Magnetron-Sputtered Polytetrafluoroethylene-Stabilized Silver Nanoisland Surface for Surface-Enhanced Fluorescence
Abstract
:1. Introduction
2. Materials and Methods
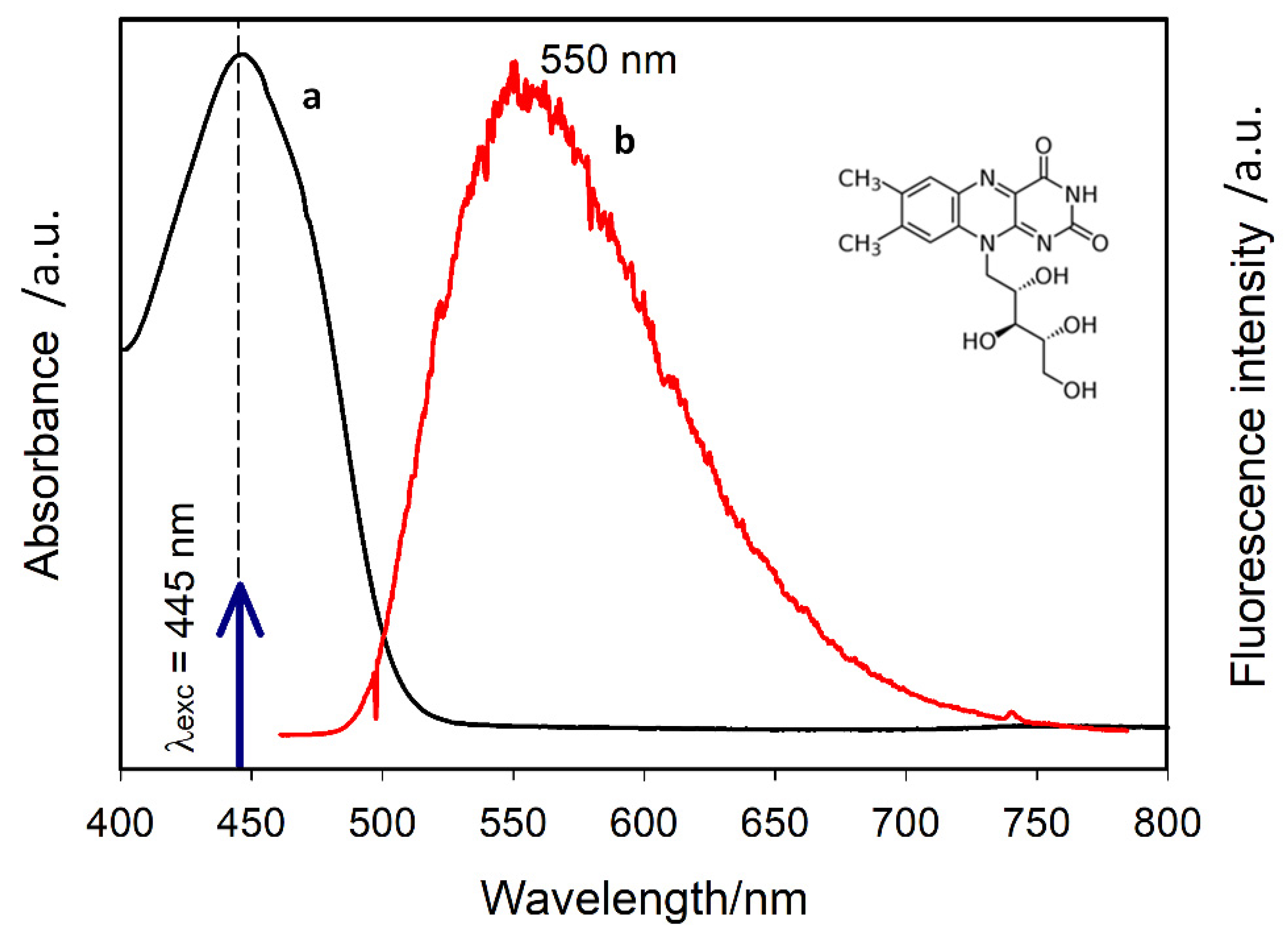
2.1. Studied Biomolecule
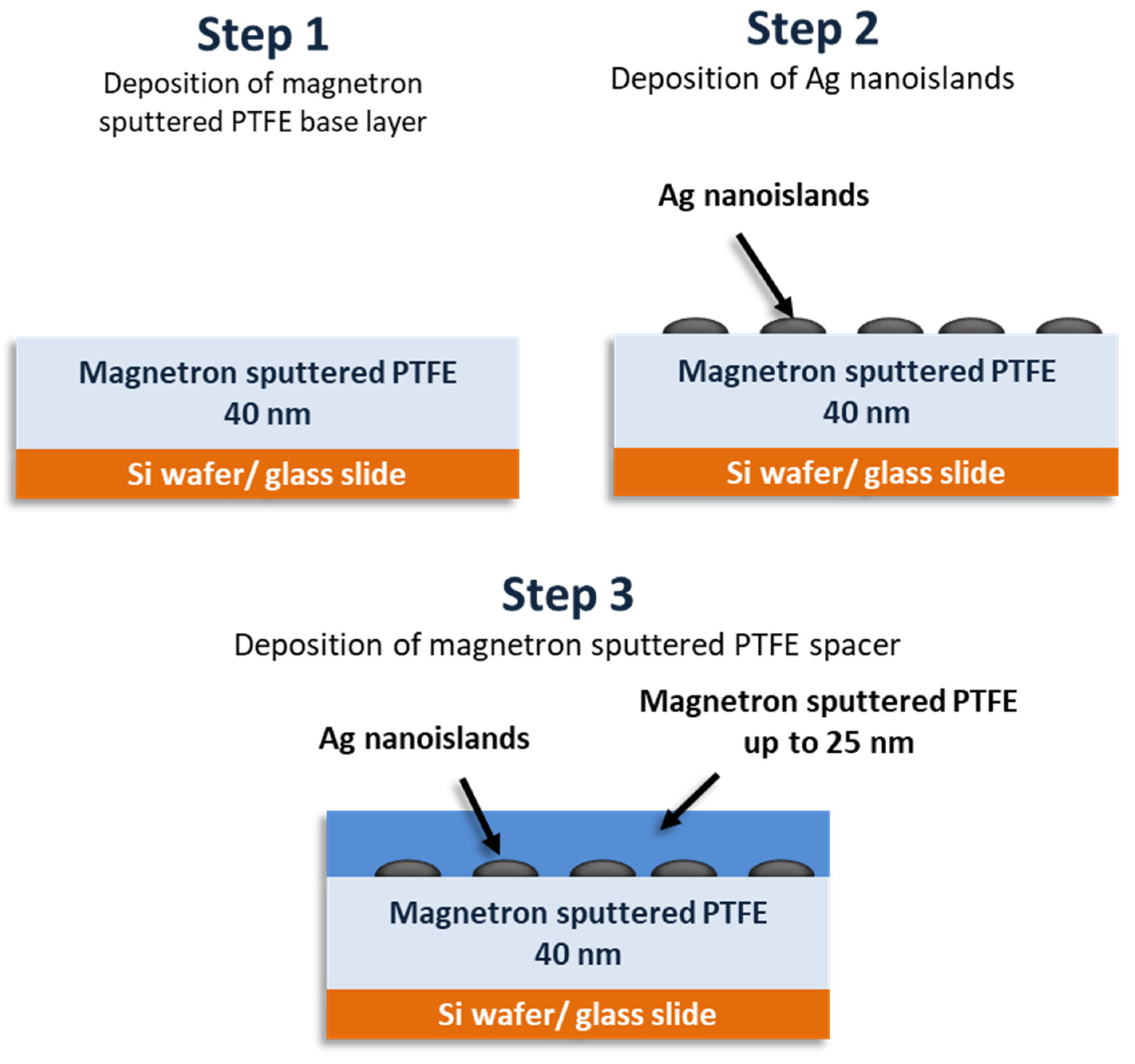
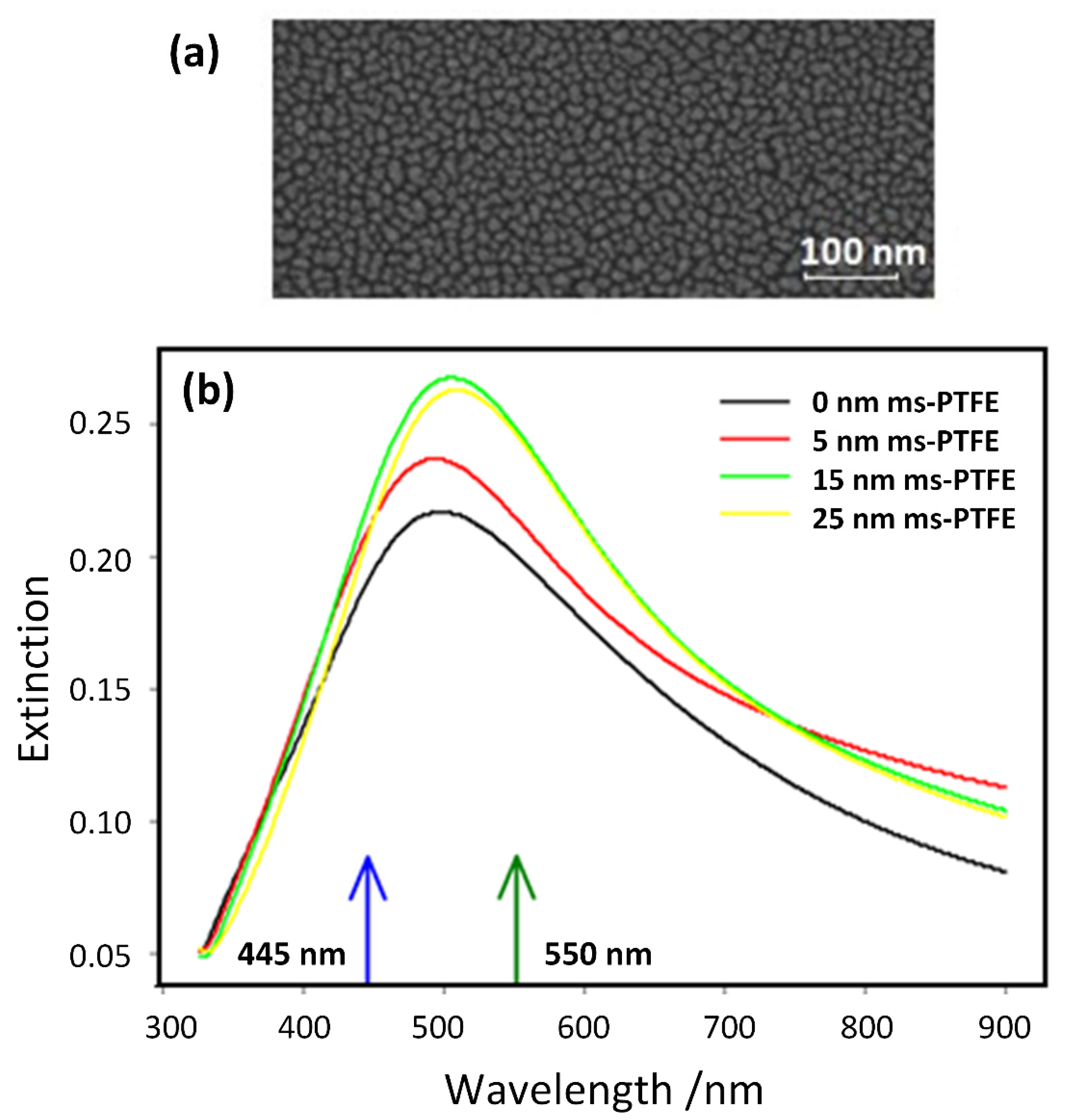
2.2. Fabrication of the SEF Nanostructures
2.3. Fluorescence Measurements
3. Results and Discussion
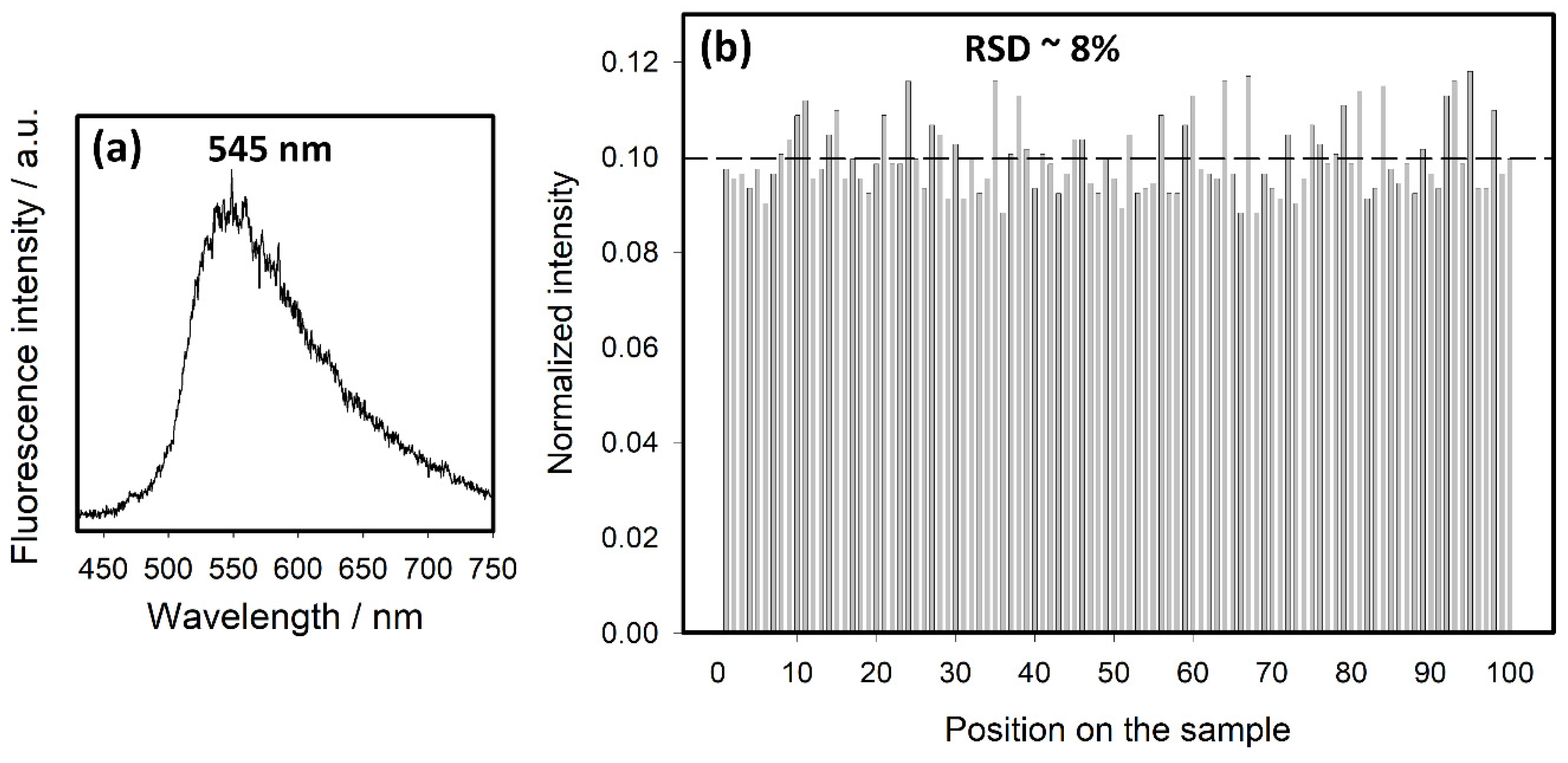
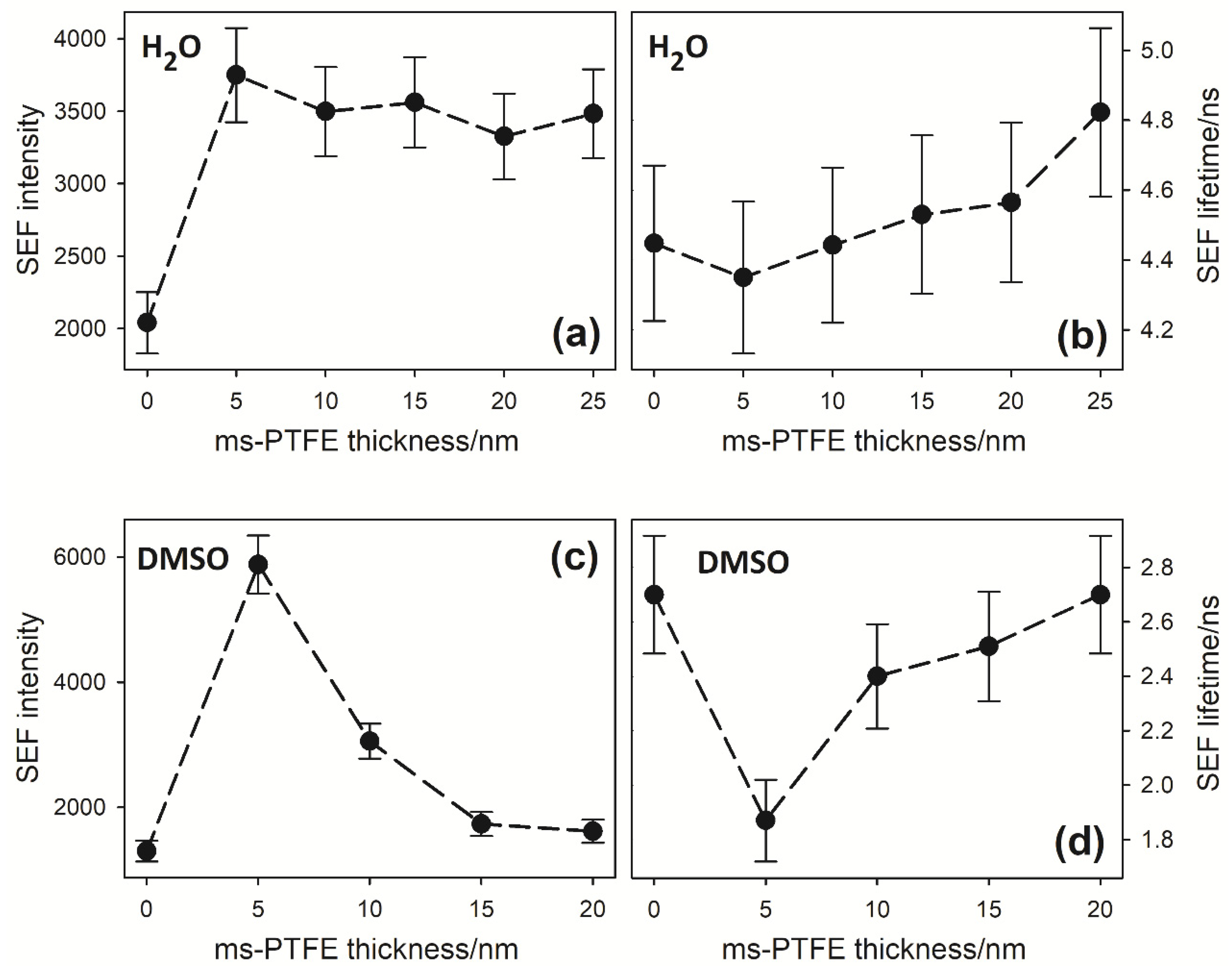
3.1. SEF Measurements
3.2. SEF Intensities and Lifetimes
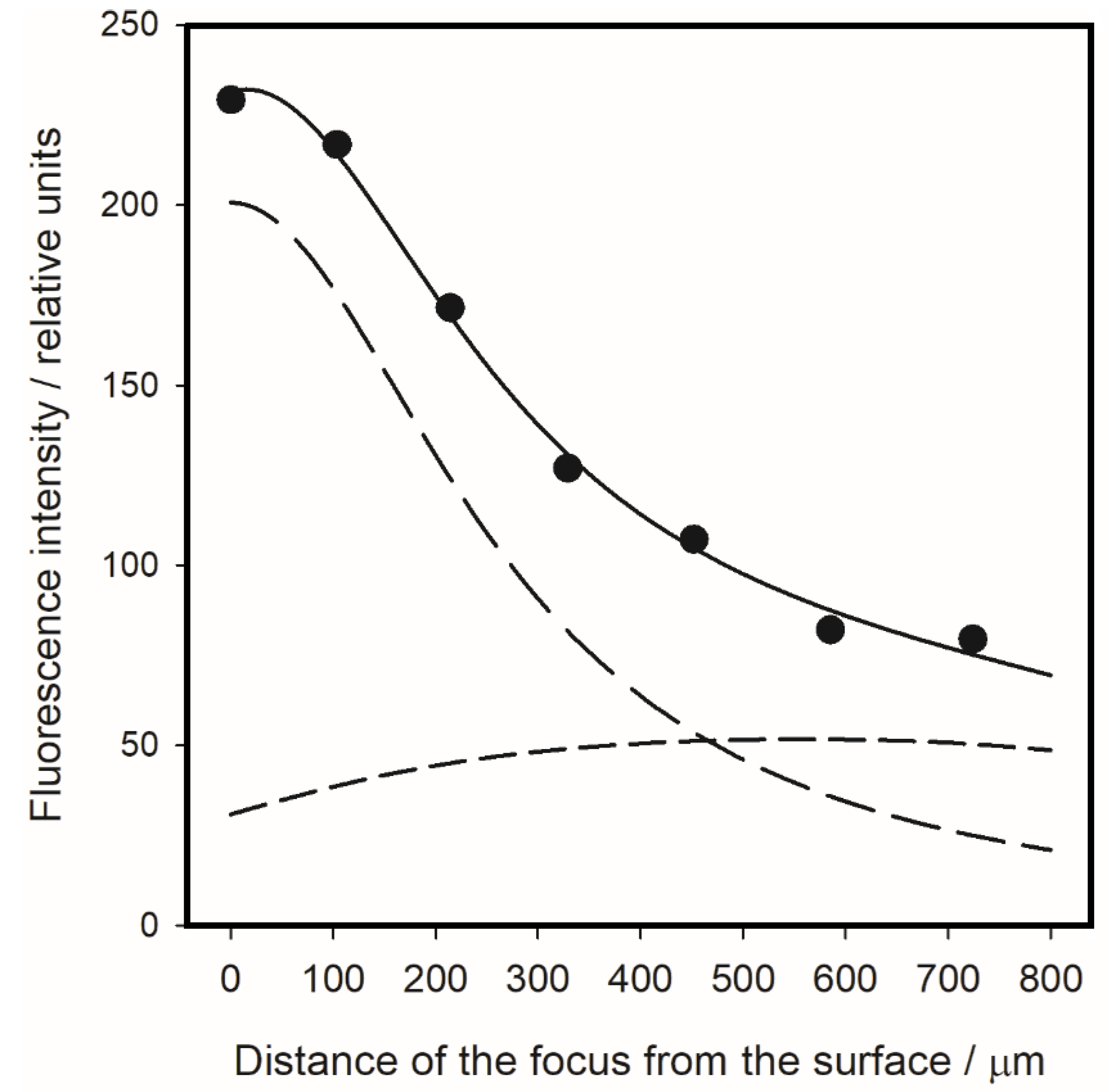
3.3. The Droplet Geometry
3.4. Estimation of the Amount of the Riboflavin Molecules Adsorbed on the Surface
3.5. Estimation of the Ratio of Fluorescence Signals from the Surface Area and the Volume of the Droplet
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Singapore, 2006. [Google Scholar]
- Procházka, M. Surface-Enhanced Raman Spectroscopy: Bioanalytical, Biomolecular and Medical Applications; Springer: Heidelberg, Switzerland, 2016. [Google Scholar]
- Aroca, R.F. Plasmon enhanced spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 5355–5363. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Etchegoin, P.G. Principles of Surface-Enhanced Raman Spectroscopy and Related Plasmonic Effects; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Li, J.F.; Li, C.Y.; Aroca, R.F. Plasmon-enhanced fluorescence spectroscopy. Chem. Soc. Rev. 2017, 46, 3962–3979. [Google Scholar] [CrossRef]
- Wokaun, A.; Lutz, H.P.; King, A.P.; Wild, U.P.; Ernst, R.R. Energy-Transfer in Surface Enhanced Luminescence. J. Chem. Phys. 1983, 79, 509–514. [Google Scholar] [CrossRef]
- Weitz, D.A.; Garoff, S. The Enhancement of Raman-Scattering, Resonance Raman-Scattering, and Fluorescence from Molecules Adsorbed on a Rough Silver Surface. J. Chem. Phys. 1983, 78, 5324–5338. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative decay engineering: Biophysical and biomedical applications. Anal. Biochem. 2001, 298, 1–24. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Geddes, C.D.; Gryczynski, I.; Malicka, J.; Gryczynski, Z.; Aslan, K.; Lukomska, J.; Matveeva, E.; Zhang, J.; Badugu, R.; et al. Advances in surface-enhanced fluorescence. J. Fluoresc. 2004, 14, 425–441. [Google Scholar] [CrossRef] [Green Version]
- Fort, E.; Gresillon, S. Surface enhanced fluorescence. J. Phys. D Appl. Phys. 2008, 41, 013001. [Google Scholar] [CrossRef] [Green Version]
- Tam, F.; Goodrich, G.P.; Johnson, B.R.; Halas, N.J. Plasmonic enhancement of molecular fluorescence. Nano. Lett. 2007, 7, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Munechika, K.; Ginger, D.S. Dependence of fluorescence intensity on the spectral overlap between fluorophores and plasmon resonant single silver nanoparticles. Nano. Lett. 2007, 7, 690–696. [Google Scholar] [CrossRef]
- Stranik, O.; Nooney, R.; McDonagh, C.; MacCraith, B.D. Optimization of nanoparticle size for plasmonic enhancement of fluorescence. Plasmonics 2007, 2, 15–22. [Google Scholar] [CrossRef]
- Bharadwaj, P.; Novotny, L. Spectral dependence of single molecule fluorescence enhancement. Opt. Express 2007, 15, 14266–14274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, G.; Khurgin, J.B. Origin of giant difference between fluorescence, resonance, and nonresonance Raman scattering enhancement by surface plasmons. Phys. Rev. A 2012, 85, 063410. [Google Scholar] [CrossRef]
- Mishra, H.; Buddha, L.M.; Karolin, J.; Dragan, A.I.; Geddes, C.D. Experimental and theoretical study of the distance dependence of metal-enhanced fluorescence, phosphorescence and delayed fluorescence in a single system. Phys. Chem. Chem. Phys. 2013, 15, 19538–19544. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, J.; Libenská, H.; Khalakhan, I.; Kuzminova, A.; Kylián, O.; Biederman, H. Localized surface plasmon resonance tuning via nanostructured gradient Ag surfaces. Mater. Lett. 2017, 192, 119–122. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Liaw, J.W.; Tsai, H.Y.; Huang, C.H. Size-Dependent Surface Enhanced Fluorescence of Gold Nanorod: Enhancement or Quenching. Plasmonics 2012, 7, 543–553. [Google Scholar] [CrossRef]
- Vahl, A.; Strobel, J.; Reichstein, W.; Polonskyi, O.; Strunskus, T.; Kienle, L.; Faupel, F. Single target sputter deposition of alloy nanoparticles with adjustable composition via a gas aggregation cluster source. Nanotechnology 2017, 28, 175703. [Google Scholar] [CrossRef]
- Asselin, J.; Legros, P.; Gregoire, A.; Boudreau, D. Correlating Metal-Enhanced Fluorescence and Structural Properties in Ag@SiO2 Core-Shell Nanoparticles. Plasmonics 2016, 11, 1369–1376. [Google Scholar] [CrossRef]
- Ferreira, M.; Constantino, C.J.; Olivati, C.A.; Vega, M.L.; Balogh, D.T.; Aroca, R.F.; Faria, R.M.; Oliveira, O.N. Langmuir and Langmuir-Blodgett films of poly[2-methoxy-5-(n-hexyloxy)-p-phenylenevinylene]. Langmuir 2003, 19, 8835–8842. [Google Scholar] [CrossRef]
- Constantino, C.J.L.; Aroca, R.F.; Mendonça, C.R.; Mello, S.V.; Balogh, D.T.; Oliveira, O.N., Jr. Surface enhanced fluorescence and Raman imaging of Langmuir-Blodgett azopolymer films. Spectroc. Acta A Molec. Biomolec. Spectr. 2001, 57, 281–289. [Google Scholar] [CrossRef]
- Bardhan, R.; Grady, N.K.; Cole, J.R.; Joshi, A.; Halas, N.J. Fluorescence Enhancement by Au Nanostructures: Nanoshells and Nanorods. ACS Nano 2009, 3, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Dragan, A.I.; Bishop, E.S.; Casas-Finet, J.R.; Strouse, R.J.; McGivney, J.; Schenerman, M.A.; Geddes, C.D. Distance Dependence of Metal-Enhanced Fluorescence. Plasmonics 2012, 7, 739–744. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Chowdhury, H.; Lakowicz, J.R. Metal-enhanced single-molecule fluorescence on silver particle monomer and dimer: Coupling effect between metal particles. Nano Lett. 2007, 7, 2101–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iliut, M.; Gabudean, A.M.; Leordean, C.; Simon, T.; Teodorescu, C.M.; Astilean, S. Riboflavin enhanced fluorescence of highly reduced graphene oxide. Chem. Phys. Lett. 2013, 586, 127–131. [Google Scholar] [CrossRef]
- Šubr, M.; Petr, M.; Kylián, O.; Kratochvíl, J.; Procházka, M. Large-scale Ag nanoislands stabilized by a magnetron-sputtered polytetrafluoroethylene film as substrates for highly sensitive and reproducible surface-enhanced Raman scattering (SERS). J. Mat. Chem. C 2015, 3, 11478–11485. [Google Scholar] [CrossRef]
- Praus, P.; Sureau, F. Spectral decomposition of intracellular complex fluorescent signals using multiwavelength phase modulation lifetime determination. J. Fluoresc. 2000, 10, 361–364. [Google Scholar] [CrossRef]
- Praus, P.; Kocisova, E.; Seksek, O.; Sureau, F.; Stepanek, J.; Turpin, P.Y. Advanced microfluorescence methods in monitoring intracellular uptake of "antisense" oligonucleotides. Curr. Org. Chem. 2007, 11, 515–527. [Google Scholar] [CrossRef]
- Kočišová, E.; Praus, P.; Rosenberg, I.; Seksek, O.; Sureau, F.; Štĕpánek, J.; Turpin, P.Y. Intracellular uptake of modified oligonucleotide studied by two fluorescence techniques. Biopolymers 2004, 74, 110–114. [Google Scholar] [CrossRef]
- Kočišová, E.; Praus, P.; Bok, J.; Bonneau, S.; Sureau, F. Intracellular Monitoring of AS1411 Aptamer by Time-Resolved Microspectrofluorimetry and Fluorescence Imaging. J. Fluoresc. 2015, 25, 1245–1250. [Google Scholar] [CrossRef]
- Drossler, P.; Holzer, W.; Penzkofer, A.; Hegemann, P. Fluorescence quenching of riboflavin in aqueous solution by methionin and cystein. Chem. Phys. 2003, 286, 409–420. [Google Scholar] [CrossRef]
- Zirak, P.; Penzkofer, A.; Mathes, T.; Hegemann, P. Photo-dynamics of roseoflavin and riboflavin in aqueous and organic solvents. Chem. Phys. 2009, 358, 111–122. [Google Scholar] [CrossRef]
- Waldeck, D.H.; Alivisatos, A.P.; Harris, C.B. Nonradiative Damping of Molecular Electronic Excited-States by Metal-Surfaces. Surf. Sci. 1985, 158, 103–125. [Google Scholar] [CrossRef] [Green Version]
- Masel, R. Principles of Adsorption and Reaction on Solid Surfaces; Wiley: Hoboken, NJ, USA, 1996; p. 240. [Google Scholar]
| Water | DMSO | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ms-PTFE Thickness | Lifetime | τF,m/τF | Γm | Q/Q0 | ms-PTFE Thickness | Lifetime | τF,m/τF | Γm | Q/Q0 |
| 0 nm | 4.45 ns | 0.89 | N/A | N/A | 0 nm | 2.70 ns | 0.93 | N/A | N/A |
| 5 nm | 4.35 ns | 0.87 | 2.99 | 1.34 | 5 nm | 1.87 ns | 0.64 | 18.99 | 2.30 |
| 10 nm | 4.44 ns | 0.89 | 2.51 | 1.29 | 10 nm | 2.40 ns | 0.83 | 4.17 | 1.39 |
| 15 nm | 4.53 ns | 0.91 | 2.08 | 1.24 | 15 nm | 2.51 ns | 0.87 | 3.11 | 1.30 |
| 20 nm | 4.57 ns | 0.91 | 1.91 | 1.23 | 22 nm | 2.70 ns | 0.93 | 1.48 | 1.15 |
| 25 nm | 4.82 ns | 0.96 | 0.74 | 1.09 | |||||
| Surface | Solvent | Volume/µL | Contact Angle/° | ζ/mm−1 |
|---|---|---|---|---|
| ms-PTFE layer | DMSO | 5 | 71 ± 4 | 1.5 ± 0.1 |
| ms-PTFE layer | water | 10 | 104 ± 2 | 0.68 ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šubr, M.; Praus, P.; Kuzminova, A.; Kočišová, E.; Kylián, O.; Sureau, F.; Procházka, M.; Štěpánek, J. Magnetron-Sputtered Polytetrafluoroethylene-Stabilized Silver Nanoisland Surface for Surface-Enhanced Fluorescence. Nanomaterials 2020, 10, 773. https://doi.org/10.3390/nano10040773
Šubr M, Praus P, Kuzminova A, Kočišová E, Kylián O, Sureau F, Procházka M, Štěpánek J. Magnetron-Sputtered Polytetrafluoroethylene-Stabilized Silver Nanoisland Surface for Surface-Enhanced Fluorescence. Nanomaterials. 2020; 10(4):773. https://doi.org/10.3390/nano10040773
Chicago/Turabian StyleŠubr, Martin, Petr Praus, Anna Kuzminova, Eva Kočišová, Ondřej Kylián, Franck Sureau, Marek Procházka, and Josef Štěpánek. 2020. "Magnetron-Sputtered Polytetrafluoroethylene-Stabilized Silver Nanoisland Surface for Surface-Enhanced Fluorescence" Nanomaterials 10, no. 4: 773. https://doi.org/10.3390/nano10040773
APA StyleŠubr, M., Praus, P., Kuzminova, A., Kočišová, E., Kylián, O., Sureau, F., Procházka, M., & Štěpánek, J. (2020). Magnetron-Sputtered Polytetrafluoroethylene-Stabilized Silver Nanoisland Surface for Surface-Enhanced Fluorescence. Nanomaterials, 10(4), 773. https://doi.org/10.3390/nano10040773






