysaccharide-based polyelectrolyte multilayers. Curr. Opin. Colloid Interface Sci. 2010, 15, 417–426. [Google Scholar] [CrossRef]
- Yang, C. Enhanced physicochemical properties of collagen by using EDC/NHS-crosslinking. Bull. Mater. Sci. 2012, 35, 913–918. [Google Scholar] [CrossRef]
- Zan, X.; Su, Z. Incorporation of Nanoparticles into Polyelectrolyte Multilayers via Counterion Exchange and in situ Reduction. Langmuir 2009, 25, 12355–12360. [Google Scholar] [CrossRef]
- Zan, X.; Su, Z. Polyelectrolyte multilayer films containing silver as antibacterial coatings. Thin Solid Films 2010, 518, 5478–5482. [Google Scholar] [CrossRef]
- Wang, N.; Ji, S.; Zhang, Z.; Li, J.; Wang, L. Self-assembly of graphene oxide and polyelectrolyte complex nanohybrid membranes for nanofiltration and pervaporation. Chem. Eng. J. 2012, 213, 318–329. [Google Scholar] [CrossRef]
- Cheng, C.; Shi, Z.-Q.; Cheng, C.; Lu, H.-Q.; Zhou, M.; Sun, S.-D.; Zhao, C. Graphene oxide and sulfonated polyanion co-doped hydrogel films for dual-layered membranes with superior hemocompatibility and antibacterial activity. Biomater. Sci. 2016, 4, 1431–1440. [Google Scholar]
- Kulkarni, D.D.; Choi, I.; Singamaneni, S.; Tsukruk, V.V. Graphene Oxide−Polyelectrolyte Nanomembranes. ACS Nano 2010, 4, 4667–4676. [Google Scholar] [CrossRef] [PubMed]
- Roach, P.; Farrar, D.; Perry, C.C. Surface Tailoring for Controlled Protein Adsorption: Effect of Topography at the Nanometer Scale and Chemistry. J. Am. Chem. Soc. 2006, 128, 3939–3945. [Google Scholar] [CrossRef]
- Cai, K.; Frant, M.; Bossert, J.; Hildebrand, G.; Liefeith, K.; Jandt, K.D. Surface functionalized titanium thin films: Zeta-potential, protein adsorption and cell proliferation. Colloids Surfaces B Biointerfaces 2006, 50, 1–8. [Google Scholar] [CrossRef]
- Bailly, A.; Lautier, A.; Laurent, A.; Guiffant, G.; Dufaux, J.; Houdart, E.; Labarre, D.; Merland, J. Thrombosis of angiographic catheters in humans: Experimental study. Int. J. Artif. Organs 1999, 22, 690–700. [Google Scholar] [CrossRef]
- Zingg, W.; Neumann, A.W.; Strong, A.B.; Hum, O.S.; Absolom, D.R. Effect of surface roughness on platelet adhesion under static and under flow conditions. Can. J. Surg. 1982, 25, 16–19. [Google Scholar]
- Agnihotri, A.; Siedlecki, C.A. Time-Dependent Conformational Changes in Fibrinogen Measured by Atomic Force Microscopy. Langmuir 2004, 20, 8846–8852. [Google Scholar] [CrossRef]
- Xu, L.-C.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomater. 2007, 28, 3273–3283. [Google Scholar] [CrossRef]
- Sethuraman, A.; Han, M.; Kane, R.S.; Belfort, G. Effect of Surface Wettability on the Adhesion of Proteins. Langmuir 2004, 20, 7779–7788. [Google Scholar] [CrossRef] [PubMed]
- Mzyk, A.; Imbir, G.; Trembecka-Wojciga, K.; Lackner, J.M.; Plutecka, H.; Jasek-Gajda, E.; Kawałko, J.; Major, R. Rolling or Two-Stage Aggregation of Platelets on the Surface of Thin Ceramic Coatings under in Vitro Simulated Blood Flow Conditions. ACS Biomater. Sci. Eng. 2020, 6, 898–911. [Google Scholar] [CrossRef]
- Qiu, Y.; Brown, A.C.; Myers, D.R.; Sakurai, Y.; Mannino, R.G.; Tran, R.; Ahn, B.; Hardy, E.T.; Kee, M.F.; Kumar, S.; et al. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc. Natl. Acad. Sci. USA 2014, 111, 14430–14435. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, K.; Kudryk, B.; Coller, B. Fibrinogen Coating Density Affects the Conformation of Immobilized Fibrinogen: Implications for Platelet Adhesion and Spreading. Thromb. Haemost. 1998, 79, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Jou, C.-H.; Lin, W.-C.; Yang, M.-C. Surface modification of poly(tetramethylene adipate-co-terephthalate) membrane via layer-by-layer assembly of chitosan and dextran sulfate polyelectrolyte multiplayer. Colloids Surfaces B Biointerfaces 2007, 54, 222–229. [Google Scholar] [CrossRef]
- Kerch, G.; Chausson, M.; Gautier, S.; Meri, R.M.; Zicans, J.; Jakobsons, E.; Joner, M. Heparin-like polyelectrolyte multilayer coatings based on fungal sulfated chitosan decrease platelet adhesion due to the increased hydration and reduced stiffness. Biomater. Tissue Technol. 2017, 1, 1–5. [Google Scholar]
- Meng, S.; Liu, Z.; Shen, L.; Guo, Z.; Chou, L.L.; Du, Q.; Du, Q.; Ge, J. The effect of a layer-by-layer chitosan–heparin coating on the endothelialization and coagulation properties of a coronary stent system. Biomater. 2009, 30, 2276–2283. [Google Scholar] [CrossRef]
- Kuo, W.-H.; Wang, M.-J.; Chien, H.-W.; Wei, T.-C.; Lee, C.; Tsai, W.-B. Surface Modification with Poly(sulfobetaine methacrylate-co-acrylic acid) To Reduce Fibrinogen Adsorption, Platelet Adhesion, and Plasma Coagulation. Biomacromolecules 2011, 12, 4348–4356. [Google Scholar] [CrossRef]
- Sperling, C.; Schweiss, R.B.; Streller, U.; Werner, C. In vitro hemocompatibility of self-assembled monolayers displaying various functional groups. Biomaterials 2005, 26, 6547–6557. [Google Scholar] [CrossRef]

| Hemocompatibility Parameter | PEM Properties | |||
|---|---|---|---|---|
| Roughness | Contact Angle | Stiffness | ||
| Surface | Protein adsorption | 0.95 | 0.94 | 0.05 |
| P-selectin expression | −0.61 | −0.65 | 0.91 | |
| vWF contribution | −0.09 | −0.15 | −0.94 | |
| Platelet-monocytes aggregates | −0.32 | −0.08 | −0.24 | |
| Platelet aggregates | −0.06 | −0.14 | −0.94 | |
| Blood | P-selectin expression | 0.62 | 0.69 | 0.40 |
| PAC-1 expression | 0.60 | 0.80 | 0.20 | |
| Platelet aggregates | −0.28 | −0.11 | −0.64 | |
| Microparticles | 0.58 | 0.77 | 0.10 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imbir, G.; Mzyk, A.; Trembecka-Wójciga, K.; Jasek-Gajda, E.; Plutecka, H.; Schirhagl, R.; Major, R. Polyelectrolyte Multilayer Films Modification with Ag and rGO Influences Platelets Activation and Aggregate Formation under In Vitro Blood Flow. Nanomaterials 2020, 10, 859. https://doi.org/10.3390/nano10050859
Imbir G, Mzyk A, Trembecka-Wójciga K, Jasek-Gajda E, Plutecka H, Schirhagl R, Major R. Polyelectrolyte Multilayer Films Modification with Ag and rGO Influences Platelets Activation and Aggregate Formation under In Vitro Blood Flow. Nanomaterials. 2020; 10(5):859. https://doi.org/10.3390/nano10050859
Chicago/Turabian StyleImbir, Gabriela, Aldona Mzyk, Klaudia Trembecka-Wójciga, Ewa Jasek-Gajda, Hanna Plutecka, Romana Schirhagl, and Roman Major. 2020. "Polyelectrolyte Multilayer Films Modification with Ag and rGO Influences Platelets Activation and Aggregate Formation under In Vitro Blood Flow" Nanomaterials 10, no. 5: 859. https://doi.org/10.3390/nano10050859
APA StyleImbir, G., Mzyk, A., Trembecka-Wójciga, K., Jasek-Gajda, E., Plutecka, H., Schirhagl, R., & Major, R. (2020). Polyelectrolyte Multilayer Films Modification with Ag and rGO Influences Platelets Activation and Aggregate Formation under In Vitro Blood Flow. Nanomaterials, 10(5), 859. https://doi.org/10.3390/nano10050859







