Advances in Nickel Nanoparticle Synthesis via Oleylamine Route
Abstract
1. Introduction
2. Materials and Methods
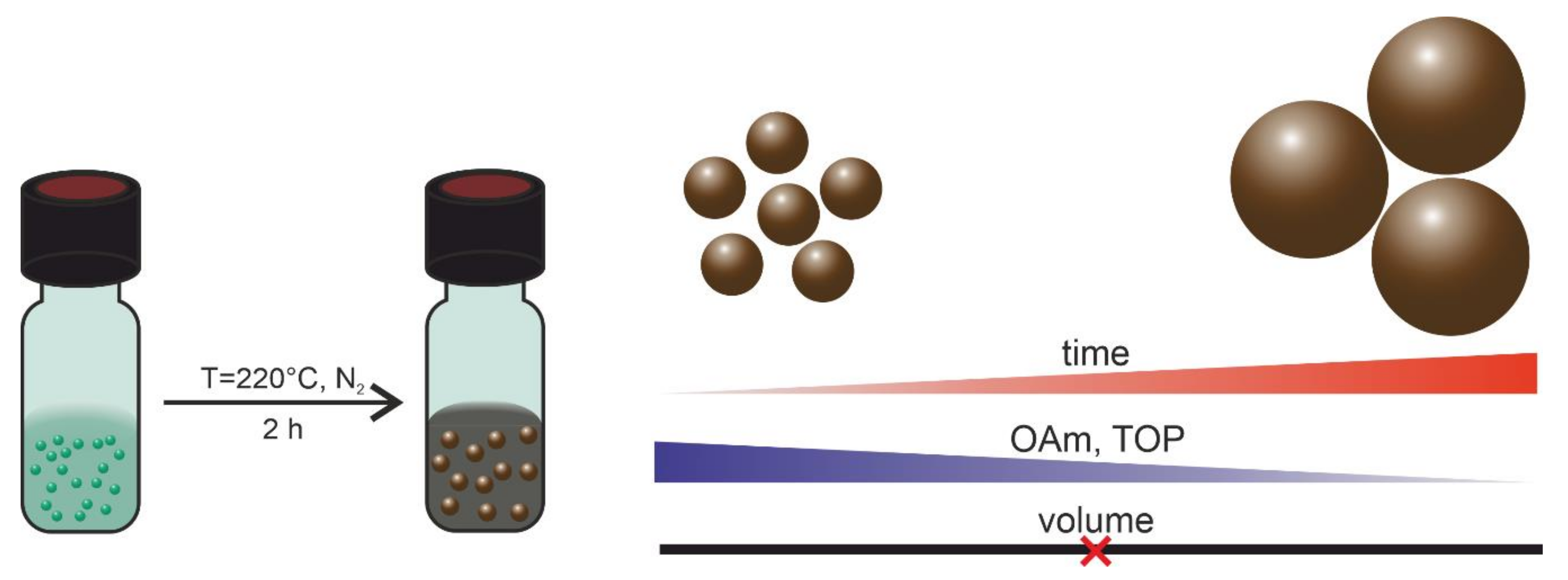
2.1. Synthesis of Nickel Nanoparticles
2.2. Analytical Methods
3. Results
- (i)
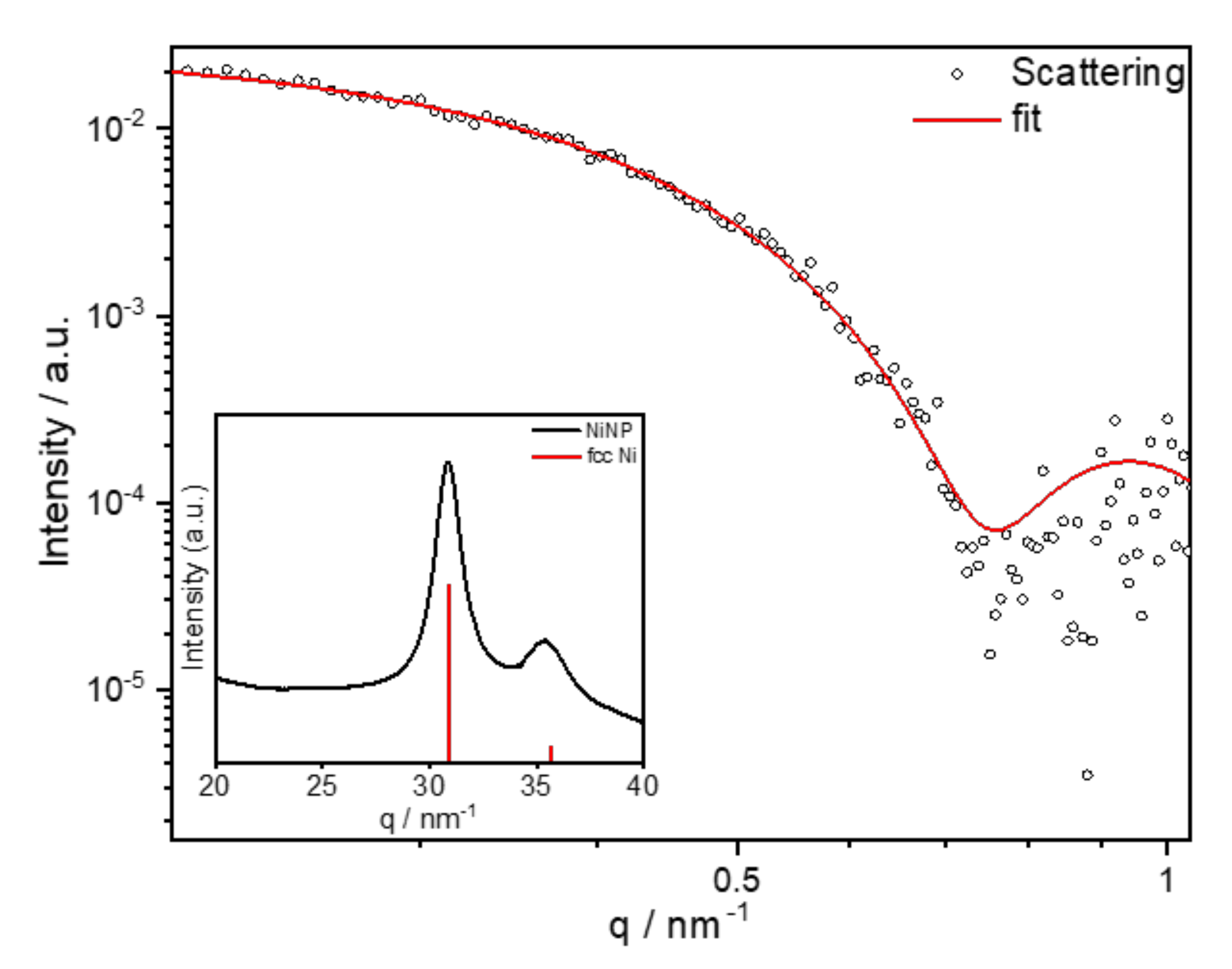
- Time dependence of particle formation: For this purpose, the reaction using 50 mmol/L Ni(acac)2 in DBE with a reducing agent OAm/Ni ratio of 10 and stabilizer TOP/Ni ratio of 2 was heated to 220 °C and stopped after 30, 60, 90, and 120 min, precipitated, and redispersed in n-hexane. The particle sizes were determined using SAXS (see Table 2). The underlying SAXS-data are shown in Figure S1. SAXS data were used for the comparison as the dispersed nanoparticles were measured in solution, giving integrated information of the whole sample. The results indicate that the final size of the particles and the minimum size distribution had already been reached after one hour at 220 °C.
- (ii)
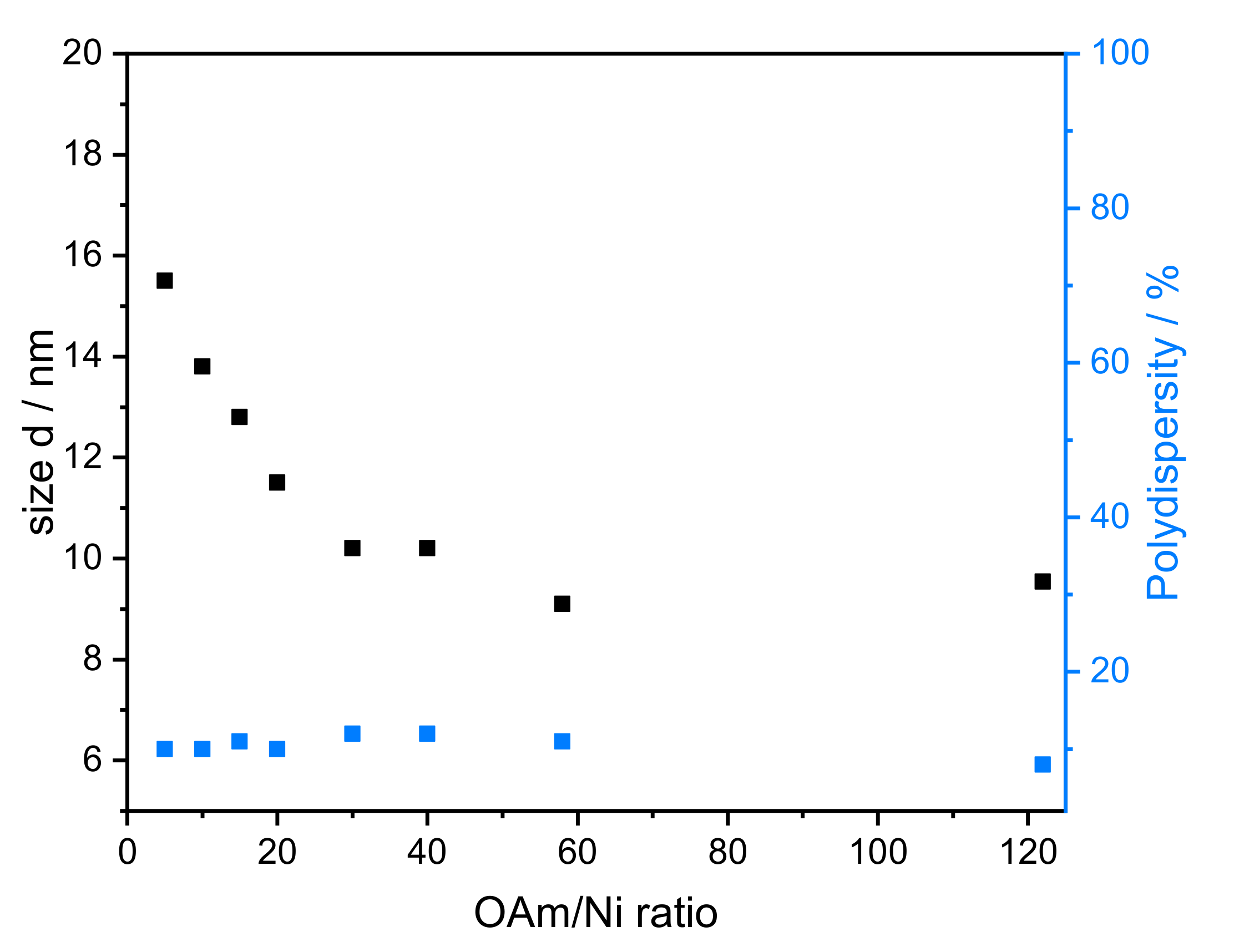
- Amount of reducing agent: Dibenzyl ether (DBE) was considered a good additional solvent to OAm. It is non-toxic, stable at high temperature, and available in high purity. Based on SAXS data, the influence of the amount of OAm on the particle size can be studied using OAm/Ni ratios of 5–122 while keeping the TOP/Ni ratio at 1.5. The underlying SAXS-data are shown in Figure S2. The diameter of the particles varied between 8.0 and 15.5 nm (Figure 4), which correlates directly with the OAm amount. There was no significant change in the polydispersity when the OAm amount was varied. When no DBE was used, NiNPs with sizes of 10 and 8 nm were obtained for the highest concentrations of OAm (OAm/Ni ratios of 58 and 122), respectively.
- (iii)
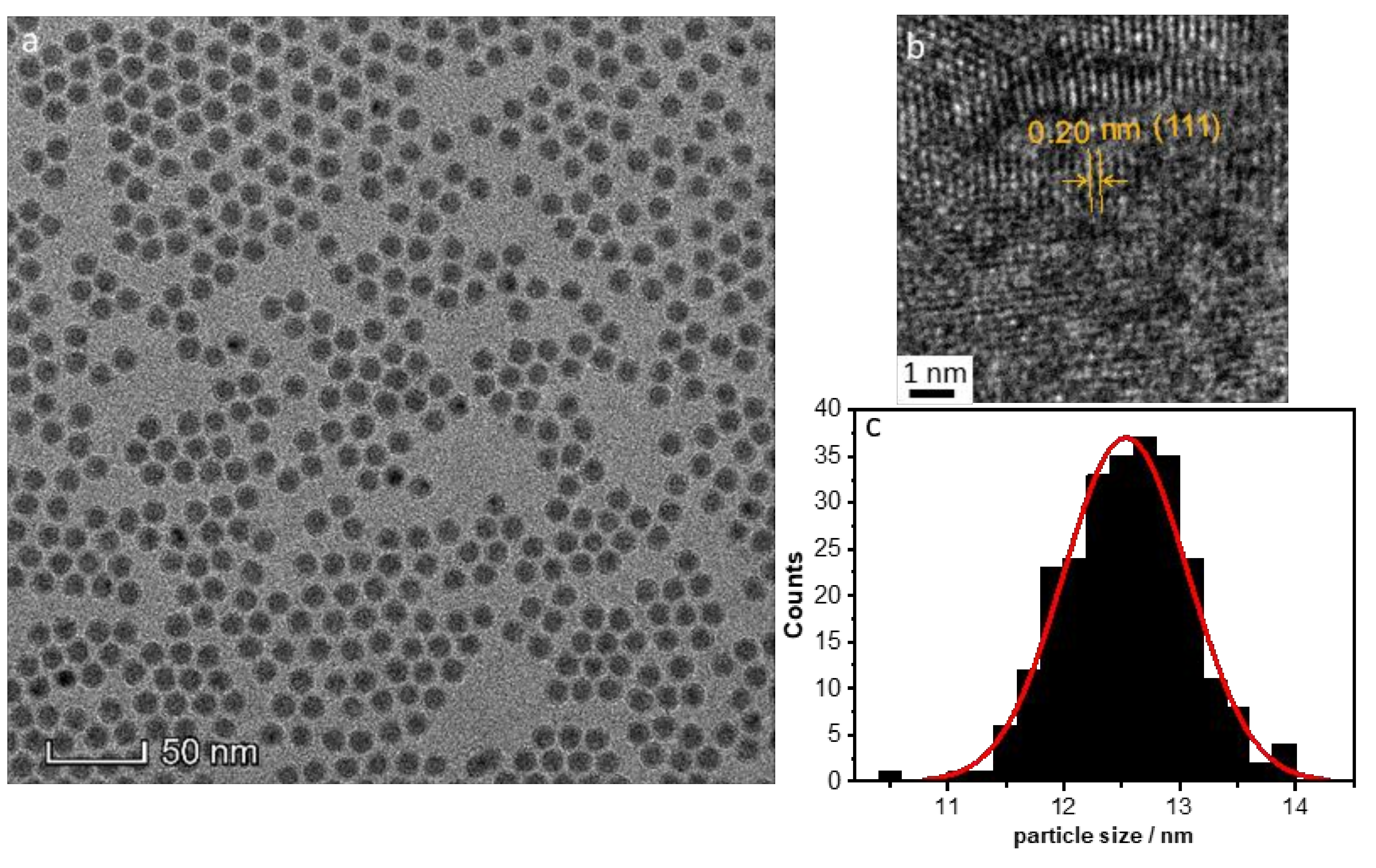
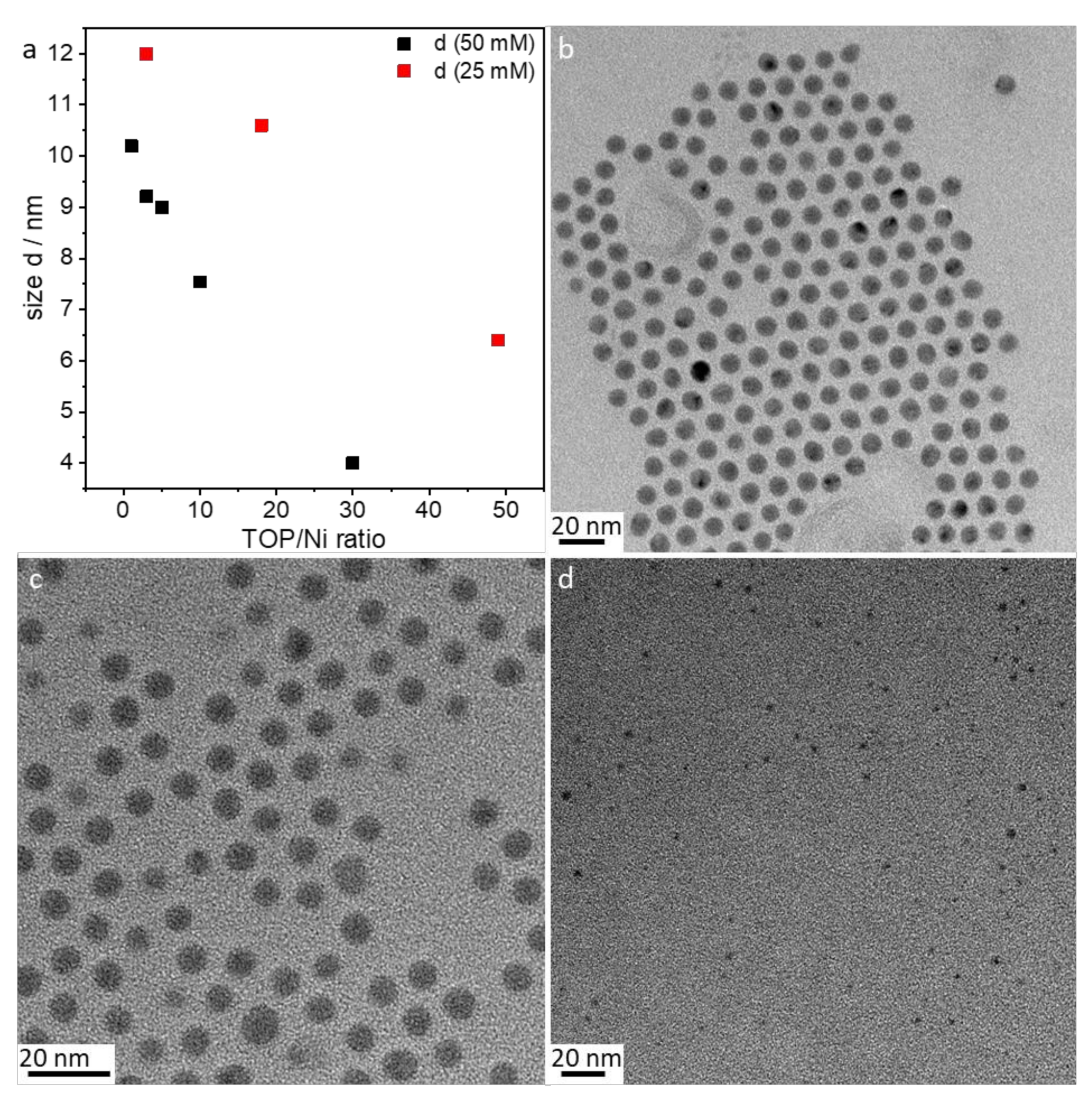
- Amount of stabilizer: The influence of the 1–50 TOP:Ni ratio was investigated using the following method based on SAXS data, which are shown in Figure S3. Two different concentrations (25 and 50 mmol/L) of Ni(acac)2 were tested. Increasing the TOP concentration resulted in both cases of decreasing particle sizes (Figure 5a). The decrease in particle size was steeper for the 50 mmol/L Ni(acac)2 concentration. TEM images revealed spherical particles narrowly distributed, well-separated, and arranged into two-dimensional networks due to the effective stabilization by TOP (Figure 5b–d). Upon increasing the TOP/Ni ratio to 30, smaller nanoparticles were formed in a significant amount, which were still well-separated but no longer formed networks.
- (iv)
- Reaction volume dependence: Standard syntheses were conducted using a 5 mL solvent and 50 mmol/L nickel concentration. Triplicate experiments conducted within several months led to equally sized nanoparticles, as shown in Table 3. The underlying SAXS-data are shown in Figure S4. Increasing the reaction volume to 20 mL showed negligible effects on the nanoparticle size and size distribution.
- (v)
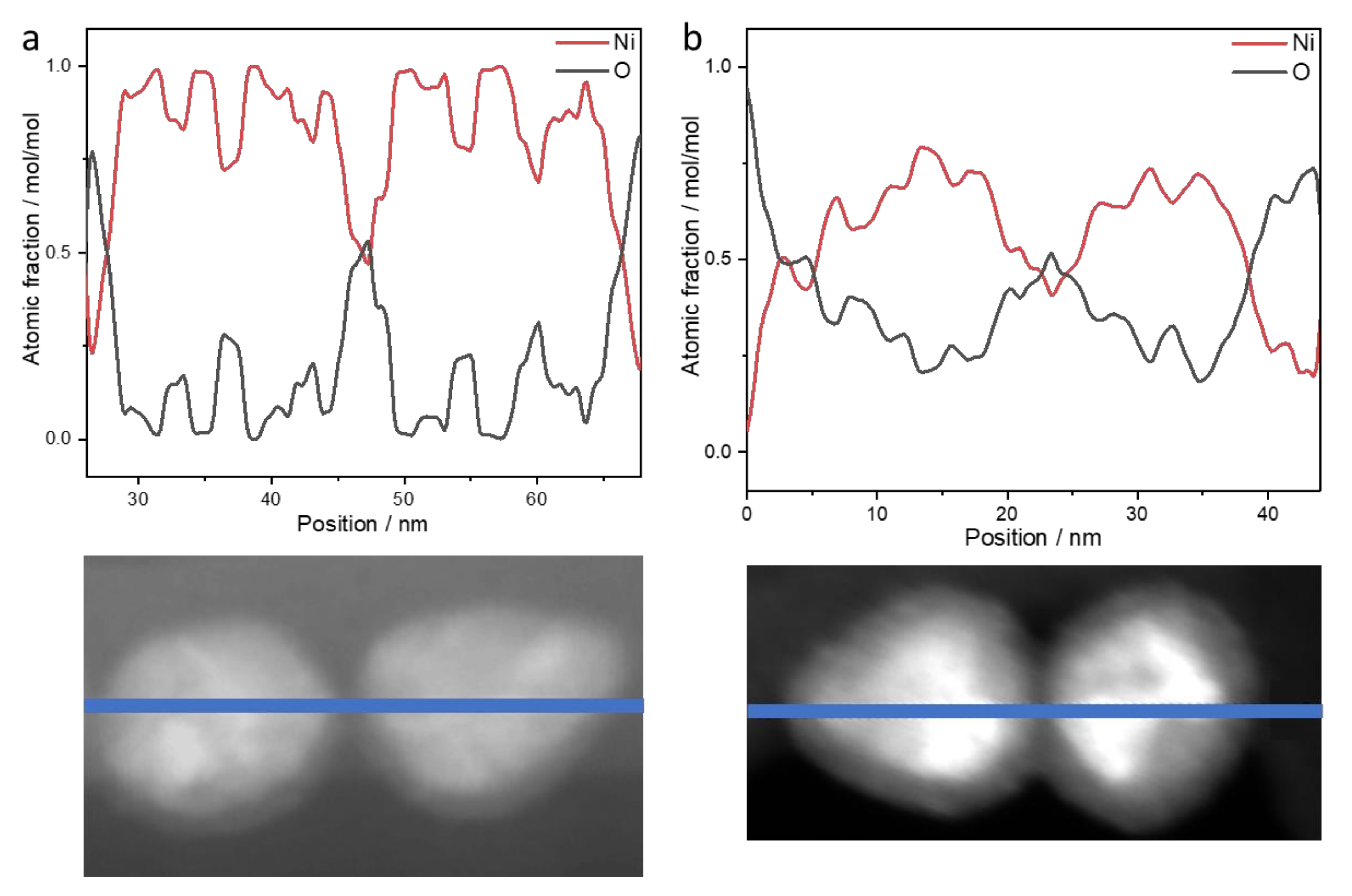
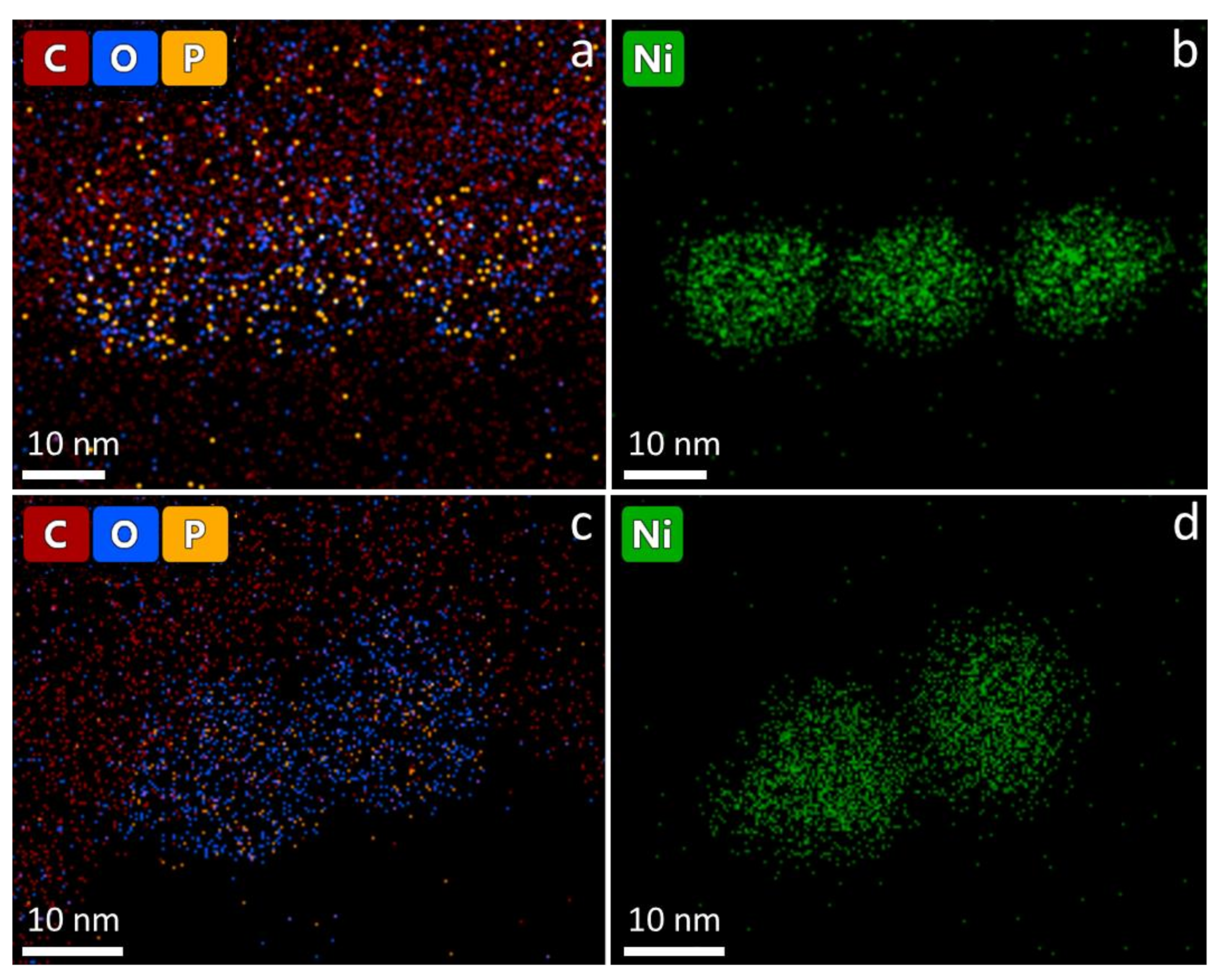
- Long-term stability of the nanoparticles: NiNPs prepared with 50 mmol/L Ni(acac)2, TOP/Ni = 3, and OAm/Ni = 61 for 2 h at 220 °C were investigated using STEM and EDS. Figure 6 shows the distribution of elements (line scan indicated by blue line, K-lines) of nickel and oxygen on the nanoparticles two weeks after synthesis (Figure 6a) and after nine weeks of storage (Figure 6b) on the TEM grid. The expected distribution of nickel within the NiNPs is aligned with the contrast differences in the STEM image. Due to the lower local concentration after synthesis, the oxygen distribution around the nanoparticles demonstrates the ongoing oxidation process on the particle surface by showing higher intensities at the outer regions of the particles and less intensity at the inner regions of the particles. The overall atomic fraction of nickel in the particles decreases over time, indicated by a maximum amount of 100 mol/mol in the fresh nanoparticles in comparison to a maximum of 80 mol/mol in the altered particles. The carbon distribution is not analyzed in the line scan, as it shows strong intensities around the particles assignable to the lacey carbon film on the used copper TEM grid, but it is shown in the mapping image (Figure 7 left). The phosphorus distribution confirms the presence of stabilizer TOP on the particle surface leading to well-separated and organized nanoparticles.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kawi, S.; Kathiraser, Y.; Ni, J.; Oemar, U.; Li, Z.W.; Saw, E.T. Progress in Synthesis of Highly Active and Stable Nickel-Based Catalysts for Carbon Dioxide Reforming of Methane. ChemSusChem 2015, 8, 3556–3575. [Google Scholar] [CrossRef] [PubMed]
- Bedwell, T.S.; Whitcombe, M.J. Analytical applications of MIPs in diagnostic assays: Future perspectives. Anal. Bioanal. Chem. 2016, 408, 1735–1751. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 954–965. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Cha, B.S.; Kim, S.; Park, K.S. Eco-friendly synthesis and biomedical applications of gold nanoparticles: A review. Microchem. J. 2020, 152, 9. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Naseem, K.; Begum, R.; Farooqi, Z.H. Platinum nanoparticles fabricated multiresponsive microgel composites: Synthesis, characterization, and applications. Polym. Compos. 2018, 39, 2167–2180. [Google Scholar] [CrossRef]
- Yu, L.; Li, N. Noble Metal Nanoparticles-Based Colorimetric Biosensor for Visual Quantification: A Mini Review. Chemosensors 2019, 7, 23. [Google Scholar] [CrossRef]
- Bae, S.Y.; Mahmood, J.; Jeon, I.Y.; Baek, J.B. Recent advances in ruthenium-based electrocatalysts for the hydrogen evolution reaction. Nanoscale Horiz. 2020, 5, 43–56. [Google Scholar] [CrossRef]
- Guo, H.Y.; Fang, Z.W.; Li, H.; Fernandez, D.; Henkelman, G.; Humphrey, S.M.; Yu, G.H. Rational Design of Rhodium-Iridium Alloy Nanoparticles as Highly Active Catalysts for Acidic Oxygen Evolution. ACS Nano 2019, 13, 13225–13234. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 28. [Google Scholar] [CrossRef]
- Maroufmashat, A.; Fowler, M. Transition of Future Energy System Infrastructure; through Power-to-Gas Pathways. Energies 2017, 10, 22. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Mills, G.A.; Steffgen, F.W. Catalytic Methanation. Catal. Rev. 1974, 8, 159–210. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Pitchumani, K. Clay entrapped nickel nanoparticles as efficient and recyclable catalysts for hydrogenation of olefins. Tetrahedron Lett. 2008, 49, 1818–1823. [Google Scholar] [CrossRef]
- Saxena, A.; Kumar, A.; Mozumdar, S. Ni-nanoparticles: An efficient green catalyst for chemo-selective oxidative coupling of thiols. J. Mol. Catal. Chem. 2007, 269, 35–40. [Google Scholar] [CrossRef]
- Gong, M.; Wang, D.-Y.; Chen, C.-C.; Hwang, B.-J.; Dai, H. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2016, 9, 28–46. [Google Scholar] [CrossRef]
- Eluri, R.; Paul, B. Microwave assisted greener synthesis of nickel nanoparticles using sodium hypophosphite. Mat. Lett. 2012, 76, 36–39. [Google Scholar] [CrossRef]
- Logutenko, O.A.; Titkov, A.I.; Vorob’ev, A.M.; Shundrina, I.K.; Yukhin, Y.M.; Lyakhov, N.Z. Synthesis of Nickel Nanoparticles by the Reduction of Its Salts Using the Modified Polyol Method in the Presence of Sodium Polyacrylates with Various Molecular Weights. Russ. J. Gen. Chem. 2018, 88, 288–294. [Google Scholar] [CrossRef]
- Chen, D.-H.; Wu, S.-H. Synthesis of nickel nanoparticles in water-in-oil microemulsions. Chem. Mater. 2000, 12, 1354–1360. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Ingham, B.; Toney, M.F.; Tilley, R.D. Effect of Surfactant Concentration and Aggregation on the Growth Kinetics of Nickel Nanoparticles. J. Phys. Chem. C 2013, 117, 16709–16718. [Google Scholar] [CrossRef]
- Liu, S.; Mei, J.; Zhang, C.; Zhang, J.; Shi, R. Synthesis and magnetic properties of shuriken-like nickel nanoparticles. J. Mater. Sci. Technol. 2018, 34, 836–841. [Google Scholar] [CrossRef]
- Wang, Z.C.; Chen, Y.Z.; Zeng, D.Q.; Zhang, Q.F.; Peng, D.L. Solution synthesis of triangular and hexagonal nickel nanosheets with the aid of tungsten hexacarbonyl. CrystEngComm 2016, 18, 1295–1301. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Ingham, B.; Cheong, S.; Williams, G.V.M.; Dotzler, C.; Toney, M.F.; Jefferson, D.A.; Corbos, E.C.; Bishop, P.T.; Cookson, J.; et al. Synthesis, Alignment, and Magnetic Properties of Monodisperse Nickel Nanocubes. J. Am. Chem. Soc. 2012, 134, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kang, E.; Son, S.U.; Park, H.M.; Lee, M.K.; Kim, J.; Kim, K.W.; Noh, H.J.; Park, J.H.; Bae, C.J.; et al. Monodisperse Nanoparticles of Ni and NiO: Synthesis, Characterization, Self-Assembled Superlattices, and Catalytic Applications in the Suzuki Coupling Reaction. Adv. Mat. 2005, 17, 429–434. [Google Scholar] [CrossRef]
- Donegan, K.P.; Godsell, J.F.; Otway, D.J.; Morris, M.A.; Roy, S.; Holmes, J.D. Size-tuneable synthesis of nickel nanoparticles. J. Nanopart. Res. 2012, 14. [Google Scholar] [CrossRef]
- Carenco, S.; Boissiére, C.D.; Nicole, L.; Sanchez, C.M.; Le Floch, P.; Mézailles, N. Controlled Design of Size-Tunable Monodisperse Nickel Nanoparticles. Chem. Mater. 2010, 22, 1340–1349. [Google Scholar] [CrossRef]
- Chen, G.; Desinan, S.; Rosei, R.; Rosei, F.; Ma, D. Synthesis of Ni-Ru Alloy Nanoparticles and Their High Catalytic Activity in Dehydrogenation of Ammonia Borane. Chem. Eur. J. 2012, 18, 7925–7930. [Google Scholar] [CrossRef]
- Carenco, S.; Labouille, S.; Bouchonnet, S.; Boissiere, C.; Le Goff, X.F.; Sanchez, C.; Mezailles, N. Revisiting the Molecular Roots of a Ubiquitously Successful Synthesis: Nickel(0) Nanoparticles by Reduction of [Ni(acetylacetonate)(2)]. Chem. Eur. J. 2012, 18, 14165–14173. [Google Scholar] [CrossRef]
- Breßler, I.; Kohlbrecher, J.; Thünemann, A.F. SASfit: A tool for small-angle scattering data analysis using a library of analytical expressions. J. Appl. Crystallogr. 2015, 48, 1587–1598. [Google Scholar] [CrossRef]
- Kohlbrecher, J. SASfit: A Program. for Fitting Simple Structural Models to Small Angle Scattering Data; Paul Scherrer Institut, Laboratory for Neutron Scattering: Villingen, Germany, 2018. [Google Scholar]
- Paris, O.; Li, C.; Siegel, S.; Weseloh, G.; Emmerling, F.; Riesemeier, H.; Erko, A.; Fratzl, P. A new experimental station for simultaneous X-ray microbeam scanning for small-and wide-angle scattering and fluorescence at BESSY II. J. Appl. Crystallogr. 2007, 40, s466–s470. [Google Scholar] [CrossRef]
- Wolf, S.E.; Leiterer, J.; Kappl, M.; Emmerling, F.; Tremel, W. Early homogenous amorphous precursor stages of calcium carbonate and subsequent crystal growth in levitated droplets. J. Am. Chem. Soc. 2008, 130, 12342–12347. [Google Scholar] [CrossRef] [PubMed]
- Hammersley, A.; Brown, K.; Burmeister, W.; Claustre, L.; Gonzalez, A.; McSweeney, S.; Mitchell, E.; Moy, J.-P.; Svensson, S.; Thompson, A. Calibration and application of an X-ray image intensifier/charge-coupled device detector for monochromatic macromolecular crystallography. J. Synchrotron Radiat. 1997, 4, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Kishi, H.; Yamaguchi, S.; Matsumura, D.; Tamura, K.; Hori, A.; Horiuchi, Y.; Serov, A.; Artyushkova, K.; Atanassov, P.; et al. Mechanism Study of Hydrazine Electrooxidation Reaction on Nickel Oxide Surface in Alkaline Electrolyte by In Situ XAFS. J. Electrochem. Soc. 2016, 163, H951–H957. [Google Scholar] [CrossRef]
- Winnischofer, H.; Rocha, T.C.; Nunes, W.C.; Socolovsky, L.M.; Knobel, M.; Zanchet, D. Chemical synthesis and structural characterization of highly disordered Ni colloidal nanoparticles. ACS Nano 2008, 2, 1313–1319. [Google Scholar] [CrossRef]
- Ishizaki, T.; Yatsugi, K.; Akedo, K. Effect of Particle Size on the Magnetic Properties of Ni Nanoparticles Synthesized with Trioctylphosphine as the Capping Agent. Nanomaterials 2016, 6, 172. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Liz-Marzán, L.M. Oleylamine in Nanoparticle Synthesis. Chem. Mater. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Cheng, G.; Puntes, V.F.; Guo, T. Synthesis and self-assembled ring structures of Ni nanocrystals. J. Colloid Interf. Sci. 2006, 293, 430–436. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, J.; Chow, G.; Ran, M.; Yi, J. Engineering magnetic properties of Ni nanoparticles by non-magnetic cores. Chem. Mater. 2009, 21, 5222–5228. [Google Scholar] [CrossRef]

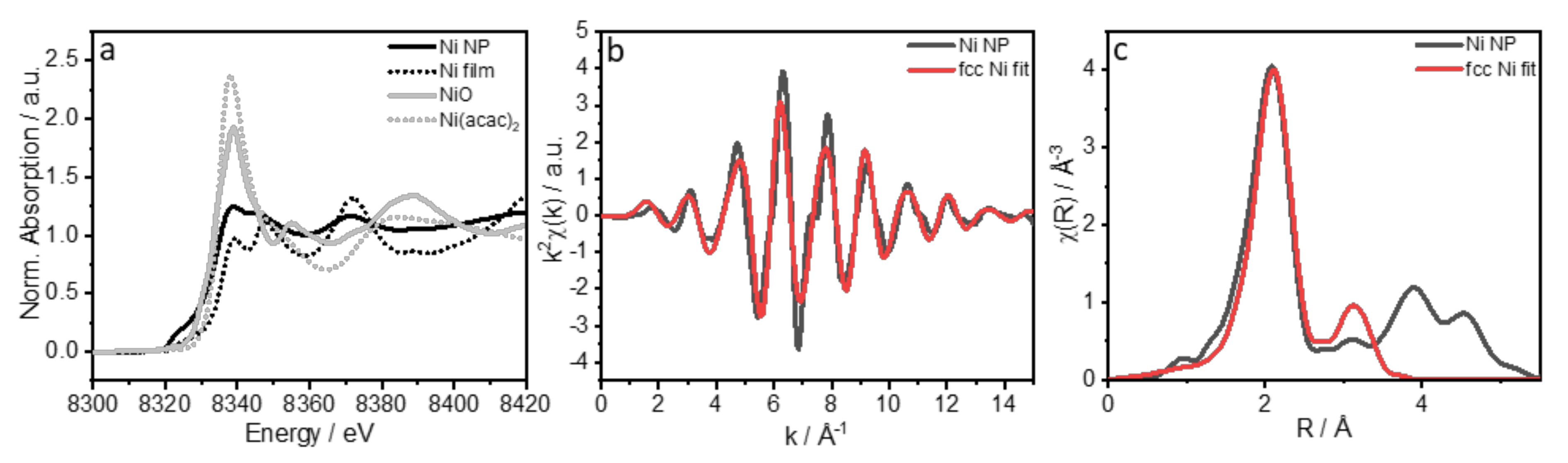
| Coordination Number | Distance R/Å | |
|---|---|---|
| NiNPs | Ni fcc | |
| 12 | 2.495 | 2.491 |
| 6 | 3.527 | 3.523 |
| 48 | 3.740 | 3.736 |
| Time/min | Size d/nm 1 | Polydispersity/% 1 |
|---|---|---|
| 30 | 9.6 | 18 |
| 60 | 10.0 | 12 |
| 90 | 10.0 | 12 |
| 120 | 10.0 | 12 |
| Volume/mL | Size d/nm | Polydispersity/% |
|---|---|---|
| 5 | 9.2 | 8 |
| 5 | 9.4 | 8 |
| 5 | 8.8 | 11 |
| 10 | 9.4 | 9 |
| 20 | 9.6 | 11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heilmann, M.; Kulla, H.; Prinz, C.; Bienert, R.; Reinholz, U.; Guilherme Buzanich, A.; Emmerling, F. Advances in Nickel Nanoparticle Synthesis via Oleylamine Route. Nanomaterials 2020, 10, 713. https://doi.org/10.3390/nano10040713
Heilmann M, Kulla H, Prinz C, Bienert R, Reinholz U, Guilherme Buzanich A, Emmerling F. Advances in Nickel Nanoparticle Synthesis via Oleylamine Route. Nanomaterials. 2020; 10(4):713. https://doi.org/10.3390/nano10040713
Chicago/Turabian StyleHeilmann, Maria, Hannes Kulla, Carsten Prinz, Ralf Bienert, Uwe Reinholz, Ana Guilherme Buzanich, and Franziska Emmerling. 2020. "Advances in Nickel Nanoparticle Synthesis via Oleylamine Route" Nanomaterials 10, no. 4: 713. https://doi.org/10.3390/nano10040713
APA StyleHeilmann, M., Kulla, H., Prinz, C., Bienert, R., Reinholz, U., Guilherme Buzanich, A., & Emmerling, F. (2020). Advances in Nickel Nanoparticle Synthesis via Oleylamine Route. Nanomaterials, 10(4), 713. https://doi.org/10.3390/nano10040713








