Soft X-ray Absorption Spectroscopic Investigation of Li(Ni0.8Co0.1Mn0.1)O2 Cathode Materials
Abstract
1. Introduction
2. Experimental Details
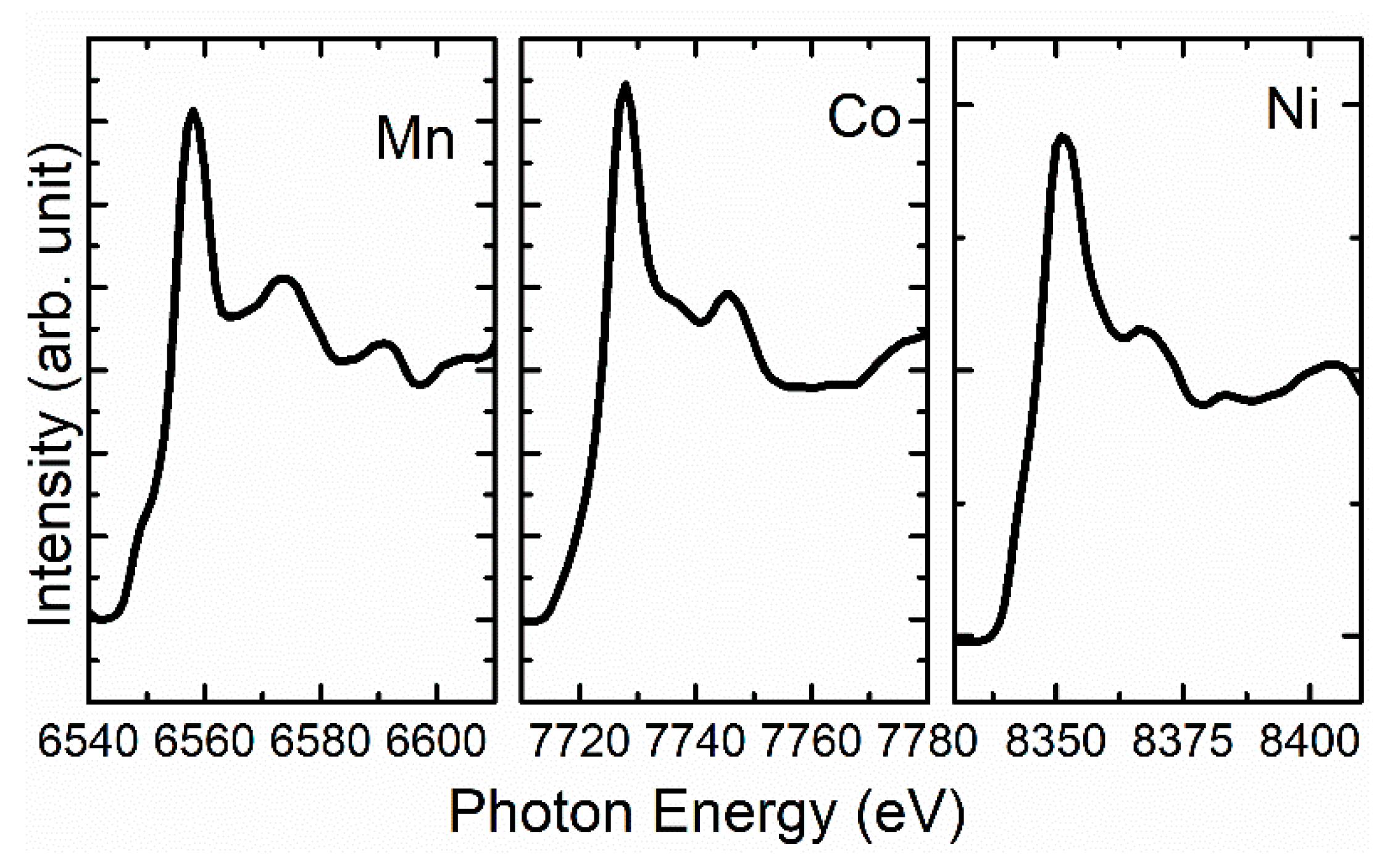
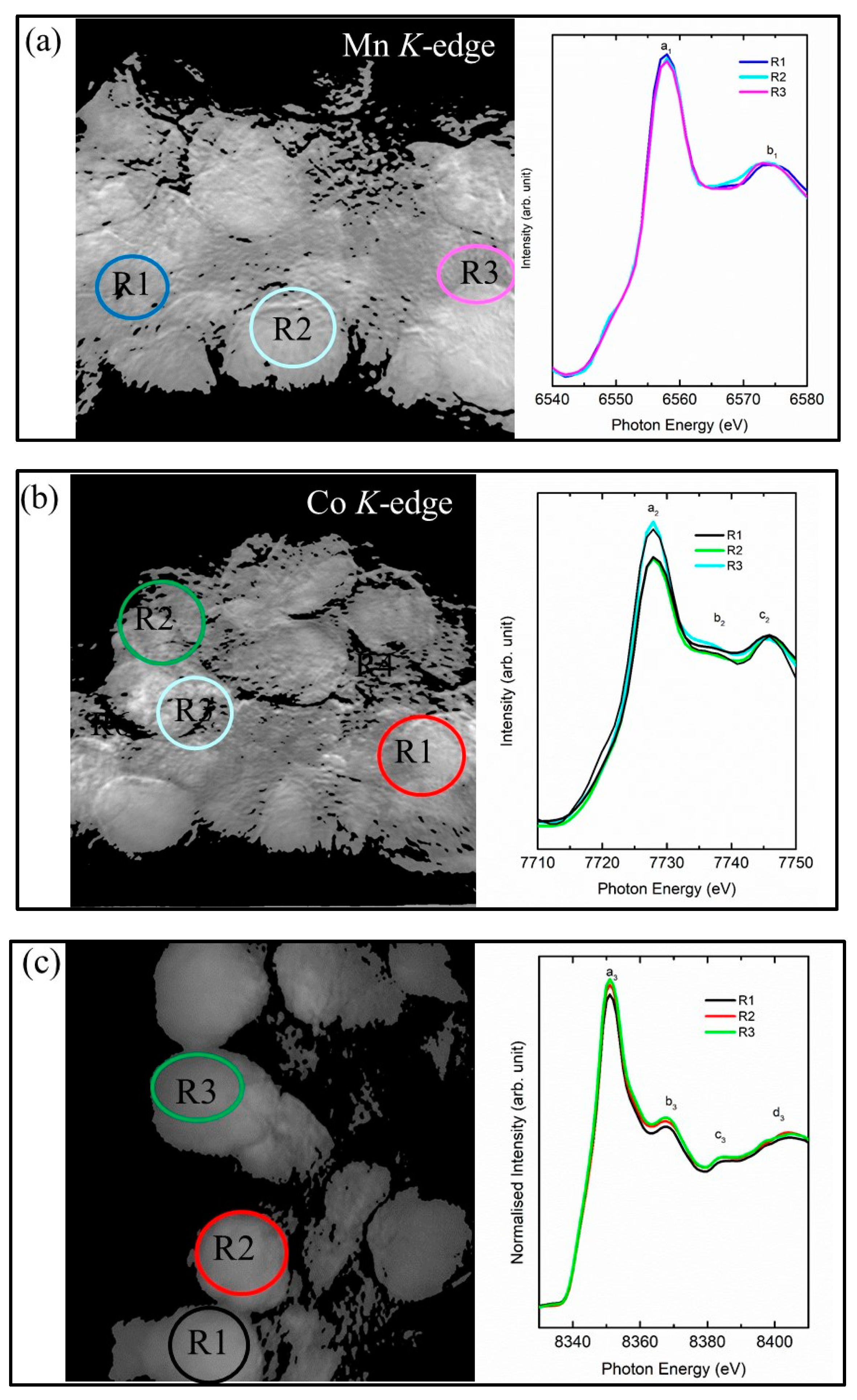
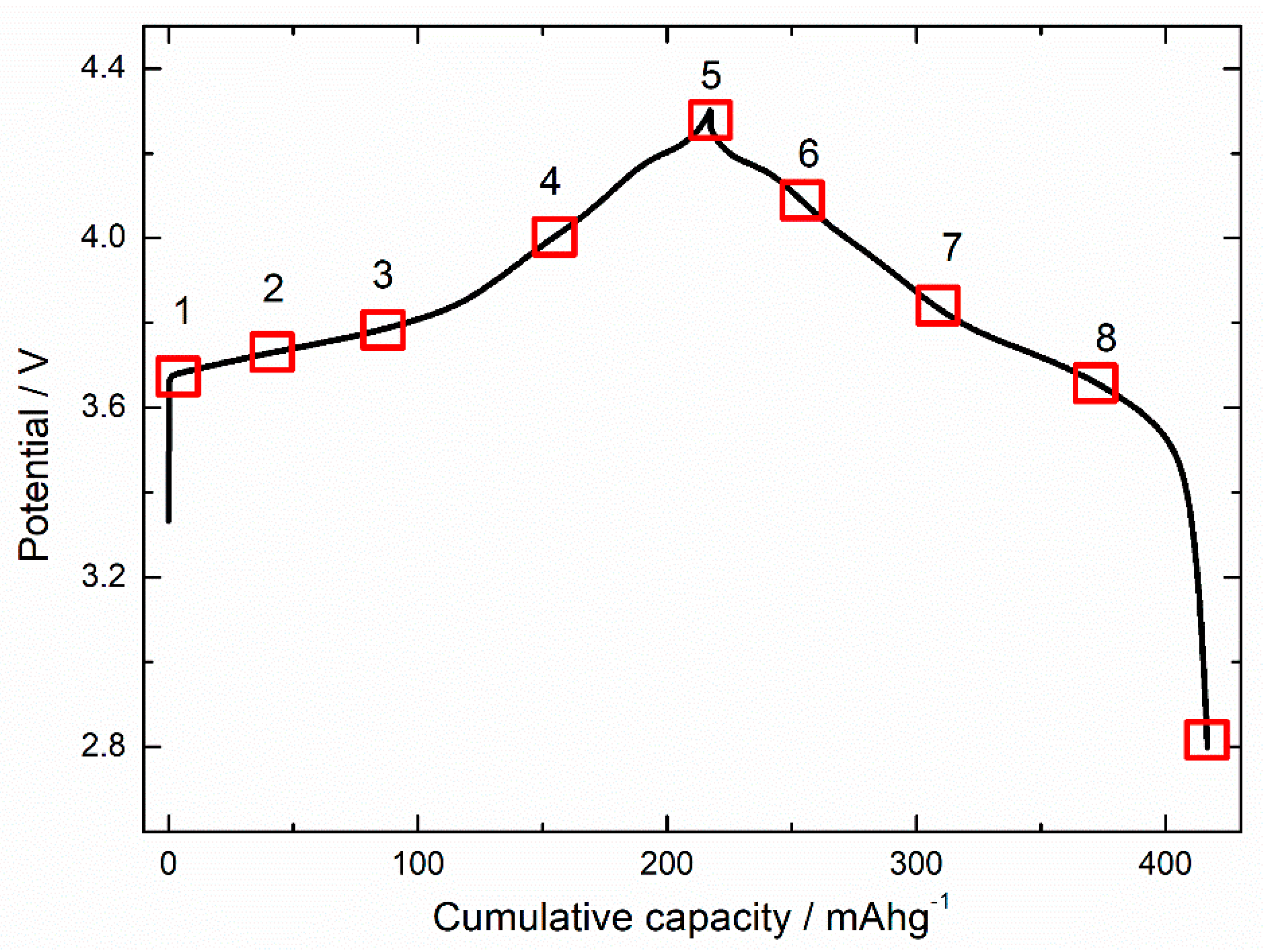
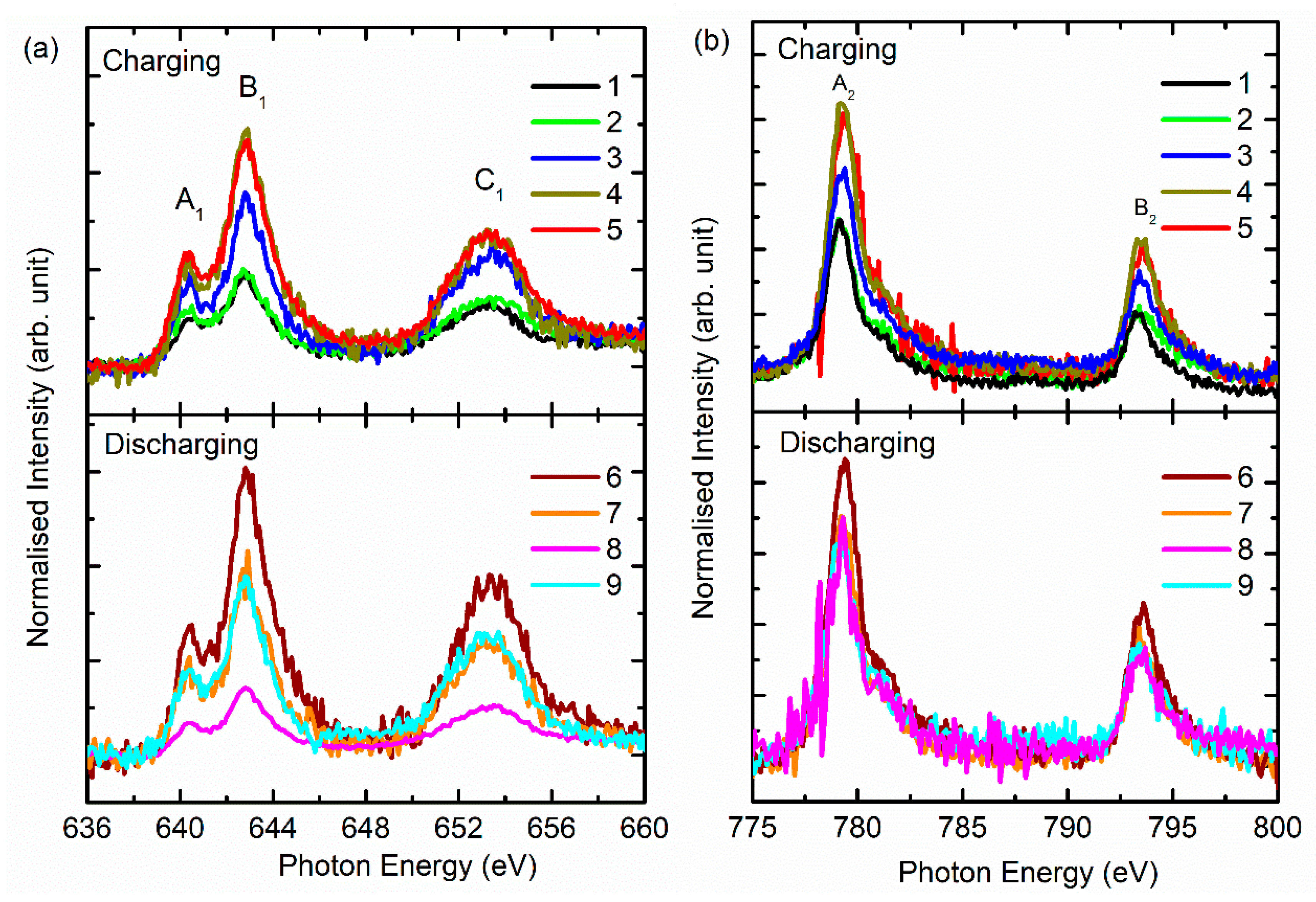
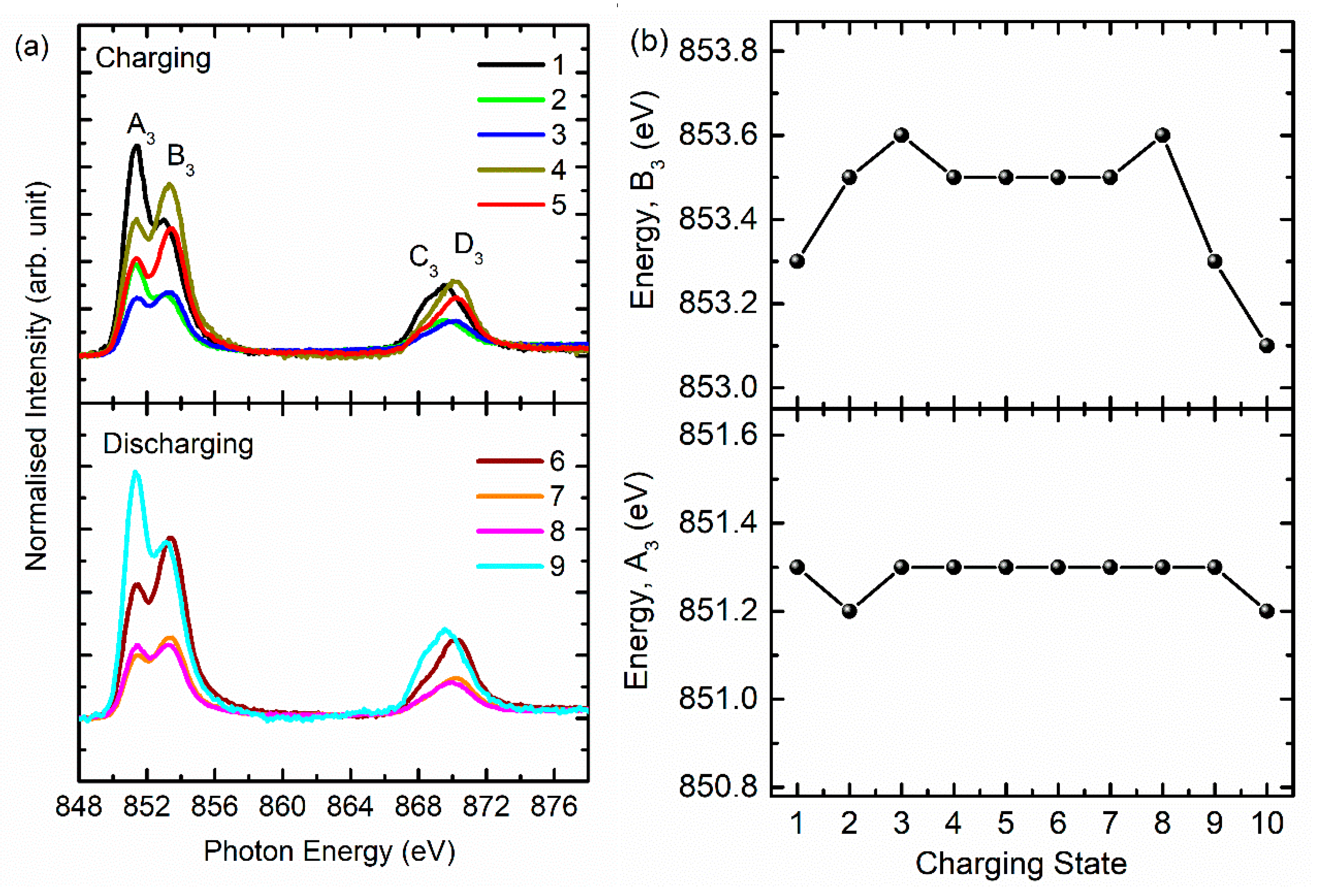
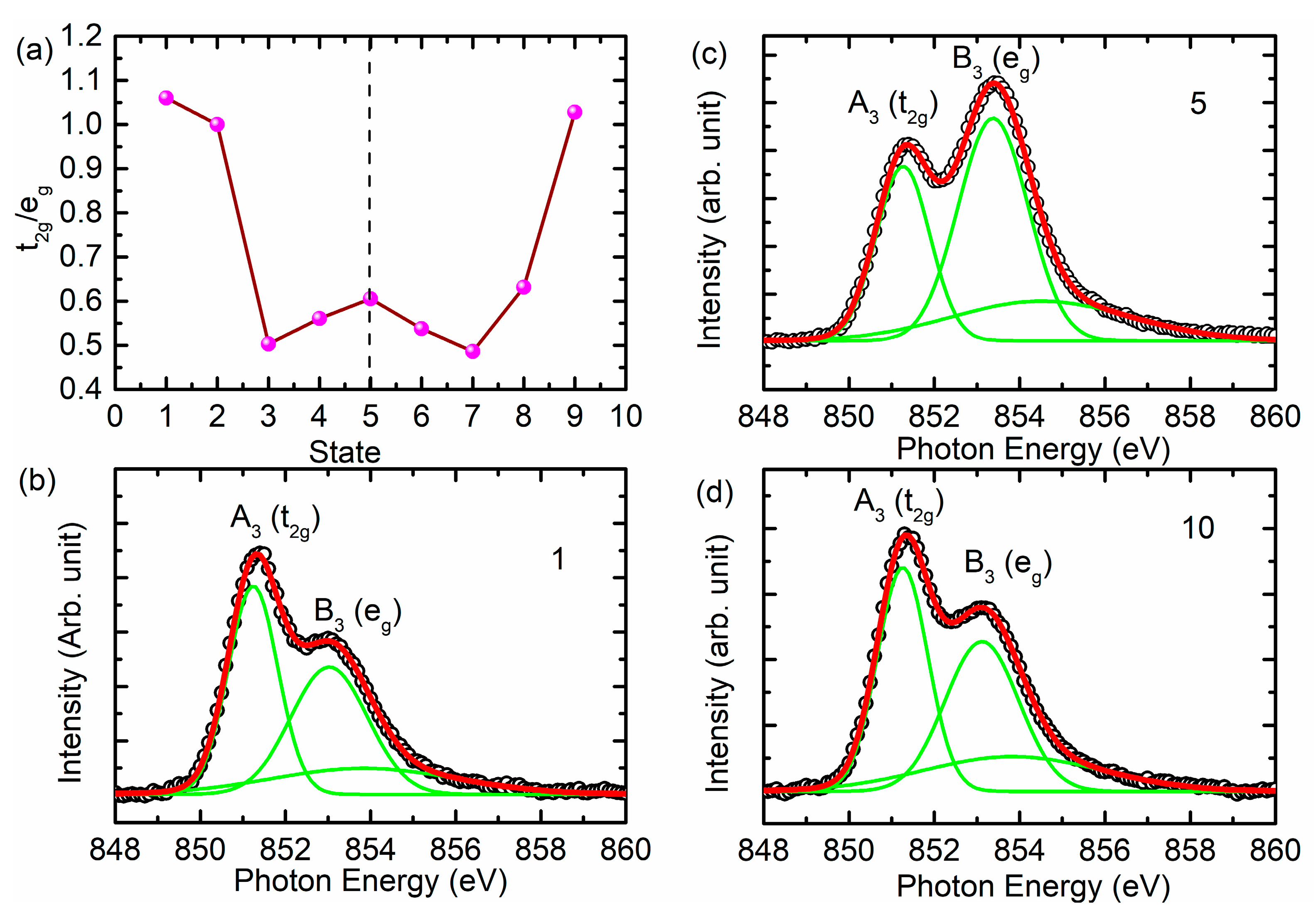
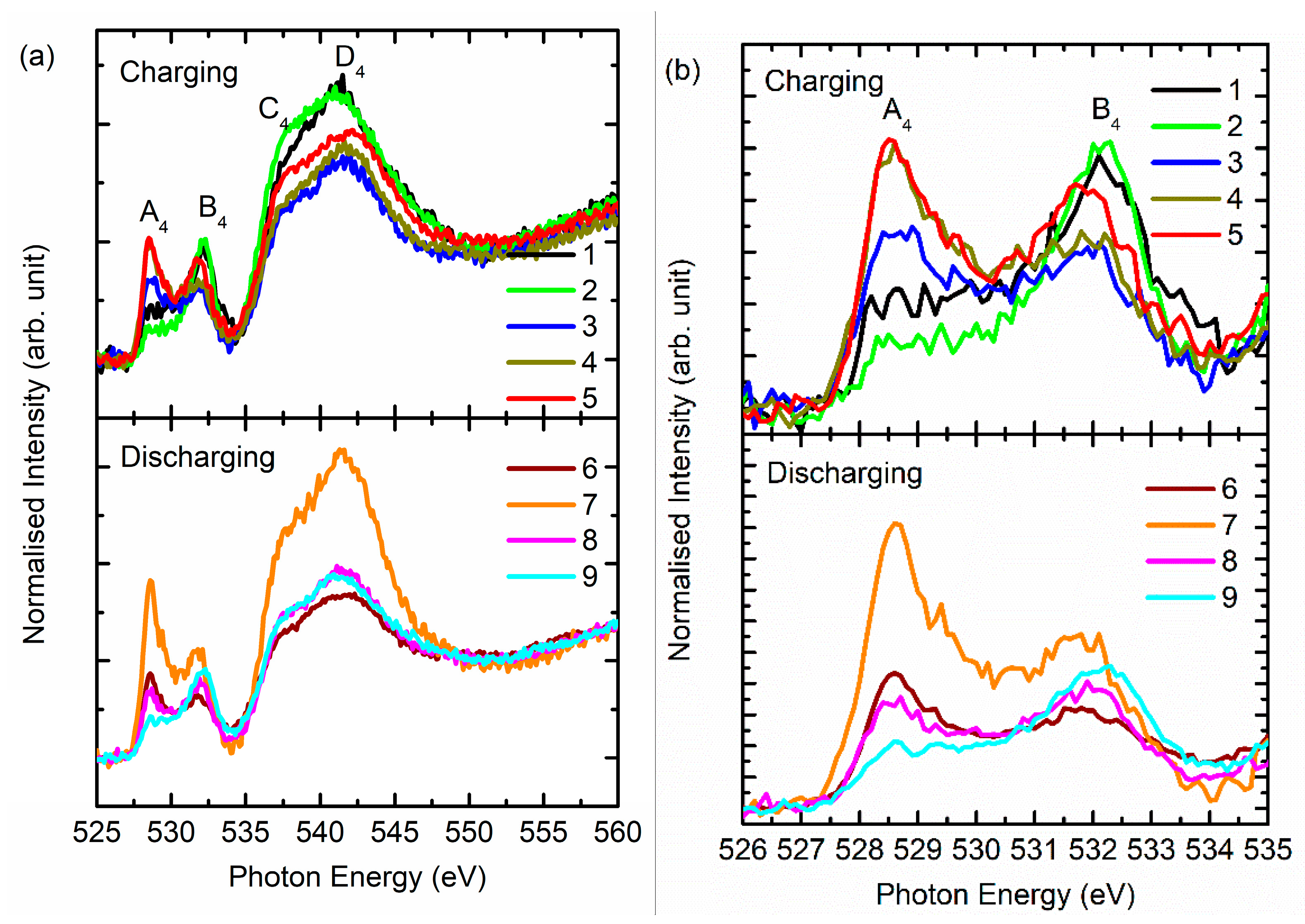
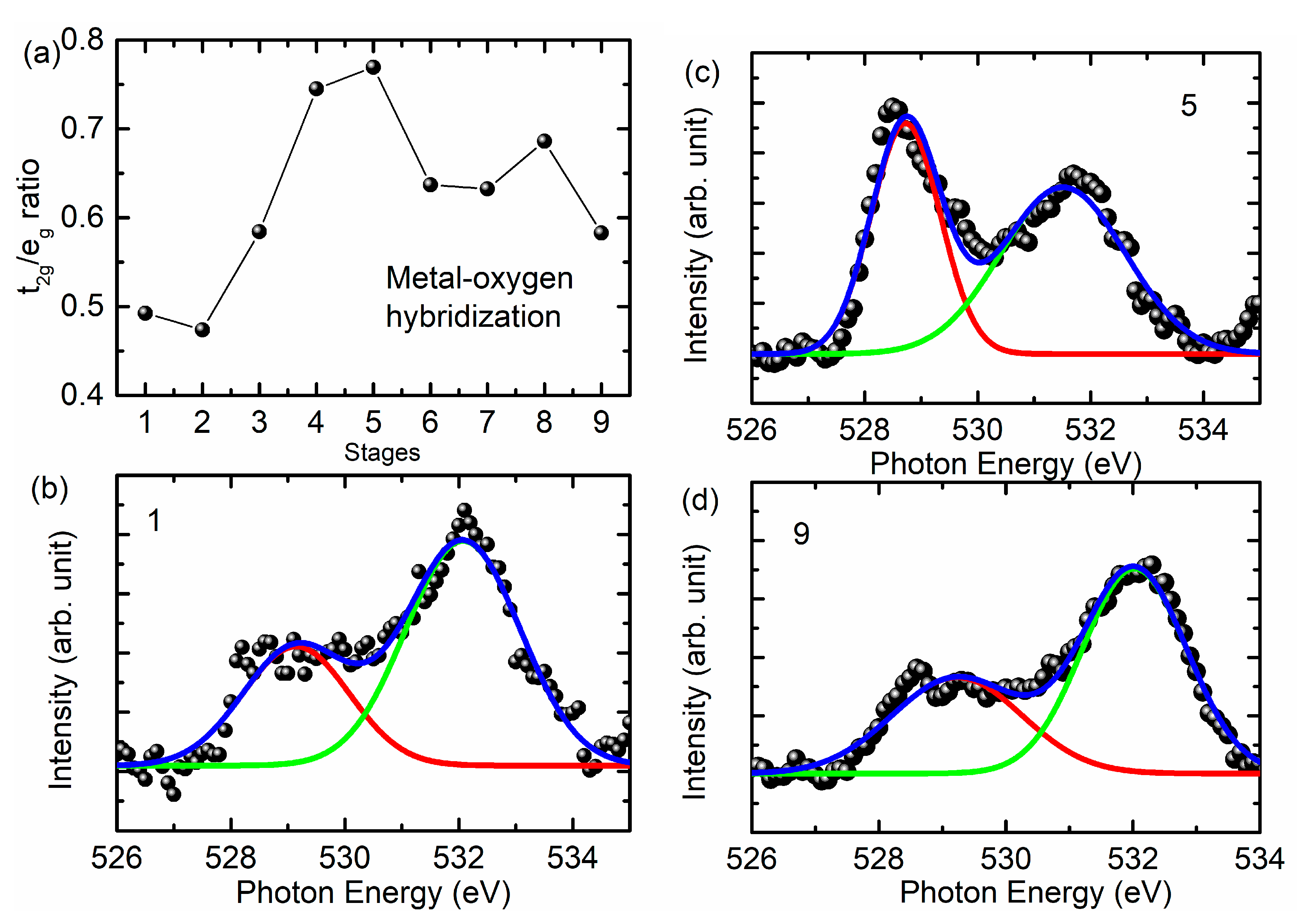
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion Battery Materials: Present and Future. Mater. Today 2015, 18, 1369–7021. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion Batteries: Outlook on Present, Future, and Hybridized Technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Güra, T.M. Review of Electrical Energy Storage Technologies, Materials and Systems: Challenges and Prospects for Large-Scale Grid Storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Biasi, L.D.; Schwarz, B.; Brezesinski, T.; Hartmann, P.; Janek, J.; Ehrenberg, H. Chemical, Structural, and Electronic Aspects of Formation and Degradation Behavior on Different Length Scales of Ni-Rich NCM and Li-Rich HE-NCM Cathode Materials in Li-Ion Batteries. Adv. Mater. 2019, 31, 1900985. [Google Scholar] [CrossRef]
- Li, Y.; Pei, A.; Yan, K.; Sun, Y.; Wu, C.-L.; Joubert, L.M.; Chin, R.; Koh, A.L.; Yu, Y.; Perrino, J.; et al. Atomic Structure of Sensitive Battery Materials and Interfaces Revealed by Cryo–Electron Microscopy. Science 2017, 358, 506–510. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, L.; Hu, Y.S.; Li, H. Atomic-Scale Structure-Property Relationships in Lithium Ion Battery Electrode Materials. Ann. Rev. Mater. Res. 2017, 47, 175–198. [Google Scholar] [CrossRef]
- Çelik, G.; Aktaş, S.; Ateş, Ş.; Özkendir, O.M.; Klysubun, W. Crystal and Electronic Structure Study of the Li2Mn1-xNdxO3 Battery Cathode. Prog. Nat. Sci. Mater. Int. 2019, 29, 119–123. [Google Scholar] [CrossRef]
- Alemu, T.; Wang, F.M. In situ Electrochemical Synchrotron Radiation for Li-ion Batteries. J. Synchrotron Rad. 2018, 25, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.J.; Tsai, Y.W.; Santhanam, R.; Wu, Y.W.; Hu, S.G.; Lee, J.F.; Liu, D.G. Evolution of Local Electronic and Atomic Structure of Co-Doped LiMn2O4 Cathode Material for Lithium Rechargeable Batteries. J. Power Sources 2003, 123, 206–215. [Google Scholar] [CrossRef]
- Ragavendran, K.R.; Mandal, P.; Yarlagadda, S. Correlation between Battery Material Performance and Cooperative Electron-Phonon Interaction in LiCoyMn2−yO4. Appl. Phys. Lett. 2017, 110, 143901. [Google Scholar] [CrossRef]
- Liu, C.; Neale, Z.G.; Cao, G. Understanding Electrochemical Potentials of Cathode Materials in Rechargeable Batteries. Mater. Today 2016, 19, 109–123. [Google Scholar] [CrossRef]
- Tokura, Y.; Nagaosa, N. Orbital Physics in Transition-Metal Oxides. Science 2000, 288, 462–468. [Google Scholar] [CrossRef]
- Singh, V.; Major, D.T. Electronic Structure and Bonding in Co-Based Single and Mixed Valence Oxides: A Quantum Chemical Perspective. Inorg. Chem. 2016, 55, 3307–3315. [Google Scholar] [CrossRef]
- Schuster, M.E.; Teschner, D.; Popovic, J.; Ohmer, N.; Girgsdies, F.; Tornow, J.; Willinger, M.G.; Samuelis, D.; Titirici, M.M.; Maier, J.; et al. Charging and Discharging Behavior of Solvothermal LiFePO4 Cathode Material Investigated by Combined EELS/NEXAFS Study. Chem. Mater. 2014, 26, 1040–1047. [Google Scholar] [CrossRef]
- House, R.A.; Maitra, U.; Jin, L.; Lozano, J.G.; Somerville, J.W.; Rees, N.H.; Naylor, A.J.; Duda, L.C.; Massel, F.; Chadwick, A.V.; et al. What Triggers Oxygen Loss in Oxygen Redox Cathode Materials? Chem. Mater. 2019, 31, 3293–3300. [Google Scholar] [CrossRef]
- Xia, Y.; Zheng, J.; Wang, C.; Gu, M. Designing Principle for Ni-Rich Cathode Materials with High Energy Density for Practical Applications. Nano Energy 2018, 49, 434–452. [Google Scholar] [CrossRef]
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.Y.; Chesneau, F.F.; Aurbach, D. Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes. J. ElectroChem. Soc. 2017, 164, A6220. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, D.; Kulshreshtha, C.; Sohn, K.-S.; Shin, N. Lithium Barium Silicate, Li2BaSiO4, from Synchrotron Powder Data. Acta Cryst. C 2009, 65, i14–i16. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Singh, J.P.; Lim, J.; Lee, S. Development of XANES Nanoscopy on BL7C at PLS-II. J. Synchrotron Rad. 2020, 27, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Kim, S.H.; Won, S.O.; Lee, I.J.; Chae, K.H. Atomic-Scale Investigation of MgO Growth on Fused Quartz Using Angle-Dependent NEXAFS Measurements. RSC Adv. 2018, 8, 31275–31286. [Google Scholar] [CrossRef]
- Lee, W.; Muhammad, S.; Kim, T.; Kim, H.; Lee, E.; Jeong, M.; Son, S.; Ryou, J.-H.; Yoon, W.-S. New Insight into Ni-Rich Layered Structure for Next-Generation Li Rechargeable Batteries. Adv. Energy Mater. 2018, 8, 1701788. [Google Scholar] [CrossRef]
- Weigel, T.; Schipper, F.; Erickson, E.M.; Susai, F.A.; Markovsky, B.; Aurbach, D. Structural and Electrochemical Aspects of LiNi0.8Co0.1Mn0.1O2 Cathode Materials Doped by Various Cations. ACS Energy Lett. 2019, 4, 508–516. [Google Scholar] [CrossRef]
- Yoshio, M.; Noguchi, H.; Itoh, J.; Okada, M.; Mouri, T. Preparation and Properties of LiCoyMnxNi1−x−yO2 as a Cathode for Lithium Ion Batteries. J. Power Sources 2000, 90, 176–181. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Nam, K.W.; Kim, M.G.; Kim, K.B. In Situ Mn K-edge X-ray Absorption Spectroscopy Studies of Electrodeposited Manganese Oxide Films for Electrochemical Capacitors. J. Phys. Chem. C 2007, 111, 749–758. [Google Scholar] [CrossRef]
- Manceau, A.; Marcus, M.A.; Grangeon, S. Determination of Mn valence states in mixed-valent manganates by XANES spectroscopy. Am. Mineral. 2012, 97, 816–827. [Google Scholar] [CrossRef]
- Okumura, T.; Yamaguchi, Y.; Shikano, M.; Kobayashia, H. Correlation of Lithium Ion Distribution and X-ray Absorption Near-Edge Structure in O3- and O2-Lithium Cobalt Oxides from First-Principle Calculation. J. Mater. Chem. 2012, 22, 17340–17348. [Google Scholar] [CrossRef]
- Ekwongsa, C.; Rujirawat, S.; Butnoi, P.; Vittayakorn, N.; Suttapun, M.; Yimnirun, R.; Kidkhunthod, P. Temperature Dependent Local Structure of LiCoO2 Determined by in-situ Co K-edge X-ray Absorption Fine Structure (EXAFS). Rad. Phys. Chem. 2019, 108545. [Google Scholar] [CrossRef]
- Bak, S.M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.J.; Kim, K.B.; Chung, K.Y.; Yang, X.Q.; Nam, K.W. Structural Changes and Thermal Stability of Charged LiNixMnyCozO2 Cathode Materials Studied by Combined In Situ Time-Resolved XRD and Mass Spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhao, K. Electronic Structure and Comparative Properties of LiNixMnyCozO2 Cathode Materials. J. Phys. Chem. C 2017, 121, 6002–6010. [Google Scholar] [CrossRef]
- Boesenberg, U.; Marcus, M.A.; Shukla, A.K.; Yi, T.; McDermott, E.; Teh, P.F.; Srinivasan, M.; Moewes, A.; Cabana, J. Asymmetric Pathways in the Electrochemical Conversion Reaction of NiO as Battery Electrode with High Storage Capacity. Sci. Rep. 2014, 4, 7133. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface Reconstruction and Chemical Evolution of Stoichiometric Layered Cathode Materials for Lithium-Ion Batteries. Nat. Commun. 2014, 5, 3529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Long, X.; Wang, Z.; Yu, P.; Han, F.; Fu, J.; Ren, G.X.; Wu, Y.; Zheng, S.; Huang, W.; et al. Mechanism Study on the Interfacial Stability of a Lithium Garnet-Type Oxide Electrolyte against Cathode Materials. ACS Appl. Energy Mater. 2018, 11, 5968–5976. [Google Scholar] [CrossRef]
- Sallis, S.; Pereira, N.; Mukherjee, P.; Quackenbush, N.F.; Faenza, N.; Schlueter, C.; Lee, T.-L.; Yang, W.L.; Cosandey, F.; Amatucci, G.G.; et al. Surface Degradation of Li1–xNi0.80Co0.15Al0.05O2 Cathodes: Correlating Charge Transfer Impedance with Surface Phase Transformations. Appl. Phys. Lett. 2016, 108, 263902. [Google Scholar] [CrossRef]
- Singh, J.P.; Kuang, H.; Lee, S.; Chae, K.H. Investigation of Metal–Oxygen Hybridization Process during the Growth of ZnFe2O4 Films on MgO (100) Substrates. (To be communicated).
- Tian, C.; Nordlund, D.; Xin, H.L.; Xu, Y.; Liu, Y.; Sokaras, D.; Lin, F.; Doeff, M.M. Depth-Dependent Redox Behavior of LiNi0.6Mn0.2Co0.2O2. J. Electrochem. Soc. 2018, 165, A696. [Google Scholar] [CrossRef]
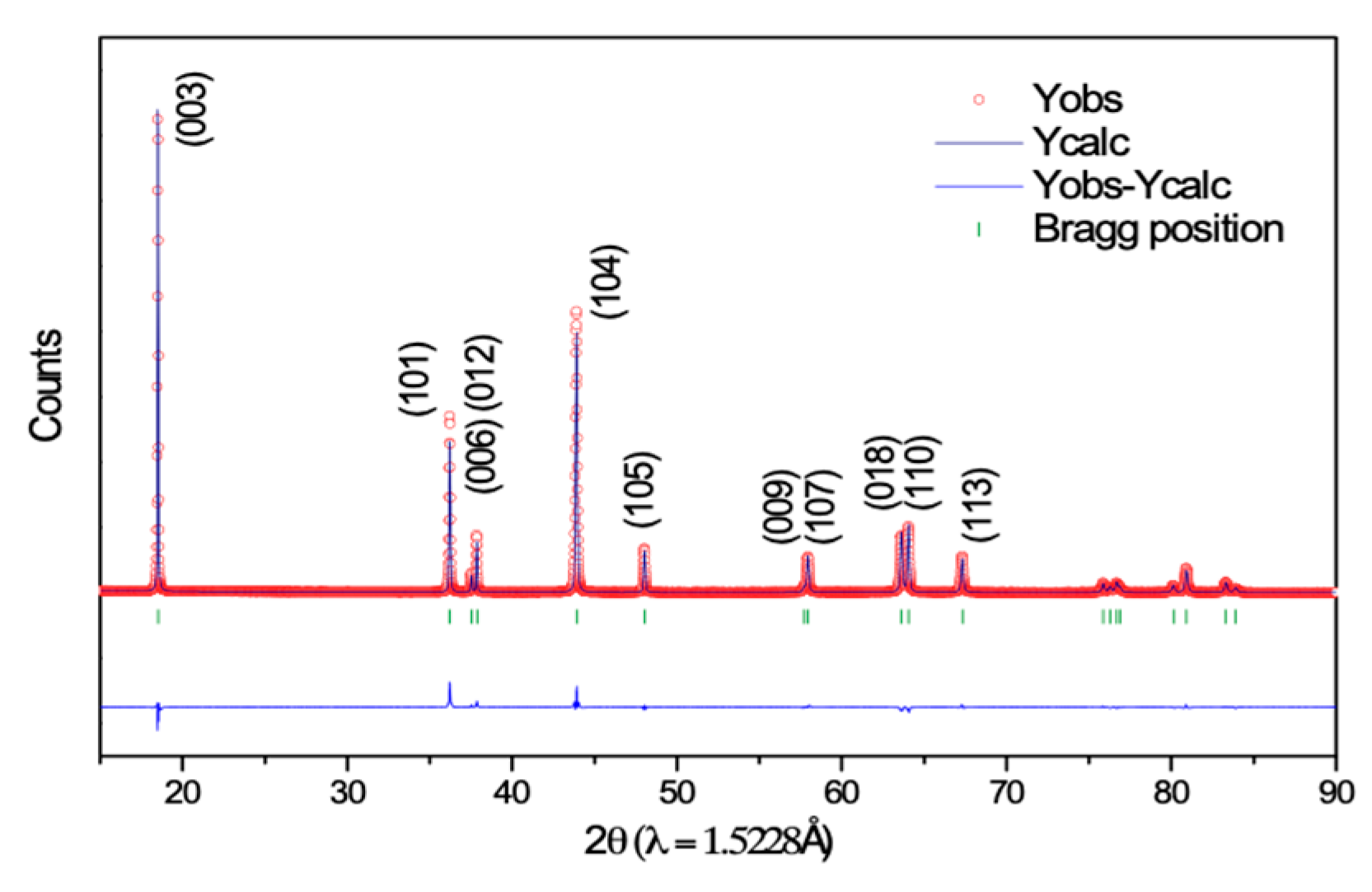
| a (Å)/c (Å) | 2.87402 (2)/14.2018 (1) | ||||
|---|---|---|---|---|---|
| Atom | Site | Wyckoff positions | Occupancy *,$ | ||
| Li | 3a | 0 | 0 | 0 | 0.0812 (2) |
| Ni2 | 3a | 0 | 0 | 0 | 0.0021 (2) |
| Ni1 | 3b | 0 | 0 | 0.5 | 0.0703 † |
| Co | 3b | 0 | 0 | 0.5 | 0.0075 † |
| Mn | 3b | 0 | 0 | 0.5 | 0.0032 † |
| O | 6c | 0 | 0 | 0.2419 (1) | 0.166 † |
| Reliability factors | Rp = 6.67%, Rwp = 9.22%, Rexp = 5.99%, S = 1.54 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, J.P.; Park, J.Y.; Chae, K.H.; Ahn, D.; Lee, S. Soft X-ray Absorption Spectroscopic Investigation of Li(Ni0.8Co0.1Mn0.1)O2 Cathode Materials. Nanomaterials 2020, 10, 759. https://doi.org/10.3390/nano10040759
Singh JP, Park JY, Chae KH, Ahn D, Lee S. Soft X-ray Absorption Spectroscopic Investigation of Li(Ni0.8Co0.1Mn0.1)O2 Cathode Materials. Nanomaterials. 2020; 10(4):759. https://doi.org/10.3390/nano10040759
Chicago/Turabian StyleSingh, Jitendra Pal, Jae Yeon Park, Keun Hwa Chae, Docheon Ahn, and Sangsul Lee. 2020. "Soft X-ray Absorption Spectroscopic Investigation of Li(Ni0.8Co0.1Mn0.1)O2 Cathode Materials" Nanomaterials 10, no. 4: 759. https://doi.org/10.3390/nano10040759
APA StyleSingh, J. P., Park, J. Y., Chae, K. H., Ahn, D., & Lee, S. (2020). Soft X-ray Absorption Spectroscopic Investigation of Li(Ni0.8Co0.1Mn0.1)O2 Cathode Materials. Nanomaterials, 10(4), 759. https://doi.org/10.3390/nano10040759






