Synergy Effect of Au and SiO2 Modification on SnO2 Sensor Properties in VOCs Detection in Humid Air
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
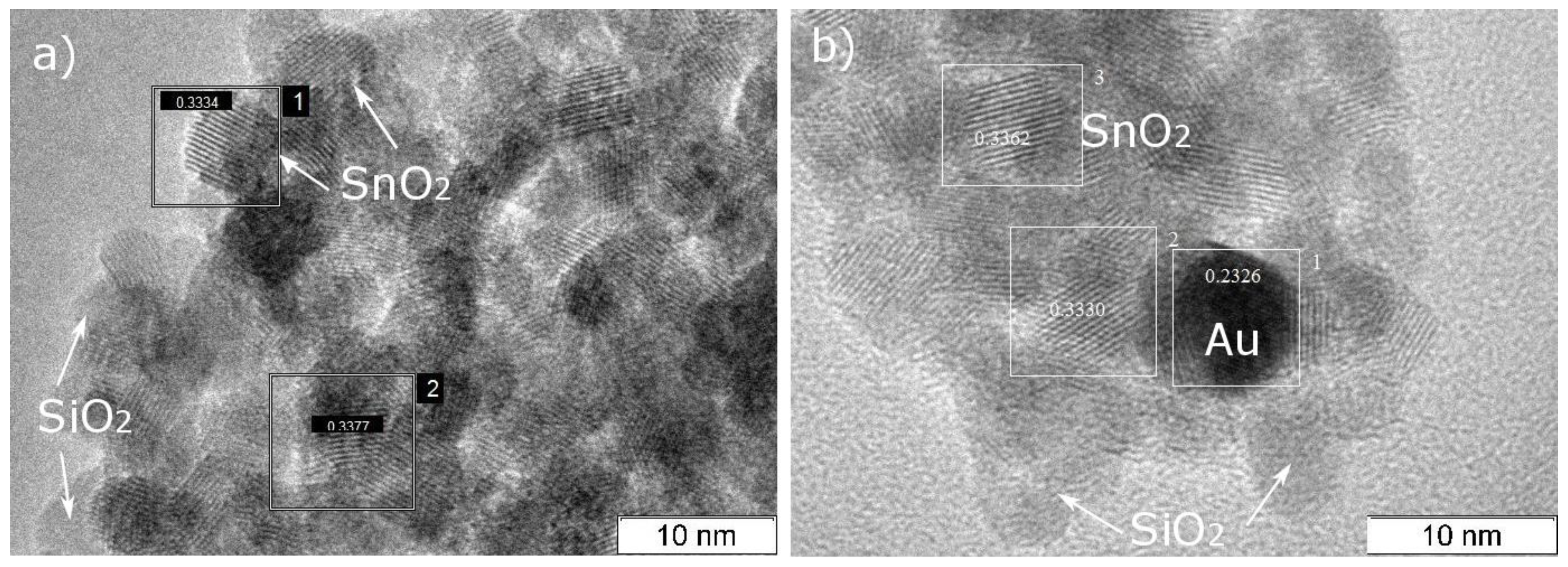
2.2. Materials Characterization
2.3. Study of Sensor Properties
3. Results
4. Conclusions
- —
- The concentration of active oxygen-containing groups on the surface of SnO2/SiO2-Au, directly involved in the oxidation of ethanol and benzene, is the highest among all the samples, which is confirmed by XPS and TPR-H2;
- —
- The addition of silicon dioxide provides long-term stability of the sensor at high temperatures, which is especially necessary for the detection of benzene;
- —
- The addition of SiO2 increases the concentration of Sn3+ defects in tin dioxide, which, in turn, provide the formation of cationic forms Au+ and Au3+, and silanol groups are additionally involved in their stabilization. These forms are characterized by a higher catalytic activity than Au0;
- —
- Similar effective ionic radii provide the possibility of Au3+ substitution in Sn4c positions that leads to a change in the electron density distribution on the Sn-O bond. This effect also leads to the involvement of the SnO2 lattice oxygen in the SnO2/SiO2-Au nanocomposite in the benzene oxidation by the Mars-van Krevelen mechanism.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neri, G. First Fifry Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Park, C.O.; Akbar, S.A. Ceramics for chemical sensing. J. Mater. Sci. 2003, 38, 4611–4637. [Google Scholar] [CrossRef]
- Matsuura, Y.; Takahata, K. Stabilization of SnO2 sintered gas sensors. Sens. Actuat. B 1991, 5, 205–209. [Google Scholar] [CrossRef]
- Gulevich, D.; Rumyantseva, M.; Gerasimov, E.; Marikutsa, A.; Krivetskiy, V.; Shatalova, T.; Khmelevsky, N.; Gaskov, A. Nanocomposites SnO2/SiO2 for CO gas sensors: Microstructure and reactivity in the interaction with the gas phase. Materials 2019, 12, 1096. [Google Scholar] [CrossRef] [PubMed]
- Gulevich, D.; Rumyantseva, M.; Marikutsa, A.; Shatalova, T.; Gerasimov, E.; Gaskov, A. Nanocomposites SnO2/SiO2: SiO2 Impact on the Active Centers and Conductivity Mechanism. Materials 2019, 12, 3618. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Bunpang, K.; Wisitsoraat, A.; Tuantranont, A.; Singkammo, S.; Phanichphant, S.; Liewhiran, C. Highly selective and sensitive CH4 gas sensors based on flame-spray-made Cr-doped SnO2 particulate films. Sens. Actuat. B Chem. 2019, 291, 177–191. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors for detection of benzene, toluene and xylene (BTX) gases: A review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Becker, G.; Domshke, G.; Fangchenel, E.; Fisher, M.; Gewald, K.; Mayer, R.; Pafel, D.; Schmidt, G.; Shvetlik, K.; Berger, V.; et al. Organikum. Practical Work on Organic Chemistry. T2; Translation from German. Mir: Moscow, Russia, 1992; p. 474. [Google Scholar]
- Patnaik, P. A Comprehensive Guide to the Hazardous Properties of Chemical Substances, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; p. 1059. [Google Scholar]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L. Functional gas sensing materials. Appl. Phys. Rev. 2020, 7, 021301. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N., Jr.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, praphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. Ordered mesoporous Ag-doped TiO2/SnO2 nanocomposite based highly sensitive and selective VOC sensors. J. Mater. Chem. A 2016, 4, 1033–1043. [Google Scholar] [CrossRef]
- Haruta, M. Gold as a Novel Catalyst in the 21st Century: Preparation, Working Mechanism and Applications. Gold Bull. 2004, 37, 27–36. [Google Scholar] [CrossRef]
- Scirè, S.; Minicò, S.; Crisafulli, C.; Satriano, C.; Pistone, A. Catalytic combustion of volatile organic compounds on gold/cerium oxide catalysts. Appl. Catal. B Environ. 2003, 40, 43–49. [Google Scholar] [CrossRef]
- Haruta, M. When Gold Is Not Noble: Catalysis by Nanoparticles. Chem. Rec. 2003, 3, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Takei, T.; Iguchi, N.; Haruta, M. Support effect in the gas phase oxidation of ethanol over nanoparticlate gold catalysts. New J. Chem. 2011, 35, 2227–2233. [Google Scholar] [CrossRef]
- Ishida, T.; Koga, H.; Okumura, M.; Haruta, M. Advances in Gold Catalysis and Understanding the Catalytic Mechanism. Chem. Rec. 2016, 16, 2278–2293. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K.; Gulina, L.; Tolstoy, V. SnO2 thin films modified by the SnO2–Au nanocomposites: Response to reducing gases. Sens. Actuat. B 2009, 141, 610–616. [Google Scholar] [CrossRef]
- Hutchings, G.J. New Directions in Gold Catalysis. Gold Bull. 2004, 37, 3–11. [Google Scholar] [CrossRef]
- Takei, T.; Akita, T.; Nakamura, I.; Fujitani, T.; Okumura, M.; Okazaki, K.; Huang, J.; Ishida, T.; Haruta, M. Heterogeneous Catalysis by Gold. In Advances in Catalysis, 1st ed.; Gates, B.C., Jentoft, F.C., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 55, pp. 1–126. [Google Scholar] [CrossRef]
- Fujita, T.; Horikawa, M.; Takei, T.; Murayama, T.; Haruta, M. Correlation between catalytic activity of supported gold catalysts for carbon monoxide oxidation and metal–oxygen binding energy of the support metal oxides. Chin. J. Catal. 2016, 37, 1651–1655. [Google Scholar] [CrossRef]
- Date, M.; Okumura, M.; Tsubota, S.; Haruta, M. Vital Role of Moisture in the Catalytic Activity of Supported Gold Nanoparticles. Angew. Chem. 2004, 43, 2129–2132. [Google Scholar] [CrossRef]
- Katoch, A.; Byun, J.-H.; Choi, S.-W.; Kim, S.S. One-pot synthesis of Au-loaded SnO2 nanofibers and their gas sensing properties. Sens. Actuat. B 2014, 202, 38–45. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Huang, J.; Wang, Y.; Kong, F.; Wu, S.; Zhang, S.; Huang, W. Preparation and CO gas-sensing behavior of Au-doped SnO2 sensors. Vacuum 2006, 81, 394–397. [Google Scholar] [CrossRef]
- Zanella, R. Characterization and reactivity in CO oxidation of gold nanoparticles supported on TiO2 prepared by deposition-precipitation with NaOH and urea. J. Catal. 2004, 222, 357–367. [Google Scholar] [CrossRef]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold Catalysts Prepared by Coprecipitation for Low-Temperature Oxidation of Hydrogen and of Carbon Monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Grisel, R.; Weststrate, K.-J.; Gluhoi, A.; Nieuwenhuys, B.E. Catalysis by Gold Nanoparticles. Gold Bull. 2002, 35, 39–45. [Google Scholar] [CrossRef]
- Haruta, M.; Daté, M. Advances in the catalysis of Au nanoparticles. Appl. Catal. A Gen. 2001, 222, 427–437. [Google Scholar] [CrossRef]
- Jeon, J.; Shim, Y.; Han, S.D.; Kim, D.H.; Kim, Y.H.; Kang, C.; Kim, J.; Kim, M.; Jang, H.W. Vertically ordered SnO2 nanobamboos for substantially improved detection of volatile reducing gases. J. Mater. Chem. A 2015, 3, 17939–179452. [Google Scholar] [CrossRef]
- Liu, C.; Kuang, Q.; Xie, Z.; Zheng, L. The effect of noble metal (Au, Pd and Pt) nanoparticles on the gas sensing performance of SnO2-based sensors: A case study on the {221} high-index faceted SnO2 octahedra. Cryst. Eng. Comm. 2015, 17, 6308–6313. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, M.; Li, X.; Wang, B.; Wang, R. Investigation on synthesis and excellent gas-sensing properties of hierarchical Au-loaded SnO2 nanoflowers. J. Mater. Res. 2019, 34, 2944–2954. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Gong, H.; Ju, D.; Cao, B. Au nanoparticle-functionalized 3D SnO2 microstructures for high performance gas sensor. Sens. Actuator. B Chem. 2016, 226, 266–272. [Google Scholar] [CrossRef]
- Kim, J.-H.; Wu, P.; Kim, H.W.; Kim, S.S. Highly Selective Sensing of CO, C6H6, and C7H8 Gases by Catalytic Functionalization with Metal Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 7173–7183. [Google Scholar] [CrossRef]
- Kim, J.-H.; Zheng, Y.; Mirzaei, A.; Kim, S.S. Excellent Carbon Monoxide Sensing Performance of Au-Decorated SnO2 Nanofibers. Korean J. Mater. Res. 2016, 26. [Google Scholar] [CrossRef]
- Moon, Y.K.; Jeong, S.-Y.; Kang, Y.C.; Lee, J.-H. Metal Oxide Gas Sensors with Au Nanocluster Catalytic Overlayer: Toward Tuning Gas Selectivity and Response Using a Novel Bilayer Sensor Design. ACS Appl. Mater. Interfaces 2019, 11, 32169–32177. [Google Scholar] [CrossRef]
- Kim, J.-H.; Katoch, A.; Kim, H.W.; Kim, S.S. Realization of ppm-level CO detection with exceptionally high sensitivity using reduced graphene oxide-loaded SnO2 nanofibers with simultaneous Au functionalization. Chem. Comm. 2016, 52, 3832–3835. [Google Scholar] [CrossRef]
- Grunwaldt, J.-D.; Kiener, C.; Wogerbauer, C.; Baiker, A. Preparation of Supported Gold Catalysts for Low-Temperature CO Oxidation via “Size-Controlled” Gold Colloids. J. Catal. 1999, 181, 223–232. [Google Scholar] [CrossRef]
- Michaelson, H.B. The work function of the elements and its periodicity. J. Appl. Phys. 1977, 48, 4729–4733. [Google Scholar] [CrossRef]
- Orlandi, M.O. Tin Oxide Materials. Synthesis, Properties and Applications. In Metal Oxides Series, 1st ed.; Korotcenkov, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; p. 666. [Google Scholar]
- Overbury, S.; Schwartz, V.; Mullins, D.; Yan, W.; Dai, S. Evaluation of the Au size effect: CO oxidation catalyzed by Au/TiO2. J. Catal. 2006, 241, 56–65. [Google Scholar] [CrossRef]
- Green, I.X.; Tang, W.; Neurock, M.; Yates, J.T. Spectroscopic Observation of Dual Catalytic Sites during Oxidation of CO on a Au/TiO2 Catalyst. Science 2011, 333, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, T.; Nakamura, I.; Haruta, M. Role of Water in CO Oxidation on Gold Catalysts. Catal. Lett. 2014, 144, 1475–1486. [Google Scholar] [CrossRef]
- Thermo Scientific XPS Simplified. Available online: https://xpssimplified.com/elements (accessed on 1 March 2019).
- Cabot, A.; Arbiol, J.; Morante, J.R.; Weimar, U.; Barsan, N.; Gopel, W. Analysis of the noble metal catalytic additives introduced by impregnation of as obtained SnO2 sol–gel nanocrystals for gas sensors. Sens. Actuator. B 2000, 70, 87–100. [Google Scholar] [CrossRef]
- Yinga, F.; Wang, S.; Au, C.-T.; Lai, S.-Y. Effect of the oxidation state of gold on the complete oxidation of isobutane on Au/CeO2 catalysts. Gold Bull. 2010, 43, 241–251. [Google Scholar] [CrossRef][Green Version]
- XPS Data. Available online: https://www.xpsdata.com/ (accessed on 15 January 2020).
- Cybulski, A.; Moulinjn, J.A.; Stankewicz, A. Novel concepts in Catalysis and Chemical Reactors: Improving the Efficiency for the Future; Wiley-VCH: Weinheim, Germany, 2010; 398p. [Google Scholar]
- Oh, H.-S.; Yang, J.H.; Costello, C.K.; Wang, Y.M.; Bare, S.R.; Kung, H.H.; Kung, M.C. Selective Catalytic Oxidation of CO: Effect of Chloride on Supported Au Catalysts. J. Catal. 2002, 210, 375–386. [Google Scholar] [CrossRef]
- Guzman, J.; Gates, B.C. Simultaneous Presence of Cationic and Reduced Gold in Functioning MgO-Supported CO Oxidation Catalysts: Evidence from X-ray Absorption Spectroscopy. J. Phys. Chem. B 2002, 106, 7659–7665. [Google Scholar] [CrossRef]
- Wang, D.; Hao, Z.; Cheng, D.; Shi, X. Influence of the calcination temperature on the Au/FeOx/Al2O3 catalyst. J. Chem. Technol. Biotechnol. 2006, 81, 1246–1251. [Google Scholar] [CrossRef]
- Gazsi, A.; Koós, A.; Bánsági, T.; Solymosi, F. Adsorption and decomposition of ethanol on supported Au catalysts. Catal. Today 2011, 160, 70–78. [Google Scholar] [CrossRef]
- Sheng, P.-Y.; Bowmaker, G.A.; Idriss, H. The Reactions of Ethanol over Au/CeO2. Appl. Catal. A Gen. 2004, 261, 171–181. [Google Scholar] [CrossRef]
- Shankar, P.; Bosco, J.; Rayappan, B. Gas sensing mechanism of metal oxides: The role of ambient atmosphere, type of semiconductor and gases—A review. Sci. Lett. J. 2015, 4, 126. [Google Scholar]
- Elavarasana, M.; Umab, K.; Yang, T.C.K. Photocatalytic oxidation of ethanol using ultrasonic modified TiO2; an in situ diffuse reflectance infrared spectroscopy study. Results Phys. 2019, 13. [Google Scholar] [CrossRef]
- Goto, T.; Itoh, T.; Akamatsu, T.; Shin, W. CO Sensing Performance of a Micro Thermoelectric Gas Sensor with AuPtPd/SnO2 Catalyst and Effects of a Double Catalyst Structure with Pt/α-Al2O3. Sensors 2015, 15, 31687–31698. [Google Scholar] [CrossRef]
- Su, H.-Y.; Yang, M.-M.; Bao, X.-H.; Li, W.-X. The Effect of Water on the CO Oxidation on Ag(111) and Au(111) Surfaces: A First-Principle Study. J. Phys. Chem. C 2008, 112, 17303–17310. [Google Scholar] [CrossRef]
- Bongiorno, A.; Landman, U. Water-Enhanced Catalysis of CO Oxidation on Free and Supported Gold Nanoclusters. Phys. Rev. Lett. 2005, 95. [Google Scholar] [CrossRef] [PubMed]
- Dat´e, M.; Haruta, M. Moisture Effect on CO Oxidation over Au/TiO2 Catalyst. J. Catal. 2001, 201, 221–224. [Google Scholar] [CrossRef]
- Grossmann, K.; Pavelko, R.G.; Barsan, N.; Weimar, U. Interplay of H2, water vapor and oxygenat the surface of SnO2 based gas sensors—An operando investigation utilizing deuterated gases. Sens. Actuator. B 2012, 166–167, 787–793. [Google Scholar] [CrossRef]
- Vladimirova, S.; Krivetskiy, V.; Rumyantseva, M.; Gaskov, A.; Mordvinova, N.; Lebedev, O.; Martyshov, M.; Forsh, P. Co3O4 as p-type material for CO sensing in humid air. Sensors 2017, 17, 2216. [Google Scholar] [CrossRef]
- Kohl, D. Surface Processes In the Detection of Reducing Gases with SnO2-Based Devices. Sens. Actuator. 1989, 18, 71–113. [Google Scholar] [CrossRef]
- Jiang, W.; Pang, Y.; Gu, L.; Yao, Y.; Su, Q.; Ji, W.; Au, C.-T. Structurally defined SnO2 substrates, nanostructured Au/SnO2 interfaces, and their distinctive behavior in benzene and methanol oxidation. J. Catal. 2017, 349, 183–196. [Google Scholar] [CrossRef]

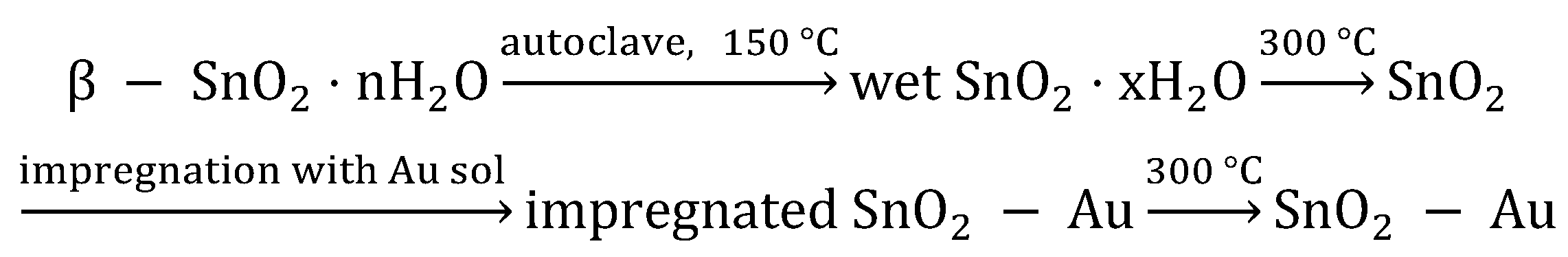

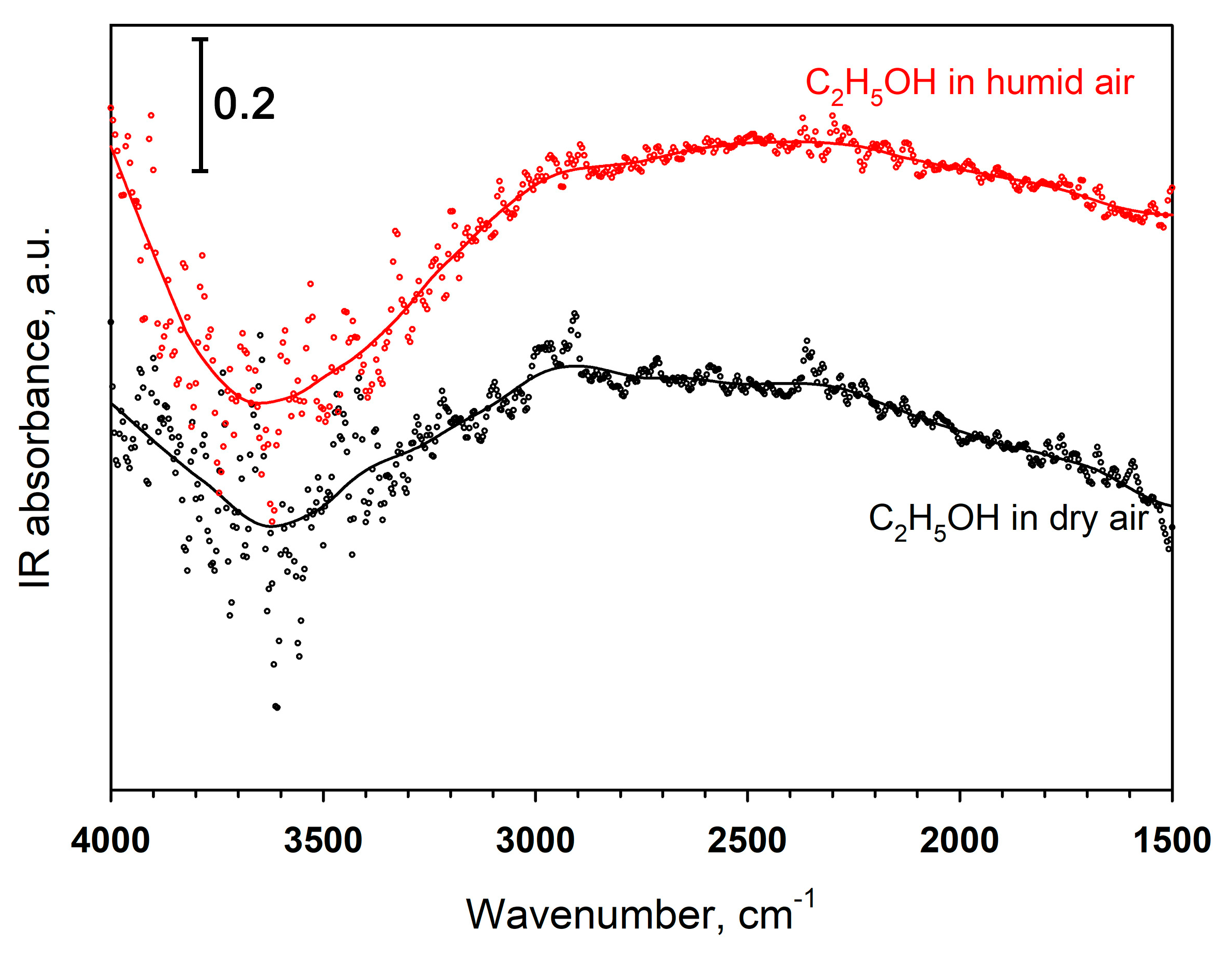
| Material Type | Au Modification Method | Gas | CGas, ppm | Tmes., °C | Sensor Signal | Reference |
|---|---|---|---|---|---|---|
| nanorods | Physical vapor deposition (PVD) | C2H5OH | 50 | 350 | 98 | [30] |
| bamboo-like nanorods | multistep alternate PVD of SnO2 and Au | 339 | ||||
| powders | deposition-precipitation | C2H5OH | 200 | 350 | 158 | [31] |
| nanoflowers | impregnation | C2H5OH | 100 | 200 | 123 | [32] |
| nanoflowers | deposition-precipitation | C2H5OH | 150 | 340 | 30 | [33] |
| nanowires | γ-ray radiolysis | C6H6 | 1 | 300 | 3 | [34] |
| nanofibres | electrosputtering | C6H6 | 10 | 300 | 2 | [35] |
| hollow spheres | electronic beam evaporation | C6H6 | 5 | 350 | 22 | [36] |
| C2H5OH | 10 | |||||
| nanofibres | UV-radiation + electrospinning | C6H6 | 2 | 400 | 4.5 | [37] |
| Sample | Au, wt.% 1 | SiO2, mol.% 2 | Phase Composition | dXRD ± 0.5 nm | Ssurf ± 5 m2/g | Resistance in Air at 150oC, RH = 1%, 106 Ohm |
|---|---|---|---|---|---|---|
| SnO2-300 | - | - | SnO2 | 3.1 | 94 | 9 |
| SnO2-Au | 2.44 ± 0.03 | - | SnO2 | 3.0 | 91 | 60 |
| SnO2/SiO2 | - | 13.0 ± 0.8 | SnO2 | 2.8 | 105 | 50 |
| SnO2/SiO2-Au | 3.64 ± 0.05 | 13.0 ± 0.8 | SnO2 | 3.3 | 100 | 300 |
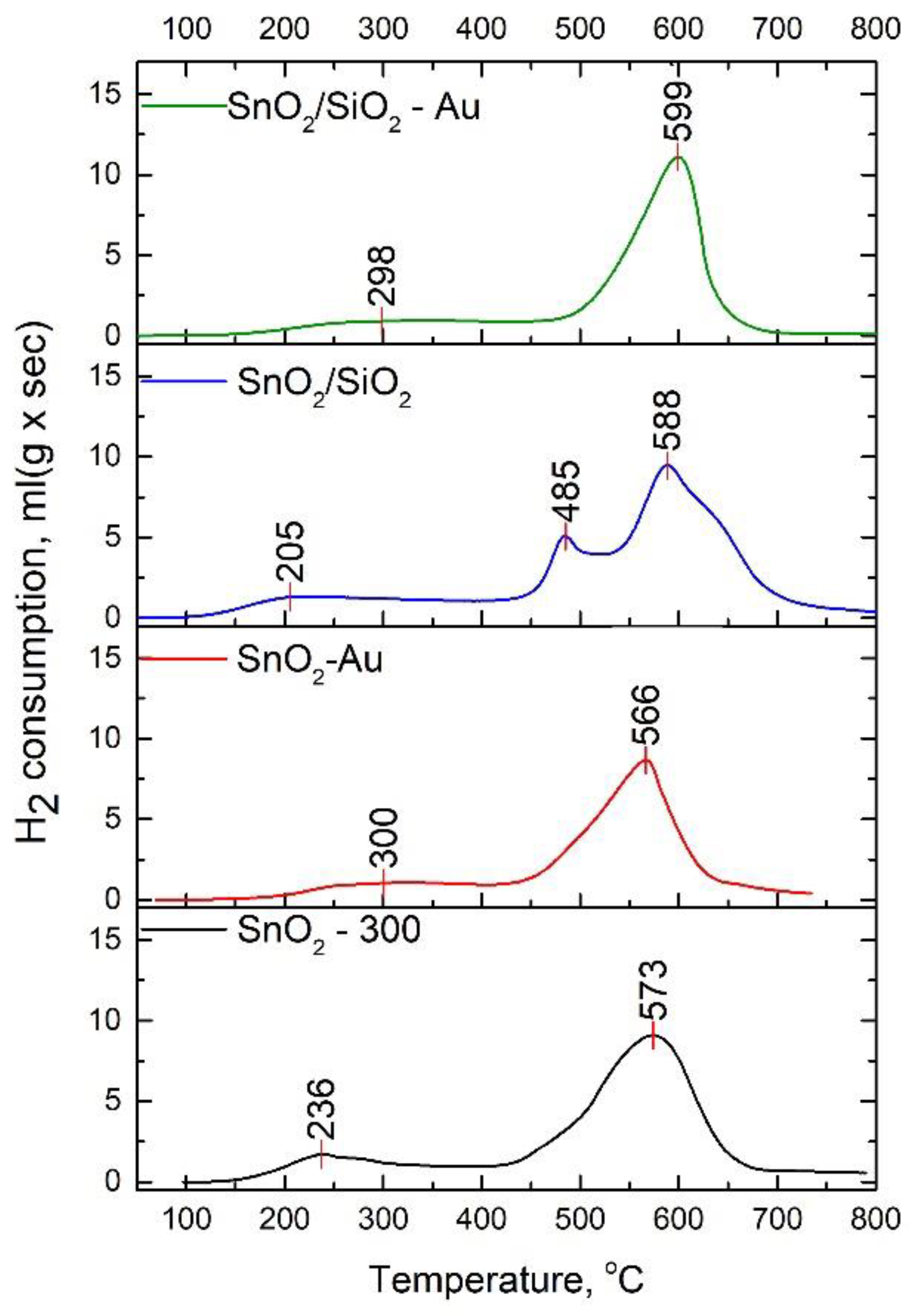
| Sample | Hydrogen Consumption, mol H2 per 1 mol SnO2 | ||
|---|---|---|---|
| Total | at 25 °C–400 °C | at 400 °C–800 °C | |
| SnO2-300 | 1.9 | 0.2 | 1.7 |
| SnO2-Au | 1.6 | 0.1 | 1.5 |
| SnO2/SiO2 | 2.3 | 0.3 | 2.0 |
| SnO2/SiO2-Au | 2.4 | 0.4 | 2.0 |
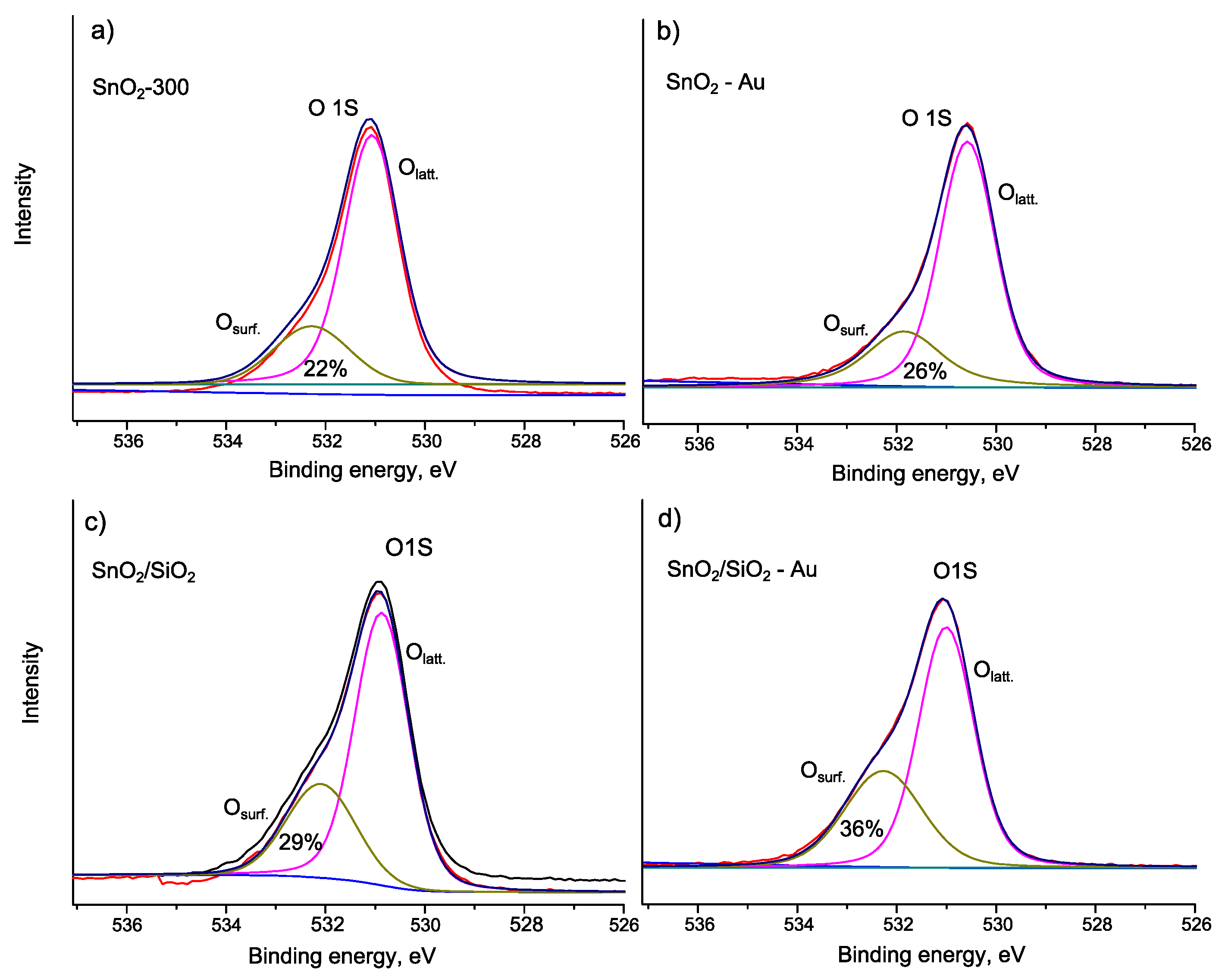
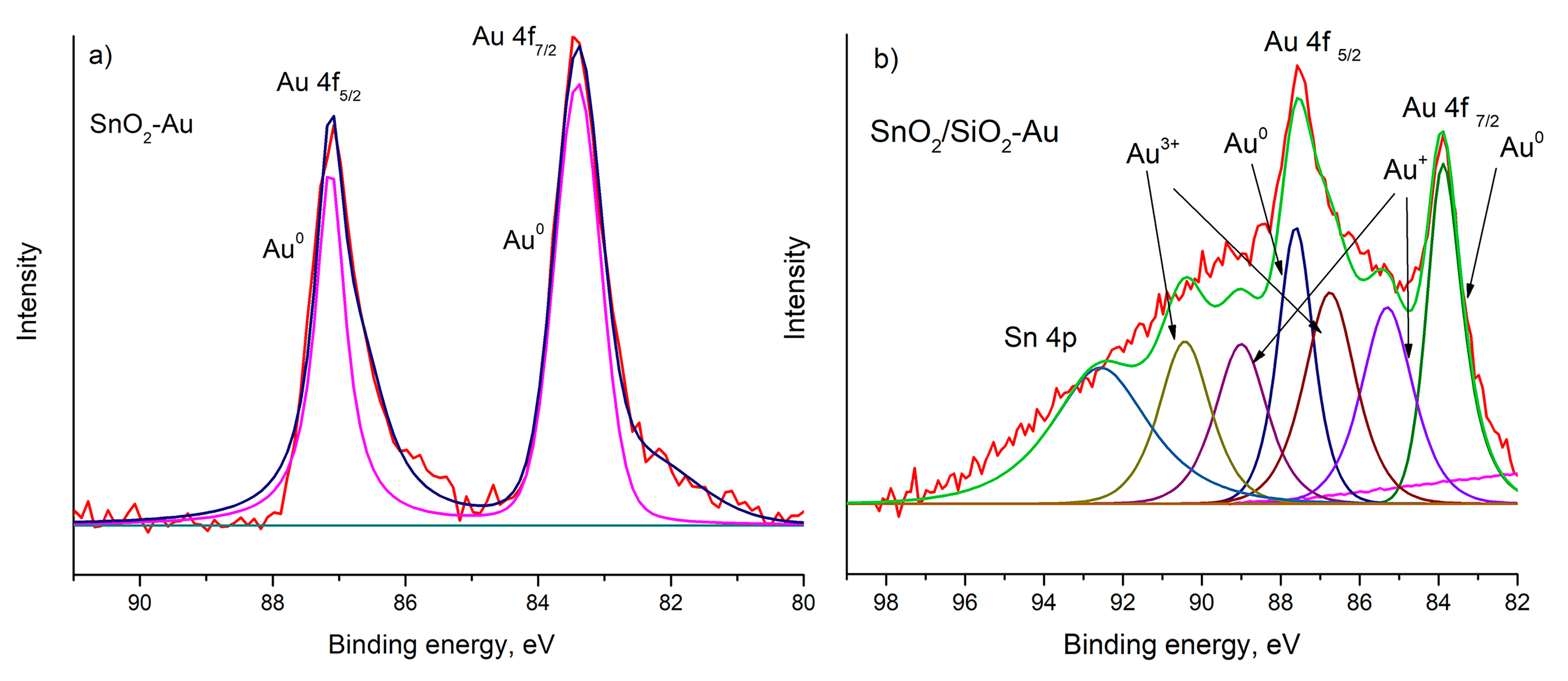
| Sample | Osurf/Olat | Binding Energy, eV | ΔE (Sn 3d5/2-O1s), eV | ||
|---|---|---|---|---|---|
| Sn 3d5/2 | Au 4f7/2 | O 1s (lat) | |||
| SnO2-300 | 0.28 | 487.2 | - | 531.1 | 43.9 |
| SnO2-Au | 0.35 | 486.7 | 83.4 | 530.6 | 43.9 |
| SnO2/SiO2 | 0.36 | 487.1 | - | 531.1 | 44.0 |
| SnO2/SiO2-Au | 0.56 | 487.2 | 83.9 | 530.1 | 42.9 |
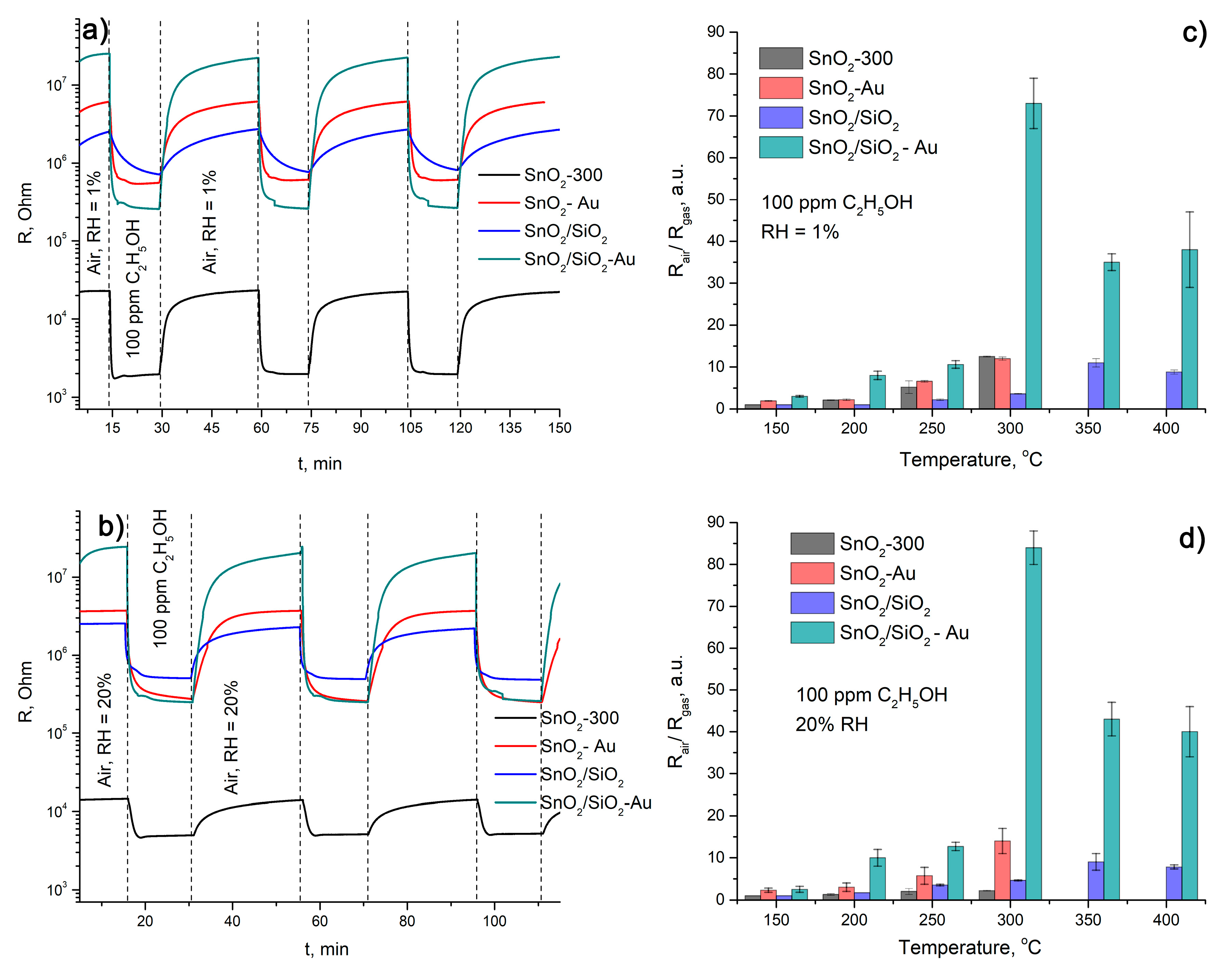
| Sample | Sensor Response | t, resp., s | t rec., s | |||
|---|---|---|---|---|---|---|
| RH = 1% | RH = 20% | RH = 1% | RH = 20% | RH = 1% | RH = 20% | |
| SnO2-300 | 12.5 ± 0.1 | 2.2 ± 0.1 | 83 ± 3 | 133 ± 2 | 167 ± 6 | 195 ± 4 |
| SnO2-Au | 12 ± 1 | 14 ± 3 | 88 ± 2 | 124 ± 4 | 164 ± 3 | 178 ± 6 |
| SnO2/SiO2 | 3.6 ± 0.1 | 4.6 ± 0.1 | 352 ± 5 | 119 ± 3 | 184 ± 7 | 124 ± 3 |
| SnO2/SiO2-Au | 73 ± 6 | 84 ± 4 | 93 ± 3 | 90 ± 2 | 159 ± 5 | 168 ± 3 |
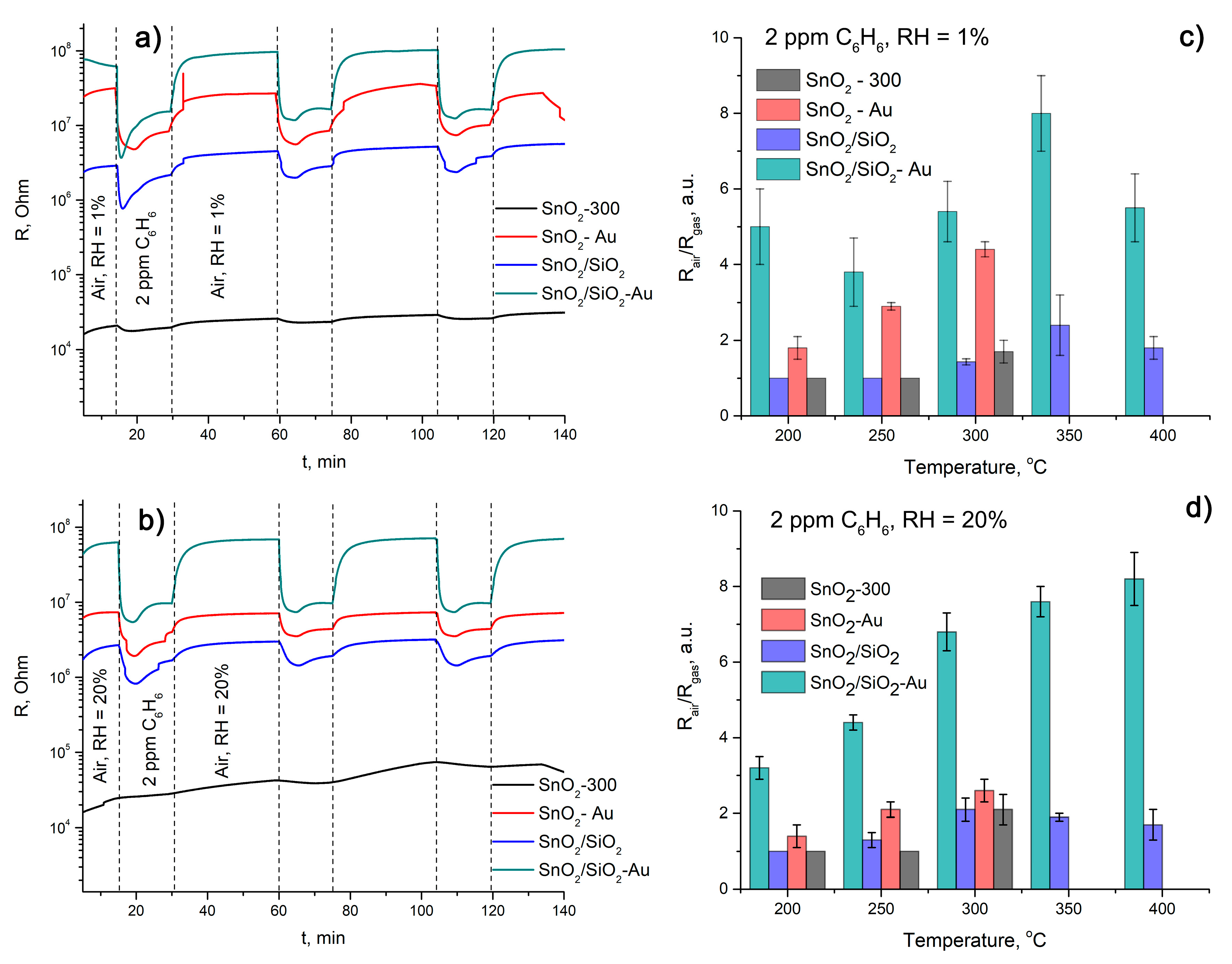
| Sample | Sensor Response | t, resp., s | t rec., s | |||
|---|---|---|---|---|---|---|
| RH = 1% | RH = 20% | RH = 1% | RH = 20% | RH = 1% | RH = 20% | |
| SnO2-300 | 1.4 ± 0.3 | 1 | - | - | - | - |
| SnO2-Au | 4.4 ± 0.1 | 2.6 ± 0.3 | 97 ± 4 | 84 ± 8 | 161 ± 3 | 137 ± 8 |
| SnO2/SiO2 | 1.2 ± 0.1 | 2.1 ± 0.3 | 73 ± 7 | 105 ± 6 | 142 ± 5 | 129 ± 4 |
| SnO2/SiO2-Au | 8.2 ± 0.8 | 6.8 ± 0.5 | 62 ± 5 | 75 ± 4 | 125 ± 3 | 128 ± 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulevich, D.; Rumyantseva, M.; Gerasimov, E.; Khmelevsky, N.; Tsvetkova, E.; Gaskov, A. Synergy Effect of Au and SiO2 Modification on SnO2 Sensor Properties in VOCs Detection in Humid Air. Nanomaterials 2020, 10, 813. https://doi.org/10.3390/nano10040813
Gulevich D, Rumyantseva M, Gerasimov E, Khmelevsky N, Tsvetkova E, Gaskov A. Synergy Effect of Au and SiO2 Modification on SnO2 Sensor Properties in VOCs Detection in Humid Air. Nanomaterials. 2020; 10(4):813. https://doi.org/10.3390/nano10040813
Chicago/Turabian StyleGulevich, Dayana, Marina Rumyantseva, Evgeny Gerasimov, Nikolay Khmelevsky, Elena Tsvetkova, and Alexander Gaskov. 2020. "Synergy Effect of Au and SiO2 Modification on SnO2 Sensor Properties in VOCs Detection in Humid Air" Nanomaterials 10, no. 4: 813. https://doi.org/10.3390/nano10040813
APA StyleGulevich, D., Rumyantseva, M., Gerasimov, E., Khmelevsky, N., Tsvetkova, E., & Gaskov, A. (2020). Synergy Effect of Au and SiO2 Modification on SnO2 Sensor Properties in VOCs Detection in Humid Air. Nanomaterials, 10(4), 813. https://doi.org/10.3390/nano10040813








