Down-Shifting and Anti-Reflection Effect of CsPbBr3 Quantum Dots/Multicrystalline Silicon Hybrid Structures for Enhanced Photovoltaic Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results
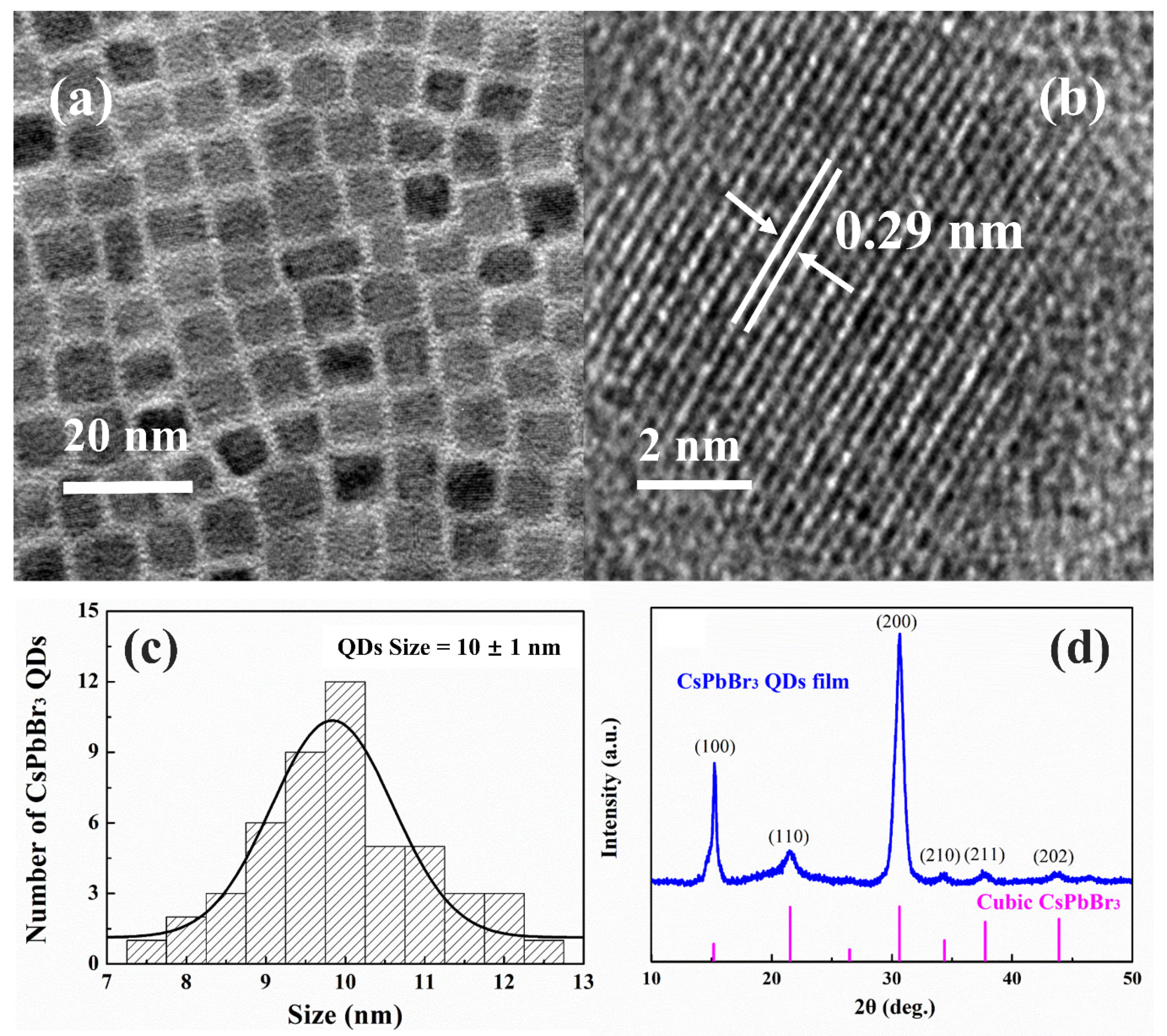
3.1. Structural Characterizations of CsPbBr3 QDs
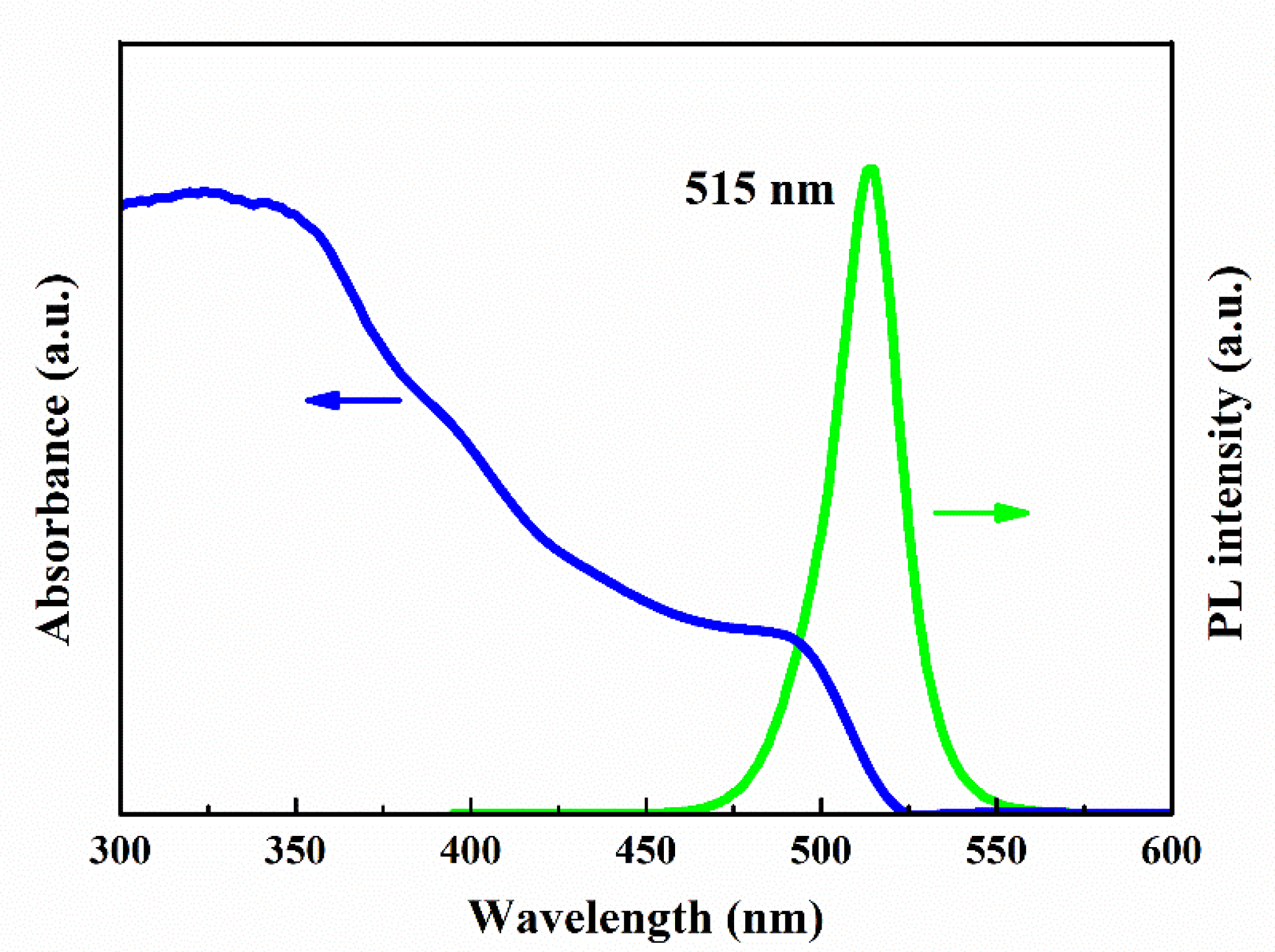
3.2. Optical Properties of CsPbBr3 QDs
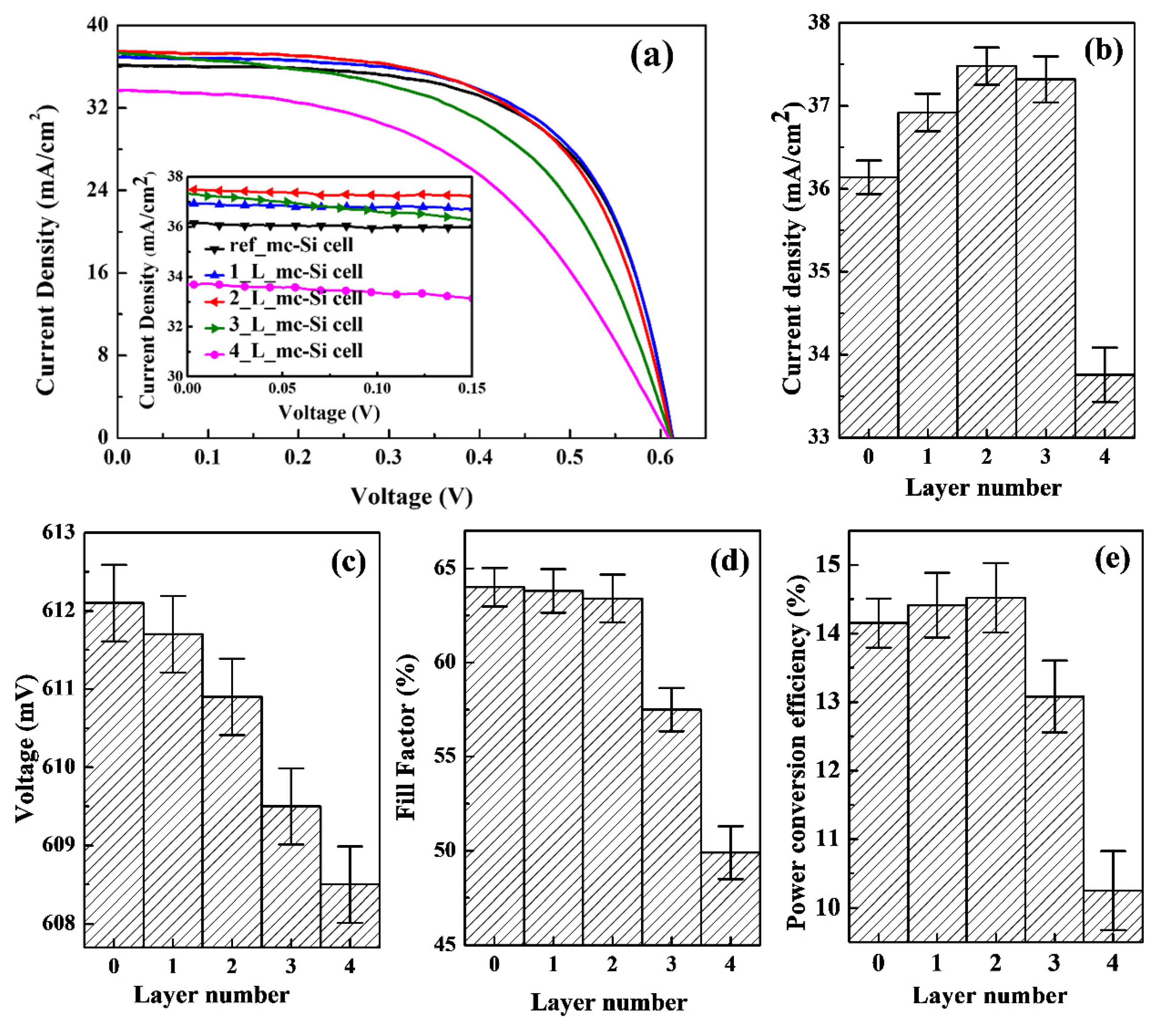
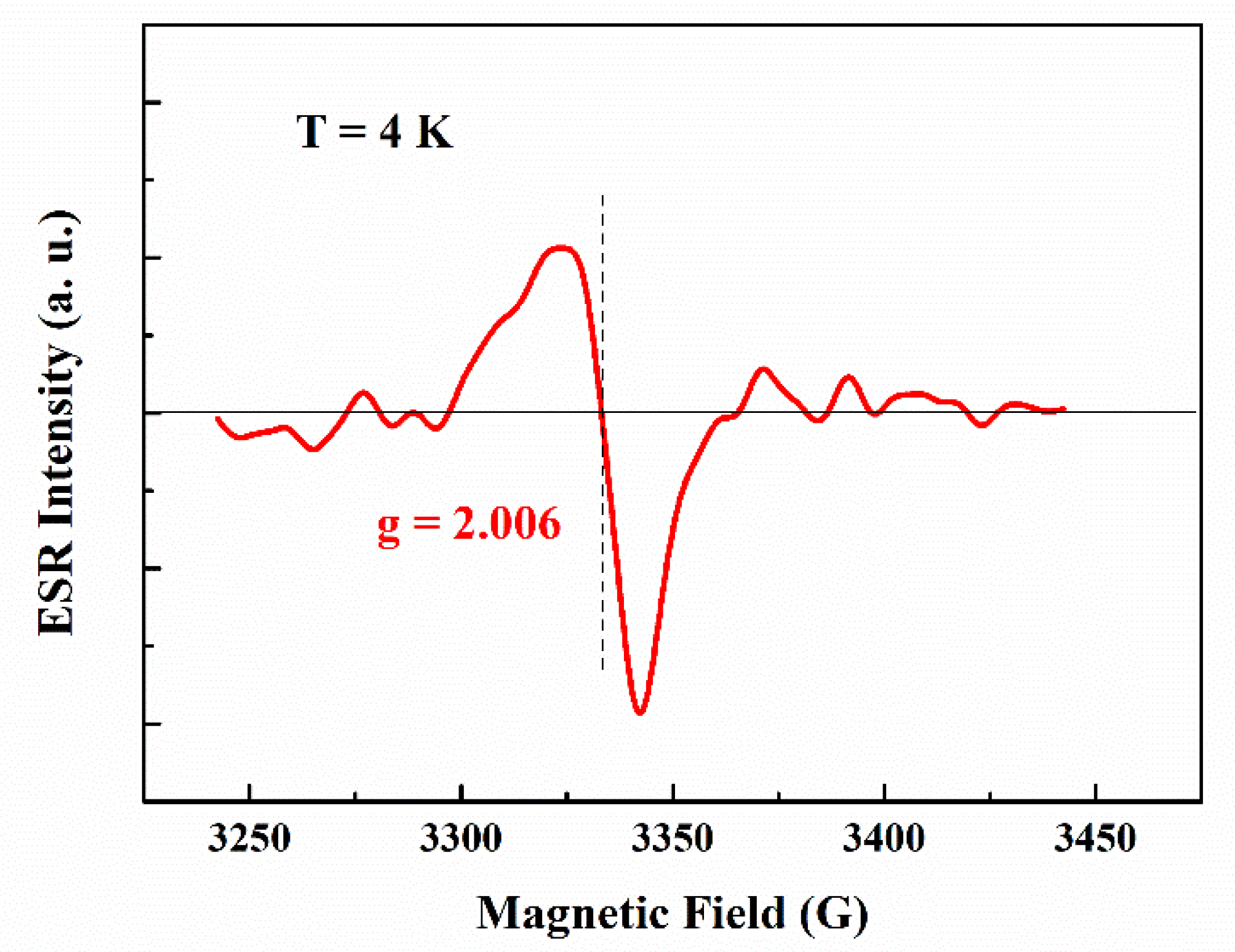
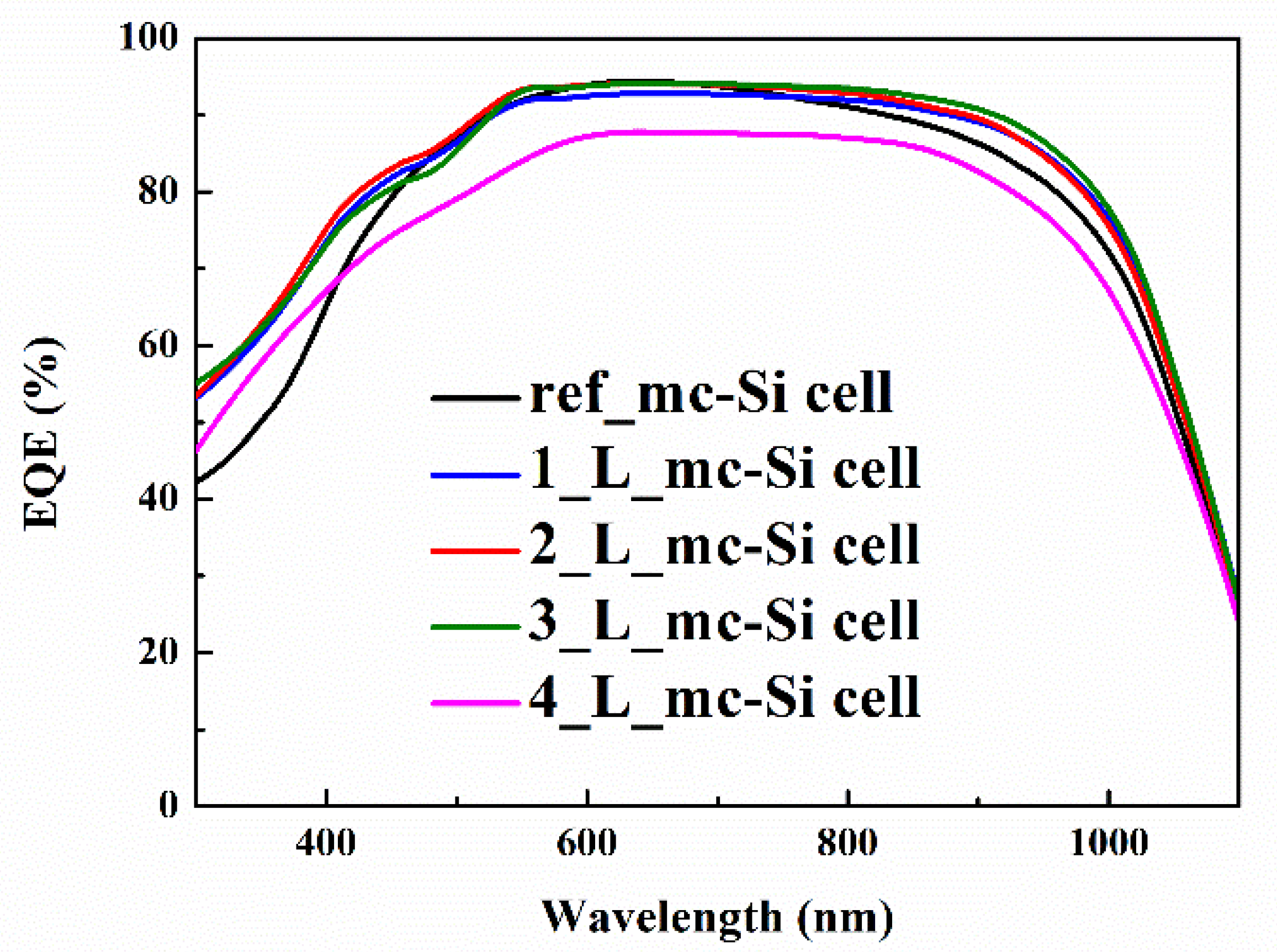
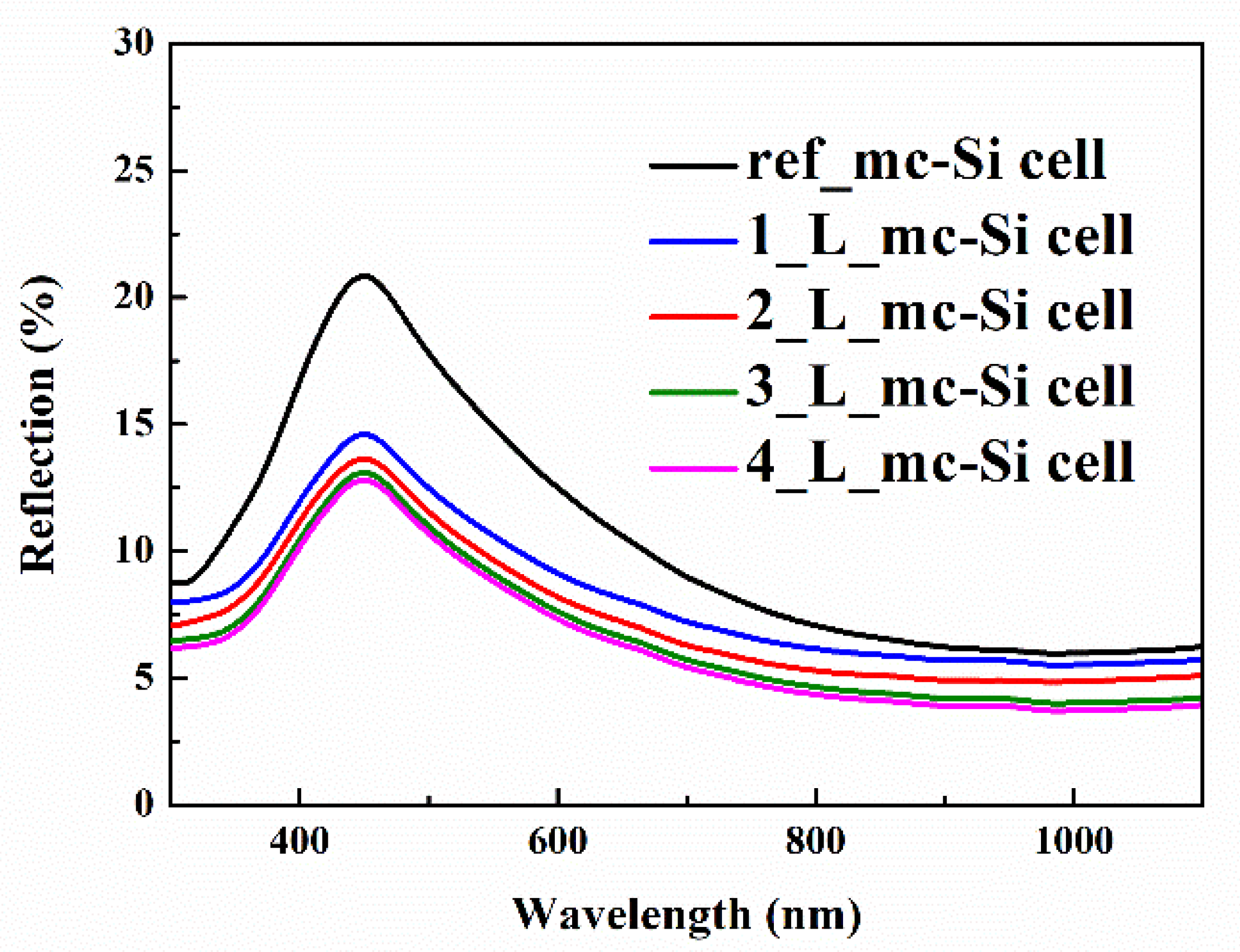
3.3. Photovoltaic Properties of CsPbBr3 QDs/mc-Si Hybrid Structured Solar Cells
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, J.H.; Wang, A.H.; Green, M.A. 24.5% Efficiency silicon PERT cells on MCZ substrates and 24.7% efficiency PERL cells on FZ substrates. Prog. Photovolt. Res. Appl. 1999, 7, 471–474. [Google Scholar] [CrossRef]
- Takatsuka, H.; Noda, M.; Yonekura, Y.; Takeuchi, Y.; Yamauchi, Y. Development of high efficiency large area silicon thin film modules using VHF-PECVD. Sol. Energy 2004, 77, 951–960. [Google Scholar] [CrossRef]
- Hu, L.; Chen, G. Analysis of optical absorption in silicon nanowire arrays for photovoltaic applications. Nano Lett. 2007, 7, 3249–3252. [Google Scholar] [CrossRef]
- Clement, F.; Menkoe, M.; Erath, D.; Kubera, T.; Hoenig, R.; Kwapil, W.; Wolke, W.; Biro, D.; Preu, R. High throughput via-metallization technique for multi-crystalline metal wrap through (MWT) silicon solar cells exceeding 16% efficiency. Sol. Energy Mater. Sol. Cells 2010, 94, 51–56. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency of p-n junction solar cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Slooff, L.H.; Kinderman, R.; Burgers, A.R.; Bakker, N.J.; van Roosmalen, J.A.M.; Büchtemann, A.; Danz, R.; Schleusener, M. Efficiency enhancement of solar cells by application of a polymer coating containing a luminescent dye. J. Sol. Energy Eng. 2007, 129, 272–276. [Google Scholar] [CrossRef]
- Huang, C.Y.; Wang, D.Y.; Wang, C.H.; Chen, Y.T.; Wang, Y.T.; Jiang, Y.T.; Yang, Y.J.; Chen, C.C.; Chen, Y.F. Efficient light harvesting by photon downconversion and light trapping in hybrid ZnS nanoparticles/Si nanotips solar cells. ACS Nano 2010, 4, 5849–5854. [Google Scholar] [CrossRef]
- Stupca, M.; Alsalhi, M.; Saud, T.A.; Almuhanna, A.; Nayfeh, M.H. Enhancement of polycrystalline silicon solar cells using ultrathin films of silicon nanoparticle. Appl. Phys. Lett. 2007, 91, 063107. [Google Scholar] [CrossRef]
- Cheng, Z.; Su, F.; Pan, L.; Cao, M.; Sun, Z. CdS quantum dot-embedded silica film as luminescent down-shifting layer for crystalline Si solar cells. J. Alloys Compd. 2010, 494, 7–10. [Google Scholar] [CrossRef]
- Sheng, X.; Corcoran, C.J.; He, J.W.; Shen, L.; Kim, S.; Park, J.; Nuzzo, R.G.; John, A.; Rogers, J.A. Enhanced ultraviolet responses in thin-film InGaP solar cells by down-shifting. Phys. Chem. Chem. Phys. 2013, 15, 20434–20437. [Google Scholar] [CrossRef]
- Nam, W.I.; Kang, E.K.; Park, H.G.; Jun, D.H.; Song, Y.M. Luminescent coverglass for improved absorption efficiency in III–V photovoltaic modules. Electron. Lett. 2016, 52, 1891–1892. [Google Scholar] [CrossRef]
- Yoon, J.; Li, L.; Semichaevsky, A.; Ryu, J.H.; Johnson, H.T.; Nuzzo, R.G.; Rogers, J.A. Flexible concentrator photovoltaics based on microscale silicon solar cells embedded in luminescent waveguides. Nat. Commun. 2011, 2, 343. [Google Scholar] [CrossRef]
- Van Sark, W.; Meijerink, A.; Schropp, R.; van Roosmalen, J.; Lysen, E.H. Enhancing solar cell efficiency by using spectral converters. Sol. Energy Mater. Sol. Cells 2005, 87, 395–409. [Google Scholar] [CrossRef]
- Pi, X.D.; Zhang, L.; Yang, D.R. Enhancing the efficiency of multicrystalline silicon solar cells by the inkjet printing of silicon-quantum-dot ink. J. Phys. Chem. C 2012, 116, 21240–21243. [Google Scholar] [CrossRef]
- Zhu, M.; Peng, X.; Wang, Z.; Bai, Z.; Chen, B.; Wang, Y.; Hao, H.; Shao, Z.; Zhong, H. Highly transparent and colour-tunable composite films with increased quantum dot loading. J. Mater. Chem. C 2014, 2, 10031–10036. [Google Scholar] [CrossRef]
- Priolo, F.; Gregorkiewicz, T.; Galli, M.; Krauss, T.F. Silicon nanostructures for photonics and photovoltaics. Nat. Nanotechnol. 2014, 9, 19–32. [Google Scholar] [CrossRef]
- Prezioso, S.; Anopchenko, A.; Gaburro, Z.; Pavesi, L.; Pucker, G.; Vanzetti, L.; Bellutti, P. Electrical conduction and electroluminescence in nanocrystalline silicon-based light emitting devices. J. Appl. Phys. 2008, 104, 063103. [Google Scholar] [CrossRef]
- Luo, J.W.; Li, S.S.; Sychugov, I.; Pevere, F.; Linnros, J.; Zunger, A. Absence of redshift in the direct bandgap of silicon nanocrystals with reduced size. Nat. Nanotechnol. 2017, 12, 930–932. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Lin, H.; Huang, H.; Reckmeier, C.; Zhang, Y.; Choy, W.C.H.; Rogach, A.L. Enhancing the brightness of cesium lead halide perovskite nanocrystal based green light-emitting devices through the interface engineering with perfluorinated ionomer. Nano Lett. 2016, 16, 1415–1420. [Google Scholar] [CrossRef]
- Bao, J.; Hadjiev, V.G. Origin of luminescent centers and edge states in low-dimensional lead halide perovskites: Controversies, challenges and instructive approaches. Nano-Micro Lett. 2019, 11, 26. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef]
- Yakunin, S.; Protesescu, L.; Krieg, F.; Bodnarchuk, M.I.; Nedelcu, G.; Humer, M.; Luca, G.D.; Fiebig, M.; Heiss, W.; Kovalenko, M.V. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of cesium lead halide perovskites. Nat. Commun. 2015, 6, 8056. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.M.; Song, J.Z.; Xiao, L.; Zeng, H.B.; Sun, H.D. All-inorganic colloidal perovskite quantum dots: A new class of lasing materials with favorable characteristics. Adv. Mater. 2015, 2, 7101–7108. [Google Scholar] [CrossRef]
- Hu, F.R.; Zhang, H.C.; Sun, C.; Yin, C.Y.; Lv, B.H.; Zhang, C.F.; Yu, W.W.; Wang, X.Y.; Zhang, Y.; Xiao, M. Superior optical properties of perovskite nanocrystals as single photon emitters. ACS Nano 2015, 9, 12410–12416. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.K.; Deng, H.; Qiao, K.K.; Farooq, U.; Ishaq, M.; Yi, F.; Liu, H.; Tang, J.; Song, H.S. Low-dimensional halide perovskites and their advanced optoelectronic applications. Nano-Micro Lett. 2017, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.H.; Lim, D.H.; Ra, H.S.; Ramasamy, P.; Lee, J.S. High performance hybrid graphene-CsPbBr3-xIx perovskite nanocrystal photodetector. RSC Adv. 2016, 6, 65252–65256. [Google Scholar] [CrossRef]
- Ramasamy, P.; Lim, D.H.; Kim, B.; Lee, S.H.; Lee, M.S.; Lee, J.S. All-inorganic cesium lead halide perovskite nanocrystals for photodetector applications. Chem. Commun. 2016, 52, 2067–2070. [Google Scholar] [CrossRef] [PubMed]
- Sadhanala, A.; Ahmad, S.; Zhao, B.; Giesbrecht, N.; Pearce, P.M.; Deschler, F.; Hoye, R.L.Z.; Gödel, C.; Bein, T.; Docampo, P.; et al. Blue-green color tunable solution processable organolead chloride-bromide mixed halide perovskites for optoelectronic applications. Nano Lett. 2015, 15, 6095–6101. [Google Scholar] [CrossRef]
- Hossain, M.I.; Qarony, W.; Ma, S.; Zeng, L.H.; Knipp, D.; Tsang, Y.H. Perovskite/silicon tandem solar cells: From detailed balance limit calculations to photon management. Nano-Micro Lett. 2019, 11, 58. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef]
- Mastronardi, M.L.; Flaig, F.M.; Faulkner, D.; Henderson, E.J.; Kubel, C.; Lemmer, U.; Ozin, G.A. Size-dependent absolute quantum yields for size-separated colloidally-stable Silicon nanocrystals. Nano Lett. 2012, 12, 337. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Yu, T.; Qiao, X.; Gresback, R.; Pi, X.; Yang, D. Optimum quantum yield of the light emission from 2 to 10 nm hydrosilylated Silicon quantum dots. Part. Part. Syst. Charact. 2016, 33, 44–52. [Google Scholar] [CrossRef]
- Li, X.M.; Wu, Y.; Zhang, S.L.; Cai, B.; Gu, Y.; Song, J.Z.; Zeng, H.B. CsPbX3 quantum dots for lighting and displays: Room temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 15, 2435–2445. [Google Scholar] [CrossRef]
- Kim, Y.C.; Jeong, H.J.; Kim, S.T.; Song, Y.H.; Kim, B.Y.; Kim, J.P.; Kang, B.K.; Yun, J.Y.; Jang, J.H. Luminescent down-shifting CsPbBr3 perovskite nanocrystals for flexible Cu(In,Ga)Se2 solar cells. Nanoscale 2020, 12, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Stoumpos, C.C.; Malliakas, C.D.; Peters, J.A.; Liu, Z.; Sebastian, M.; Im, J.; Chasapis, T.C.; Wibowo, A.C.; Chung, D.Y.; Freeman, A.J.; et al. Crystal growth of the perovskite semiconductor CsPbBr3: A new material for high-energy radiation detection. Cryst. Growth Des. 2013, 13, 2722–2727. [Google Scholar] [CrossRef]
- Palazon, F.; Di Stasio, F.; Lauciello, S.; Krahne, R.; Prato, M.; Manna, L. Evolution of CsPbBr3 nanocrystals upon post-synthesis annealing under an inert atmosphere. J. Mater. Chem. C 2016, 4, 9179–9182. [Google Scholar] [CrossRef]
- Mao, X.L.; Qiu, T.; Zhang, S.F.; Ma, H.; Hu, Y.Q.; Bai, F.; Wu, Z.C. Air-stable CsPb1-xBixBr3 (0 ≤ x. J. Mater. Chem. C 2017, 5, 4931–4939. [Google Scholar]
- Zhang, H.J.; Liu, X.; Dong, J.P.; Yu, H.; Zhou, C.; Zhang, B.B.; Xu, Y.D.; Jie, W.Q. Centimeter-szed inorganic lead halide perovskite CsPbBr3 crystals grown by an improved solution method. Cryst. Growth Des. 2017, 17, 6426–6431. [Google Scholar] [CrossRef]
- Gong, X.; Guan, L.; Pan, H.P.; Sun, Q.; Zhao, X.J.; Li, H.; Pan, H.; Shen, Y.; Shao, Y.; Sun, L.J.; et al. Highly efficient perovskite solar cells via Nickel passivation. Adv. Funct. Mater. 2018, 28, 1804286. [Google Scholar] [CrossRef]
- Náfrádi, B.; Szirmai, P.; Spina, M.; Lee, H.; Yazyev, O.; Arakcheeva, A.; Chernyshov, D.; Gibert, M.; Forró, L.; Horváth, E. Optically switched magnetism in photovoltaic perovskite CH3NH3(Mn:Pb)I3. Nat. Commun. 2016, 7, 13406. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yong, Z.J.; Zhang, K.C.; Liu, B.M.; Wang, Z.W.; Hou, J.S.; Fang, Y.Z.; Zhou, Y.; Sun, H.T.; Song, B. Ultrabroad photoluminescence and electroluminescence at new wavelengths from doped organometal halide perovskites. J. Phys. Chem. Lett. 2016, 7, 2735–2741. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.D.; Li, Q.; Li, D.S.; Yang, D.R. Spin-coating silicon-quantum-dot ink to improve solar cell efficiency. Sol. Energy Mater. Sol. Cells 2011, 95, 2941–2945. [Google Scholar] [CrossRef]
- Jiang, Y.; Qiu, L.B.; Juarez-Perez, E.J.; Ono, L.K.; Hu, Z.H.; Liu, Z.H.; Wu, Z.F.; Meng, L.Q.; Wang, Q.J.; Qi, Y.B. Reduction of lead leakage from damaged lead halide perovskite solar modules using self-healing polymer-based encapsulation. Nat. Energy 2019, 4, 585–593. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; He, H.Y.; Berry, J.J.; Zhu, K.; Xu, T. On-device lead sequestration for perovskite solar cells. Nature 2020, 578, 555–558. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Wu, D.; Zhu, P.; Shan, D.; Zeng, X.; Xu, J. Down-Shifting and Anti-Reflection Effect of CsPbBr3 Quantum Dots/Multicrystalline Silicon Hybrid Structures for Enhanced Photovoltaic Properties. Nanomaterials 2020, 10, 775. https://doi.org/10.3390/nano10040775
Cao Y, Wu D, Zhu P, Shan D, Zeng X, Xu J. Down-Shifting and Anti-Reflection Effect of CsPbBr3 Quantum Dots/Multicrystalline Silicon Hybrid Structures for Enhanced Photovoltaic Properties. Nanomaterials. 2020; 10(4):775. https://doi.org/10.3390/nano10040775
Chicago/Turabian StyleCao, Yunqing, Dong Wu, Ping Zhu, Dan Shan, Xianghua Zeng, and Jun Xu. 2020. "Down-Shifting and Anti-Reflection Effect of CsPbBr3 Quantum Dots/Multicrystalline Silicon Hybrid Structures for Enhanced Photovoltaic Properties" Nanomaterials 10, no. 4: 775. https://doi.org/10.3390/nano10040775
APA StyleCao, Y., Wu, D., Zhu, P., Shan, D., Zeng, X., & Xu, J. (2020). Down-Shifting and Anti-Reflection Effect of CsPbBr3 Quantum Dots/Multicrystalline Silicon Hybrid Structures for Enhanced Photovoltaic Properties. Nanomaterials, 10(4), 775. https://doi.org/10.3390/nano10040775




