Cellulose Nanocrystals Loaded with Thiamethoxam: Fabrication, Characterization, and Evaluation of Insecticidal Activity against Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Insect Culture
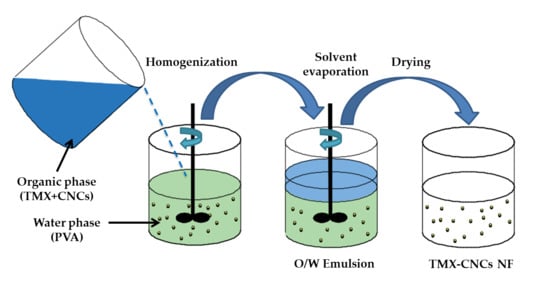
2.2. Preparation of TMX-NF
2.2.1. Preparation of CNCs
2.2.2. Preparation of TMX-Loaded CNCs
2.3. Characterization of CNCs and TMX-CNCs
2.4. Determination of Entrapment Efficiency of TMX in the NF
2.5. In Vitro Release of TMX
2.6. Bioassay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of TMX-NF
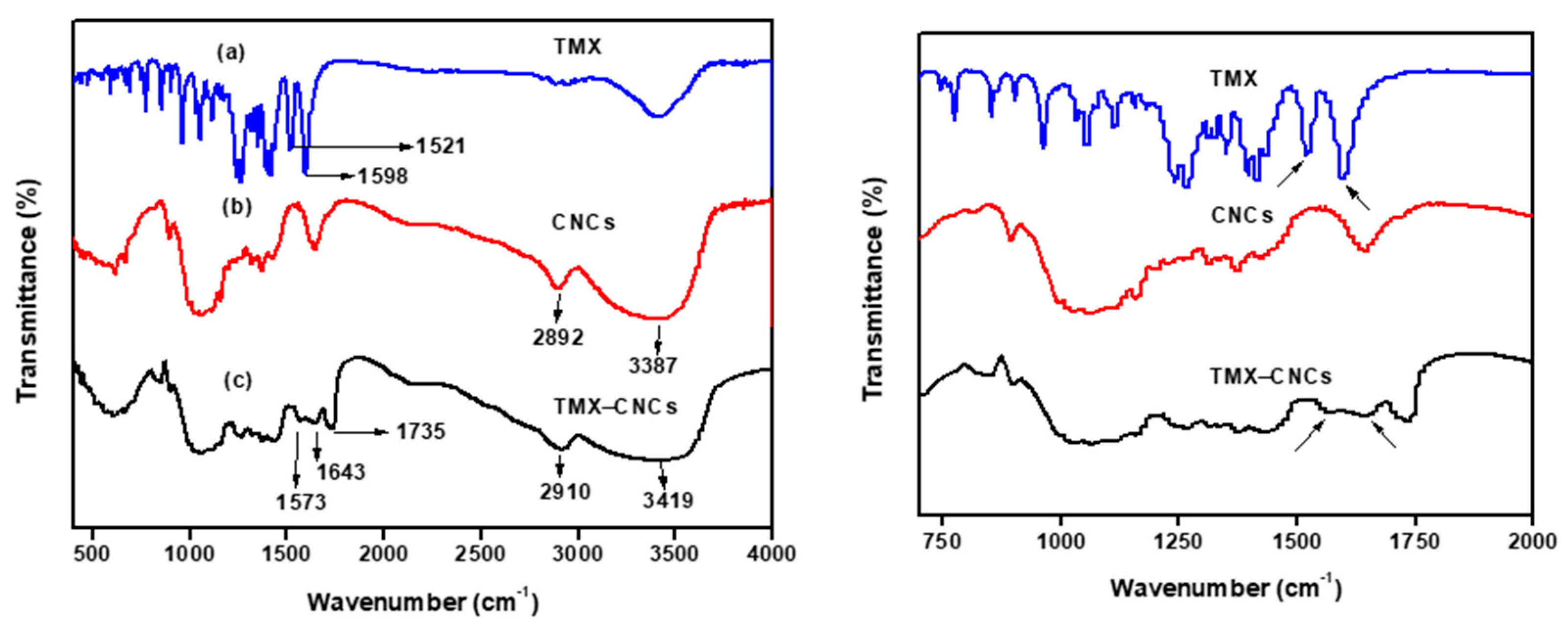
3.1.1. FT-IR Spectroscopy
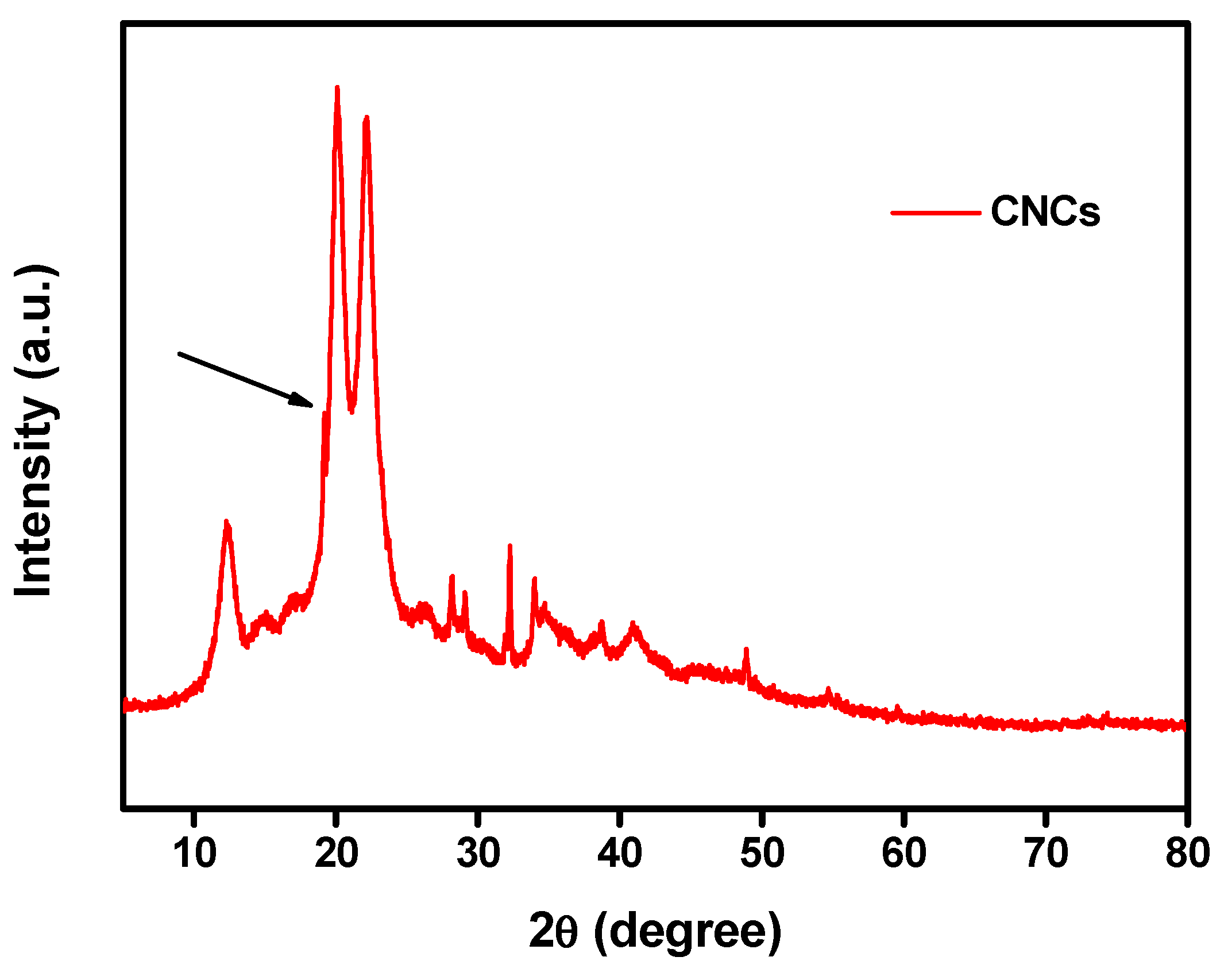
3.1.2. X-ray Diffraction Analysis
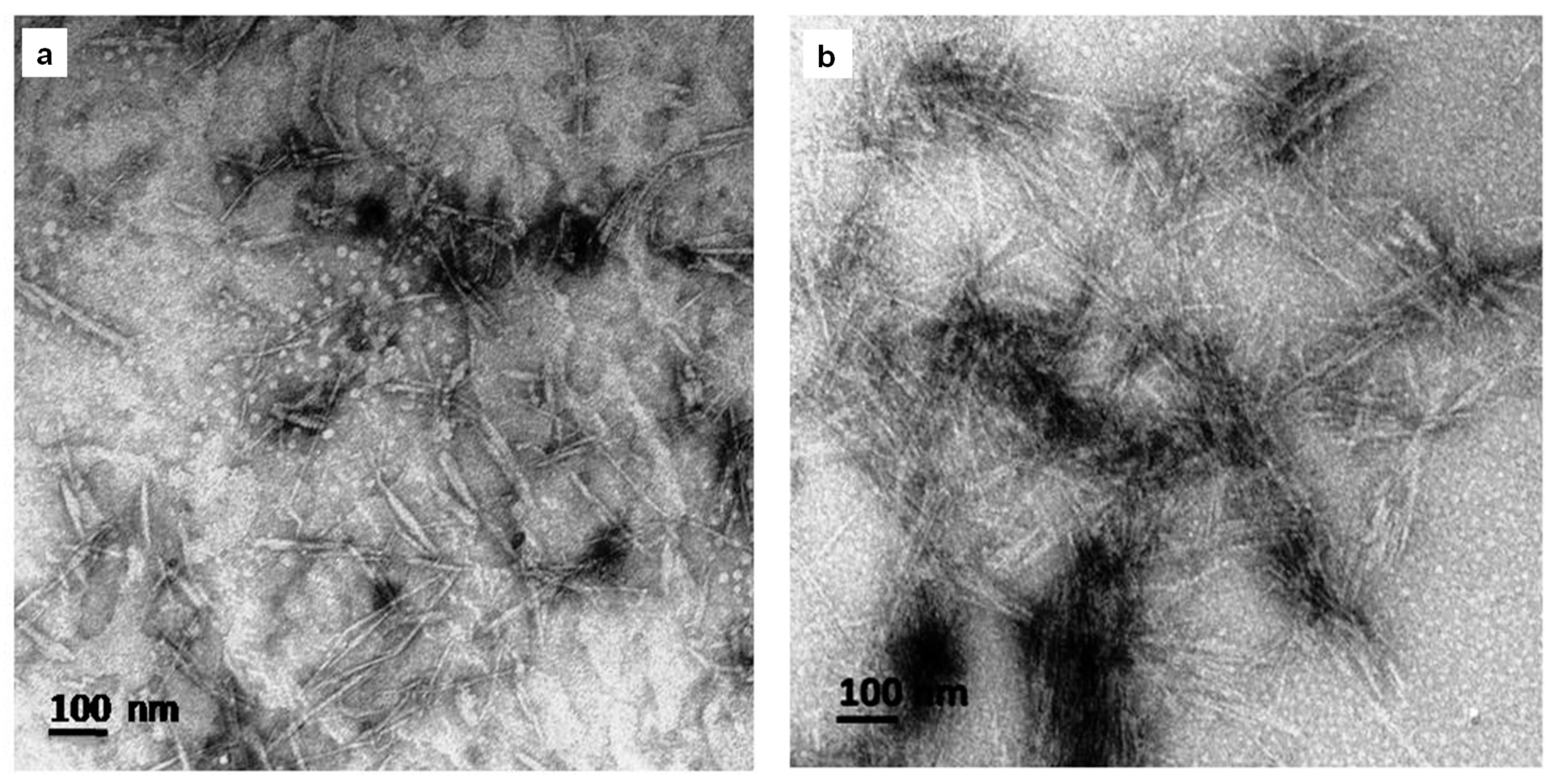
3.1.3. TEM Analysis
3.1.4. Particle Size and Zeta Potential
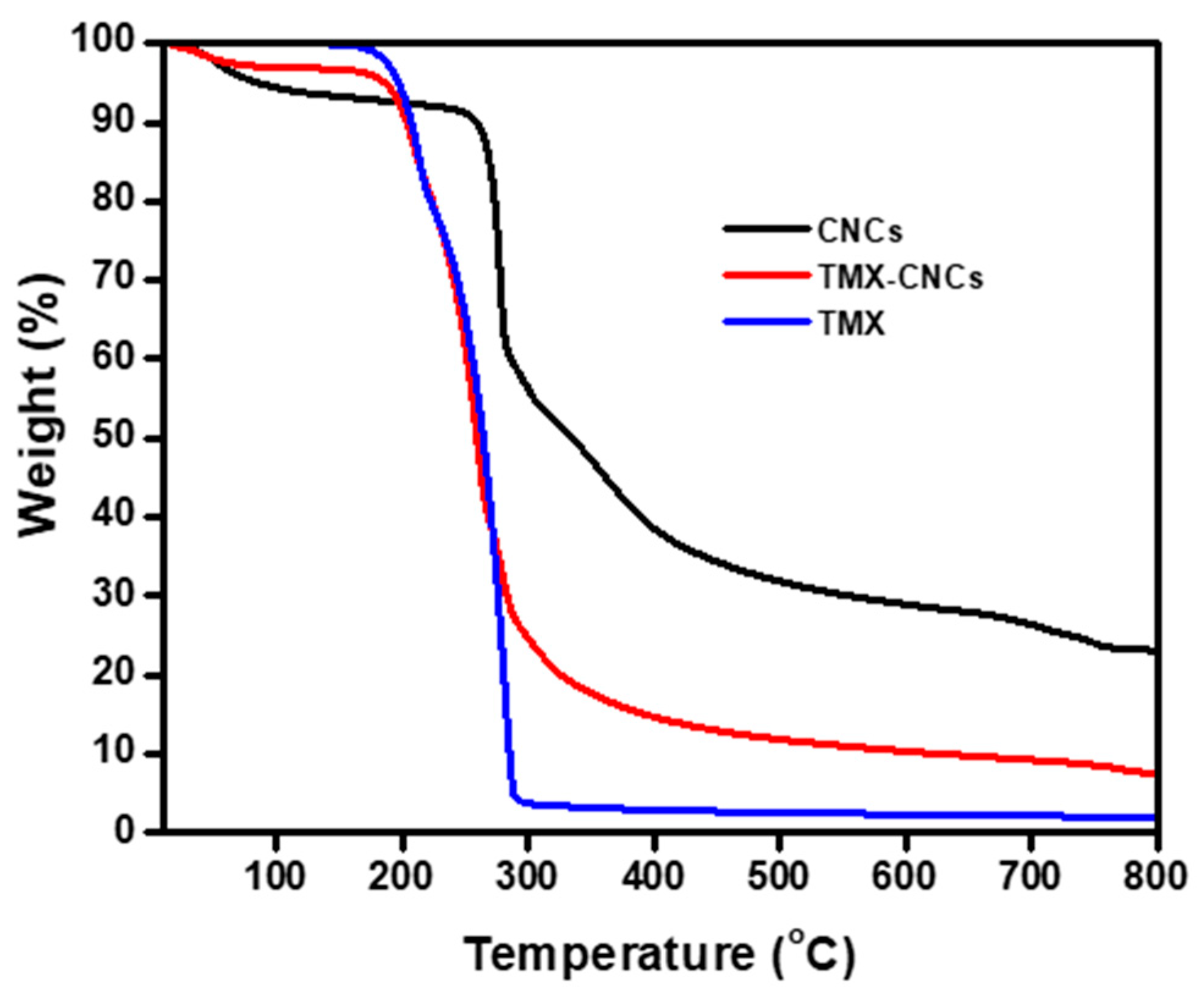
3.1.5. Thermogravimetric Analysis (TGA)
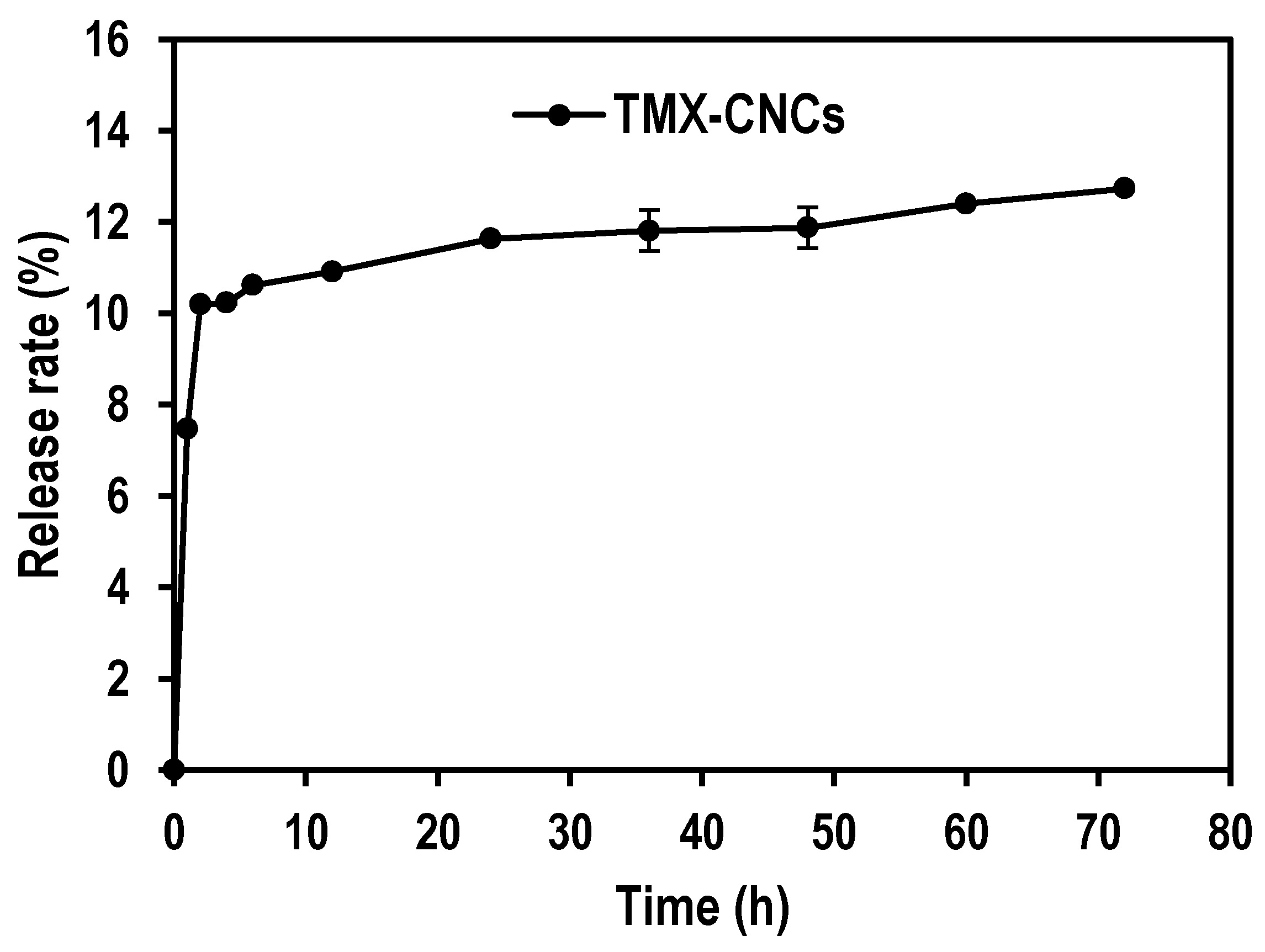
3.2. In Vitro Release of TMX
3.3. Bioassay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Cui, H.; Wang, Y.; Sun, C.; Cui, B.; Zeng, Z. Development strategies and prospects of nano-based smart pesticide formulation. J. Agric. Food Chem. 2017, 66, 6504–6512. [Google Scholar] [CrossRef]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current challenges and trends in the discovery of agrochemicals. Science 2013, 341, 742–746. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, Q.; Cui, G.; Guo, X.; Wei, B.; Gan, C.; Li, W.; Mo, D.; Lu, R.; Cui, J. Release-controlled microcapsules of thiamethoxam encapsulated in beeswax and their application in field. J. Environ. Sci. Health B 2019, 55, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, S.; Wen, L.; Liu, F.; Liu, A.; Wang, Q.; Sun, H.; Yu, W.; Chen, J. Controlled release of avermectin from porous hollow silica nanoparticles: Influence of shell thickness on loading efficiency, UV-shielding property and release. J. Control. Release 2006, 111, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef]
- Massinon, M.; De Cock, N.; Forster, W.A.; Nairn, J.J.; McCue, S.W.; Zabkiewicz, J.A.; Lebeau, F. Spray droplet impaction outcomes for different plant species and spray formulations. Crop Prot. 2017, 99, 65–75. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, N.; Song, J.; Cai, D.; Wu, Z. Micro-nanopores fabricated by high-energy electron beam irradiation: Suitable structure for controlling pesticide loss. J. Agric. Food Chem. 2013, 61, 5215–5219. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, M.; Sun, X.; Cai, D.; Wu, Z. Controlling pesticide loss through nanonetworks. ACS Sustain. Chem. Eng. 2014, 2, 918–924. [Google Scholar] [CrossRef]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Kah, M.; Beulke, S.; Tiede, K.; Hofmann, T. Nanopesticides: State of knowledge, environmental fate, and exposure modeling. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1823–1867. [Google Scholar] [CrossRef]
- Assalin, M.R.; dos Santos, L.D.L.; de Souza, D.R.C.; Rosa, M.A.; Duarte, R.R.M.; Castanha, R.F.; Donaire, P.P.R.; Durán, N. Nanoformulation as a tool for improvement of thiamethoxam encapsulation and evaluation of ecotoxicological impacts. Energy Ecol. Environ. 2019, 4, 310–317. [Google Scholar] [CrossRef]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Kohli, P. The emerging field of nanotube biotechnology. Nat. Rev. Drug Discov. 2003, 2, 29. [Google Scholar] [CrossRef]
- Liu, F.; Wen, L.X.; Li, Z.Z.; Yu, W.; Sun, H.Y.; Chen, J.F. Porous hollow silica nanoparticles as controlled delivery system for water-soluble pesticide. Mater. Res. Bull. 2006, 41, 2268–2275. [Google Scholar] [CrossRef]
- Scott, N.R.; Chen, H.; Cui, H. Nanotechnology applications and implications of agrochemicals toward sustainable agriculture and food systems. J. Agric. Food Chem. 2018, 26, 6451–6456. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, G.; Chi, Y.; Cai, D.; Wu, Z. Fabrication of a controllable nanopesticide system with magnetic collectability. Chem. Eng. J. 2017, 328, 320–330. [Google Scholar] [CrossRef]
- Pascoli, M.; Lopes-Oliveira, P.J.; Fraceto, L.F.; Seabra, A.B.; Oliveira, H.C. State of the art of polymeric nanoparticles as carrier systems with agricultural applications: A minireview. Energy Ecol. Environ. 2018, 3, 137–148. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; He, S.; Xiao, Y.; Qin, X.; Zhang, Y.; Li, D.; Ma, H.; You, H.; Li, J. Fabrication of a hollow mesoporous silica hybrid to improve the targeting of a pesticide. Chem. Eng. J. 2019, 364, 361–369. [Google Scholar] [CrossRef]
- Grillo, R.; Abhilash, P.C.; Fraceto, L.F. Nanotechnology applied to bio-encapsulation of pesticides. J. Nanosci. Nanotechnol. 2016, 16, 1231–1234. [Google Scholar] [CrossRef]
- Mishra, S.; Keswani, C.; Abhilash, P.; Fraceto, L.F.; Singh, H.B. Integrated approach of agri-nanotechnology: Challenges and future trends. Front. Plant Sci. 2017, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Huang, J.; Dufresne, A.J.N. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef] [PubMed]
- Abu-Danso, E.; Srivastava, V.; Sillanpää, M.; Bhatnagar, A. Pretreatment assisted synthesis and characterization of cellulose nanocrystals and cellulose nanofibers from absorbent cotton. Int. J. Biol. Macromol. 2017, 102, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, Y.; Long, Y.; An, X.; Shen, J.; Ni, Y. Anchoring 20 (R)-ginsenoside Rg3 onto cellulose nanocrystals to increase the hydroxyl radical scavenging activity. ACS Sustain. Chem. Eng. 2017, 5, 7507–7513. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Venditti, R.A.; Rojas, O. Pickering emulsions stabilized by cellulose nanocrystals grafted with thermo-responsive polymer brushes. J. Colloid Interface Sci. 2012, 369, 202–209. [Google Scholar] [CrossRef]
- Tang, C.; Spinney, S.; Shi, Z.; Tang, J.; Peng, B.; Luo, J.; Tam, K.C. Amphiphilic cellulose nanocrystals for enhanced Pickering emulsion stabilization. Langmuir 2018, 34, 12897–12905. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45. [Google Scholar] [CrossRef]
- Tang, C.; Li, Y.; Pun, J.; Osman, A.S.M.; Tam, K.C. Polydopamine microcapsules from cellulose nanocrystal stabilized Pickering emulsions for essential oil and pesticide encapsulation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 403–413. [Google Scholar] [CrossRef]
- Maienfisch, P.; Huerlimann, H.; Rindlisbacher, A.; Gsell, L.; Dettwiler, H.; Haettenschwiler, J.; Sieger, E.; Walti, M. The discovery of thiamethoxam: A second-generation neonicotinoid. Pest Manag. Sci. 2001, 57, 165–176. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Tosi, S.; Nieh, J. A common neonicotinoid pesticide, thiamethoxam, alters honey bee activity, motor functions, and movement to light. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gajbhiye, V.; Gupta, R. Soil dissipation and leaching behavior of a neonicotinoid insecticide thiamethoxam. Bull. Environ. Contam. Toxicol. 2008, 80, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Bernardes, J.S.; Fávaro, W.J.; Paula, A.J.; Durán, N. Cellulose nanocrystals as carriers in medicine and their toxicities: A review. Carbohydr. Polym. 2018, 181, 514–527. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef]
- Zhang, S.F.; Chen, P.H.; Zhang, F.; Yang, Y.F.; Liu, D.K.; Wu, G. Preparation and physicochemical characteristics of polylactide microspheres of emamectin benzoate by modified solvent evaporation/extraction method. J. Agric. Food Chem. 2013, 61, 12219–12225. [Google Scholar] [CrossRef] [PubMed]
- Elabasy, A.; Shoaib, A.; Waqas, M.; Jiang, M.; Shi, Z. Synthesis, characterization, and pesticidal activity of emamectin benzoate nanoformulations against Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Molecules 2019, 24, 2801. [Google Scholar] [CrossRef]
- Venkatesan, J.; Alam, M.S.; Hong, E.J.; Kim, S.K.; Shim, M.S. Preparation of piperlongumine-loaded chitosan nanoparticles for safe and efficient cancer therapy. RSC Adv. 2016, 6, 79307–79316. [Google Scholar] [CrossRef]
- Popat, A.; Liu, J.; Hu, Q.; Kennedy, M.; Peters, B.; Lu, G.Q.M.; Qiao, S.Z. Adsorption and release of biocides with mesoporous silica nanoparticles. Nanoscale 2012, 4, 970–975. [Google Scholar] [CrossRef]
- Afzal, M.B.S.; Shad, S.A.; Abbas, N.; Ayyaz, M.; Walker, W.B. Cross-resistance, the stability of acetamiprid resistance and its effect on the biological parameters of cotton mealybug, Phenacoccus solenopsis (Homoptera: Pseudococcidae), in Pakistan. Pest Manag. Sci. 2015, 71, 151–158. [Google Scholar] [CrossRef]
- Finney, D. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971; p. 333. [Google Scholar]
- POLO-Plus, a user’s guide to Probit or Logic Analysis; LeOra Software: Berkeley, CA, USA, 2002.
- Maiti, S.; Jayaramudu, J.; Das, K.; Reddy, S.M.; Sadiku, R.; Ray, S.S.; Liu, D. Preparation and characterization of nano-cellulose with new shape from different precursor. Carbohydr. Polym. 2013, 98, 562–567. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Cordeiro, N.; Mozetic, M.; Mathew, A.P.; Oksman, K.; Faria, M.; Thomas, S.; Pothan, L.A. Utilization of various lignocellulosic biomass for the production of nanocellulose: A comparative study. Cellulose 2015, 22, 1075–1090. [Google Scholar] [CrossRef]
- Naduparambath, S.; Jinitha, T.; Shaniba, V.; Sreejith, M.; Balan, A.K.; Purushothaman, E. Isolation and characterisation of cellulose nanocrystals from sago seed shells. Carbohydr. Polym. 2018, 180, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.J.; Kumar, J.; Shakil, N.; Walia, S. Release kinetics of controlled release formulations of thiamethoxam employing nano-ranged amphiphilic PEG and diacid based block polymers in soil. J. Environ. Sci. Health A 2012, 47, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Jameel, M.; Shoeb, M.; Khan, M.T.; Ullah, R.; Mobin, M.; Farooqi, M.K.; Adnan, S.M. Enhanced insecticidal activity of thiamethoxam by zinc oxide nanoparticles: A novel nanotechnology approach for pest control. ACS Omega 2020, 5, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Lani, N.; Ngadi, N.; Johari, A.; Jusoh, M. Isolation, characterization, and application of nanocellulose from oil palm empty fruit bunch fiber as nanocomposites. J. Nanomater. 2014, 2014, 702538. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, L.; Yu, J. Effect of polysaccharide nanocrystals on structure, properties, and drug release kinetics of alginate-based microspheres. Colloids Surf. B Biointerfaces 2011, 85, 270–279. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Akhlaghi, S.P.; Berry, R.C.; Tam, K.C. Surface modification of cellulose nanocrystal with chitosan oligosaccharide for drug delivery applications. Cellulose 2013, 20, 1747–1764. [Google Scholar] [CrossRef]
- Chauhan, N.; Dilbaghi, N.; Gopal, M.; Kumar, R.; Kim, K.-H.; Kumar, S. Development of chitosan nanocapsules for the controlled release of hexaconazole. Int. J. Biol. Macromol. 2017, 97, 616–624. [Google Scholar] [CrossRef]
- Anjali, C.; Sharma, Y.; Mukherjee, A.; Chandrasekaran, N. Neem oil (Azadirachta indica) nanoemulsion—A potent larvicidal agent against Culex quinquefasciatus. Pest Manag. Sci. 2012, 68, 158–163. [Google Scholar] [CrossRef]
- Feng, J.; Shi, Y.; Yu, Q.; Sun, C.; Yang, G. Effect of emulsifying process on stability of pesticide nanoemulsions. Colloids. Surf. A Physicochem. Eng. Asp. 2016, 497, 286–292. [Google Scholar] [CrossRef]
- Saini, P.; Gopal, M.; Kumar, R.; Srivastava, C. Development of pyridalyl nanocapsule suspension for efficient management of tomato fruit and shoot borer (Helicoverpa armigera). J. Environ. Sci. Health B 2014, 49, 344–351. [Google Scholar] [CrossRef]
- Wang, C.; Cui, B.; Guo, L.; Wang, A.; Zhao, X.; Wang, Y.; Sun, C.; Zeng, Z.; Zhi, H.; Chen, H. Fabrication and evaluation of lambda-cyhalothrin nanosuspension by one-step melt emulsification technique. Nanomaterials 2019, 9, 145. [Google Scholar] [CrossRef] [PubMed]

| Sample | Nanoformulation | |||
|---|---|---|---|---|
| ζ (mV) a | Size (nm) | PdI b | EE (%) c | |
| CNCs | −39.0 ± 1.8 | 124.5 ± 0.5 | 0.3 ± 0.0 | - |
| TMX-CNCs | −23.6 ± 0.3 | 798.0 ± 149 | 1.0 ± 0.0 | 83.7 ± 1.8 |
| Formulation | Time (h) | LC50 (95% CL, µg/mL) | Slope ± SE | χ2 |
|---|---|---|---|---|
| TMX-CNCs | 24 | 0.70 (0.60–0.85) | 3.34 ± 0.60 | 2.63 |
| 48 | 0.37 (0.24–0.48) | 1.88 ± 0.44 | 2.35 | |
| 72 | 0.25 (0.11–0.35) | 1.75 ± 0.44 | 2.96 | |
| TMX (TC) | 24 | 0.91 (0.51–1.83) | 0.89 ± 0.25 | 0.19 |
| 48 | 0.44 (0.15–0.78) | 0.85 ± 0.25 | 0.12 | |
| 72 | 0.28 (0.10–0.45) | 1.09 ± 0.27 | 1.83 | |
| TMX 25% WDG | 24 | 2.11 (1.60–3.71) | 1.80 ± 0.47 | 1.45 |
| 48 | 1.02 (0.53–1.47) | 1.34 ± 0.42 | 0.77 | |
| 72 | 0.55 (0.15–0.83) | 1.45 ± 0.44 | 1.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elabasy, A.; Shoaib, A.; Waqas, M.; Shi, Z.; Jiang, M. Cellulose Nanocrystals Loaded with Thiamethoxam: Fabrication, Characterization, and Evaluation of Insecticidal Activity against Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Nanomaterials 2020, 10, 788. https://doi.org/10.3390/nano10040788
Elabasy A, Shoaib A, Waqas M, Shi Z, Jiang M. Cellulose Nanocrystals Loaded with Thiamethoxam: Fabrication, Characterization, and Evaluation of Insecticidal Activity against Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Nanomaterials. 2020; 10(4):788. https://doi.org/10.3390/nano10040788
Chicago/Turabian StyleElabasy, Asem, Ali Shoaib, Muhammad Waqas, Zuhua Shi, and Mingxing Jiang. 2020. "Cellulose Nanocrystals Loaded with Thiamethoxam: Fabrication, Characterization, and Evaluation of Insecticidal Activity against Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae)" Nanomaterials 10, no. 4: 788. https://doi.org/10.3390/nano10040788
APA StyleElabasy, A., Shoaib, A., Waqas, M., Shi, Z., & Jiang, M. (2020). Cellulose Nanocrystals Loaded with Thiamethoxam: Fabrication, Characterization, and Evaluation of Insecticidal Activity against Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae). Nanomaterials, 10(4), 788. https://doi.org/10.3390/nano10040788