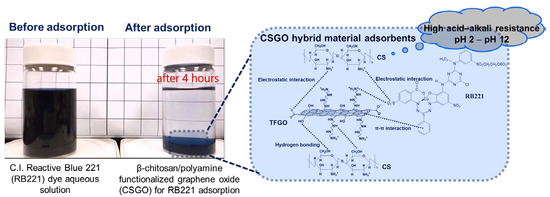
Adsorption Performance for Reactive Blue 221 Dye of β-Chitosan/Polyamine Functionalized Graphene Oxide Hybrid Adsorbent with High Acid–Alkali Resistance Stability in Different Acid–Alkaline Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
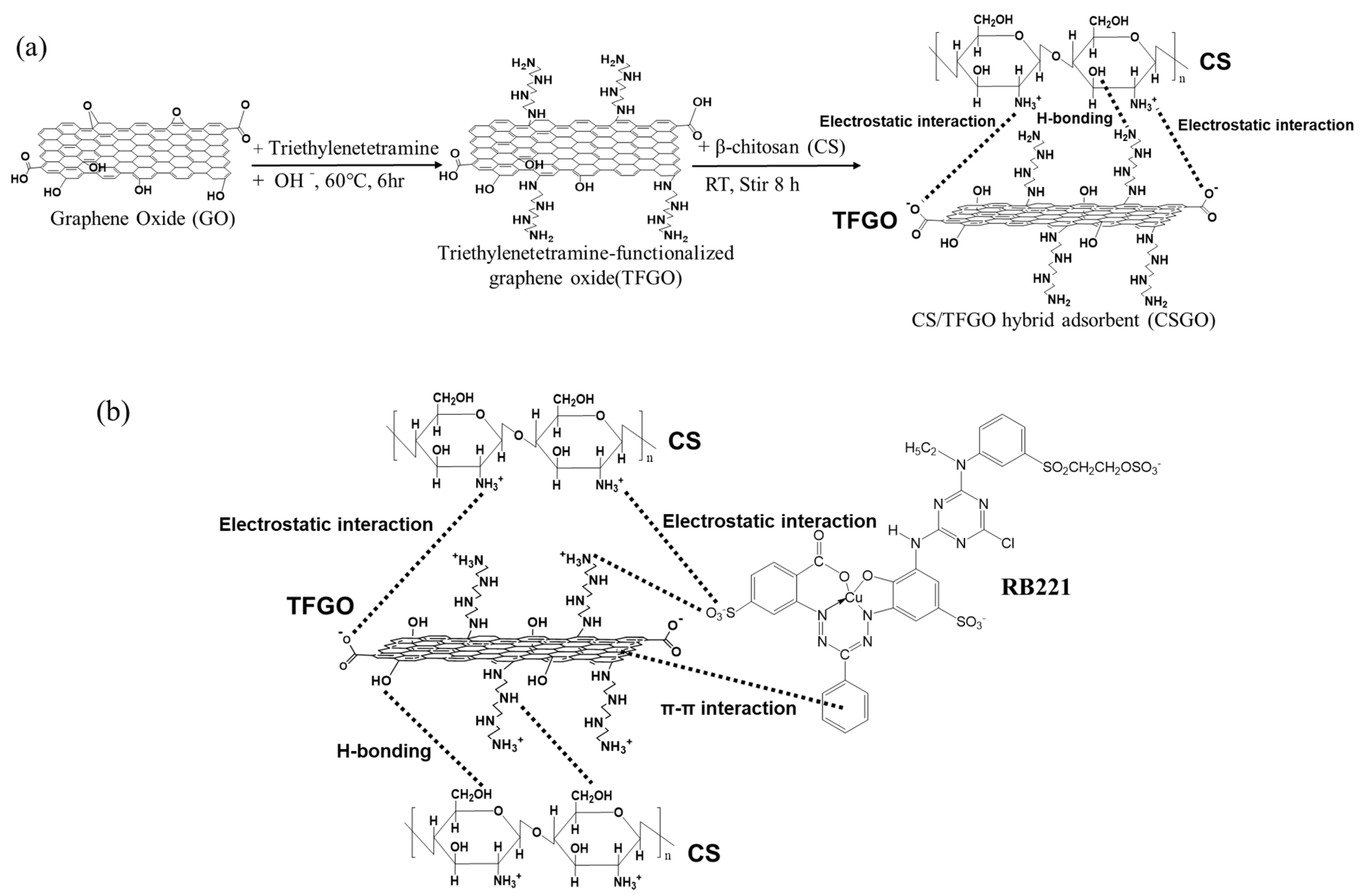
2.2. Synthesis of TFGO and CS/TFGO Hybrid Adsorbent (CSGO)
2.3. Test of Reactive Dye Adsorption
2.4. Characterization and Measurements
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.2. Ultraviolet–Visible (UV–Vis) Spectroscopy
2.4.3. Elemental Analysis (EA)
2.4.4. Transmission Electron Microscopy (TEM)
2.4.5. Raman Spectroscopy
2.4.6. Field-Emission Scanning Electron Microscopy (FE-SEM)
3. Results and Discussion
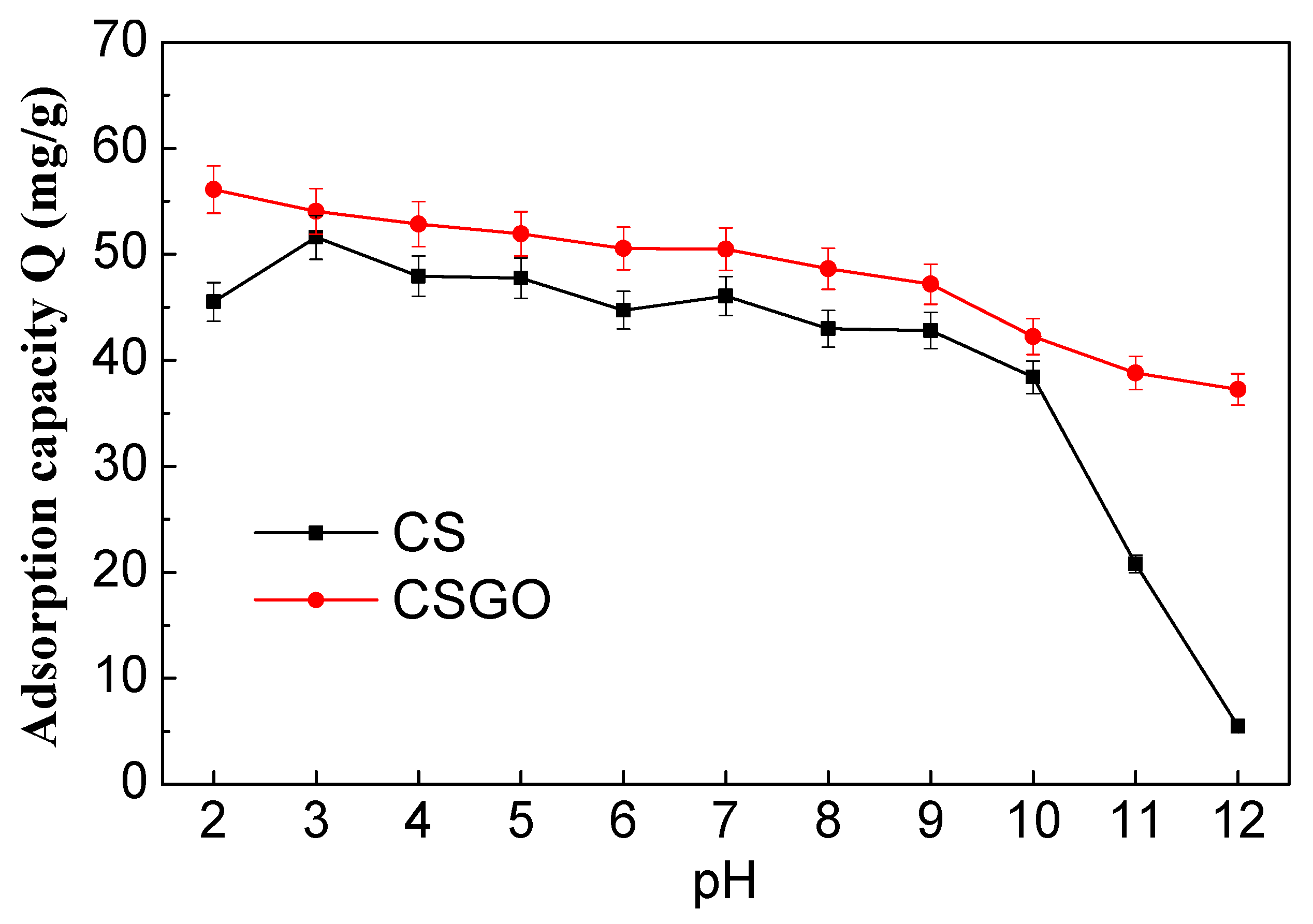
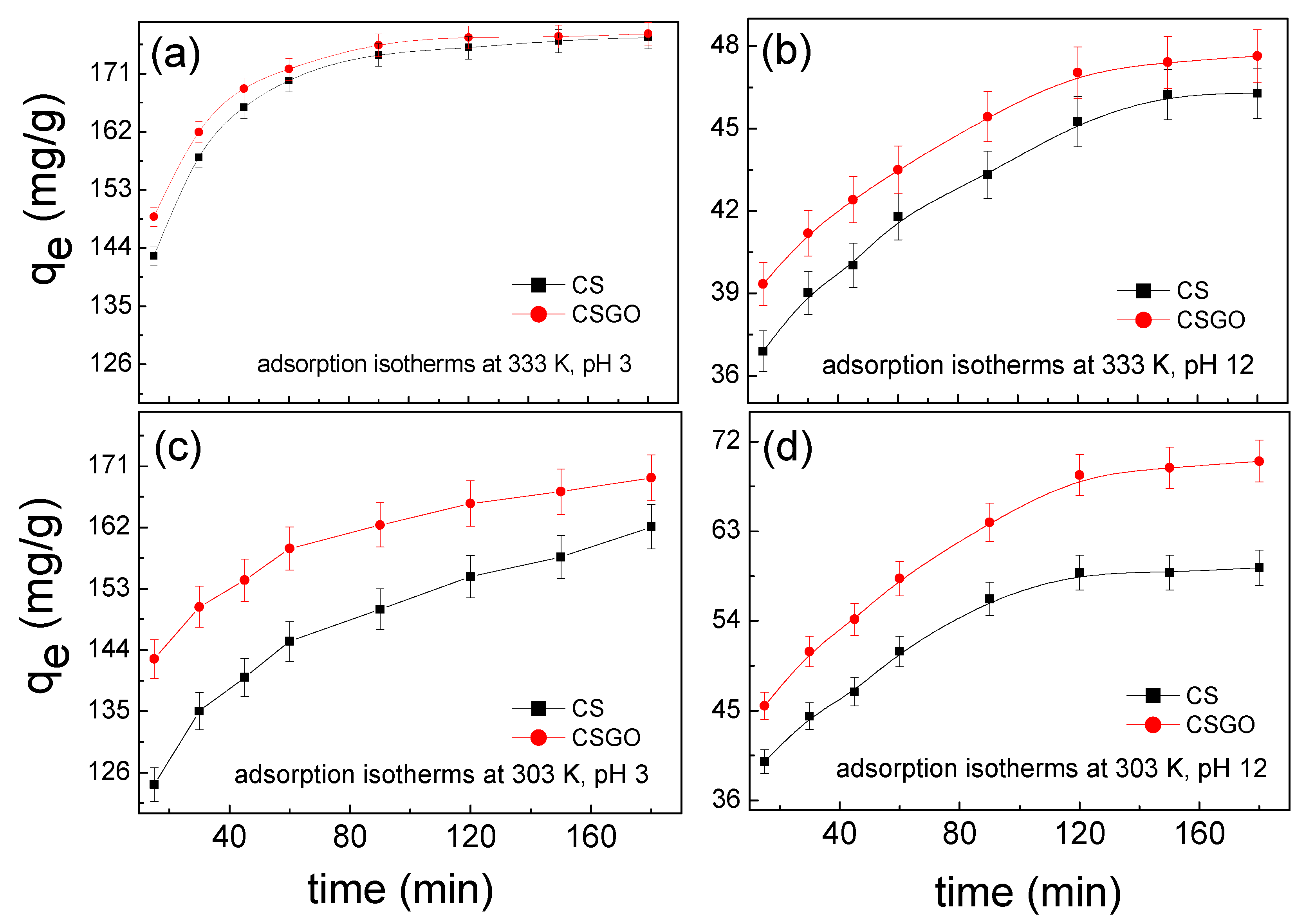
3.1. Adsorption Capacities of Adsorbents for RB221 Affected by Changing pH Values

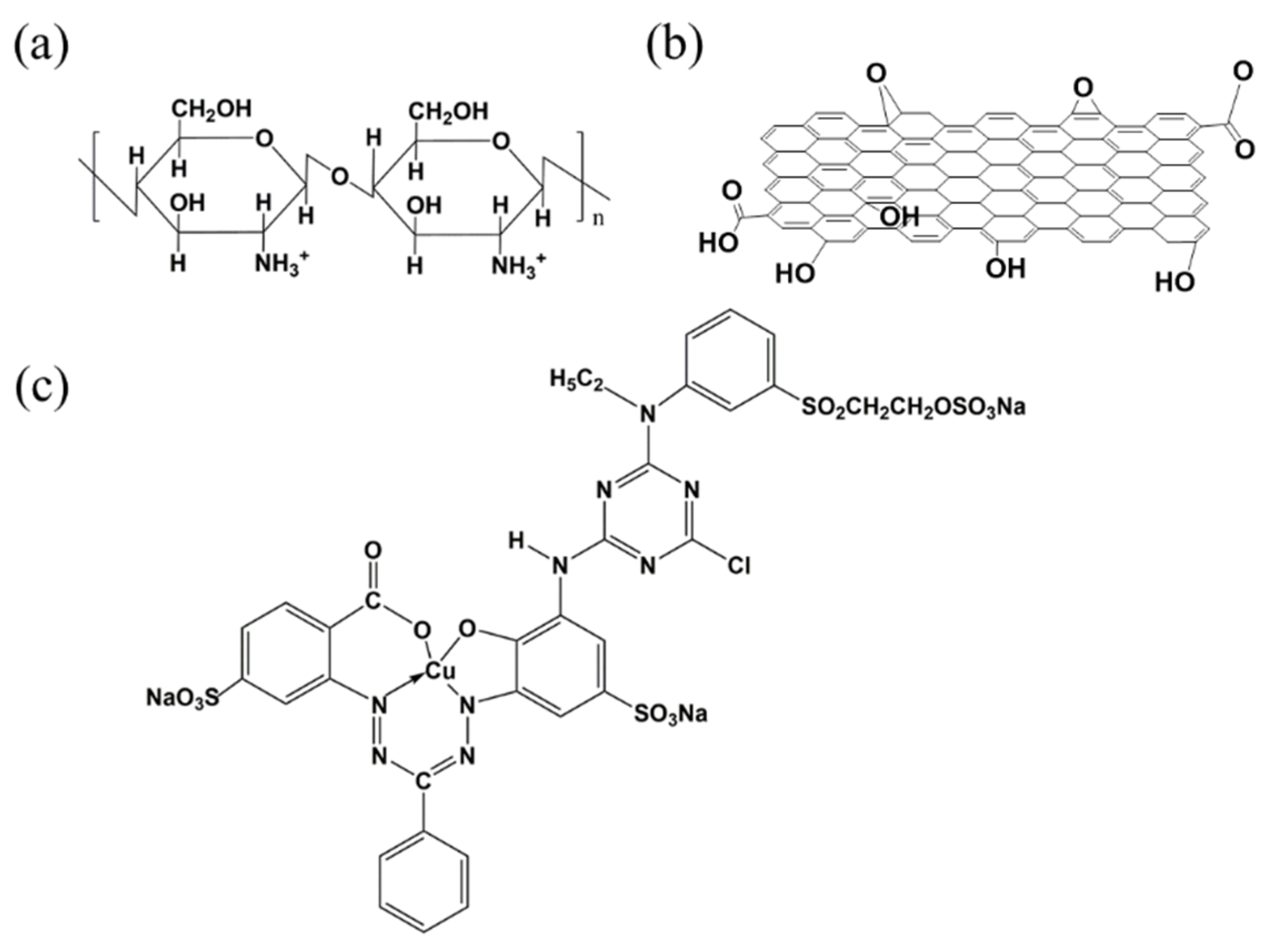
3.2. Adsorption Mechanism of CSGO for RB221
3.2.1. H Bonding Forces
3.2.2. FTIR Analysis
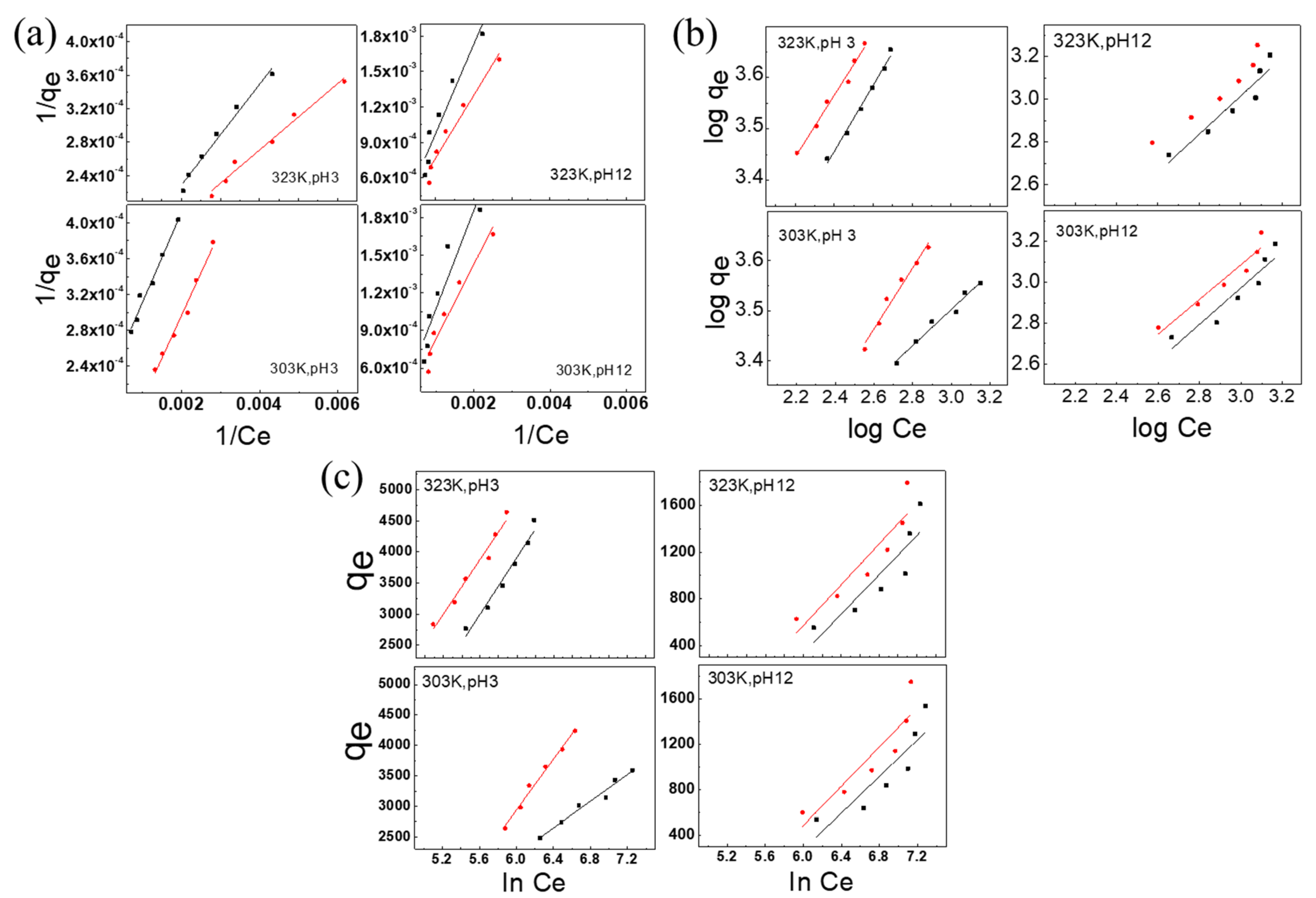
3.3. Discussion of Isothermal Adsorption Models
3.3.1. Langmuir Adsorption Isotherm
3.3.2. Freundlich Adsorption Isotherm
3.3.3. Temkin Adsorption Isotherm
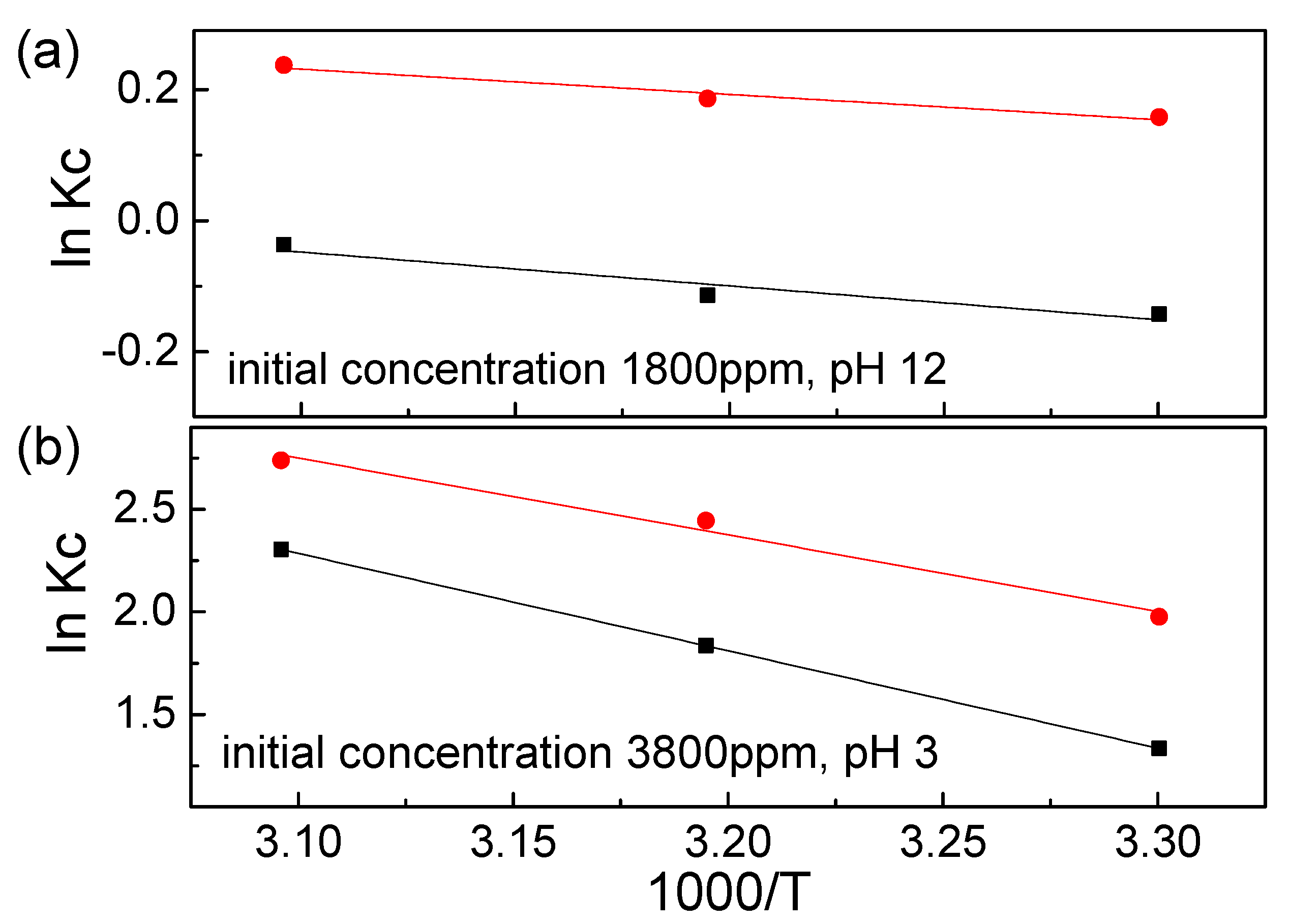
3.4. Adsorption Thermodynamics
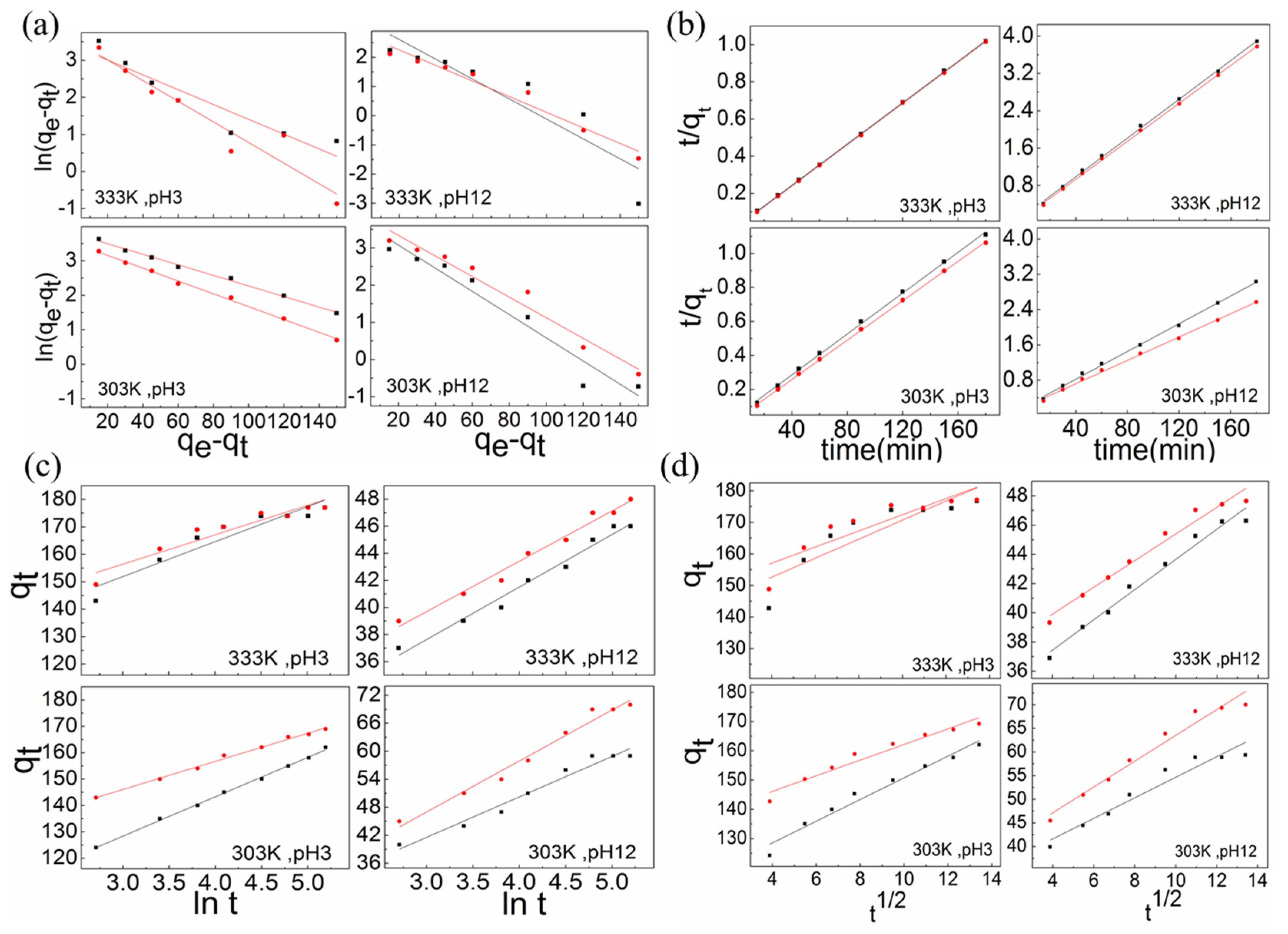
3.5. Adsorption Kinetics
3.5.1. Pseudo-First-Order Rate Model
3.5.2. Pseudo-Second-Order Rate Model
3.5.3. Elovich Model
3.5.4. Weber–Morris Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Belpaire, C.; Reyns, T.; Geeraerts, C.; Loco, J.V. Toxic textile dyes accumulate in wild european eel anguilla anguilla. Chemosphere 2015, 138, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Letašiová, S.; Medveďová, A.; Šovčíková, A.; Dušinská, M.; Volkovová, K.; Mosoiu, C.; Bartonová, A. Bladder cancer, a review of the environmental risk factors. Environ. Health 2012, 11, S11. [Google Scholar] [CrossRef] [PubMed]
- Golka, K.; Wiese, A.; Assennato, G.; Bolt, H.M. Occupational exposure and urological cancer. World J. Urol. 2004, 21, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Games, L.M.; Hites, R.A. Composition, treatment efficiency, and environmental significance of dye manufacturing plant effluents. Anal. Chem. 1977, 49, 1433–1440. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour. Technol. 2003, 87, 129–132. [Google Scholar] [CrossRef]
- Suryavathi, V.; Sharma, S.; Sharma, S.; Saxena, P.; Pandey, S.; Grover, R.; Kumar, S.; Sharma, K.P. Acute toxicity of textile dye wastewaters (untreated and treated) of sanganer on male reproductive systems of albino rats and mice. Reprod. Toxicol. 2005, 19, 547–556. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, K.; Cheng, X.; Xu, Y.; Jia, Q. Study on optimizing production scheduling for water-saving in textile dyeing industry. J. Clean. Prod. 2017, 141, 721–727. [Google Scholar] [CrossRef]
- Wu, J.; Ma, L.; Chen, Y.; Cheng, Y.; Liu, Y.; Zha, X. Catalytic ozonation of organic pollutants from bio-treated dyeing and finishing wastewater using recycled waste iron shavings as a catalyst: Removal and pathways. Water Res. 2016, 92, 140–148. [Google Scholar] [CrossRef]
- Yukseler, H.; Uzal, N.; Sahinkaya, E.; Kitis, M.; Dilek, F.B.; Yetis, U. Analysis of the best available techniques for wastewaters from a denim manufacturing textile mill. J. Environ. Manag. 2017, 203, 1118–1125. [Google Scholar] [CrossRef]
- Wua, H.; Wang, S. Impacts of operating parameters on oxidatio-reduction potential and pretreatment efficacy in the pretreatment of printing and dyeing wastewater by fenton process. J. Hazard. Mater. 2012, 243, 86–94. [Google Scholar] [CrossRef]
- Rosales, E.; Meijidea, J.; Tavares, T.; Pazosa, M.; Sanromán, M.A. Grapefruit peelings as a promising biosorbent for the removal of leather dyes and hexavalent chromium. Process Saf. Environ. Protect. 2016, 101, 61–71. [Google Scholar] [CrossRef]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of pinus radiata. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Paz, A.; Carballo, J.; Pérez, M.J.; Domínguez, J.M. Domínguez, biological treatment of model dyes and textile wastewaters. Chemosphere 2017, 181, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Kale, P.C.; Chaudhari, P.L. Removal of reactive blue 221 dye from textile waste water by using zinc peroxide nanoparticles. Int. J. Sci. Res. Manag. 2017, 5, 5700–5709. [Google Scholar] [CrossRef]
- Homaeigohar, S. The nanosized dye adsorbents for water treatment. Nanomaterials 2020, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Birajdar, B. Effective La-Na Co-doped TiO2 nano-particles for dye adsorption: Synthesis, characterization and study on adsorption kinetics. Nanomaterials 2019, 9, 400. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, C.; Kim, S. Water recycling from desalination and purification process of reactive dye manufacturing industry by combined membrane filtration. J. Clean. Prod. 2005, 13, 779–786. [Google Scholar] [CrossRef]
- Nadeem, K.G.; Guyer, T.; Keskinler, B.; Dizge, N. Investigation of segregated wastewater streams reusability with membrane process for textile industry. J. Clean. Prod. 2019, 228, 1437–1445. [Google Scholar] [CrossRef]
- Radi, M.A.; Nasirizadeh, N.; Rohani Moghadam, M.; Dehghani, M. The comparison of sonochemistry, electrochemistry and sonoelectrochemistry techniques on decolorization of C.I reactive blue 49. Ultrason. Sonochem. 2015, 27, 609–615. [Google Scholar] [CrossRef]
- Van, H.T.; Nguyen, L.H.; Hoang, T.K.; Tranc, T.P.; Vod, A.T.; Phame, T.T.; Nguyen, X.C. Using FeO-constituted iron slag wastes as heterogeneous catalyst for fenton and ozonation processes to degrade reactive red 24 from aqueous solution. Sep. Purif. Technol. 2019, 224, 431–442. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous fenton catalytic reactivity: A review. Appl. Catal. B 2019, 255, 117739. [Google Scholar] [CrossRef]
- Sivagami, K.; Sakthivel, K.P.; Nambi, I.M. Advanced oxidation processes for the treatment of tannery wastewater. J. Environ. Chem. Eng. 2018, 6, 3656–3663. [Google Scholar] [CrossRef]
- Di, L.; Yang, H.; Xian, T.; Liu, X.; Chen, X. Photocatalytic and photo-fenton catalytic degradation activities of z-scheme Ag2S/BiFeO3 heterojunction composites under visible-light irradiation. Nanomaterials 2019, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Mohanty, P.; Maiti, A. Bacterial mediated bio-decolourization of wastewater containing mixed reactive dyes using jack-fruit seed as co-substrate: Process optimization. J. Clean. Prod. 2019, 235, 21–33. [Google Scholar] [CrossRef]
- Ahmad, A.; Siti, H.M.S.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumard, R. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, M.; Xu, W.; He, S.; Men, Y. Natural polysaccharides-modified graphene oxide for adsorption of organic dyes from aqueous solutions. J. Colloid Interface Sci. 2017, 486, 84–96. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, C.; Chu, W.; Vipin, A.K.; Sun, L. Environmental remediation applications of carbon nanotubes and graphene oxide: Adsorption and catalysis. Nanomaterials 2019, 9, 439. [Google Scholar] [CrossRef]
- Suárez-Iglesias, O.; Collado, S.; Oulego, P.; Díaz, M. Graphene-family nanomaterials in wastewater treatment plants. Chem. Eng. J. 2017, 313, 121–135. [Google Scholar] [CrossRef]
- Abdi, G.; Alizadeh, A.; Amirian, J.; Rezaei, S.; Sharma, G. Polyamine-modified magnetic graphene oxide surface: Feasible adsorbent for removal of dyes. J. Mol. Liq. 2019, 289, 111118. [Google Scholar] [CrossRef]
- Chiu, C.W.; Lin, C.A.; Hong, P.D. Melt-spinning and thermal stability behavior of TiO2 nanoparticle/polypropylene nanocomposite fibers. J. Polym. Res. 2011, 18, 367–372. [Google Scholar] [CrossRef]
- Azizi, A.; Moniri, E.; Hassani, A.H.; Ahmad Panahi, H.; Miralinaghi, M. Polymerization of graphene oxide with polystyrene: Non-linear isotherms and kinetics studies of anionic dyes. Microchem. J. 2019, 145, 559–565. [Google Scholar] [CrossRef]
- Gogoi, J.; Choudhury, A.D.; Chowdhury, D. Graphene oxide clay nanocomposite as an efficient photo-catalyst for degradation of cationic dye. Mater. Chem. Phys. 2019, 232, 438–445. [Google Scholar] [CrossRef]
- Morsi, R.E.; Al-Sabagh, A.M.; Moustafa, Y.M.; ElKholy, S.G.; Sayed, M.S. Polythiophene modified chitosan/magnetite nanocomposites for heavy metals and selective mercury removal. Egypt. J. Pet. 2018, 27, 1077–1085. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater—A short review. Adv. Colloid Interface Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef]
- Shajahan, A.; Shankar, S.; Sathiyaseelan, A.; Narayan, K.S.; Narayanan, V.; Kaviyarasan, V.; Ignacimuthu, S. Comparative studies of chitosan and its nanoparticles for the adsorption efficiency of various dyes. Int. J. Biol. Macromol. 2017, 104, 1449–1458. [Google Scholar] [CrossRef]
- Wu, M.T.; Tsai, Y.L.; Chiu, C.W.; Cheng, C.C. Synthesis, characterization, and highly acid resistant properties of crosslinking β-chitosan with polyamines for heavy metal ion adsorption. RSC Adv. 2016, 6, 104754–104762. [Google Scholar] [CrossRef]
- Chiu, C.W.; Wu, M.T.; Lee, J.C.M.; Cheng, T.Y. Isothermal adsorption properties for the adsorption and removal of reactive blue 221 dye from aqueous solutions by cross-linked β-chitosan glycan as acid-resistant adsorbent. Polymers 2018, 10, 1328. [Google Scholar] [CrossRef]
- Ren, Q.; Feng, L.; Fan, R.; Ge, X.; Sun, Y. Water-dispersible triethylenetetramine-functionalized graphene: Preparation, characterization and application as an amperometric glucose sensor. Mater. Sci. Eng. C 2016, 68, 308–316. [Google Scholar]
- Staron, P.; Chwastowski, J.; Banach, M. Sorption and desorption studies on silver ions from aqueous solution by coconut fiber. J. Clean. Prod. 2017, 149, 290–301. [Google Scholar] [CrossRef]
- Schuurmann, G.; Funar-Timofei, S. Multilinear regression and comparative molecular field analysis (CoMFA) of azo dye−fiber affinities. 2. inclusion of solution-phase molecular orbital descriptors. J. Chem. Inf. Comput. Sci. 2003, 43, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Ibrahim, M.H.; Abdullah, A.Z. Elimination of reactive blue 4 from aqueous solutions using 3-aminopropyl triethoxysilane modified chitosan beads. Carbohydr. Polym. 2015, 132, 89–96. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.O.; Park, S.; Park, H.S. Adsorption isotherms and kinetics of cationic and anionic dyes on three-dimensional reduced graphene oxide macrostructure. J. Ind. Eng. Chem. 2015, 21, 1191–1196. [Google Scholar] [CrossRef]
- Alkan, M.; Demirbaş, O.; Çelikçapa, S.; Doğan, M. Sorption of acid red 57 from aqueous solution onto sepiolite. J. Hazard. Mater. 2004, 116, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Haitham, K.; Razak, S.; Nawi, M.A. Kinetics and isotherm studies of methyl orange adsorption by a highly recyclable immobilized polyaniline on a glass plate. Arab. J. Chem. 2019, 12, 1595–1606. [Google Scholar] [CrossRef]
- Yamaki, S.B.; Barros, D.S.; Garcia, C.M.; Socoloski, P.; Oliveira, O.N.; Atvars, T.D.Z. Spectroscopic studies of the intermolecular interactions of congo red and tinopal cbs with modified cellulose fibers. Langmuir 2005, 21, 5414–5420. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, B.; Cheng, R.; Li, Y. Behaviors and mechanism of acid dyes sorption onto diethylenetriamine-modified native and enzymatic hydrolysis starch. J. Hazard. Mater. 2010, 183, 224–232. [Google Scholar] [CrossRef]
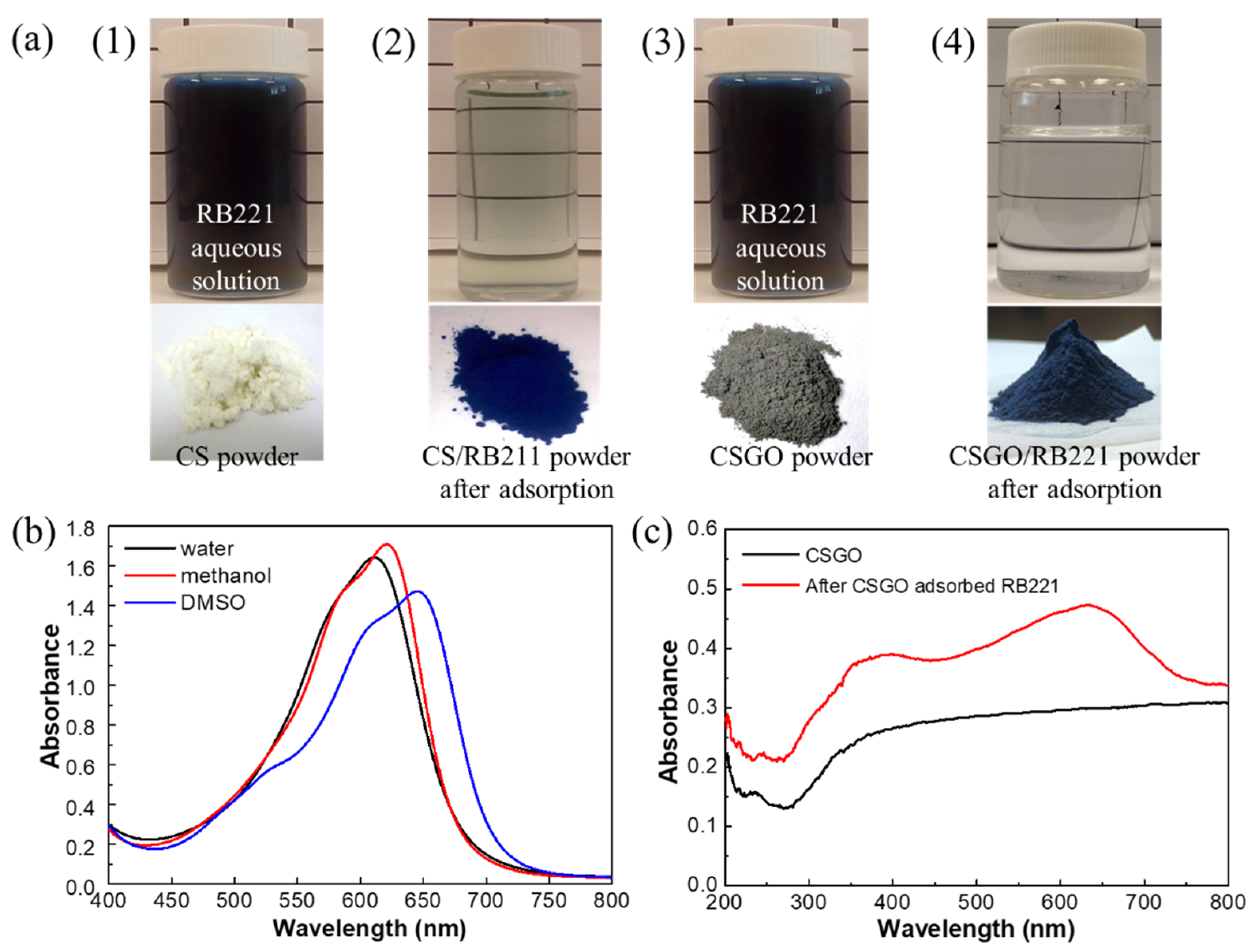
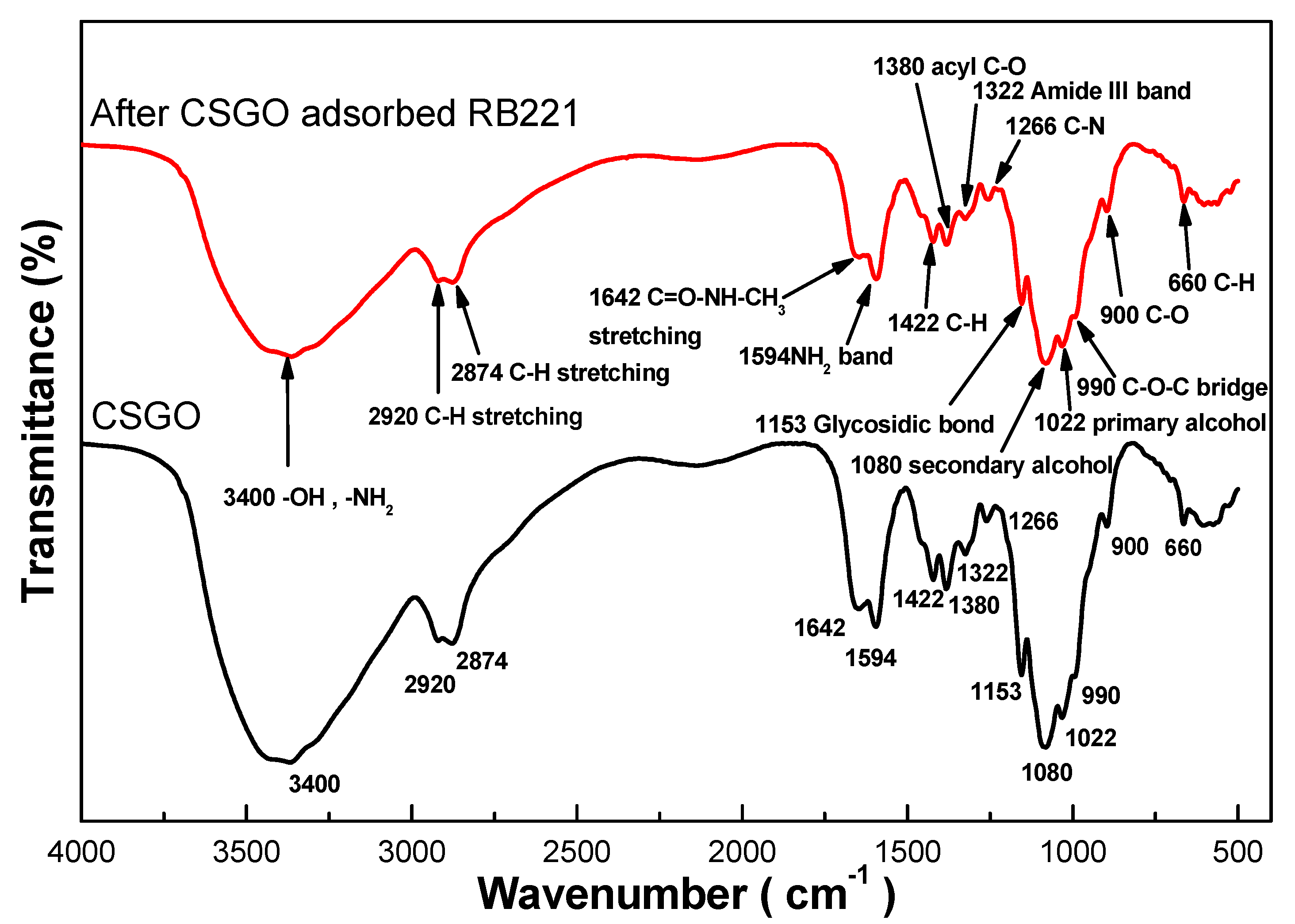
| pH Value | pH 3 | pH 12 | ||||||
|---|---|---|---|---|---|---|---|---|
| Adsorbents type | CS | CSGO | CS | CSGO | ||||
| Temperature (K) | 303 | 323 | 303 | 323 | 303 | 323 | 303 | 323 |
| Langmuir Isotherm | ||||||||
| Q0 (mg/g) | 9.94 | 16.61 | 10.53 | 25.32 | 1.27 | 1.32 | 1.68 | 1.89 |
| B (L/mg) | 503.00 | 602.00 | 950.00 | 395.00 | 2625.00 | 3789.50 | 2976.50 | 2649.50 |
| RL | 6.6 × 10−7 | 5.5 × 10−7 | 3.5 × 10−7 | 8.4 × 10−7 | 3.8 × 10−7 | 2.6 × 10−7 | 3.36 × 10−7 | 3.77 × 10−7 |
| R2 | 0.9782 | 0.9801 | 0.9821 | 0.9810 | 0.8730 | 0.9277 | 0.9460 | 0.9587 |
| Freundlich Isotherm | ||||||||
| 1/n | 0.363 | 0.642 | 0.613 | 0.602 | 0.892 | 0.895 | 0.848 | 0.827 |
| N | 2.755 | 1.558 | 1.631 | 1.662 | 1.121 | 1.117 | 1.179 | 1.209 |
| kf (mg/g) | 259.24 | 82.47 | 74.11 | 132.13 | 1.99 | 2.13 | 3.49 | 4.37 |
| R2 | 0.9731 | 0.9882 | 0.9732 | 0.9835 | 0.8885 | 0.9084 | 0.9278 | 0.9482 |
| Temkin Isotherm | ||||||||
| Kt (dm3/g) | 1.286 | 1.263 | 1.243 | 1.298 | 1.193 | 1.195 | 1.202 | 1.206 |
| b (J/mol) | 0.580 | 0.277 | 0.263 | 0.320 | 0.553 | 0.568 | 0.537 | 0.574 |
| R2 | 0.9729 | 0.9690 | 0.9876 | 0.9712 | 0.7943 | 0.8064 | 0.8244 | 0.8572 |
| Adsorbent Type | pH Value | Initial Concentration, C0 (mg/L) | Final Concentration, Ce (mg/L) | T (K) | Final Dye Removal, CAe (mg/L) | Kc | Thermodynamic Parameter | ||
|---|---|---|---|---|---|---|---|---|---|
| ΔG0 (KJ/mol) | ΔH0 (KJ/mol) | ΔS0 (J/mol·K) | |||||||
| CS | 3 | 3800 | 792.475 | 303 | 3007.525 | 3.795 | −3.360 | −39.491 | 141.421 |
| 522.732 | 313 | 3277.268 | 6.269 | −4.777 | |||||
| 344.89 | 323 | 3455.101 | 10.018 | −6.188 | |||||
| CSGO | 462.676 | 303 | 3337.324 | 7.213 | −4.978 | −31.099 | 119.264 | ||
| 303.329 | 313 | 3496.671 | 11.528 | −6.362 | |||||
| 230.772 | 323 | 3569.228 | 15.466 | −7.354 | |||||
| CS | 12 | 1800 | 964.196 | 303 | 835.804 | 0.867 | 0.360 | −4.277 | 12.858 |
| 951.055 | 313 | 848.945 | 0.893 | 0.296 | |||||
| 916.759 | 323 | 883.241 | 0.963 | 0.100 | |||||
| CSGO | 828.886 | 303 | 971.114 | 1.172 | −0.399 | −3.200 | 11.840 | ||
| 816.744 | 313 | 983.256 | 1.204 | −0.483 | |||||
| 793.715 | 323 | 1006.285 | 1.268 | −0.637 | |||||
| pH Value | pH 3 | pH 12 | ||||||
|---|---|---|---|---|---|---|---|---|
| Adsorbents Type | CS | CSGO | CS | CSGO | ||||
| Temperature (K) | 303 | 333 | 303 | 333 | 303 | 333 | 303 | 333 |
| Pseudo First Order Model | ||||||||
| k1 (min−1) | 0.9848 | 0.9802 | 0.9816 | 0.9726 | 0.9693 | 0.9665 | 0.9727 | 0.9737 |
| R2 | 0.9954 | 0.8826 | 0.9968 | 0.9215 | 0.9475 | 0.8361 | 0.9650 | 0.9645 |
| Pseudo Second Order Model | ||||||||
| h (mg/g·min) | 166.6667 | 178.5714 | 172.4138 | 181.8182 | 63.6943 | 48.0769 | 75.7576 | 49.2611 |
| k2 (g/mg·min) | 0.3573 | 0.0818 | 0.1248 | 0.0583 | 2.5072 | 1.1715 | 2.8896 | 0.7465 |
| R2 | 0.9990 | 0.9999 | 0.9998 | 0.9999 | 0.9984 | 0.9991 | 0.9978 | 0.9995 |
| Elovich Model | ||||||||
| A (mg/g·min) | 4.44 × 103 | 9.14 × 104 | 4.34 × 104 | 1.03 × 106 | 52 | 1.985 × 103 | 43 | 1.104 × 104 |
| β (g/mg) | 0.0677 | 0.0783 | 0.0932 | 0.0925 | 0.1152 | 0.2450 | 0.0925 | 0.2759 |
| R2 | 0.9973 | 0.9053 | 0.9969 | 0.9114 | 0.9714 | 0.9817 | 0.9807 | 0.9839 |
| Weber-Morris Model | ||||||||
| kdif (mg/g·min) | 3.7064 | 3.0179 | 2.6706 | 2.5610 | 2.1707 | 1.0364 | 2.7270 | 0.9157 |
| C | 113.7400 | 140.5400 | 135.4800 | 146.8100 | 32.9690 | 33.2940 | 36.2720 | 36.2440 |
| R2 | 0.9723 | 0.7822 | 0.9556 | 0.7923 | 0.9402 | 0.9800 | 0.9668 | 0.9727 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-W.; Wu, M.-T.; Lin, C.-L.; Li, J.-W.; Huang, C.-Y.; Soong, Y.-C.; Lee, J.C.-M.; Lee Sanchez, W.A.; Lin, H.-Y. Adsorption Performance for Reactive Blue 221 Dye of β-Chitosan/Polyamine Functionalized Graphene Oxide Hybrid Adsorbent with High Acid–Alkali Resistance Stability in Different Acid–Alkaline Environments. Nanomaterials 2020, 10, 748. https://doi.org/10.3390/nano10040748
Chiu C-W, Wu M-T, Lin C-L, Li J-W, Huang C-Y, Soong Y-C, Lee JC-M, Lee Sanchez WA, Lin H-Y. Adsorption Performance for Reactive Blue 221 Dye of β-Chitosan/Polyamine Functionalized Graphene Oxide Hybrid Adsorbent with High Acid–Alkali Resistance Stability in Different Acid–Alkaline Environments. Nanomaterials. 2020; 10(4):748. https://doi.org/10.3390/nano10040748
Chicago/Turabian StyleChiu, Chih-Wei, Ming-Tsung Wu, Chih-Lung Lin, Jia-Wun Li, Chen-Yang Huang, Yu-Chian Soong, Jimmy Chi-Min Lee, William Anderson Lee Sanchez, and Hsuan-Yu Lin. 2020. "Adsorption Performance for Reactive Blue 221 Dye of β-Chitosan/Polyamine Functionalized Graphene Oxide Hybrid Adsorbent with High Acid–Alkali Resistance Stability in Different Acid–Alkaline Environments" Nanomaterials 10, no. 4: 748. https://doi.org/10.3390/nano10040748
APA StyleChiu, C.-W., Wu, M.-T., Lin, C.-L., Li, J.-W., Huang, C.-Y., Soong, Y.-C., Lee, J. C.-M., Lee Sanchez, W. A., & Lin, H.-Y. (2020). Adsorption Performance for Reactive Blue 221 Dye of β-Chitosan/Polyamine Functionalized Graphene Oxide Hybrid Adsorbent with High Acid–Alkali Resistance Stability in Different Acid–Alkaline Environments. Nanomaterials, 10(4), 748. https://doi.org/10.3390/nano10040748