Incorporating Aminated Nanodiamonds to Improve the Mechanical Properties of 3D-Printed Resin-Based Biomedical Appliances
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Purified and Aminated ND-Incorporated Nanocomposites
2.3. Characterizing the ND- and A-ND-Incorporated Nanocomposites
2.4. Water Contact Angle and Hydrophilicity
2.5. Water Sorption and Solubility
2.6. Flexural Strength and Modulus
2.7. Impact Strength
2.8. Surface Hardness and Wear Resistance
2.9. Hydro-Thermal Fatigue Testing
2.10. Trueness
2.11. Statistical Analysis
3. Results
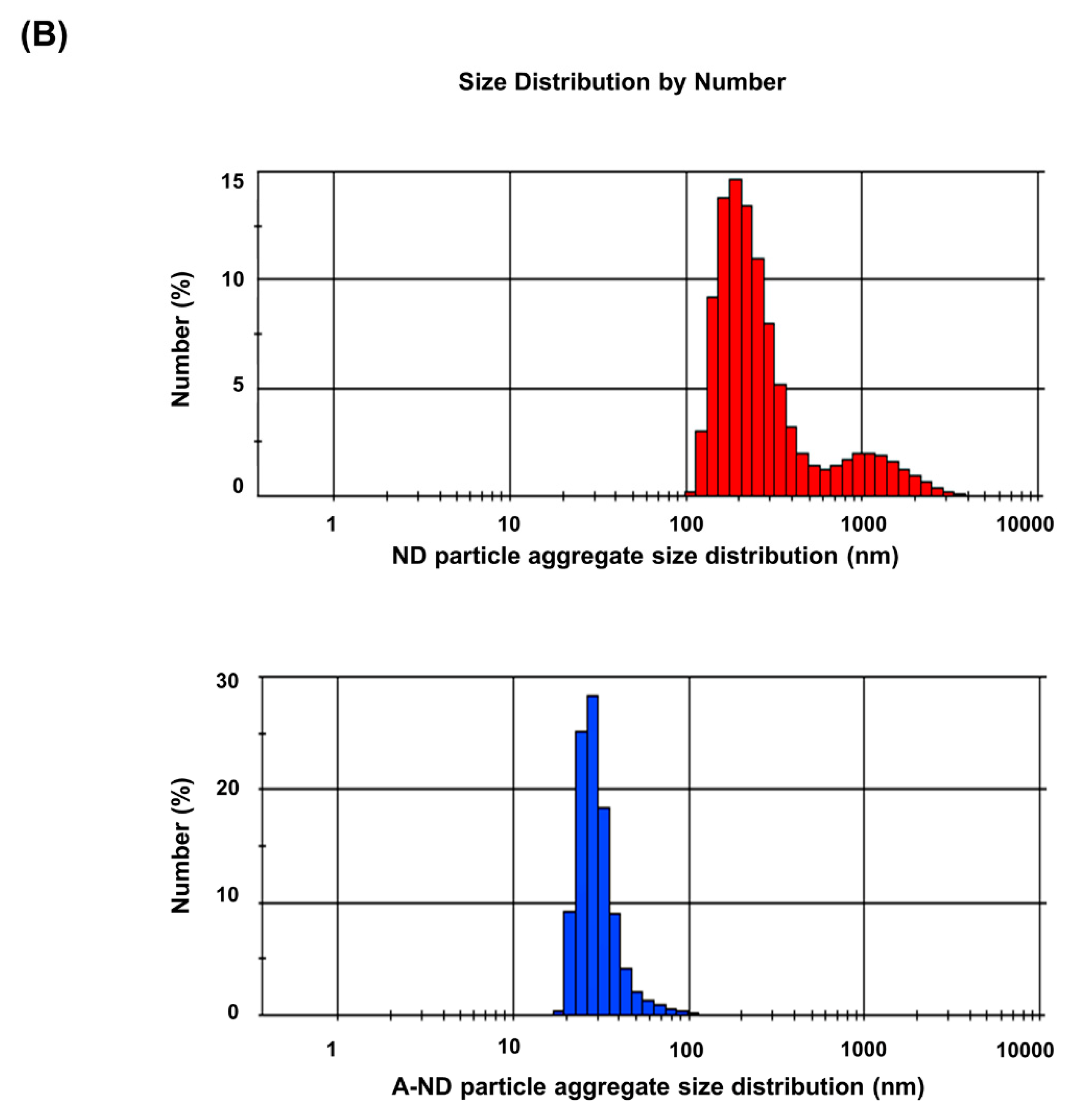
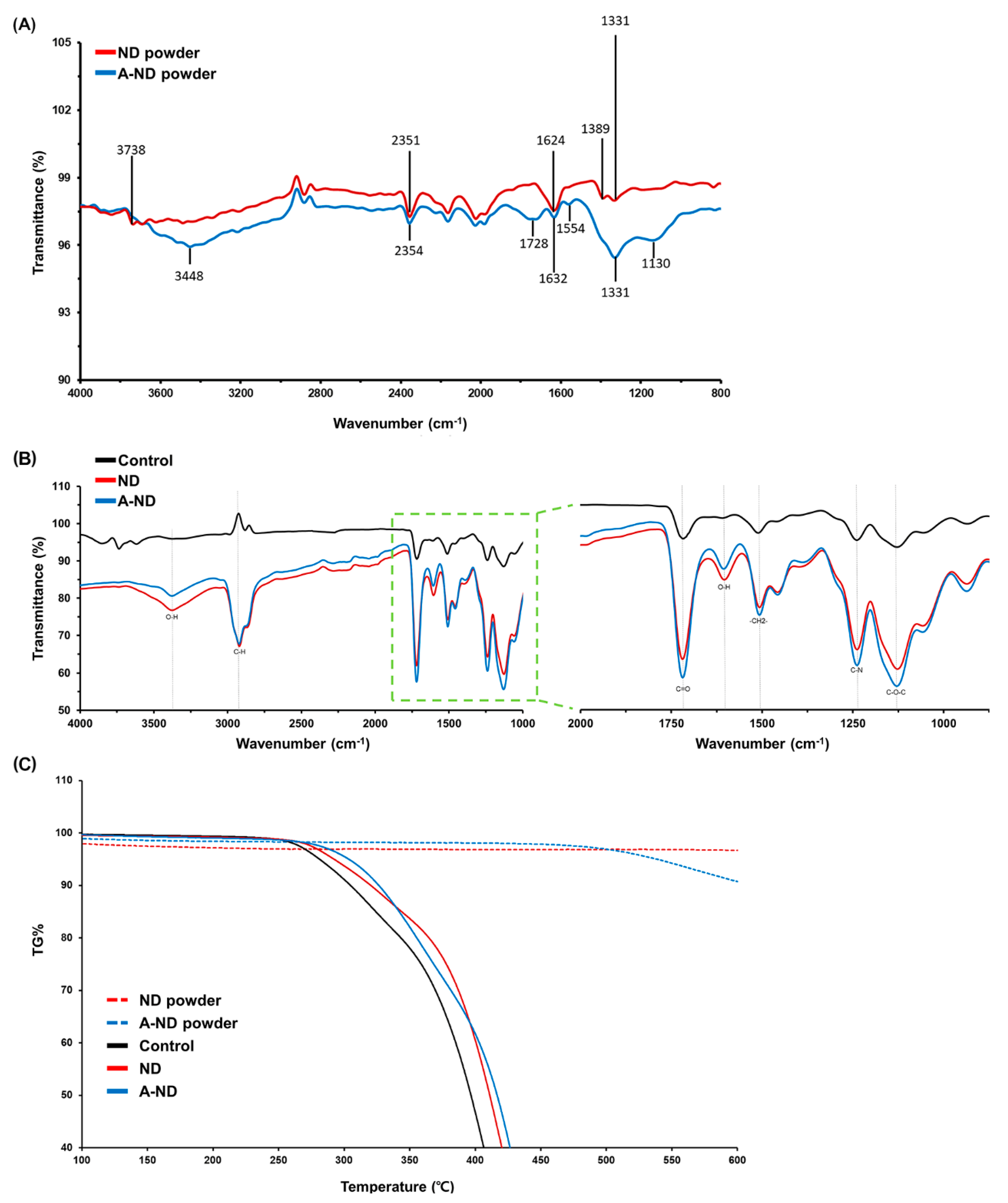
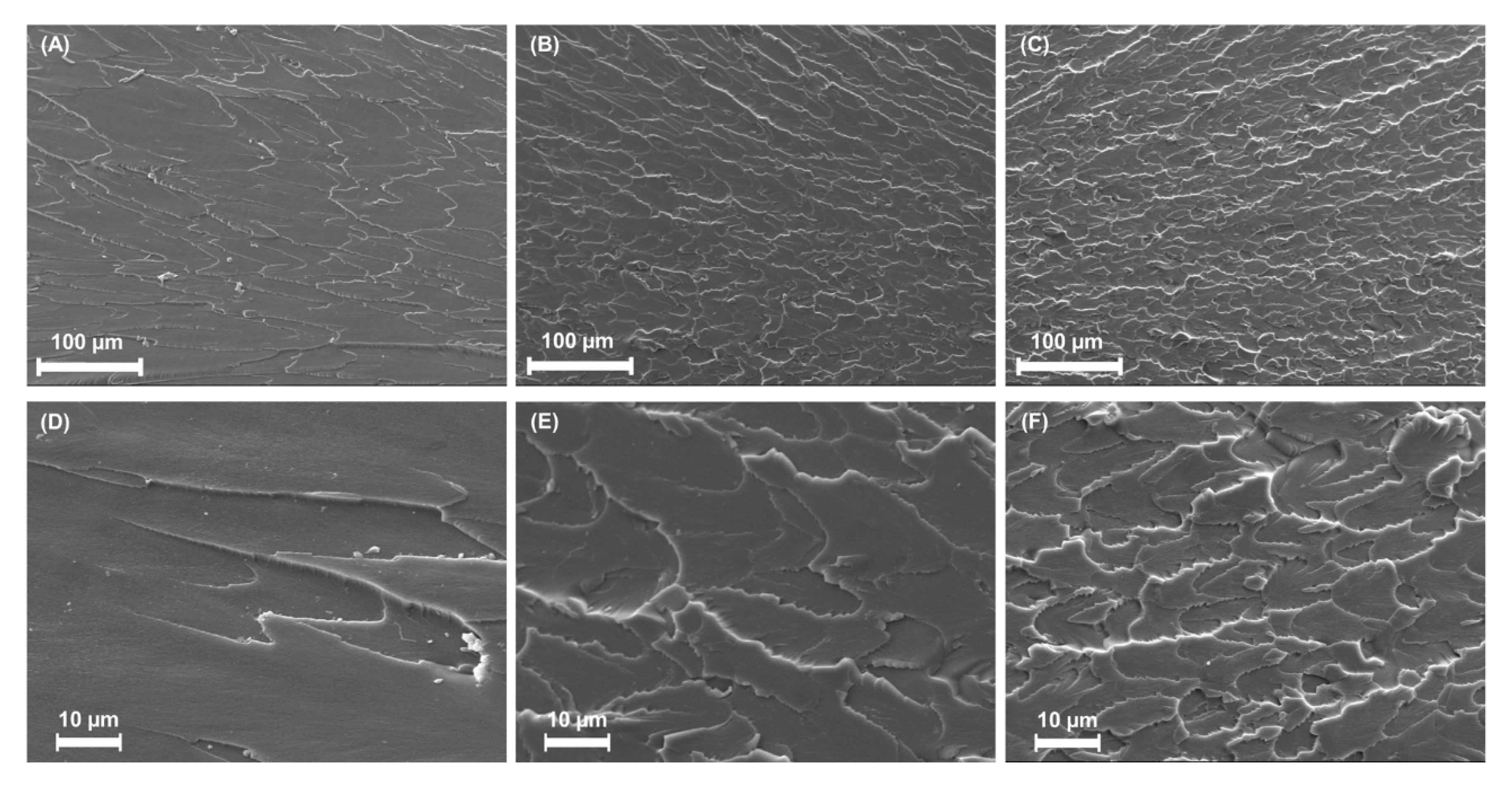
3.1. Characterizing ND- and A-ND-Incorporated Nanocomposites
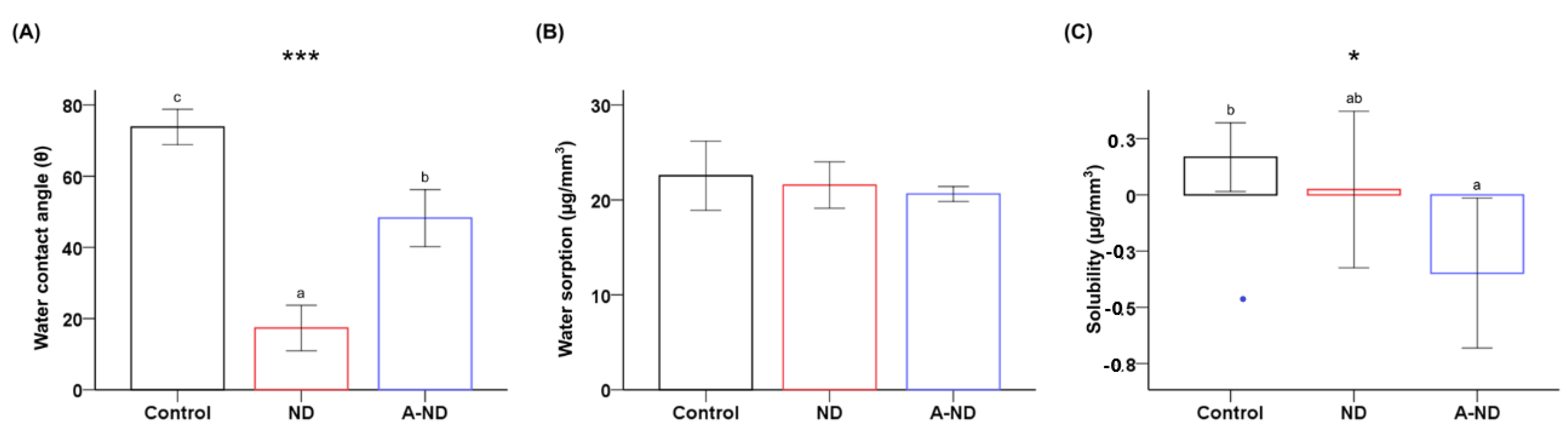
3.2. Hydrophilicity, Water Sorption, and Solubility
3.3. Mechanical Properties of the Nanocomposites
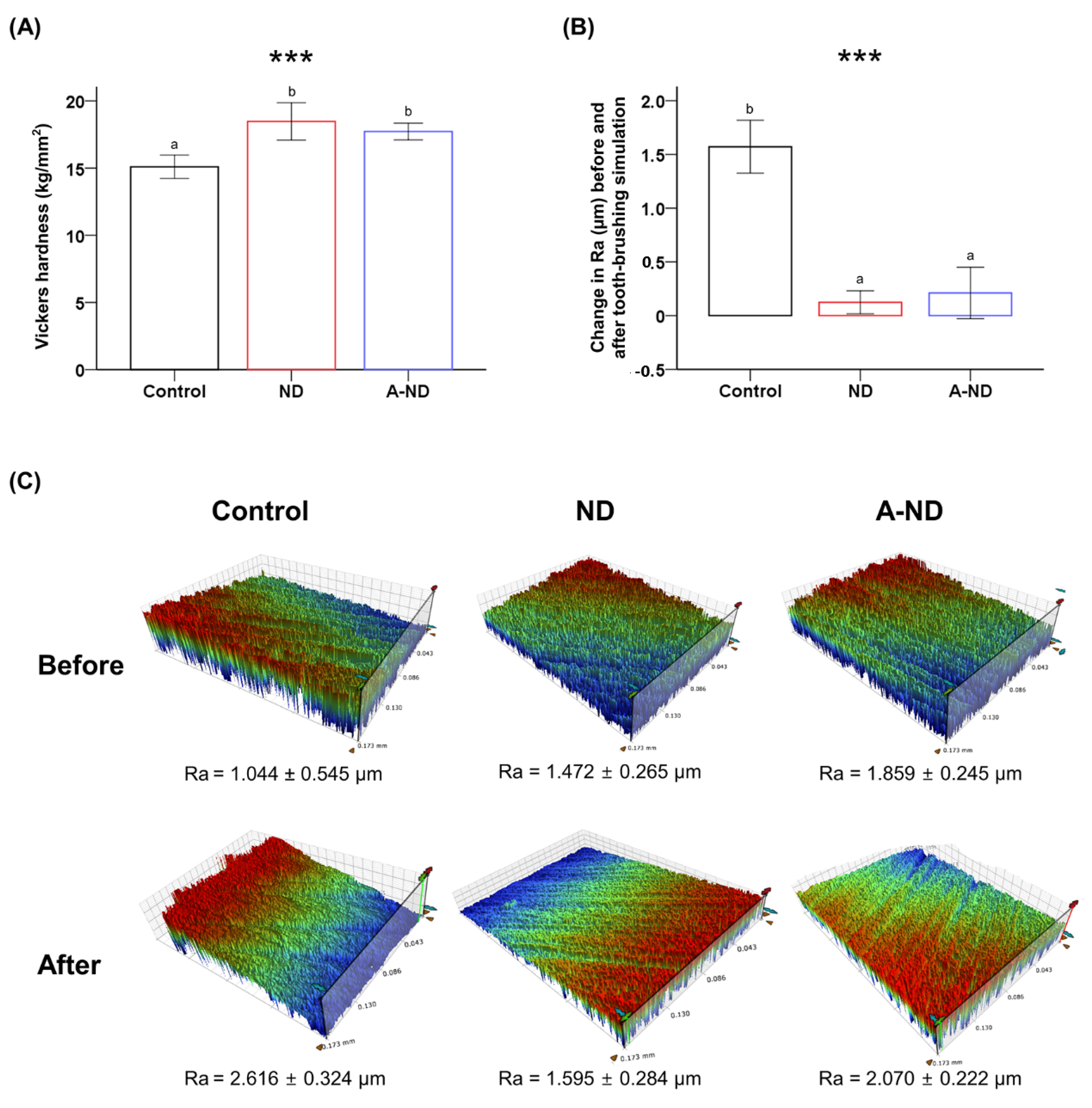
3.4. Surface Microhardness and Wear Resistance
3.5. Response to Hydro-Thermal Fatigue
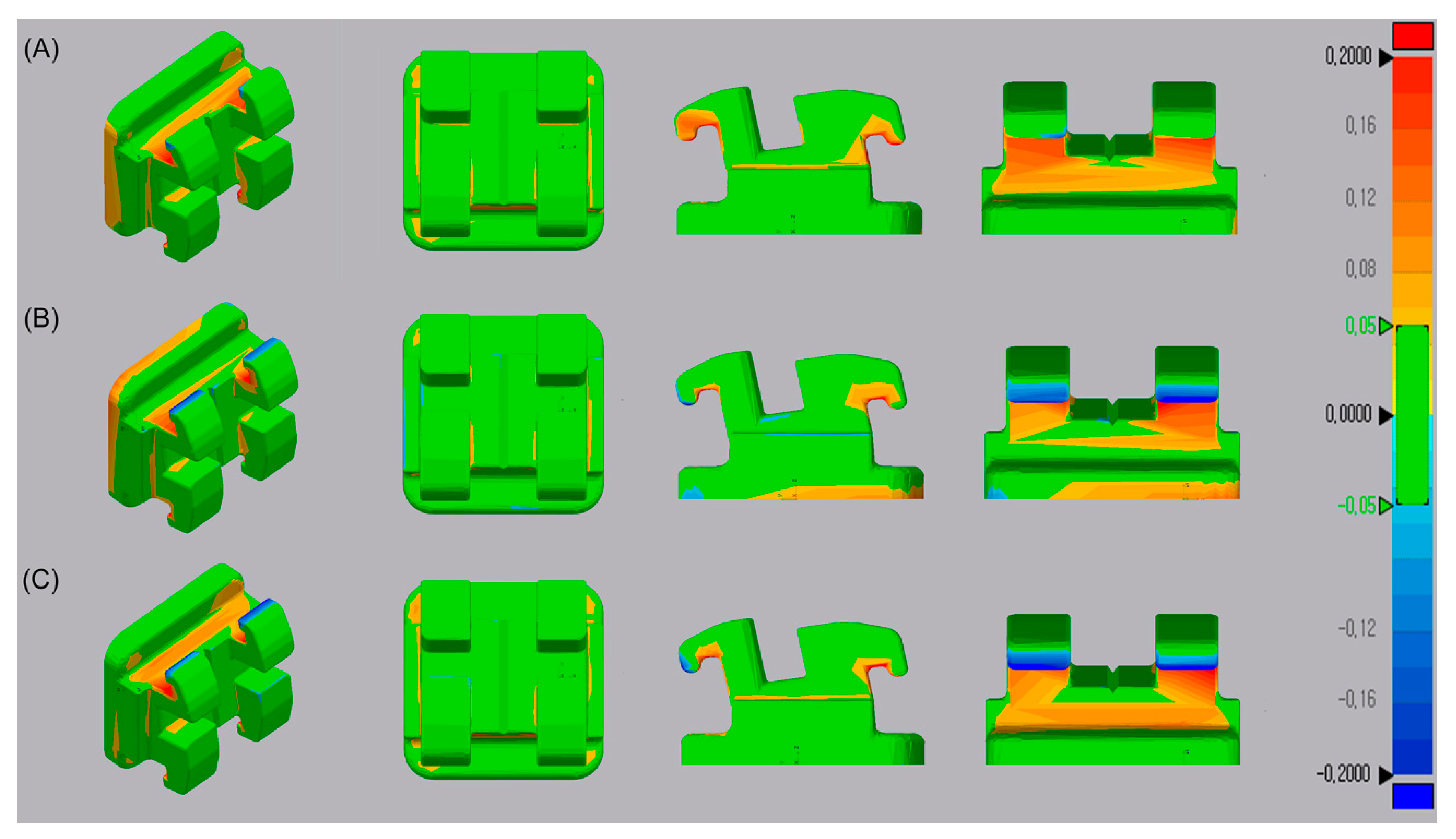
3.6. Trueness
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Attaran, M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.Y.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Krey, K.F.; Darkazanly, N.; Kuhnert, R.; Ruge, S. 3D-printed orthodontic brackets-proof of concept. Int. J. Comput. Dent. 2016, 19, 351–362. [Google Scholar] [PubMed]
- Choi, S.H.; Kang, D.Y.; Hwang, C.J. Surface roughness of three types of modern plastic bracket slot floors and frictional resistance. Angle Orthod. 2014, 84, 177–183. [Google Scholar] [CrossRef]
- Karami, P.; Khasraghi, S.S.; Hashemi, M.; Rabiei, S.; Shojaei, A. Polymer/nanodiamond composites-a comprehensive review from synthesis and fabrication to properties and applications. Adv. Colloid Interfac 2019, 269, 122–151. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.Y.; Li, Y.; Hao, L.; Yang, Y.H.; Tang, T.; Tang, D.N.; Xiong, W. Graphene-Reinforced Biodegradable Resin Composites for Stereolithographic 3D Printing of Bone Structure Scaffolds. J. Nanomater. 2019, 2019, 9710264. [Google Scholar] [CrossRef]
- Ho, D.A.; Wang, C.H.K.; Chow, E.K.H. Nanodiamonds: The intersection of nanotechnology, drug development, and personalized medicine. Sci. Adv. 2015, 1, e1500439. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Mangal, U.; Kim, J.Y.; Seo, J.Y.; Kwon, J.S.; Choi, S.H. Novel Poly(Methyl Methacrylate) Containing Nanodiamond to Improve the Mechanical Properties and Fungal Resistance. Materials 2019, 12, 3438. [Google Scholar] [CrossRef]
- Protopapa, P.; Kontonasaki, E.; Bikiaris, D.; Paraskevopoulos, K.M.; Koidis, P. Reinforcement of a PMMA resin for fixed interim prostheses with nanodiamonds. Dent. Mater. J. 2011, 30, 222–231. [Google Scholar] [CrossRef]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Mochalin, V.N.; Neitzel, I.; Knoke, I.Y.; Han, J.J.; Klug, C.A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Fluorescent PLLA-nanodiamond composites for bone tissue engineering. Biomaterials 2011, 32, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Neitzel, I.; Mochalin, V.; Bares, J.A.; Carpick, R.W.; Erdemir, A.; Gogotsi, Y. Tribological Properties of Nanodiamond-Epoxy Composites. Tribol. Lett. 2012, 47, 195–202. [Google Scholar] [CrossRef]
- Rakha, S.A.; Ali, N.; Haleem, Y.A.; Alam, F.; Khurram, A.A.; Munir, A. Comparison of Mechanical Properties of Acid and UV Ozone Treated Nanodiamond Epoxy Nanocomposites. J. Mater. Sci. Technol. 2014, 30, 753–758. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Buron, M.; Gude, M.R.; Chaos-Moran, R.; Campo, M.; Urena, A. Effects of dispersion techniques of carbon nanofibers on the thermo-physical properties of epoxy nanocomposites. Compos. Sci. Technol. 2008, 68, 2722–2730. [Google Scholar] [CrossRef]
- Lee, M.J.; Kwon, J.S.; Kim, J.Y.; Ryu, J.H.; Seo, J.Y.; Jang, S.; Kim, K.M.; Hwang, C.J.; Choi, S.H. Bioactive resin-based composite with surface pre-reacted glass-ionomer filler and zwitterionic material to prevent the formation of multi-species biofilm. Dent. Mater. 2019, 35, 1331–1341. [Google Scholar] [CrossRef]
- Kritika, S.; Jothimani, B.; Vidhya, S.; Sanjeev, K.; Mahalaxmi, S.; Venkatachalapathy, B.; Sureshkumar, S. Incorporation of hydrophobic nanochitosan improves wear resistance of dental sealants. Int. J. Polym. Mater. Polym. Biomater. 2020, 1–9. [Google Scholar] [CrossRef]
- Kim, B.I.; Jeong, S.H.; Jang, S.O.; Kim, K.N.; Kwon, H.K.; Park, Y.D. Tooth whitening effect of toothpastes containing nano-hydroxyapatite. Key Eng. Mater. 2006, 309–311, 541–544. [Google Scholar]
- Gale, M.S.; Darvell, B.W. Thermal cycling procedures for laboratory testing of dental restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, Y.S.; Jung, H.D.; Hwang, C.J.; Baik, H.S.; Cha, J.Y. Precision and trueness of dental models manufactured with different 3-dimensional printing techniques. Am. J. Orthod. Dentofac. 2018, 153, 144–153. [Google Scholar] [CrossRef]
- Miller, F.A. Amides, Carboxylate Ion, and C-O Single Bonds. In Course Notes on the Interpretation of Infrared and Raman Spectra; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Mochalin, V.N.; Neitzel, I.; Etzold, B.J.M.; Peterson, A.; Palmese, G.; Gogotsi, Y. Covalent incorporation of aminated nanodiamond into an epoxy polymer network. ACS Nano 2011, 5, 7494–7502. [Google Scholar] [CrossRef] [PubMed]
- Pentecost, A.; Gour, S.; Mochalin, V.; Knoke, I.; Gogotsi, Y. Deaggregation of nanodiamond powders using salt- and sugar-assisted milling. ACS Appl. Mater. Interfaces 2010, 2, 3289–3294. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, S.; Kausar, A.; Muhammad, B.; Gul, S.; Farooq, M. A Review on Polymeric Nanocomposites of Nanodiamond, Carbon Nanotube, and Nanobifiller: Structure, Preparation and Properties. Polym.-Plast. Technol. 2015, 54, 1379–1409. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Sarabia-Sainz, A.; Fleitas-Salazar, N.; Santacruz-Gomez, K.; Acosta-Elias, M.; Pedroza-Montero, M.; Riera, R. Deagglomeration and characterization of detonation nanodiamonds for biomedical applications. J. Appl. Biomed. 2017, 15, 15–21. [Google Scholar] [CrossRef]
- Lai, L.; Barnard, A.S. Stability of Nanodiamond Surfaces Exposed to N, NH, and NH2. J. Phys. Chem. C 2011, 115, 6218–6228. [Google Scholar] [CrossRef]
- Liu, M.Y.; Xu, D.Z.; Wang, K.; Deng, F.J.; Wan, Q.; Zeng, G.J.; Huang, Q.; Zhang, X.Y.; Wei, Y. Nanodiamond based supermolecular nanocomposites: Preparation and biocompatibility evaluation. RSC Adv. 2015, 5, 96983–96989. [Google Scholar] [CrossRef]
- Kalsoom, U.; Peristyy, A.; Nesterenko, P.N.; Paull, B. A 3D printable diamond polymer composite: A novel material for fabrication of low cost thermally conducting devices. RSC Adv. 2016, 6, 38140–38147. [Google Scholar] [CrossRef]
- Garg, P.; Singh, B.P.; Kumar, G.; Gupta, T.; Pandey, I.; Seth, R.K.; Tandon, R.P.; Mathur, R.B. Effect of dispersion conditions on the mechanical properties of multi-walled carbon nanotubes based epoxy resin composites. J. Polym. Res. 2011, 18, 1397–1407. [Google Scholar] [CrossRef]
- Suave, J.; Coelho, L.A.F.; Amico, S.C.; Pezzin, S.H. Effect of sonication on thermo-mechanical properties of epoxy nanocomposites with carboxylated-SWNT. Mater. Sci. Eng. A-Struct. 2009, 509, 57–62. [Google Scholar] [CrossRef]
- Ayatollahi, M.R.; Alishahi, E.; Doagou-R, S.; Shadlou, S. Tribological and mechanical properties of low content nanodiamond/epoxy nanocomposites. Compos. Part B-Eng. 2012, 43, 3425–3430. [Google Scholar] [CrossRef]
- Yamamoto, B.E.; Trimble, A.Z.; Minei, B.; Nejhad, M.N.G. Development of multifunctional nanocomposites with 3-D printing additive manufacturing and low graphene loading. J Thermoplast. Compos. 2019, 32, 383–408. [Google Scholar] [CrossRef]
- Choi, W.; Jin, J.; Park, S.; Kim, J.Y.; Lee, M.J.; Sun, H.; Kwon, J.S.; Lee, H.; Choi, S.H.; Hong, J. Quantitative Interpretation of Hydration Dynamics Enabled the Fabrication of a Zwitterionic Antifouling Surface. ACS Appl. Mater. Interfaces 2020, 12, 7951–7965. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Cabot, J.M.; Smejkal, P.; Farajikhah, S.; Sayyar, S.; Innis, P.C.; Beirne, S.; Barnsley, G.; Lewis, T.W.; Breadmore, M.C.; et al. Three-Dimensional Printing of Abrasive, Hard, and Thermally Conductive Synthetic Microdiamond-Polymer Composite Using Low-Cost Fused Deposition Modeling Printer. ACS Appl. Mater. Interfaces 2019, 11, 4353–4363. [Google Scholar] [CrossRef] [PubMed]
- Lechleitner, T.; Klauser, F.; Seppi, T.; Lechner, J.; Jennings, P.; Perco, P.; Mayer, B.; Steinmuller-Nethl, D.; Preiner, J.; Hinterdorfer, P.; et al. The surface properties of nanocrystalline diamond and nanoparticulate diamond powder and their suitability as cell growth support surfaces. Biomaterials 2008, 29, 4275–4284. [Google Scholar] [CrossRef]
- Lopes, L.G.; Jardim Filho Ada, V.; de Souza, J.B.; Rabelo, D.; Franco, E.B.; de Freitas, G.C. Influence of pulse-delay curing on sorption and solubility of a composite resin. J. Appl. Oral Sci. 2009, 17, 27–31. [Google Scholar] [CrossRef]
- Tuna, S.H.; Keyf, F.; Gumus, H.O.; Uzun, C. The evaluation of water sorption/solubility on various acrylic resins. Eur. J. Dent. 2008, 2, 191–197. [Google Scholar] [CrossRef]
- Neitzel, I.; Mochalin, V.; Knoke, I.; Palmese, G.R.; Gogotsi, Y. Mechanical properties of epoxy composites with high contents of nanodiamond. Compos. Sci. Technol. 2011, 71, 710–716. [Google Scholar] [CrossRef]
- Jee, A.Y.; Lee, M. Surface functionalization and physicochemical characterization of diamond nanoparticles. Curr. Appl. Phys. 2009, 9, E144–E147. [Google Scholar] [CrossRef]
- Galehdari, N.A.; Kelkar, A.D. Effect of neutron radiation on the mechanical and thermophysical properties of nanoengineered polymer composites. J. Mater. Res. 2017, 32, 426–434. [Google Scholar] [CrossRef]
- McGrath, L.M.; Parnas, R.S.; King, S.H.; Schroeder, J.L.; Fischer, D.A.; Lenhart, J.L. Investigation of the thermal, mechanical, and fracture properties of alumina-epoxy composites. Polymer 2008, 49, 999–1014. [Google Scholar] [CrossRef]
- Jee, A.Y.; Lee, M. Mechanical Properties of Polycarbonate and Poly(Methyl Methacrylate) Films Reinforced with Surface-Functionalized Nanodiamonds. J. Nanosci. Nanotechno. 2011, 11, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Carlyle, W.C.; De Smedt, P.; Abolfathi, A.; Kuo, E.E.; Tricca, R.E.; Chen, J.C.; Wu, B.M. Systems and Methods for Intra-Oral Drug Delivery. U.S. Patent No. 8,439,674, 14 May 2013. [Google Scholar]
- Bonda, D.J.; Manjila, S.; Selman, W.R.; Dean, D. The Recent Revolution in the Design and Manufacture of Cranial Implants. Neurosurgery 2015, 77, 814–824. [Google Scholar] [CrossRef]
- Ilie, N.; Hilton, T.J.; Heintze, S.D.; Hickel, R.; Watts, D.C.; Silikas, N.; Stansbury, J.W.; Cadenaro, M.; Ferracane, J.L. Academy of Dental Materials guidance-Resin composites: Part I-Mechanical properties. Dent. Mater. 2017, 33, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Pieniak, D.; Przystupa, K.; Walczak, A.; Niewczas, A.M.; Krzyzak, A.; Bartnik, G.; Gil, L.; Lonkwic, P. Hydro-Thermal Fatigue of Polymer Matrix Composite Biomaterials. Materials (Basel) 2019, 12, 3650. [Google Scholar] [CrossRef] [PubMed]
- Finer, Y.; Santerre, J.P. The influence of resin chemistry on a dental composite’s biodegradation. J. Biomed. Mater. Res. Part A 2004, 69, 233–246. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Lee, W.S.; Lee, K.B. Accuracy evaluation of dental models manufactured by CAD/CAM milling method and 3D printing method. J. Adv. Prosthodont. 2018, 10, 245–251. [Google Scholar] [CrossRef]


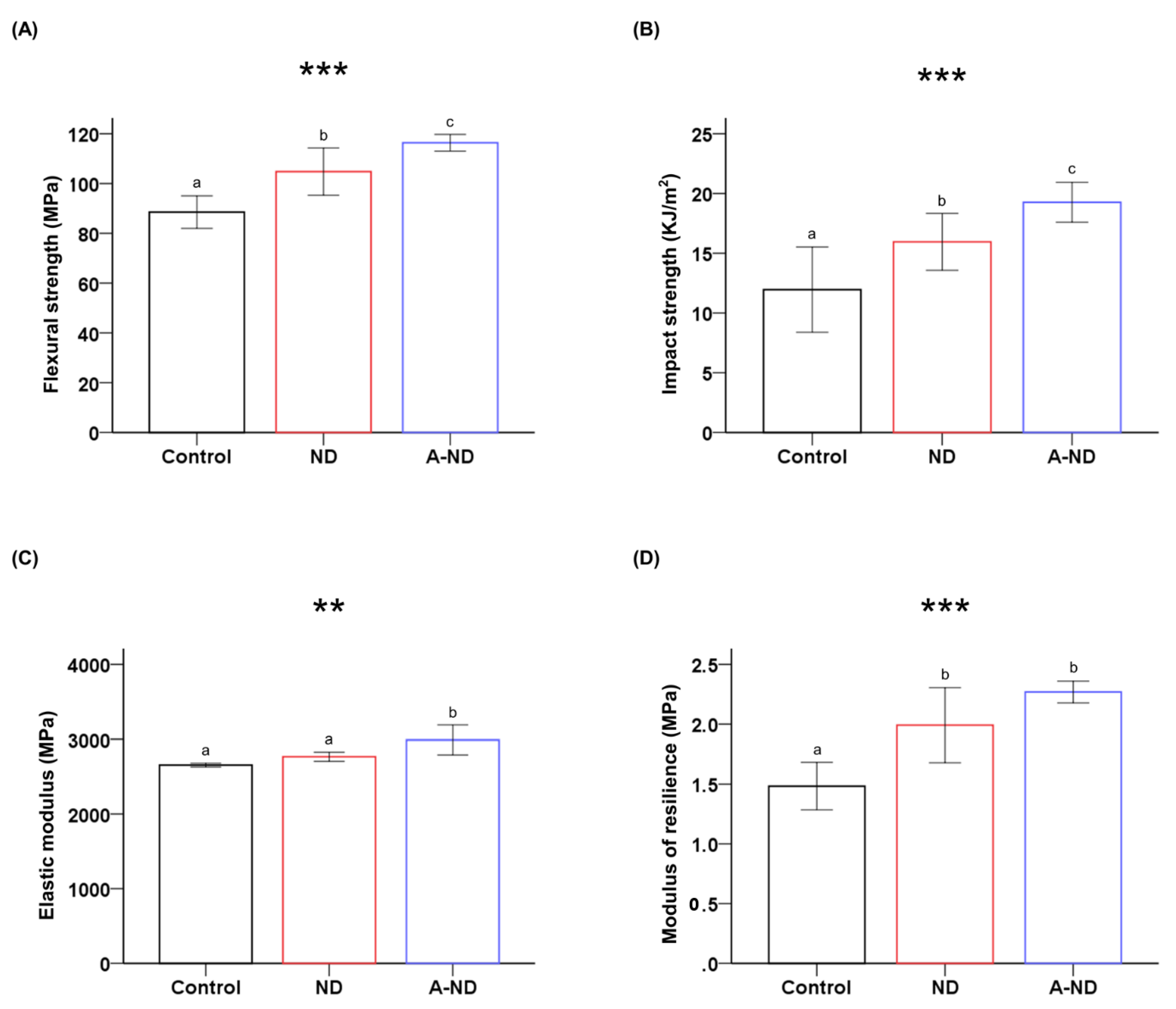
| Group | Flexural Strength (MPa) *** | Impact Strength (kJ/m2) *** | Elastic Modulus (MPa) ** | Modulus of Resilience (MPa) *** | Vickers Microhardness (kg/mm2) *** |
|---|---|---|---|---|---|
| Control | 88.53 ± 6.53 a | 11.95 ± 3.56 a | 2654.34 ± 25.23 a | 1.48 ± 0.19 a | 15.09 ± 0.87 a |
| ND | 104.78 ± 9.49 b | 15.95 ± 2.38 b | 2766.48 ± 61.03 a | 1.99 ± 0.31 b | 18.47 ±1.38 b |
| A-ND | 116.38 ± 3.36 c | 19.26 ± 1.66 c | 2989.58 ± 200.28 b | 2.26 ± 0.09 b | 17.72 ±0.62 b |
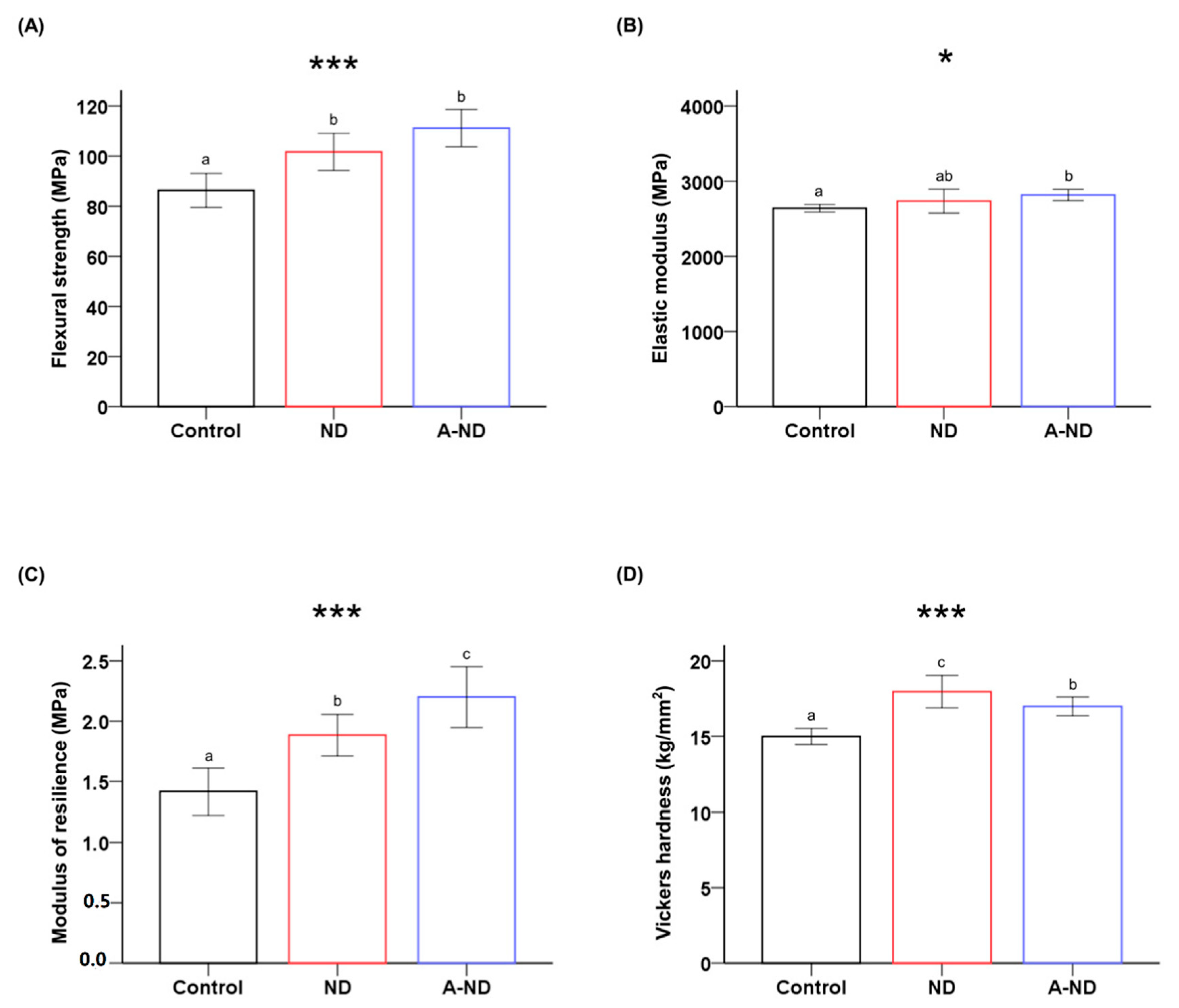
| Groups | Flexural Strength (MPa) *** | Elastic Modulus (MPa) * | Modulus of Resilience (MPa) *** | Vickers Microhardness (kg/mm2) *** |
|---|---|---|---|---|
| Control | 86.35 ± 6.78 a | 2640.00 ± 50.25 a | 1.41 ± 0.19 a | 14.98 ± 0.52 a |
| ND | 101.76 ± 7.44 b | 2735.08 ± 158.70 ab | 1.88 ± 0.17 b | 17.96 ± 1.07 c |
| A-ND | 111.21 ± 7.43 b | 2816.25 ± 73.83 b | 2.20 ± 0.25 c | 16.98 ± 0.62 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangal, U.; Seo, J.-Y.; Yu, J.; Kwon, J.-S.; Choi, S.-H. Incorporating Aminated Nanodiamonds to Improve the Mechanical Properties of 3D-Printed Resin-Based Biomedical Appliances. Nanomaterials 2020, 10, 827. https://doi.org/10.3390/nano10050827
Mangal U, Seo J-Y, Yu J, Kwon J-S, Choi S-H. Incorporating Aminated Nanodiamonds to Improve the Mechanical Properties of 3D-Printed Resin-Based Biomedical Appliances. Nanomaterials. 2020; 10(5):827. https://doi.org/10.3390/nano10050827
Chicago/Turabian StyleMangal, Utkarsh, Ji-Young Seo, Jaehun Yu, Jae-Sung Kwon, and Sung-Hwan Choi. 2020. "Incorporating Aminated Nanodiamonds to Improve the Mechanical Properties of 3D-Printed Resin-Based Biomedical Appliances" Nanomaterials 10, no. 5: 827. https://doi.org/10.3390/nano10050827
APA StyleMangal, U., Seo, J.-Y., Yu, J., Kwon, J.-S., & Choi, S.-H. (2020). Incorporating Aminated Nanodiamonds to Improve the Mechanical Properties of 3D-Printed Resin-Based Biomedical Appliances. Nanomaterials, 10(5), 827. https://doi.org/10.3390/nano10050827







