Fe/Mg-Modified Carbonate Apatite with Uniform Particle Size and Unique Transport Protein-Related Protein Corona Efficiently Delivers Doxorubicin into Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Fe/Mg-CA NPs
2.3. Turbidity Measurement of Fe/Mg-CA NPs
2.4. Particle Size and Surface Charge Measurement of Fe/Mg-CA NPs
2.5. Optical Microscopic Imaging
2.6. Fourier Transform Infrared (FTIR) Spectroscopy
2.7. Stability Test of Fe/Mg-CA NPs
2.8. Field Emission Scanning Electron Microscopy (FESEM) and Energy Dispersive X-ray (EDX) Analysis
2.8.1. FESEM Image Analysis for Fe/Mg- CA NPs in Absence of 10% FBS
2.8.2. FESEM Image Analysis for Fe/Mg-CA NPs in Presence of 10% FBS
2.8.3. FESEM Image Analysis for Fe-Carbonate Apatite (Fe-CA) and Mg-Carbonate Apatite (Mg-CA) NPs
2.8.4. Energy Dispersive X-ray (EDX) Analysis
2.9. Flame Atomic Absorption Spectroscopy (FAAS)
2.10. Binding Affinity of DOX with Fe/Mg-CA NPs
- A = total concentrations of DOX used in the experiment (i.e., 1, 2.5, 5, 7.5 and 10 µM)
- B = different concentrations of DOX (i.e., 1, 2.5, 5, 7.5 and 10 µM) bound NPs in the pellet resuspended with 10 mM EDTA in PBS.
2.11. pH Dissolution Study of Fe/Mg-CA NPs
2.12. pH Dependent Release of DOX from Fe/Mg-CA NPs
- A = NP bound drug at pH 7.5
- B = NP bound drug at different pHs (7.5, 7, 6.5, 6 and 5.5)
2.13. Cell Culture and Seeding
2.14. Cell Treatment and Preparation of Free DOX, DOX-CA and DOX-Fe/Mg-CA NPs
2.15. Cell Viability by MTT (3-4,5-Dimethylthiazol-2-yl-2,5-Diphenyltetrazolium Bromide) Assay
- CVNP bound drug = Cell Viability of NP Bound Drug
- CytotoxicityNP = 100 − Cell Viability of NP
- CytotoxicityDOX = 100 − Cell Viability of DOX
2.16. Cellular Uptake of DOX-Loaded Fe/Mg-CA NPs in MCF-7 Cells using Fluorescence Microscopy
2.17. Quantitative Analysis of Cellular Uptake for DOX-Loaded Fe/Mg-CA NPs
- FFree Drug = Initial fluorescence intensity of free DOX
- FInternalized drug = Fluorescence intensity of the amount of DOX internalized from the respective NPs into the cells
2.18. Protein Corona Analysis
2.18.1. In-Solution Digestion of CA and Fe/Mg-CA Protein Corona for Mass Spectrometric Analysis
2.18.2. Sample Preparation for Mass Spectrometry-Based Proteomics
2.18.3. High Efficiency Nanoflow Liquid Chromatography Electrospray-Ionization Coupled with Mass Spectrometry
2.18.4. Protein Quantification and Identification through Automated De Novo Sequencing (PEAKS Studio 8.0)
2.19. Animal Biodistribution Study
2.20. Blood Serum Analysis
3. Results and Discussion
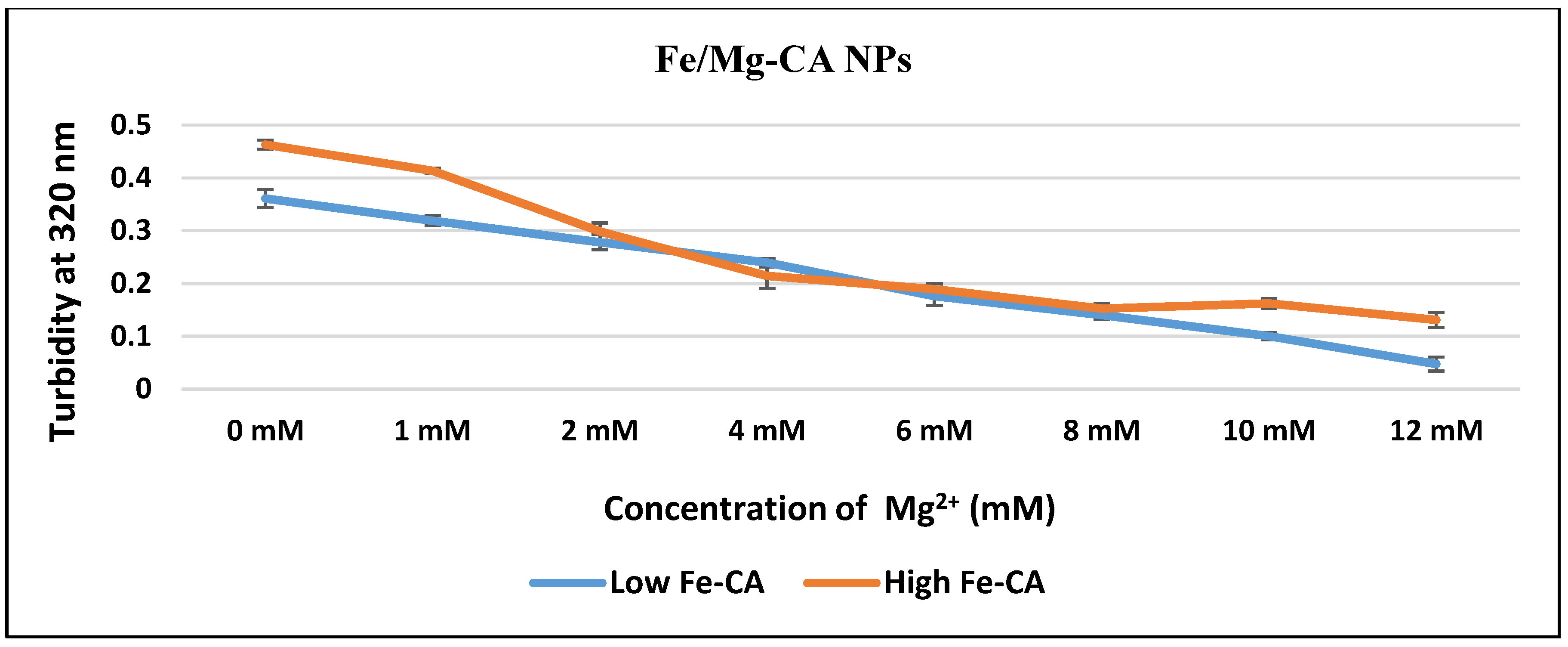
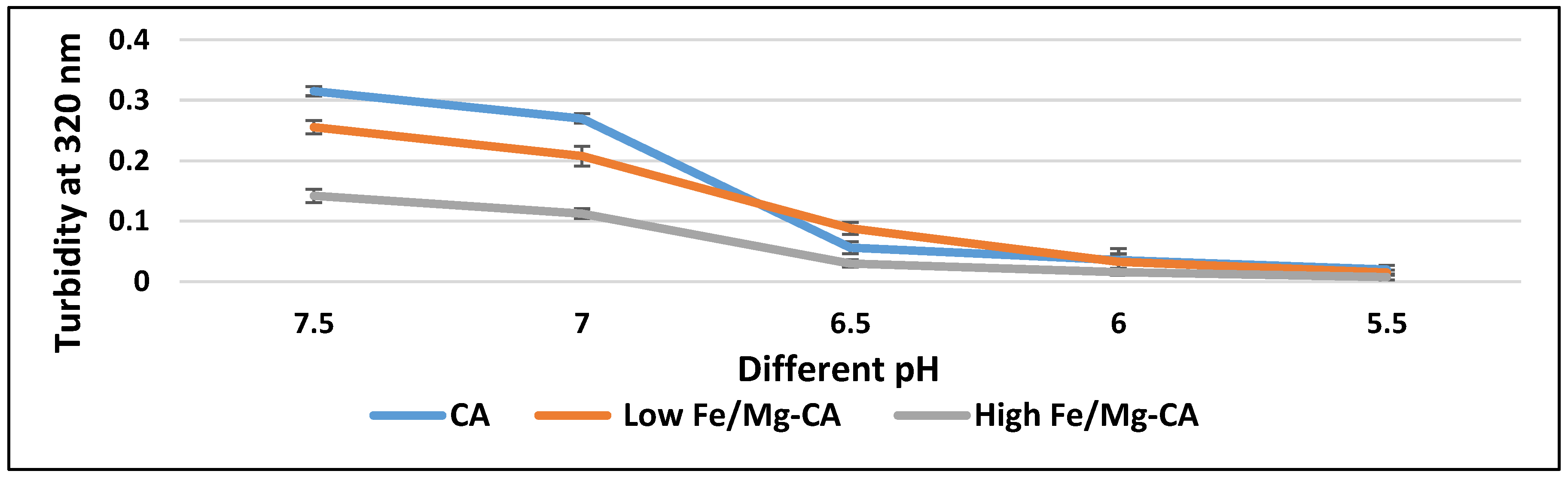
3.1. Measurement of Turbidity as a Reflection of Particle Formation
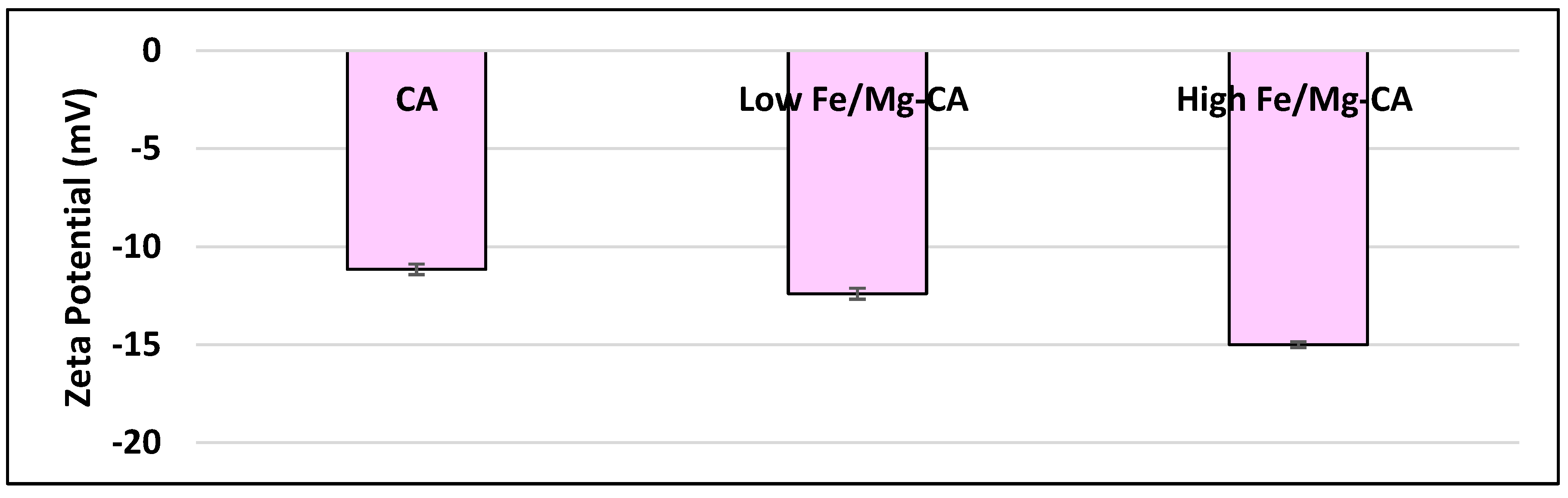
3.2. Particle Size and Surface Charge Measurement
3.3. Observation of Particle Aggregates under Optical Microscope
3.4. Characterization of Fe/Mg-CA NPs by Fourier Transform Infrared (FTIR) Spectroscopy
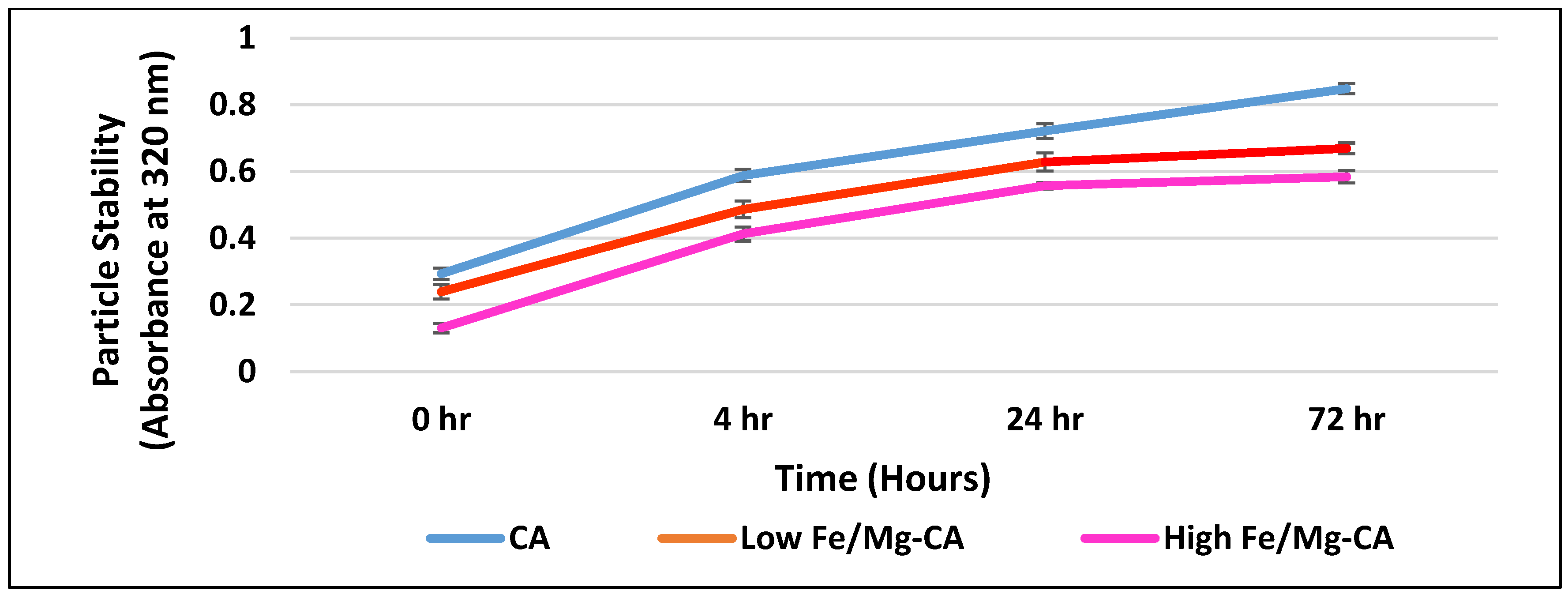
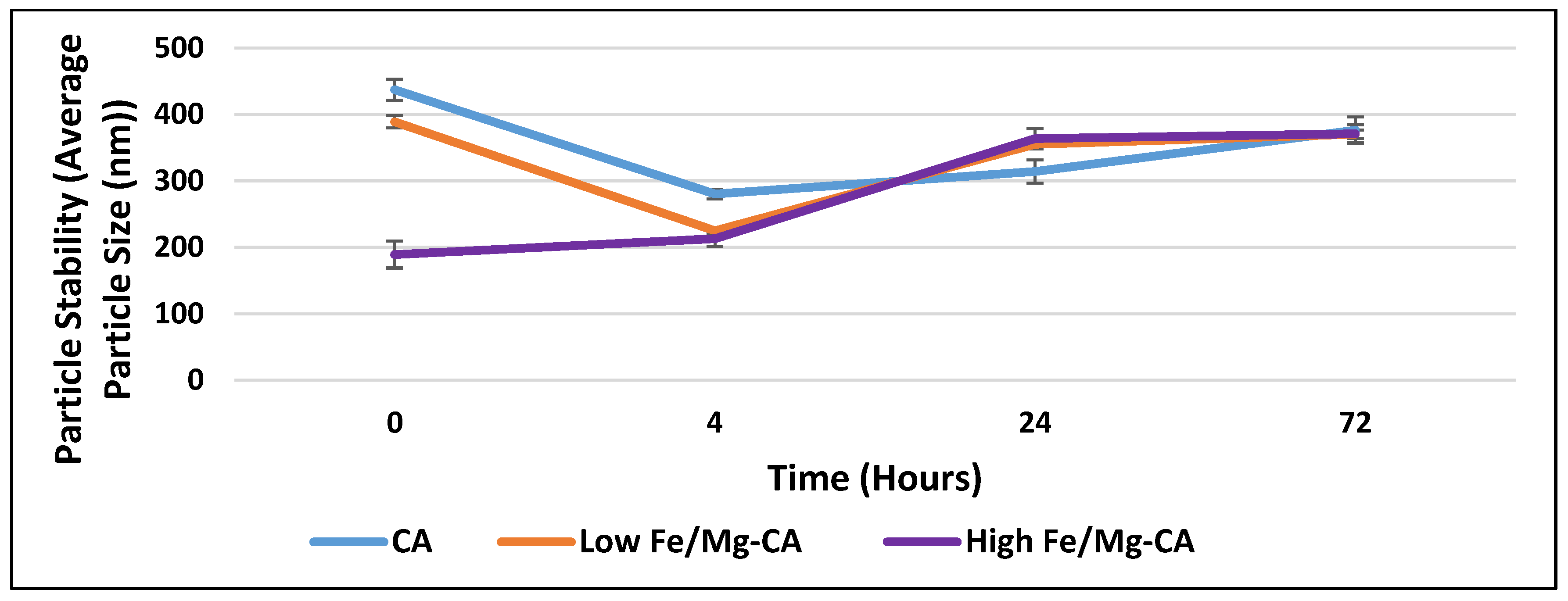
3.5. Stability Analysis of Fe/Mg-CA NPs through Turbidity Measurement and Z-Average Diameter
3.6. Characterization through FESEM and EDX
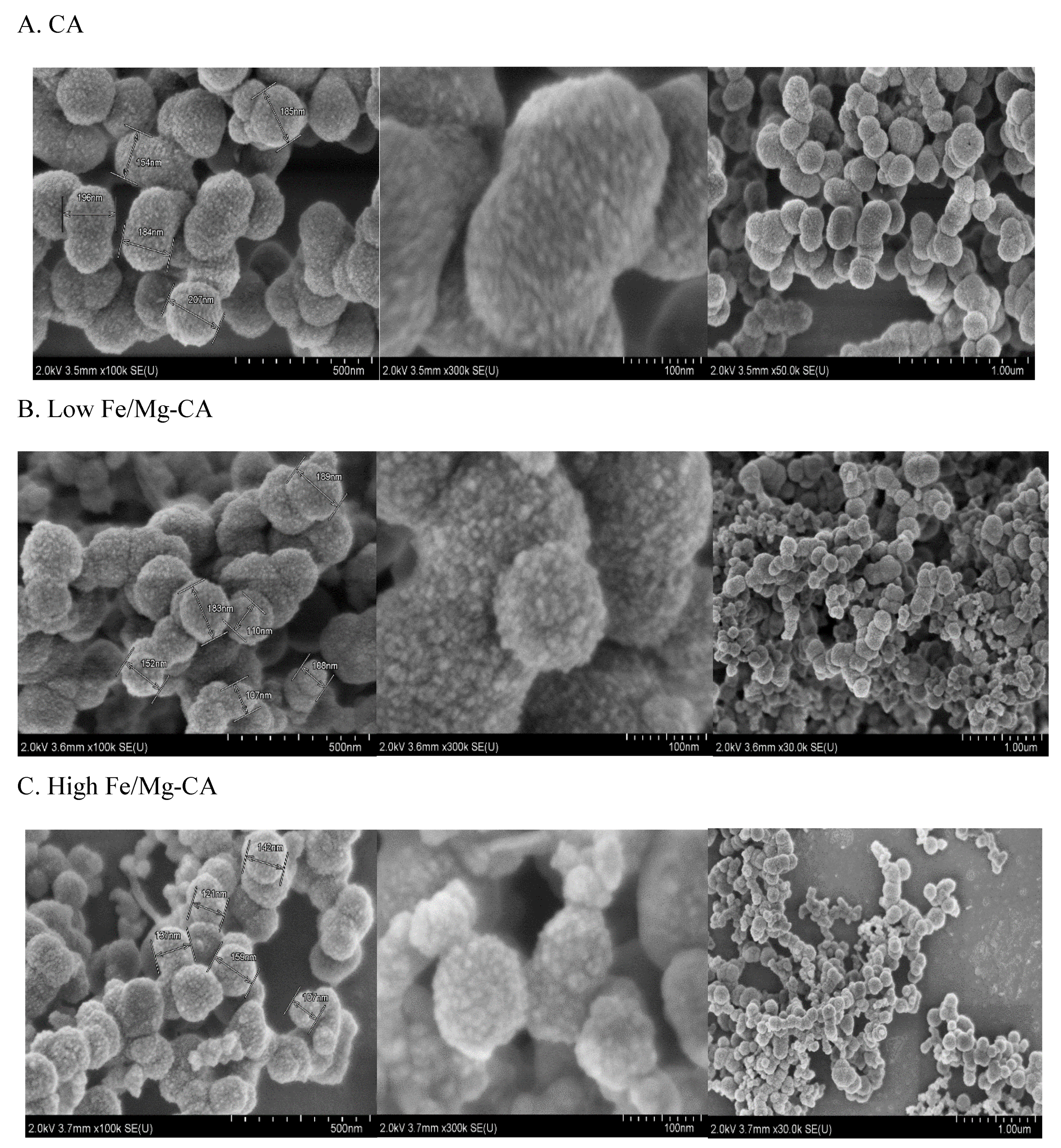
3.6.1. Morphological Analysis of Fe/Mg-CA NPs through FESEM in Absence of 10% FBS
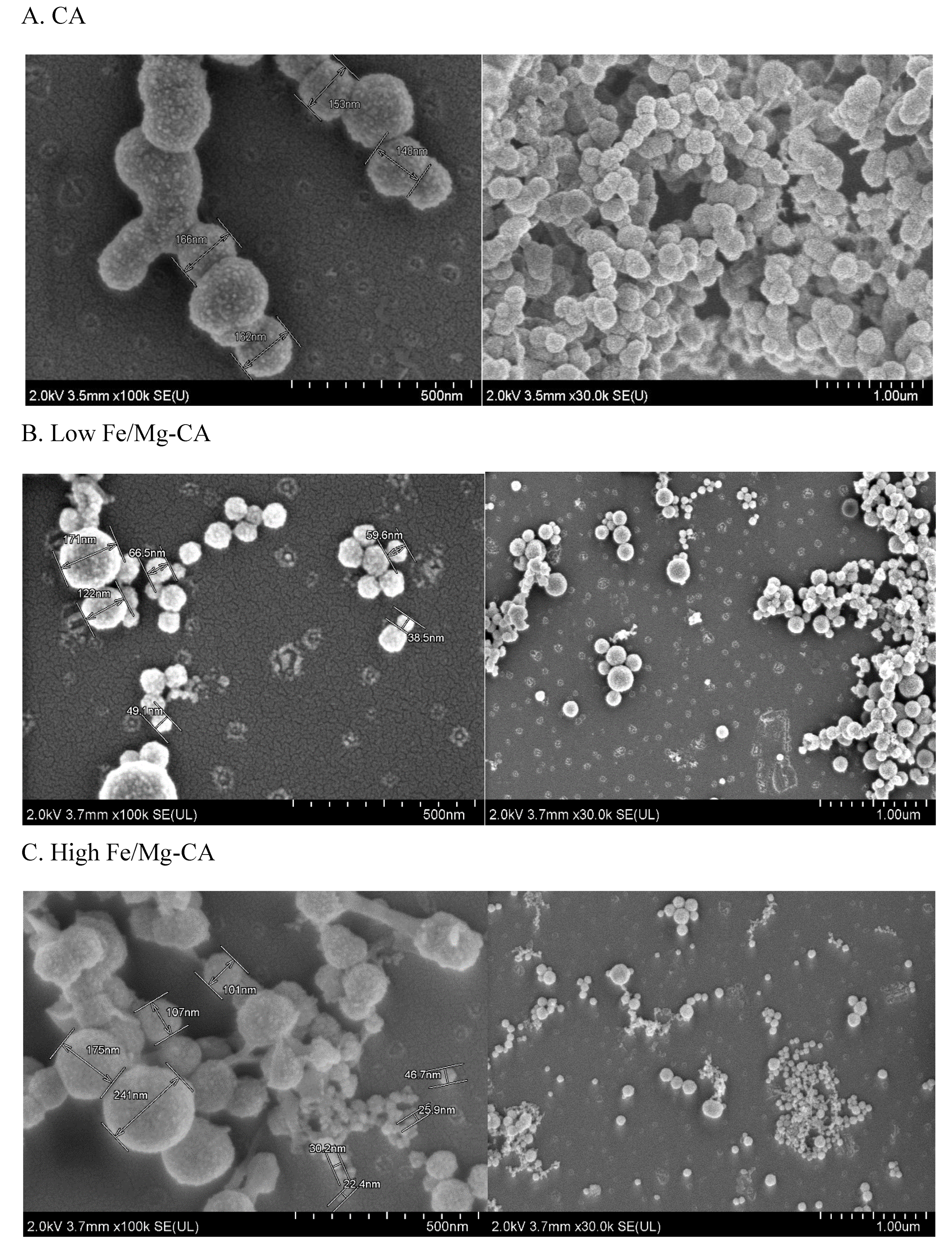
3.6.2. Morphological Analysis of Fe/Mg-CA NPs through FESEM in Presence of 10% FBS
3.6.3. Individual effects of Fe3+ and Mg2+ on CA NPs by FESEM Image Analysis
3.6.4. Elemental Analysis by Energy Dispersive X-ray (EDX)
3.7. Elemental Analysis by Flame Atomic Absorption Spectroscopy (FAAS)
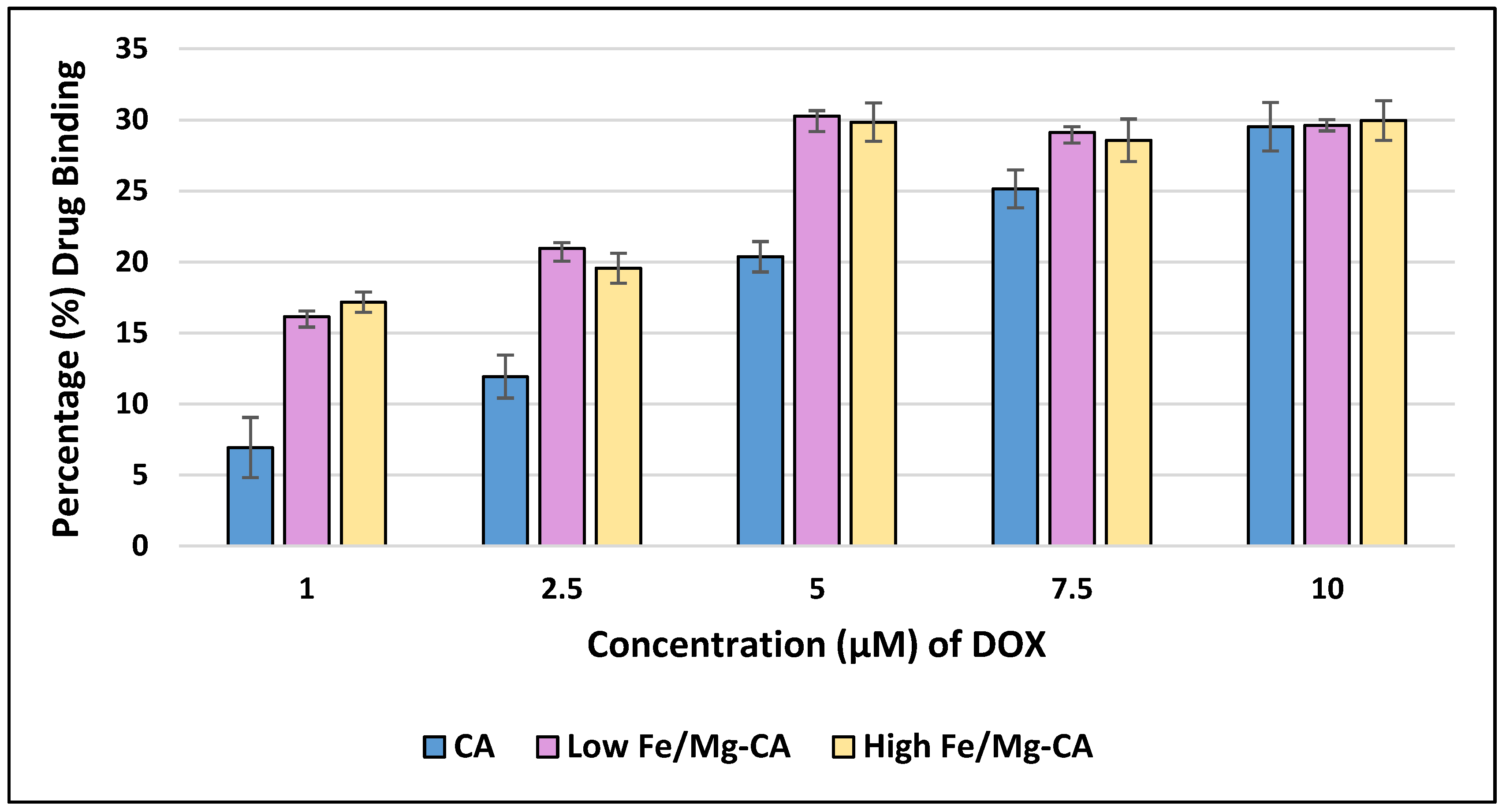
3.8. Binding Affinity of DOX Towards Fe/Mg-CA NPs
3.9. In Vitro pH-Dependent Dissolution Study of Fe/Mg-CA NPs
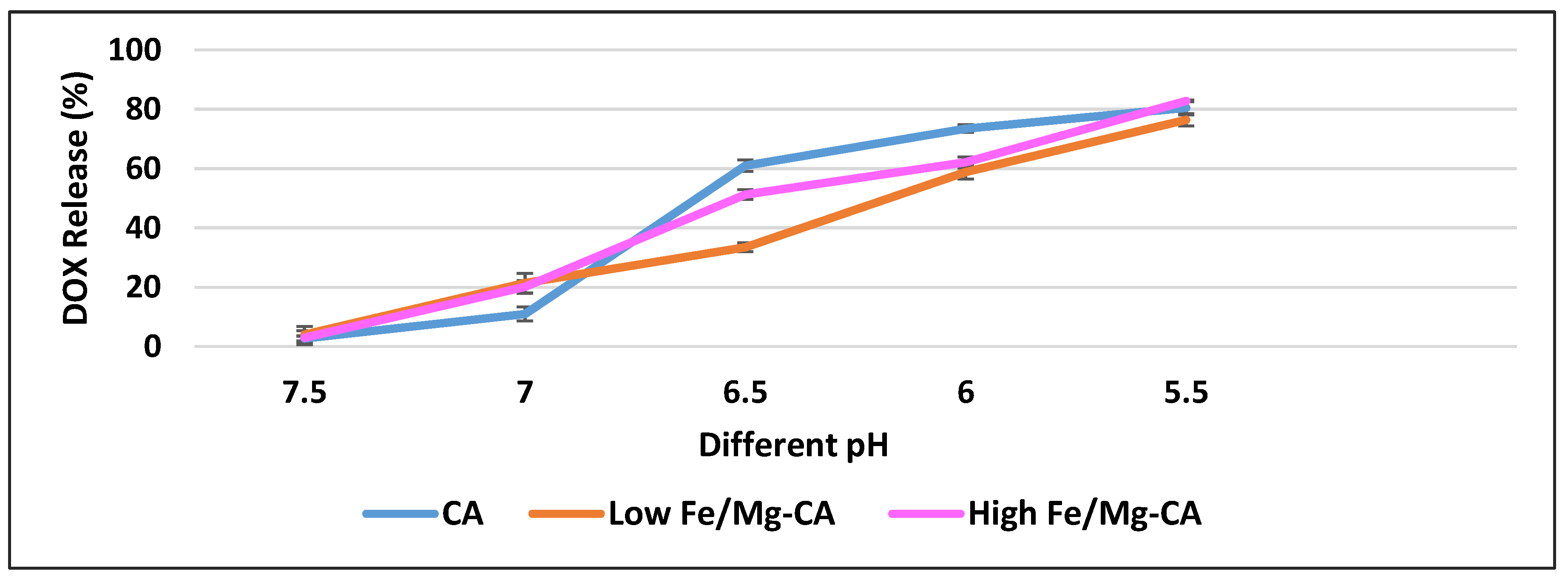
3.10. pH-Dependent DOX Release Kinetics from DOX-Loaded Fe/Mg-CA NPs
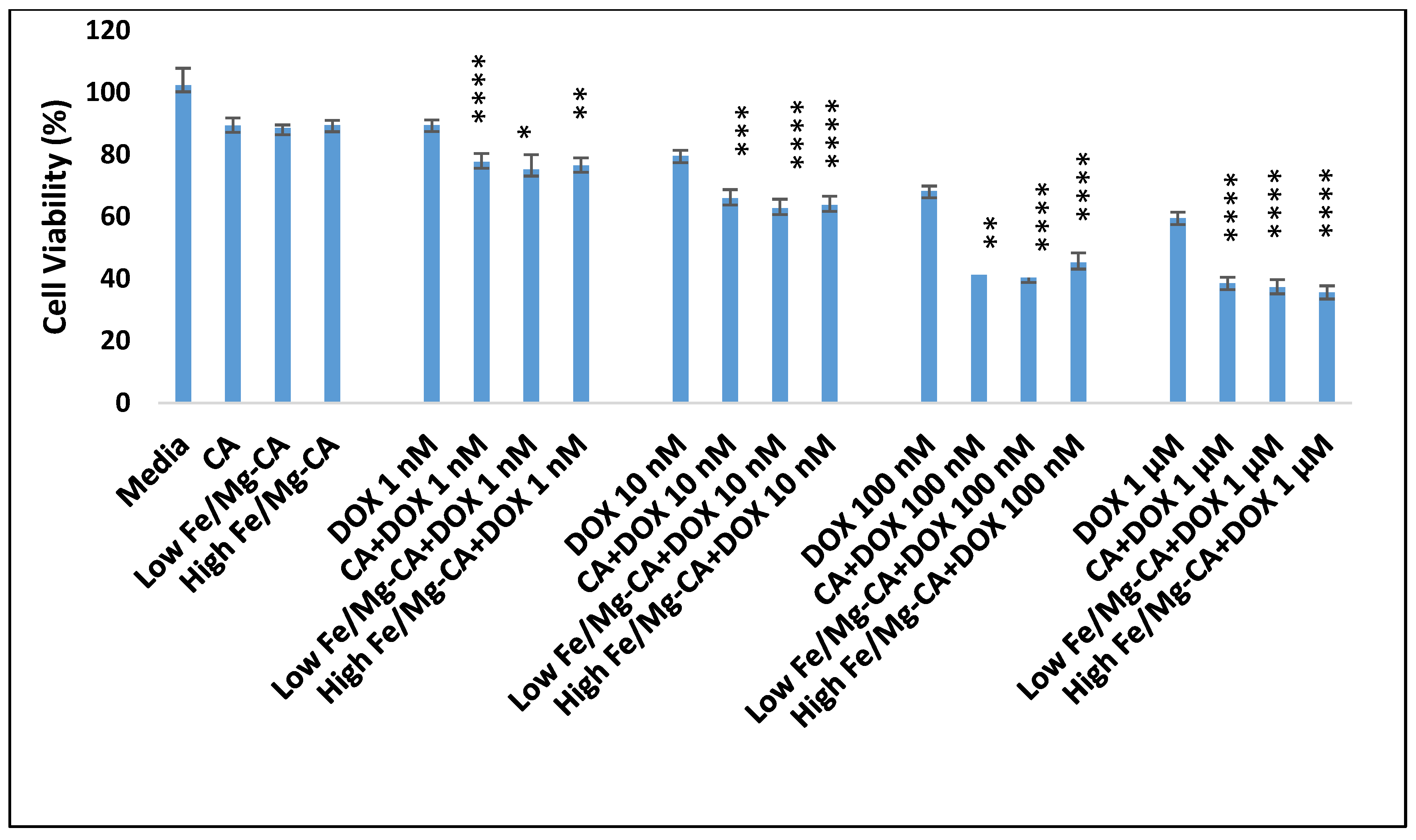
3.11. Cell Viability Assessment by MTT Assay
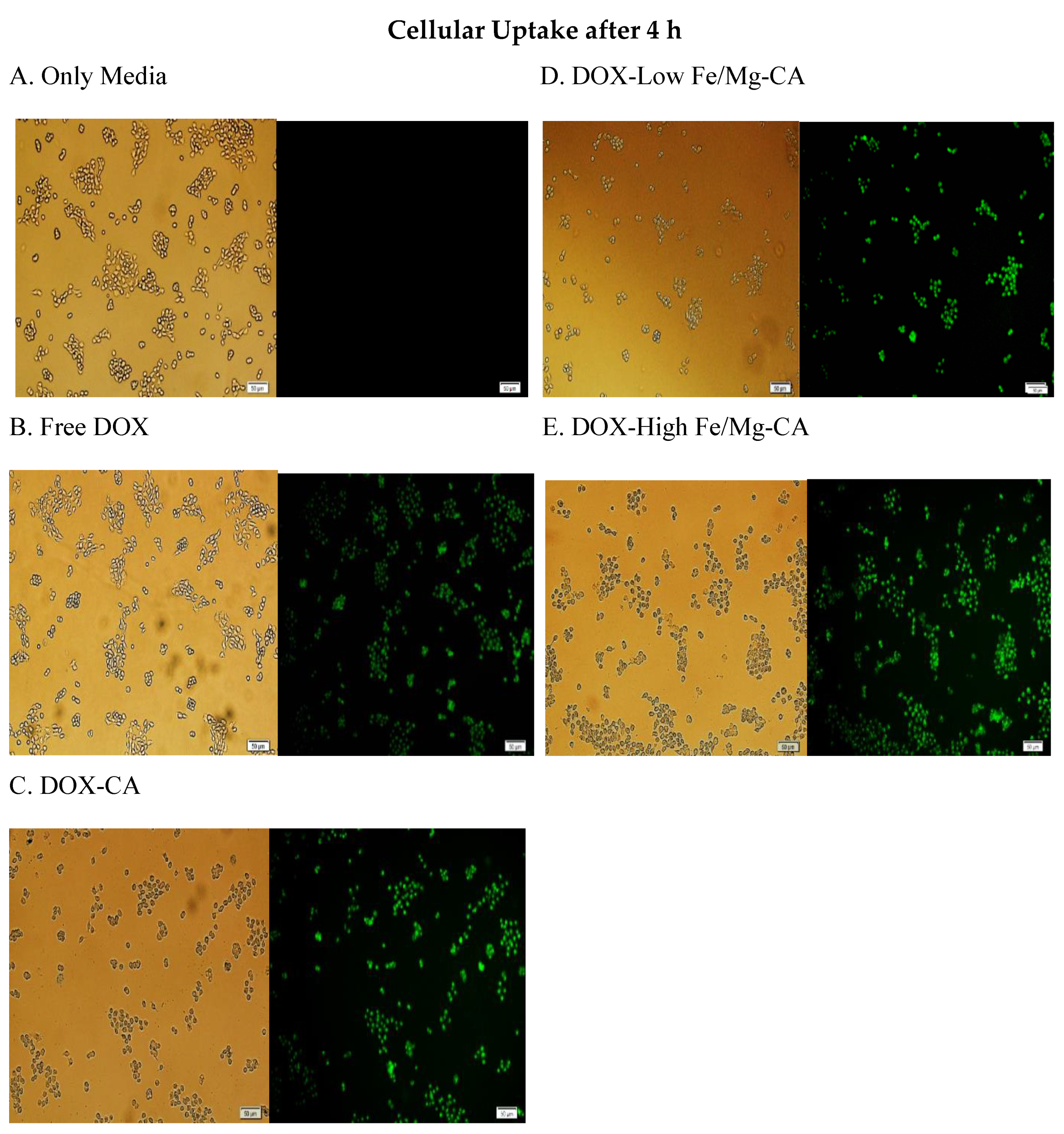
3.12. In Vitro Cellular Uptake of DOX-Loaded Fe/Mg-CA NPs in MCF-7 Cells
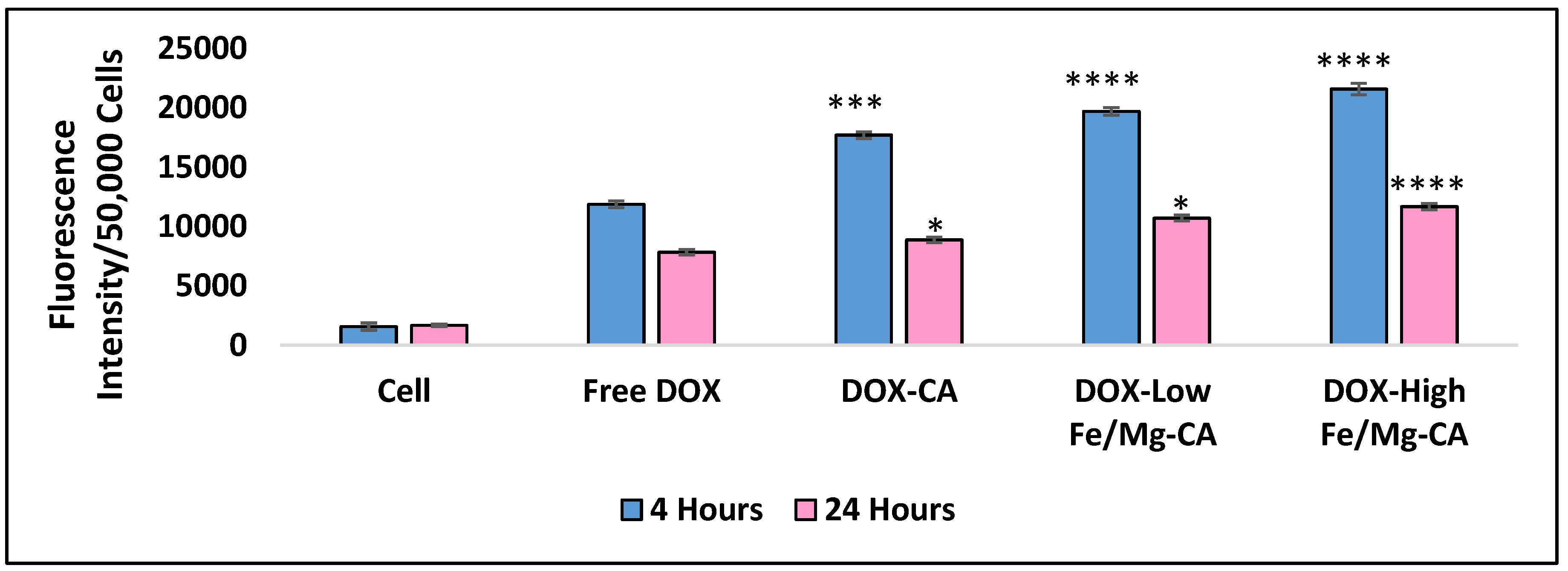
3.13. Quantitative Analysis of Cellular Uptake for DOX-Loaded Fe/Mg-CA NPs
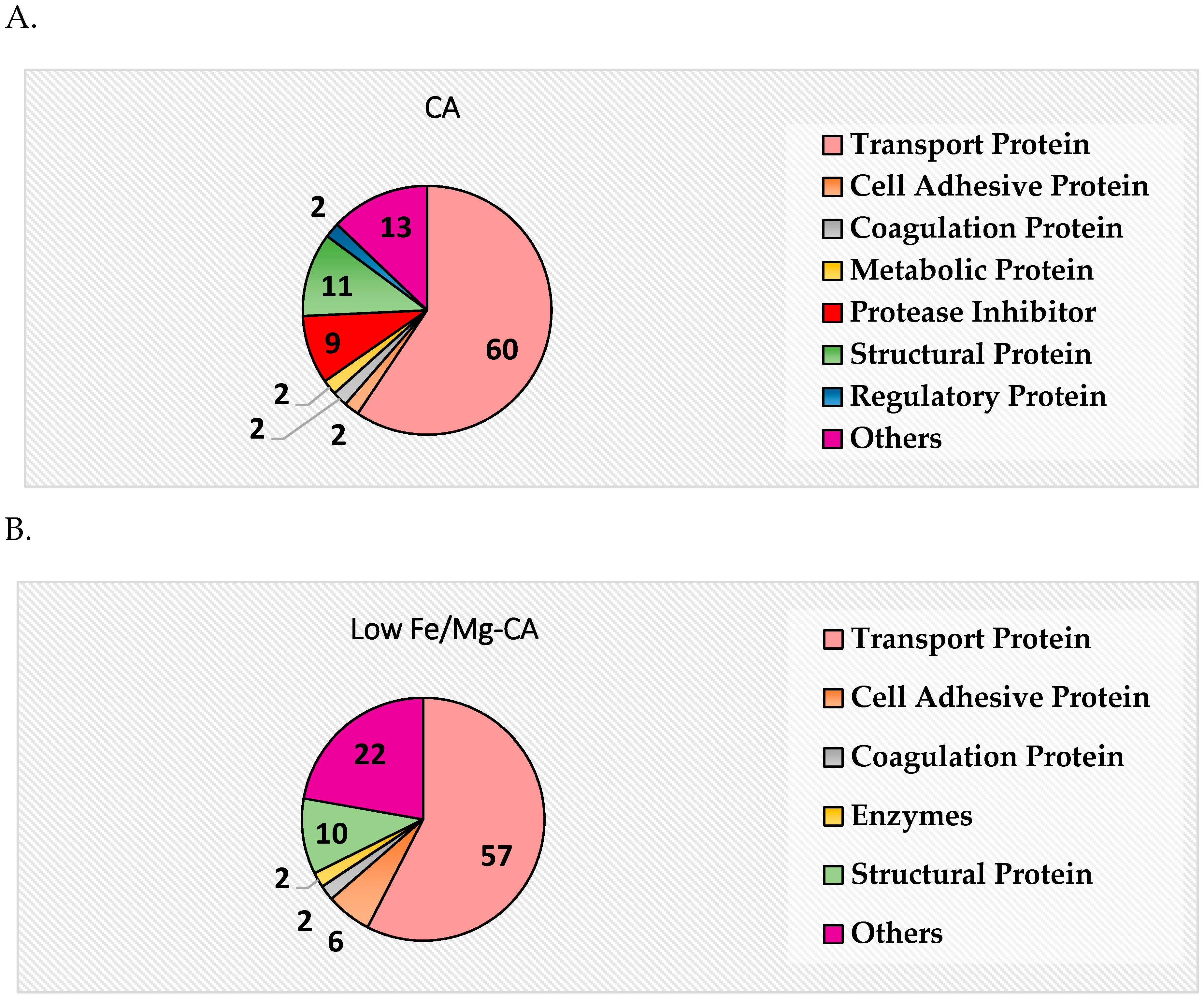
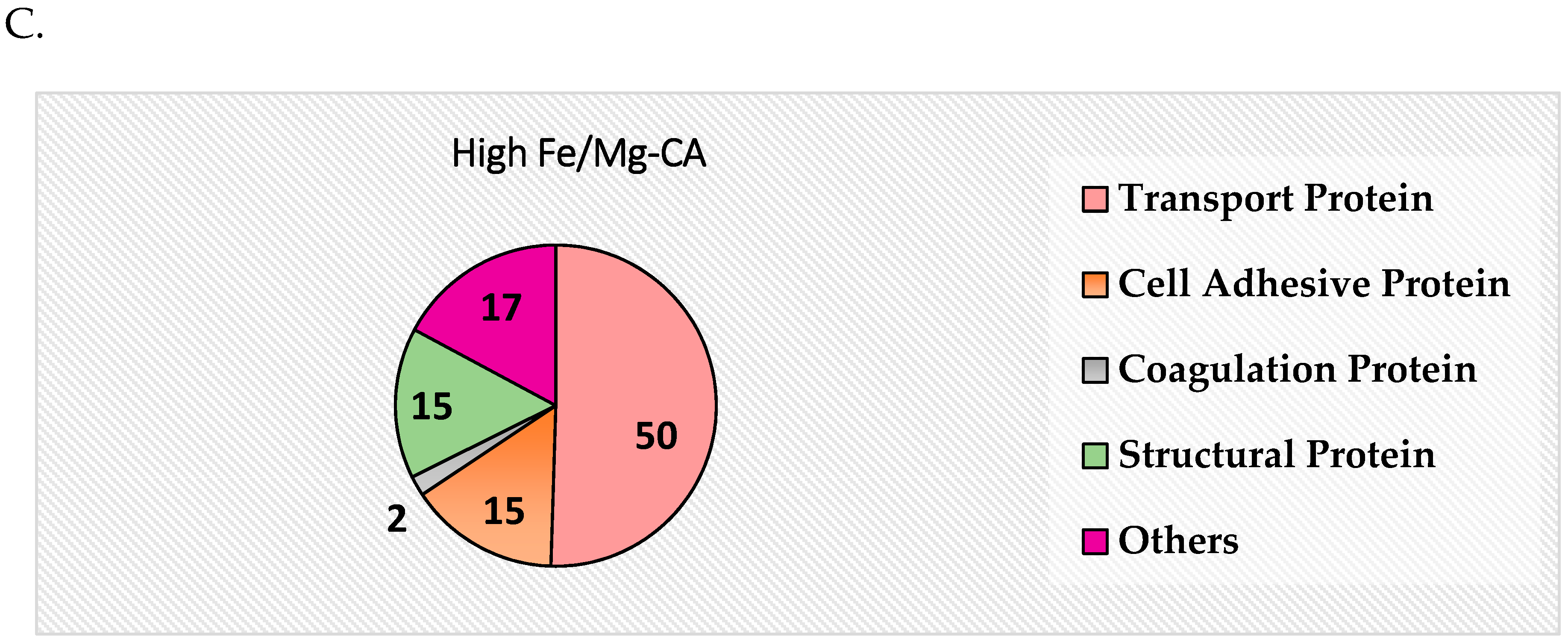
3.14. Protein Corona Analysis
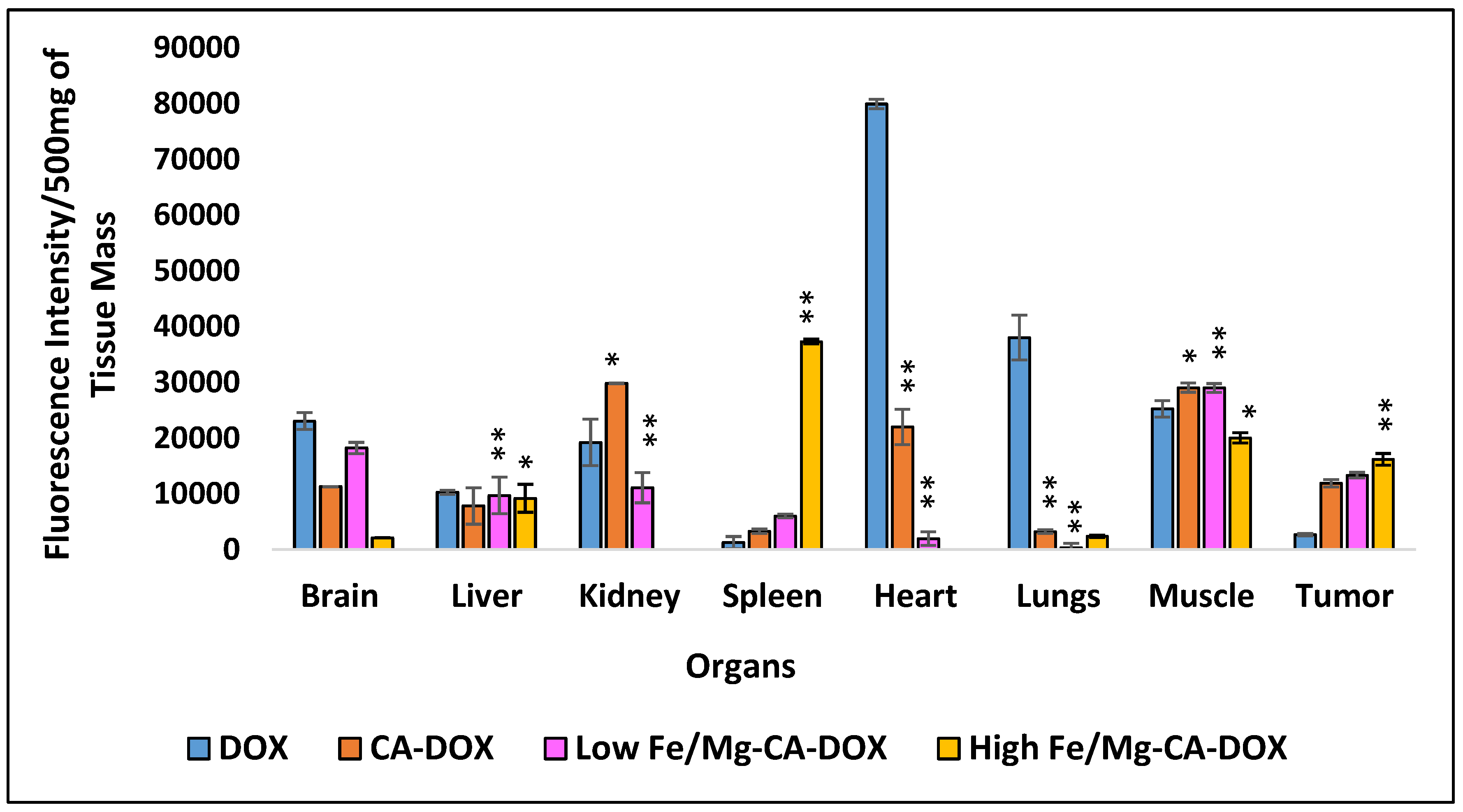
3.15. Animal Biodistribution Study of Fe/Mg-CA NPs
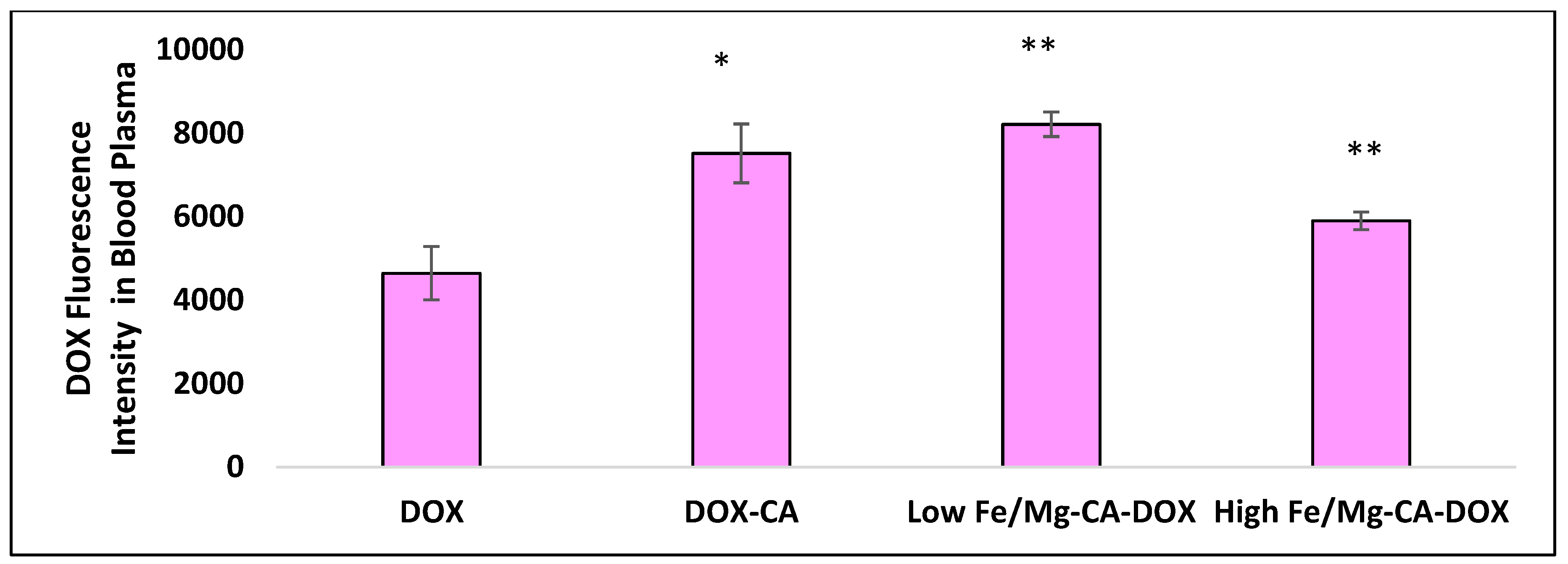
3.16. Blood Plasma Analysis of Fe/Mg-CA NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Alteri, R.; Bertaut, B.; Brooks, D.; Chambers, W.; Chang, E.; DeSantis, C.; Gansler, T.; Gapstur, S.; Gaudet, M.; Gober, K.; et al. Cancer Facts & Figures; American Cancer Society: Atlanta, GA, USA, 2015; pp. 1–52. [Google Scholar]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef]
- Shao, N.; Lu, S.; Wickstrom, E.; Panchapakesan, B. Integrated molecular targeting of IGF1R and HER2 surface receptors and destruction of breast cancer cells using single wall carbon nanotubes. Nanotechnology 2007, 18, 315101. [Google Scholar] [CrossRef]
- Panchapakesan, B.; Lu, S.; Sivakumar, K.; Taker, K.; Cesarone, G.; Wickstrom, E. Single-wall carbon nanotube nanobomb agents for killing breast cancer cells. NanoBiotechnology 2005, 1, 153–160. [Google Scholar] [CrossRef]
- Panchapakesan, B.; Book-Newell, B.; Sethu, P.; Rao, M.; Irudayaraj, J. Gold nanoprobes for theranostics. Nanomedicine 2011, 6, 1787–1811. [Google Scholar] [CrossRef] [PubMed]
- Loeian, M.S.; Mehdi Aghaei, S.; Farhadi, F.; Rai, V.; Yang, H.W.; Johnson, M.D.; Aqil, F.; Mandadi, M.; Rai, S.N.; Panchapakesan, B. Liquid biopsy using the nanotube-CTC-chip: Capture of invasive CTCs with high purity using preferential adherence in breast cancer patients. Lab Chip 2019, 19, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Watson, P.; Conway, T.A.; Lynch, J.F. Clinical/genetic features in hereditary breast cancer. Breast Cancer Res. Treat. 1990, 15, 63–71. [Google Scholar] [CrossRef]
- Dharap, S.S.; Wang, Y.; Chandna, P.; Khandare, J.J.; Qiu, B.; Gunaseelan, S.; Sinko, P.J.; Stein, S.; Farmanfarmaian, A.; Minko, T. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 12962–12967. [Google Scholar] [CrossRef]
- Etrych, T.; Jelinkova, M.; Rihova, B.; Ulbrich, K. New HPMA copolymers containing doxorubicin bound via pH-sensitive linkage: Synthesis and preliminary in vitro and in vivo biological properties. J. Control. Release 2001, 73, 89–102. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Mozar, F.S.; Chowdhury, E.H. PEGylation of Carbonate Apatite Nanoparticles Prevents Opsonin Binding and Enhances Tumor Accumulation of Gemcitabine. J. Pharm. Sci. 2018, 107, 2497–2508. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Pahle, J.; Walther, W. Vectors and strategies for nonviral cancer gene therapy. Expert Opin. Biol. Ther. 2016, 16, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Walter, W.; Stein, U. Viral vectors for gene transfer a review of their use in the treatment of human disease. Drugs 2000, 60, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.F.; Liu, Z.A.; Huang, W.B.; Tian, A.L.; Hu, S.Y. The research of nanoparticles as gene vector for tumor gene therapy. Crit. Rev. Oncol. Hematol. 2014, 89, 352–357. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef]
- Karim, M.E.; Tha, K.K.; Othman, I.; Borhan Uddin, M.; Chowdhury, E.H. Therapeutic Potency of Nanoformulations of siRNAs and shRNAs in Animal Model Cancers. Pharmaceutics 2018, 10, 65. [Google Scholar] [CrossRef]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone, C.B., 2nd; Charles, B.; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.T.; Chowdhury, E.H. Recent Progress in Delivery of Therapeutic and Imaging Agents Utilizing Organic-Inorganic Hybrid Nanoparticles. Curr. Drug Deliv. 2018, 15, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowiak, J.W.; Verduzco, D.; Schramm, K.J.; Gillies, R.J. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol. Pharm. 2011, 8, 2032–2038. [Google Scholar] [CrossRef]
- Hossain, S.; Stanislaus, A.; Chua, M.J.; Tada, S.; Tagawa, Y.I.; Chowdhury, E.H.; Akaike, T. Carbonate apatite-facilitated intracellularly delivered siRNA for efficient knockdown of functional genes. J. Control. Release 2010, 147, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Helmlinger, G.; Sckell, A.; Dellian, M.; Forbes, N.S.; Jain, R.K. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin. Cancer Res. 2002, 8, 1284–1291. [Google Scholar] [PubMed]
- Hossain, S.; Tada, S.; Akaike, T.; Chowdhury, E.H. Influences of electrolytes and glucose on formulation of carbonate apatite nanocrystals for efficient gene delivery to mammalian cells. Anal. Biochem. 2010, 397, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Chowdhury, E.H.; Akaike, T. Nanoparticles and toxicity in therapeutic delivery: The ongoing debate. Therapeutic. Deliv. 2011, 2, 125–132. [Google Scholar] [CrossRef]
- Tada, S.; Chowdhury, E.H.; Cho, C.S.; Akaike, T. pH-sensitive carbonate apatite as an intracellular protein transporter. Biomaterials 2010, 31, 1453–1459. [Google Scholar] [CrossRef]
- Kutsuzawa, K.; Akaike, T.; Chowdhury, E.H. The influence of the cell-adhesive proteins E-cadherin and fibronectin embedded in carbonate-apatite DNA carrier on transgene delivery and expression in a mouse embryonic stem cell line. Biomaterials 2008, 29, 370–376. [Google Scholar] [CrossRef]
- Chowdhury, E.H. pH-sensitive nano-crystals of carbonate apatite for smart and cell-specific transgene delivery. Expert Opin. Drug Deliv. 2007, 4, 193–196. [Google Scholar] [CrossRef]
- Chowdhury, E. pH-responsive magnesium- and carbonate-substituted apatite nano-crystals for efficient and cell-targeted delivery of transgenes. Open J. Genet. 2013, 3, 33687. [Google Scholar] [CrossRef]
- Filip, R.; Pierzynowski, S.G. The role of glutamine and α-ketoglutarate in gut metabolism and the potential application in medicine and nutrition. J. Pre-Clin. Clin. Res. 2007, 1, 9–15. [Google Scholar]
- Iafisco, M.; Drouet, C.; Adamiano, A.; Pascaud, P.; Montesi, M.; Panseri, S.; Sarda, S.; Tampieri, A. Superparamagnetic iron-doped nanocrystalline apatite as a delivery system for doxorubicin. J. Mater. Chem. B 2016, 4, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.; Rijal, N.P.; Khanal, S.; Pai, D.; Sankar, J.; Bhattarai, N. Magnesium incorporated chitosan based scaffolds for tissue engineering applications. Bioact. Mater. 2016, 1, 132–139. [Google Scholar] [CrossRef]
- Haas, J.D.; Brownlie, T.T. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J. Nutr. 2001, 131, 676S–688S. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Lynch, I.; Ejtehadi, M.R.; Monopoli, M.P.; Bombelli, F.B.; Laurent, S. Protein−Nanoparticles Interactions: Opportunities and Challenges. Chem. Rev. 2011, 111, 5610–5637. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, Y.; Shen, W.; Zhong, W. Fluorescamine Labeling for Assessment of Protein Conformational Change and Binding Affinity in Protein-Nanoparticle Interaction. Anal. Chem. 2017, 89, 12160–12167. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.M.; Liversidge, G.G. Drug nanoparticles: Formulating poorly water-soluble compounds. Toxicol. Pathol. 2008, 36, 43–48. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, p.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Zhao, N.; Woodle, M.C.; Mixson, A.J. Advances in delivery systems for doxorubicin. J. Nanomed. Nanotechnol. 2018, 9, 519. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Peetla, C.; Bhave, R.; Vijayaraghavalu, S.; Stine, A.; Kooijman, E.; Labhasetwar, V. Drug resistance in breast cancer cells: Biophysical characterization of and doxorubicin interaction with membrane lipids. Mol. Pharm. 2010, 7, 2334–2348. [Google Scholar] [CrossRef] [PubMed]
- Mehbuba Hossain, S.; Chowdhury, E.H. Citrate- and Succinate-Modified Carbonate Apatite Nanoparticles with Loaded Doxorubicin Exhibit Potent Anticancer Activity against Breast Cancer Cells. Pharmaceutics 2018, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Fatemian, T.; Chowdhury, E.H. Cytotoxicity Enhancement in Breast Cancer Cells with Carbonate Apatite-Facilitated Intracellular Delivery of Anti-Cancer Drugs. Toxics 2018, 6, 12. [Google Scholar] [CrossRef]
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The future of cancer diagnosis and therapy. Cancer J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Chowdhury, E.H.; Maruyama, A.; Kano, A.; Nagaoka, M.; Kotaka, M.; Hirose, S.; Kunou, M.; Akaike, T. pH-sensing nano-crystals of carbonate apatite: Effects on intracellular delivery and release of DNA for efficient expression into mammalian cells. Gene 2006, 376, 87–94. [Google Scholar] [CrossRef]
- Scapin, M.A.; Guilhen, S.N.; Cotrim, M.E.; Pires, M.A. Determination of Ca/P molar ratio in hydroxyapatite (HA) by X-ray fluorescence technique. In Proceedings of the INAC 2015: International Nuclear Atlantic Conference Brazilian Nuclear Program State Policy for a Sustainable World, Sao Paulo, Brazil, 4–9 October 2015. [Google Scholar]
- Wei, M.; Evans, J.H.; Bostrom, T.; Grondhal, L. Synthesis and characterization of hydroxyapatite, fluoride-substituted hydroxyapatite and fluorapatite. J. Mater. Sci. Mater. Med. 2003, 14, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Guo, D.; Xiao, K.; Wang, X.; Wang, L.; Luo, J. A drug-specific nanocarrier design for efficient anticancer therapy. Nat. Commun. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Yamamoto, H.; Chowdhury, E.H.; Wu, X.; Hirose, H.; Haque, A.; Doki, Y.; Mori, M.; Akaike, T. Fabrication and intracellular delivery of doxorubicin/carbonate apatite nanocomposites: Effect on growth retardation of established colon tumor. PLoS ONE 2013, 8, e60428. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Kong, H.; Zeng, Y.; Liu, G. Functional biomimetic nanoparticles for drug delivery and theranostic applications in cancer treatment. Sci. Technol. Adv. Mater. 2018, 19, 771–790. [Google Scholar] [CrossRef]
- Meng, H.; Xue, M.; Xia, T.; Zhao, Y.L.; Tamanoi, F.; Stoddart, J.F.; Zink, J.I.; Nel, A.E. Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J. Am. Chem. Soc. 2010, 132, 12690–12697. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Cheng, S.H.; Huang, I.P.; Souris, J.S.; Yang, C.S.; Mou, C.Y.; Lo, L.W. Intracellular pH-responsive mesoporous silica nanoparticles for the controlled release of anticancer chemotherapeutics. Angew. Chem. Int. Ed. Engl. 2010, 49, 8214–8219. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shi, J.; Shen, W.; Dong, X.; Feng, J.; Ruan, M.; Li, Y. Stimuli-responsive controlled drug release from a hollow mesoporous silica sphere/polyelectrolyte multilayer core-shell structure. Angew. Chem. Int. Ed. Engl. 2005, 44, 5083–5087. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blazquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef]
- Musteata, F.M.; Pawliszyn, J.; Qian, M.G.; Wu, J.-T.; Miwa, G.T. Determination of drug plasma protein binding by solid phase microextraction. J. Pharm. Sci. 2006, 95, 1712–1722. [Google Scholar] [CrossRef]
- Olson, R.E.; Christ, D.D. Plasma Protein Binding of Drugs. Annu. Rep. Med. Chem. 1996, 31, 327–336. [Google Scholar]
- Göppert, T.M.; Müller, R.H. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: Comparison of plasma protein adsorption patterns. J. Drug Target. 2005, 13, 179–187. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, J. Clearance pathways and tumor targeting of imaging nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [PubMed]
- Hayward, R.; Hydock, D.; Gibson, N.; Greufe, S.; Bredahl, E.; Parry, T. Tissue retention of doxorubicin and its effects on cardiac, smooth, and skeletal muscle function. J. Physiol. Biochem. 2013, 69, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Van Norren, K.; Van Helvoort, A.; Argiles, J.M.; Van Tuijl, S.; Arts, K.; Gorselink, M.; Laviano, A.; Kegler, D.; Haagsman, H.P.; Van Der Beek, E.M. Direct effects of doxorubicin on skeletal muscle contribute to fatigue. Br. J. Cancer 2009, 100, 311–314. [Google Scholar] [CrossRef] [PubMed]



| Element | Wavelength (nM) | Flame |
|---|---|---|
| Ca | 422.7 | Air- Acetylene |
| Fe | 248.3 | Air- Acetylene |
| Mg | 285.2 | Air- Acetylene |
| Sample | Wave Number (cm−1) | Ionic Groups |
|---|---|---|
| CA | 865, 1435, 1474, 1662 | CO32− |
| 668, 1012 | PO43− | |
| High Fe/Mg-CA | 862, 1436, 1488, 1520, 1652 | CO32− |
| 566, 668, 1006 | PO43− | |
| Low Fe/Mg-CA | 866, 1436, 1488, 1508, 1662 | CO32− |
| 555, 575, 668, 1017 | PO43− |
| Ratio | Low Fe/Mg-CA (wt. (%) ± stdev.) | High Fe/Mg-CA (wt. (%) ± stdev.) | CA (wt. (%) ± stdev.) |
|---|---|---|---|
| Ca/P | 3.65 ± 0.11 | 4.27 ± 0.22 | 2.00 ± 0.13 |
| Ca+Mg/P | 5.08 ± 0.13 | 5.81 ± 0.22 | 2.39 ± 0.09 |
| Sample | Mg/Ca (wt.(%) ± stdev.) |
|---|---|
| Low Fe/Mg-CA | 0.41 ± 0.06 |
| High Fe/Mg-CA | 0.41 ± 0.01 |
| CA | 0.16 ± 0.01 |
| Sample | Weight (%) of Ca ± stdev. | Weight (%) of Mg ± stdev. | Weight (%) of Fe ± stdev. |
|---|---|---|---|
| CA | 29 ± 0.07 | ||
| Low Fe/Mg-CA | 20 ± 0.16 | 1 ± 0.05 | 0.3 ± 0.08 |
| High Fe/Mg-CA | 25 ± 0.14 | 3 ± 0.11 | 2 ± 0.26 |
| Samples | Cellular Uptake (%) (4 h after Treatment) | Cellular Uptake (%) (24 h after Treatment) |
|---|---|---|
| Free DOX | 26 ± 0.28 | 17 ± 0.01 |
| DOX-loaded low Fe/Mg-CA | 44 ± 0.42 | 24 ± 0.97 |
| DOX-loaded high Fe/Mg-CA | 49 ± 0.42 | 26 ± 0.61 |
| DOX-loaded CA | 40 ± 1.14 | 19 ± 0.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, S.T.; Karim, M.E.; Abidin, S.A.Z.; Othman, I.; Holl, M.M.B.; Chowdhury, E.H. Fe/Mg-Modified Carbonate Apatite with Uniform Particle Size and Unique Transport Protein-Related Protein Corona Efficiently Delivers Doxorubicin into Breast Cancer Cells. Nanomaterials 2020, 10, 834. https://doi.org/10.3390/nano10050834
Haque ST, Karim ME, Abidin SAZ, Othman I, Holl MMB, Chowdhury EH. Fe/Mg-Modified Carbonate Apatite with Uniform Particle Size and Unique Transport Protein-Related Protein Corona Efficiently Delivers Doxorubicin into Breast Cancer Cells. Nanomaterials. 2020; 10(5):834. https://doi.org/10.3390/nano10050834
Chicago/Turabian StyleHaque, Sheikh Tanzina, Md. Emranul Karim, Syafiq Asnawi Zainal Abidin, Iekhsan Othman, Mark M. Banaszak Holl, and Ezharul Hoque Chowdhury. 2020. "Fe/Mg-Modified Carbonate Apatite with Uniform Particle Size and Unique Transport Protein-Related Protein Corona Efficiently Delivers Doxorubicin into Breast Cancer Cells" Nanomaterials 10, no. 5: 834. https://doi.org/10.3390/nano10050834
APA StyleHaque, S. T., Karim, M. E., Abidin, S. A. Z., Othman, I., Holl, M. M. B., & Chowdhury, E. H. (2020). Fe/Mg-Modified Carbonate Apatite with Uniform Particle Size and Unique Transport Protein-Related Protein Corona Efficiently Delivers Doxorubicin into Breast Cancer Cells. Nanomaterials, 10(5), 834. https://doi.org/10.3390/nano10050834







