2D/1D V2O5 Nanoplates Anchored Carbon Nanofibers as Efficient Separator Interlayer for Highly Stable Lithium–Sulfur Battery
Abstract
:1. Introduction
2. Experimental
2.1. Raw Chemicals
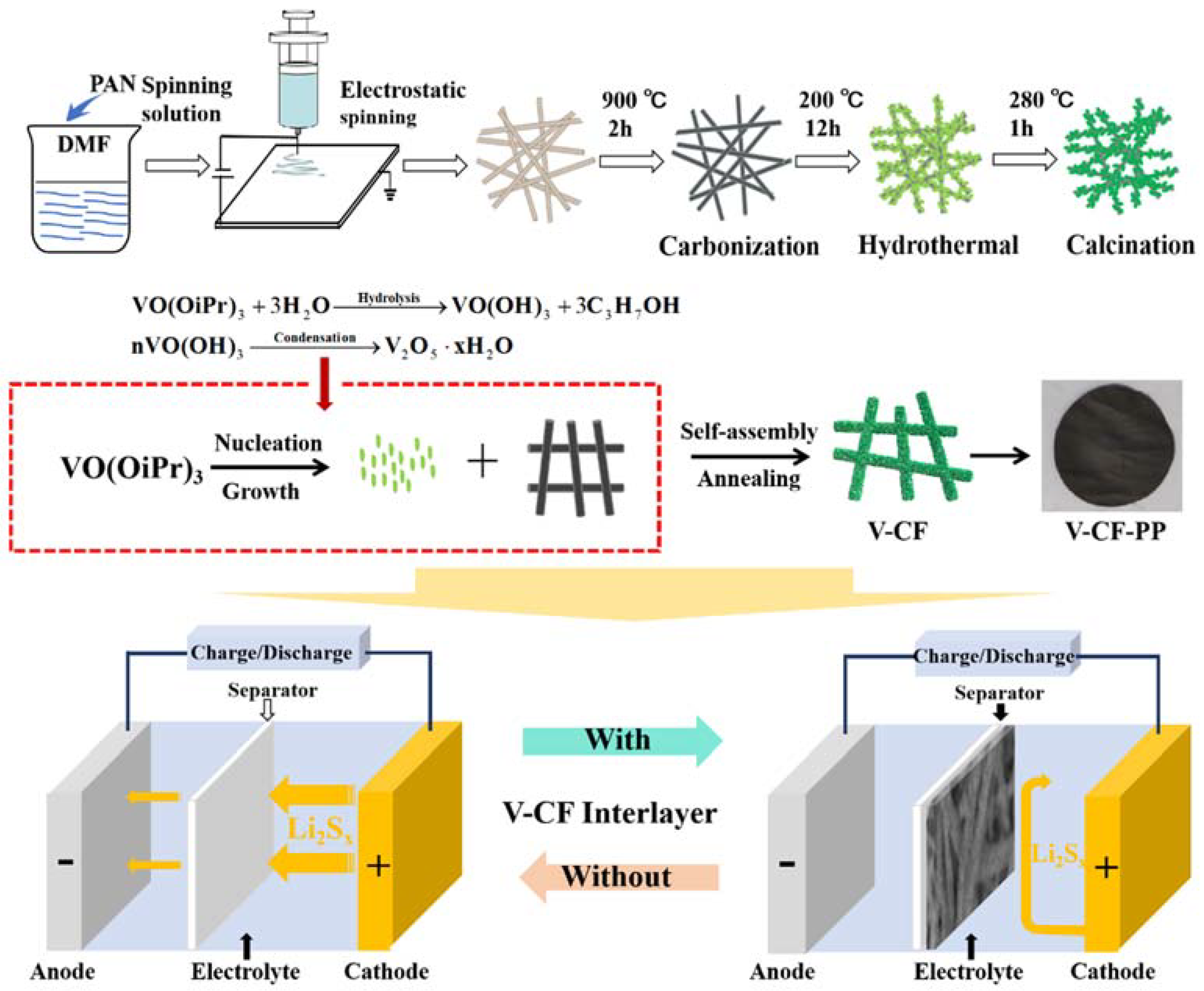
2.2. Preparation of CFs
2.3. Preparation of V2O5 Nanoplates Decorated CF
2.4. Battery Fabrication and Electrochemical Measurements
2.5. Saturated Adsorption Content Tests
2.6. Electrochemical Characterization
2.7. Characterization
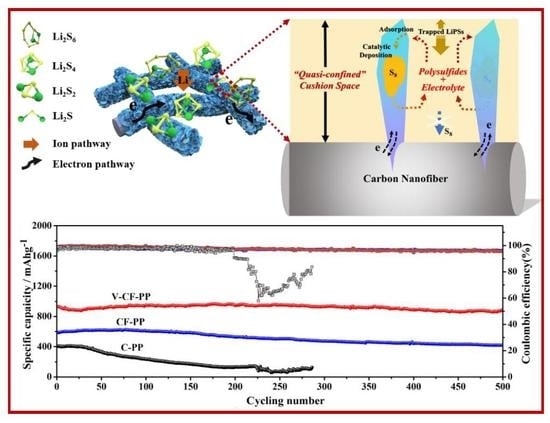
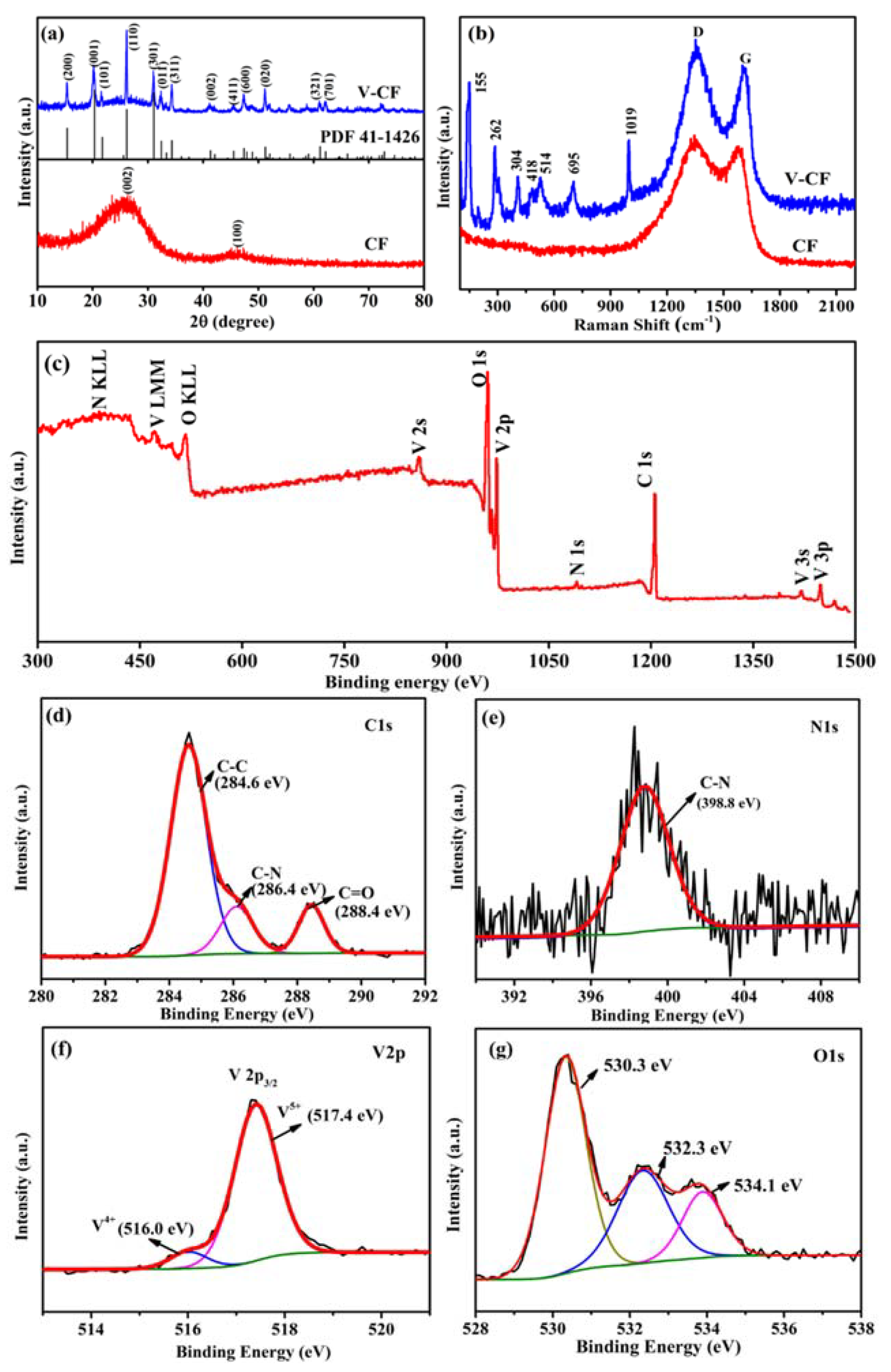
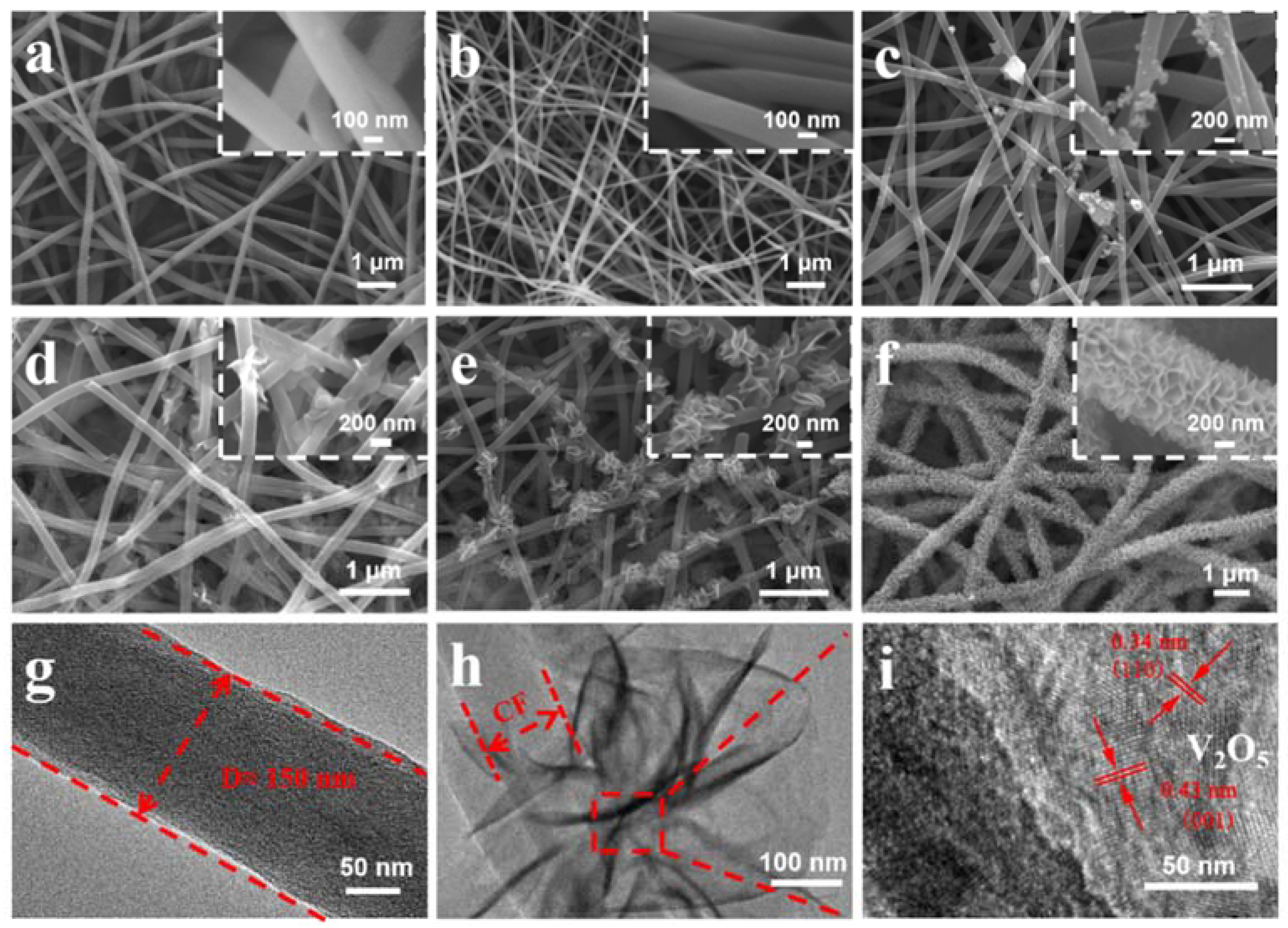
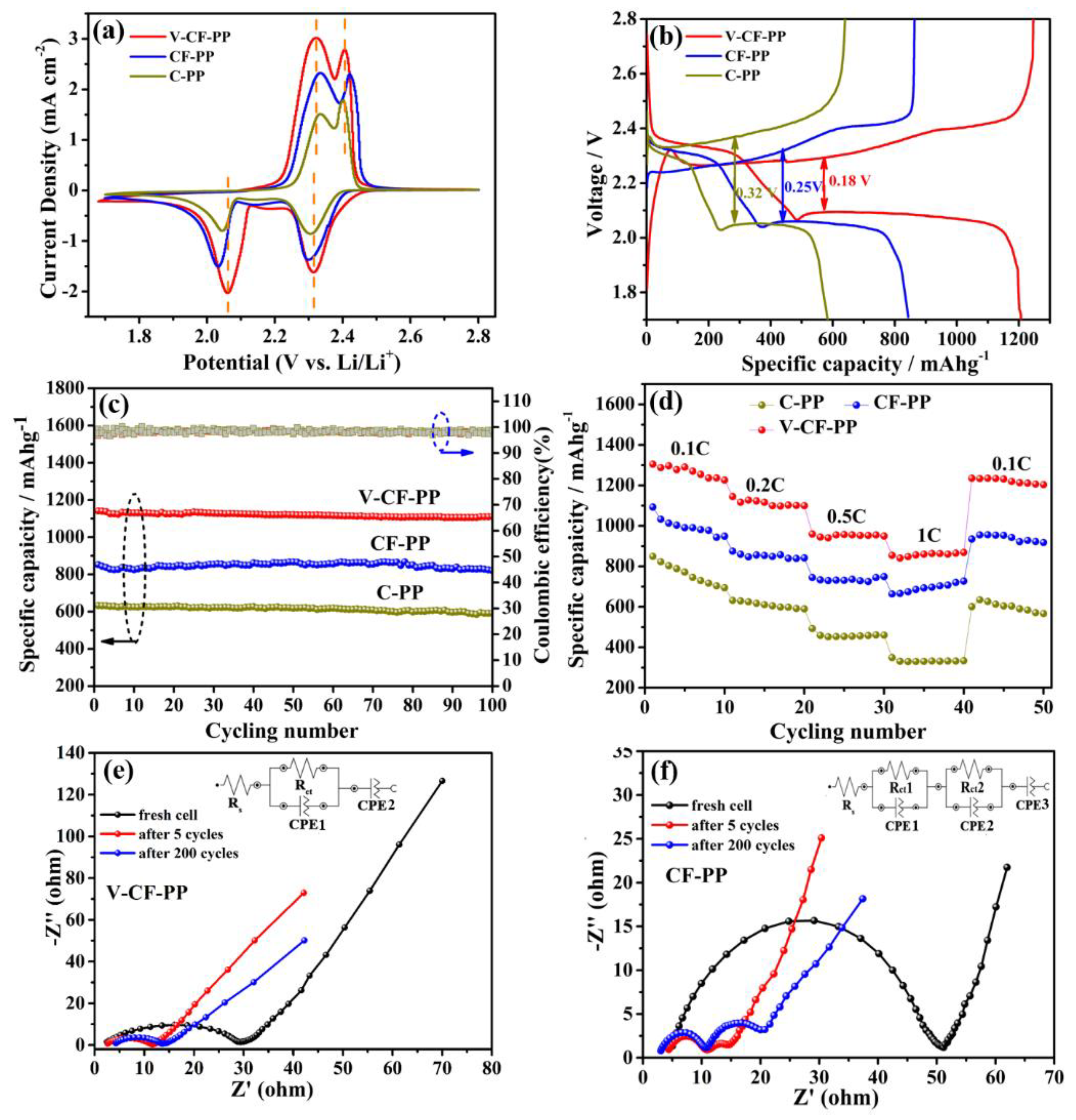
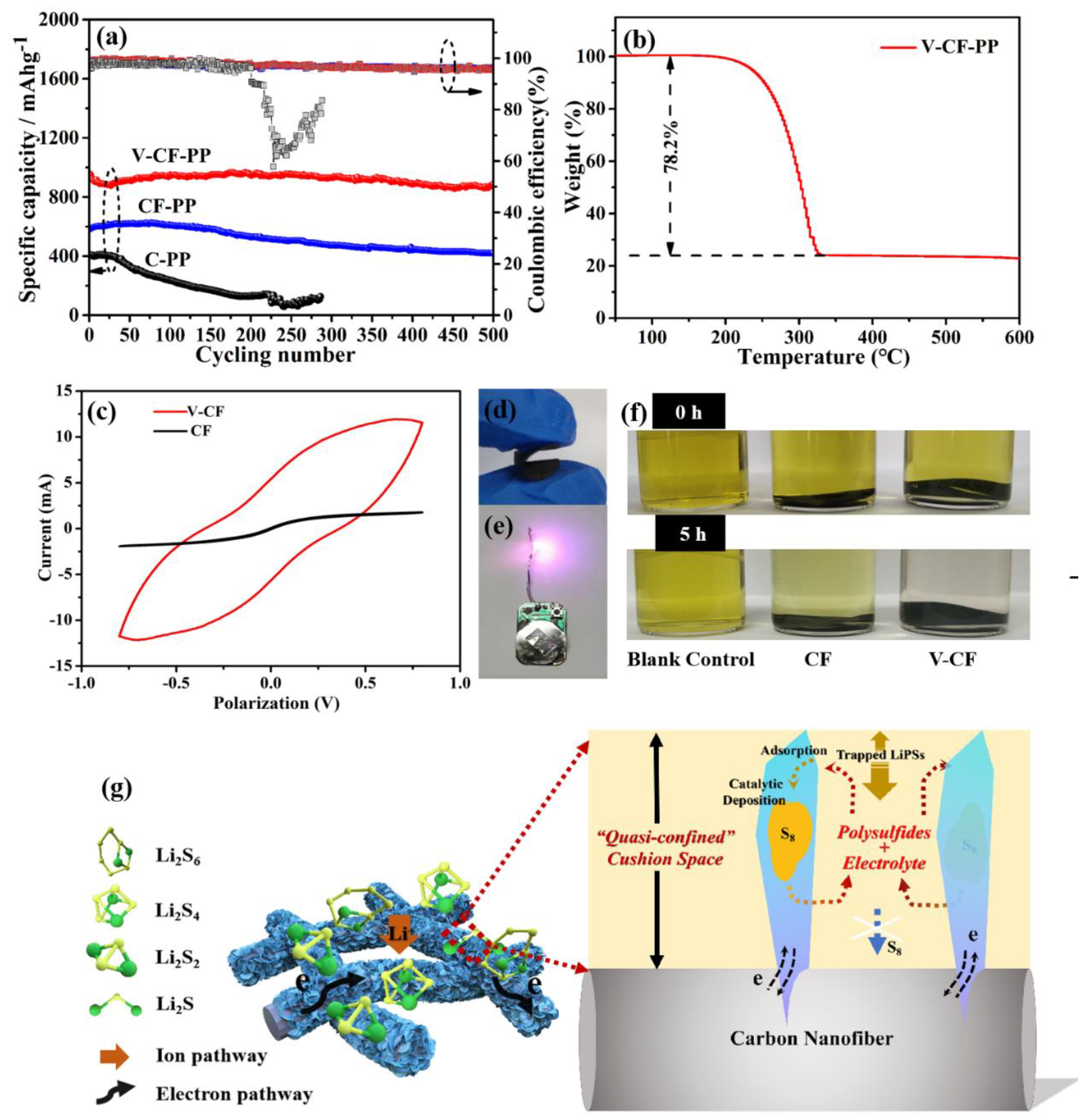
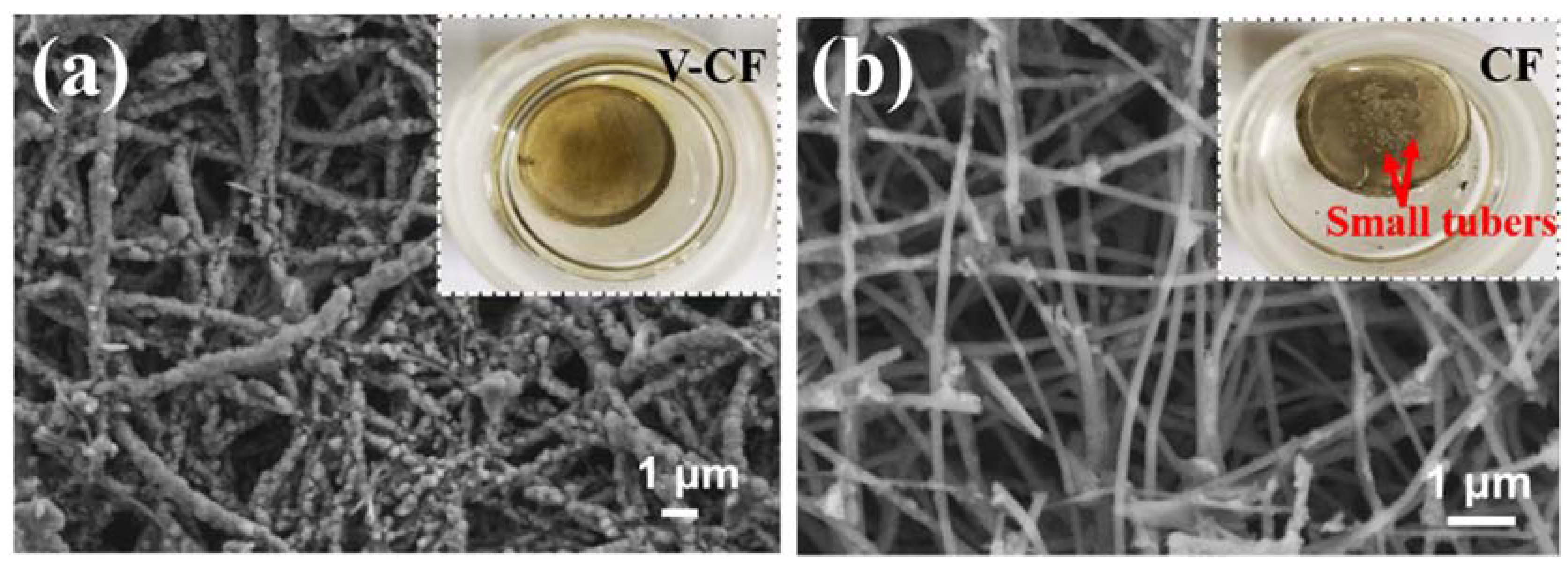
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Dong, C.; Gao, W.; Jin, B.; Jiang, Q. Advances in Cathode Materials for High-Performance Lithium-Sulfur Batteries. IScience 2018, 6, 151–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benveniste, G.; Rallo, H.; Canals Casals, L.; Merino, A.; Amante, B. Comparison of the state of Lithium-Sulphur and lithium-ion batteries applied to electromobility. J. Environ. Manag. 2018, 226, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, B.; Yuan, C.; Shen, L.; Xu, G.; Nie, P.; Zhang, X. Encapsulating Sulfur into Hierarchically Ordered Porous Carbon as a High-Performance Cathode for Lithium–Sulfur Batteries. Chem. Eur. J. 2013, 19, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Paek, E.; Hwang, G.S.; Manthiram, A. High-Performance Lithium-Sulfur Batteries with a Self-Supported, 3D Li2S-Doped Graphene Aerogel Cathodes. Adv. Energy Mater. 2016, 6, 1501355. [Google Scholar] [CrossRef]
- Zhou, G.; Yin, L.C.; Wang, D.W.; Li, L.; Pei, S.; Gentle, I.R.; Li, F.; Cheng, H.M. Fibrous Hybrid of Graphene and Sulfur Nanocrystals for High-Performance Lithium–Sulfur Batteries. ACS Nano 2013, 7, 5367–5375. [Google Scholar] [CrossRef]
- Zhou, M.G.; Lu, L.; Ma, C.Q.; Wang, S.G.; Shi, Y.; Koratkar, N.; Ren, W.C.; Li, F.; Cheng, H. A graphene foam electrode with high sulfur loading for flexible and high energy Li-S batteries. Nano Energy 2015, 11, 356–365. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Peng, H.J.; Zhao, M.; Huang, J.Q. Heterogeneous/Homogeneous Mediators for High-Energy-Density Lithium–Sulfur Batteries: Progress and Prospects. Adv. Funct. Mater. 2018, 28, 1707536. [Google Scholar] [CrossRef]
- Zheng, G.; Yang, Y.; Cha, J.J.; Hong, S.S.; Cui, Y. Hollow Carbon Nanofiber-Encapsulated Sulfur Cathodes for High Specific Capacity Rechargeable Lithium Batteries. Nano Lett. 2011, 11, 4462–4467. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.P.; Yin, Y.X.; Wan, L.J.; Guo, Y.G. Sulfur Encapsulated in Graphitic Carbon Nanocages for High-Rate and Long-Cycle Lithium–Sulfur Batteries. Adv. Mater. 2016, 28, 9539–9544. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.; Su, Y.S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.; Yi, Z.; Sun, Y.; Liu, Y.; Jiang, Y.; Shen, Y.; Xin, Y.; Zhang, Z.; Huang, Y. Insight into the Electrode Mechanism in Lithium-Sulfur Batteries with Ordered Microporous Carbon Confined Sulfur as the Cathode. Adv. Energy Mater. 2014, 4, 1301473. [Google Scholar] [CrossRef]
- Borchardt, L.; Oschatz, M.; Kaskel, S. Carbon Materials for Lithium Sulfur Batteries-Ten Critical Questions. Chem. Eur. J. 2016, 22, 7324–7351. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Chen, Y.; Yang, X.; Zhang, H.; Li, X.; Zhang, H. LiNO3-free electrolyte for Li-S battery: A solvent of choice with low Ksp of polysulfide and low dendrite of lithium. Nano Energy 2017, 39, 262–272. [Google Scholar] [CrossRef]
- Tan, L.; Li, X.; Wang, Z.; Guo, H.; Wang, J. Lightweight Reduced Graphene Oxide@MoS2 Interlayer as Polysulfide Barrier for High-Performance Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 3707–3713. [Google Scholar] [CrossRef]
- Tang, H.; Yao, S.; Xue, S.; Liu, M.; Chen, L.; Jing, M.; Shen, X.; Li, T.; Xiao, K.; Qin, S. In-situ synthesis of carbon@Ti4O7 non-woven fabric as a multi-functional interlayer for excellent lithium-sulfur battery. Electrochim. Acta 2018, 263, 158–167. [Google Scholar] [CrossRef]
- Xin, S.; Gu, L.; Zhao, N.H.; Yin, Y.X.; Zhou, L.J.; Guo, Y.G.; Wan, L.J. Smaller Sulfur Molecules Promise Better Lithium–Sulfur Batteries. J. Am. Chem. Soc. 2012, 134, 18510–18513. [Google Scholar] [CrossRef]
- Yu, M.; Ma, J.; Song, H.; Wang, A.; Tian, F.; Wang, Y.; Qiu, H.; Wang, R. Atomic layer deposited TiO2 on a nitrogen-doped graphene/sulfur electrode for high performance lithium–sulfur batteries. Energy Environ. Sci. 2016, 9, 1495–1503. [Google Scholar] [CrossRef]
- Hou, T.Z.; Chen, X.; Peng, H.J.; Huang, J.Q.; Li, B.Q.; Zhang, Q.; Li, B. Design Principles for Heteroatom-Doped Nanocarbon to Achieve Strong Anchoring of Polysulfides for Lithium–Sulfur Batteries. Small 2016, 12, 3283–3291. [Google Scholar] [CrossRef]
- Ji, X.; Evers, S.; Black, R.; Nazar, L.F. Stabilizing lithium–sulphur cathodes using polysulphide reservoirs. Nat. Commun. 2011, 2, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.L.; Chen, F.F.; Pan, H.P.; Wang, L.; Wang, D.; Jiang, Y.; Wang, L.B.; Qian, Y.T. Study on the effect of transition metal sulfide in lithium–sulfur battery. Inorg. Chem. Front. 2019, 6, 477–481. [Google Scholar] [CrossRef]
- Qian, W.; Gao, Q.; Zhang, H.; Tian, W.; Li, Z.; Tan, Y.L. Crosslinked Polypyrrole Grafted Reduced Graphene Oxide-Sulfur Nanocomposite Cathode for High Performance Li-S Battery. Electrochim. Acta 2017, 235, 32–41. [Google Scholar] [CrossRef]
- Hong, X.H.; Li, S.L.; Tang, X.N.; Sun, Z.H.; Li, F. Self-supporting porous CoS2/rGO sulfur host prepared by bottom-up assembly for lithium-sulfur batteries. J. Alloys Compd. 2018, 749, 586–593. [Google Scholar] [CrossRef]
- Luo, L.Y.; Qin, X.Y.; Wu, J.X.; Liang, G.M.; Li, Q.; Liu, M.; Kang, F.Y.; Chen, G.H.; Li, B.H. An interwoven MoO3@CNT scaffold interlayer for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 8612–8619. [Google Scholar] [CrossRef]
- Wang, Z.S.; Shen, J.D.; Liu, J.; Xu, X.J.; Liu, Z.B.; Hu, R.Z.; Yang, L.C.; Feng, Y.Z.; Liu, J.; Shi, Z.C.; et al. Self-Supported and Flexible Sulfur Cathode Enabled via Synergistic Confinement for High-Energy-Density Lithium–Sulfur Batteries. Adv. Mater. 2019, 31, 1902228. [Google Scholar] [CrossRef]
- Bai, S.; Liu, X.; Zhu, K.; Wu, S.; Zhou, H. Metal–organic framework-based separator for lithium–sulfur batteries. Nat. Energy 2016, 1, 16094. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Xiang, M.; Wu, H.; Liu, H.; Dou, S. Interwoven V2O5 nanowire/graphene nanoscroll hybrid assembled as efficient polysulfide-trapping-conversion interlayer for long-life lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 19358–19370. [Google Scholar] [CrossRef]
- Jeong, Y.C.; Kim, J.H.; Nam, S.; Park, C.R.; Yang, S.J. Rational Design of Nanostructured Functional Interlayer/Separator for Advanced Li–S Batteries. Adv. Funct. Mater. 2018, 28, 1707411. [Google Scholar] [CrossRef]
- Su, Y.S.; Manthiram, A. Lithium–sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166. [Google Scholar] [CrossRef] [Green Version]
- Schuster, J.; He, G.; Mandlmeier, B.; Yim, T.; Lee, K.T.; Bein, T.; Nazar, L.F. Spherical Ordered Mesoporous Carbon Nanoparticles with High Porosity for Lithium–Sulfur Batteries. Angew. Chem. Int. Ed. 2012, 51, 3591–3595. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xu, T.; Gordin, M.L.; Zhu, P.; Lv, D.; Jiang, Y.B.; Chen, Y.; Duan, Y.; Wang, D. Nitrogen-Doped Mesoporous Carbon Promoted Chemical Adsorption of Sulfur and Fabrication of High-Areal-Capacity Sulfur Cathode with Exceptional Cycling Stability for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2014, 24, 1243–1250. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, Z.; Wang, L.; Nie, H.; Zhong, M.E.; Lai, Q.; Xu, X.; Zhang, L.; Huang, S. A Lightweight TiO2/Graphene Interlayer, Applied as a Highly Effective Polysulfide Absorbent for Fast, Long-Life Lithium–Sulfur Batteries. Adv. Mater. 2015, 27, 2891–2898. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Yang, Z.; Yuan, H.; Zhang, J.; Yang, Y.; Liu, H. MnO2-decorated graphene aerogel with dual-polymer interpenetrating network as an efficient hybrid host for Li-S batteries. J. Alloys Compd. 2019, 791, 483–489. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, X.; Zhang, S.; Liang, G.; Kang, F.; Chen, G.; Li, B. Fe3O4-Decorated Porous Graphene Interlayer for High-Performance Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 26264–26273. [Google Scholar] [CrossRef]
- An, Q.; Zhang, P.; Xiong, F.; Wei, Q.; Sheng, J.; Wang, Q.; Mai, L. Three-dimensional porous V2O5 hierarchical octahedrons with adjustable pore architectures for long-life lithium batteries. Nano Res. 2015, 8, 481–490. [Google Scholar] [CrossRef]
- Shao, J.; Li, X.; Wan, Z.; Zhang, L.; Ding, Y.; Zhang, L.; Qu, Q.; Zheng, H. Low-Cost Synthesis of Hierarchical V2O5 Microspheres as High-Performance Cathode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 7671–7675. [Google Scholar] [CrossRef]
- Song, H.; Liu, C.; Zhang, C.; Cao, G. Self-doped V4+–V2O5 nanoflake for 2 Li-ion intercalations with enhanced rate and cycling performance. Nano Energy 2016, 22, 1–10. [Google Scholar] [CrossRef]
- Chou, S.L.; Wang, J.Z.; Sun, J.Z.; Wexler, D.; Forsyth, M.; Liu, H.K.; MacFarlane, D.R.; Dou, S.X. High Capacity, Safety, and Enhanced Cyclability of Lithium Metal Battery Using a V2O5 Nanomaterial Cathode and Room Temperature Ionic Liquid Electrolyte. Chem. Mater. 2008, 20, 7044–7051. [Google Scholar] [CrossRef]
- Ng, S.H.; Patey, T.J.; Büchel, R.; Krumeich, F.; Wang, J.Z.; Liu, H.K.; Pratsinis, S.E.; Novák, P. Flame spray-pyrolyzed vanadium oxide nanoparticles for lithium battery cathodes. Phys. Chem. Chem. Phys. 2009, 11, 3748–3755. [Google Scholar] [CrossRef] [Green Version]
- Ragupathy, P.; Shivakumara, S.; Vasan, H.N.; Munichandraiah, N. Preparation of Nanostrip V2O5 by the Polyol Method and Its Electrochemical Characterization as Cathode Material for Rechargeable Lithium Batteries. J. Phys. Chem. C 2008, 112, 16700–16707. [Google Scholar] [CrossRef]
- Sun, B.; Huang, K.; Qi, X.; Wei, X.; Zhong, J. Rational Construction of a Functionalized V2O5 Nanosphere/MWCNT Layer-by-Layer Nanoarchitecture as Cathode for Enhanced Performance of Lithium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 5633–5639. [Google Scholar] [CrossRef]
- Kim, M.S.; Shin, E.S.; Kim, J.S.; Cho, W.I.; Oh, S.H. The effect of V2O5/C additive on the suppression of polysulfide dissolution in Li-sulfur batteries. J. Electroceram. 2014, 33, 142–148. [Google Scholar] [CrossRef]
- Liang, X.; Kwok, C.Y.; Lodi-Marzano, F.; Pang, Q.; Cuisinier, M.; Huang, H.; Hart, C.J.; Houtarde, D.; Kaup, K.; Sommer, H.; et al. Tuning Transition Metal Oxide–Sulfur Interactions for Long Life Lithium Sulfur Batteries: The “Goldilocks” Principle. Adv. Energy Mater. 2016, 6, 1501636. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; She, Z.W.; Fu, Z.; Zhang, R.; Cui, Y. Understanding the Anchoring Effect of Two-Dimensional Layered Materials for Lithium–Sulfur Batteries. Nano Lett. 2015, 15, 3780–3786. [Google Scholar] [CrossRef]
- Guan, B.; Zhang, Y.; Fan, L.; Wu, X.; Wang, M.; Qiu, Y.; Zhang, N.; Sun, K. Blocking Polysulfide with Co2B@CNT via “Synergetic Adsorptive Effect” toward Ultrahigh-Rate Capability and Robust Lithium–Sulfur Battery. ACS Nano 2019, 13, 6742–6750. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Qin, X.; Liang, G.; Han, W.; Zhou, D.; He, Y.B.; Li, B.; Kang, F. Suppressing Self-Discharge and Shuttle Effect of Lithium–Sulfur Batteries with V2O5-Decorated Carbon Nanofiber Interlayer. Small 2017, 13, 1602539. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Purushothaman, K.K.; Muralidharan, G. V2O5/functionalized MWCNT hybrid nanocomposite: The fabrication and its enhanced supercapacitive performance. RSC Adv. 2014, 4, 37437–37445. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Li, B.; Wang, P.; Fan, L.; Zhang, N.; Sun, K. N doped carbon coated V2O5 nanobelt arrays growing on carbon cloth toward enhanced performance cathodes for lithium ion batteries. RSC Adv. 2018, 8, 6540–6543. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Gao, M.; Li, X.; Liu, Y.; Pan, H. Preparation of mesohollow and microporous carbon nanofiber and its application in cathode material for lithium–sulfur batteries. J. Alloys Compd. 2014, 608, 220–228. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, B.; Xin, H.L.; Yang, G.; Cai, H.; Nie, F.; Huang, H. Self-assembled V2O5 nanosheets/reduced graphene oxide hierarchical nanocomposite as a high-performance cathode material for lithium ion batteries. J. Mater. Chem. A 2013, 1, 10814–10820. [Google Scholar] [CrossRef]
- Li, W.; Chen, Z.; Li, J.; Chen, X.; Xuan, H.; Wang, X. Preparation of PAN/phenolic-based carbon/carbon composites with flexible towpreg carbon fiber. Mater. Sci. Eng. A 2008, 485, 481–486. [Google Scholar] [CrossRef]
- Xia, D.; Quan, J.; Wu, G.; Liu, X.; Zhang, Z.; Ji, H.; Chen, D.; Zhang, L.; Wang, Y.; Yi, S.; et al. Linear-Polyethyleneimine-Templated Synthesis of N-Doped Carbon Nanonet Flakes for High-performance Supercapacitor Electrodes. Nanomaterials 2019, 9, 1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minamisawa, T.; Oshida, K.; Kobayashi, N.; Ando, A.; Misawa, D.; Itaya, T.; Moriyama, M.; Osawa, K.; Hata, T.; Sugiyama, Y.; et al. Development of Electrode Materials of Lithium-Ion Battery Utilizing Nanospaces. C J. Carbon Res. 2018, 4, 23. [Google Scholar] [CrossRef] [Green Version]
- Carter, R.; Oakes, L.; Muralidharan, N.; Cohn, A.P.; Douglas, A.; Pint, C.L. Polysulfide Anchoring Mechanism Revealed by Atomic Layer Deposition of V2O5 and Sulfur-Filled Carbon Nanotubes for Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2017, 9, 7185–7192. [Google Scholar] [CrossRef]
- Ghosh, A.; Ra, E.J.; Jin, M.; Jeong, H.K.; Kim, T.H.; Biswas, C.; Lee, Y.H. High Pseudocapacitance from Ultrathin V2O5 Films Electrodeposited on Self-Standing Carbon-Nanofiber Paper. Adv. Funct. Mater. 2011, 21, 2541–2547. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, Y.J.; Yang, H.; Xu, Z.L. Super-wetting, photoactive TiO2 coating on amino-silane modified PAN nanofiber membranes for highly efficient oil-water emulsion separation application. J. Membr. Sci. 2019, 580, 40–48. [Google Scholar] [CrossRef]
- Kalybekkyzy, S.; Mentbayeva, A.; Kahraman, M.; Zhang, Y.; Bakenov, Z. Flexible S/DpAN/KB nanofiber composite as binder-free cathodes for Li-S batteries. J. Electrochem. Soc. 2019, 166, A5396–A5402. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, D.; Li, Y.; Qu, J.; Yu, Z.Z. Fabrication of PAN@TiO2/Ag nanofibrous membrane with high visible light response and satisfactory recyclability for dye photocatalytic degradation. Appl. Surf. Sci. 2017, 426, 622–629. [Google Scholar] [CrossRef]
- Zhu, H.; Du, M.; Zhang, M.; Zou, M.; Yang, T.; Fu, Y.; Yao, J. The design and construction of 3D rose-petal-shaped MoS2 hierarchical nanostructures with structure-sensitive properties. J. Mater. Chem. A 2014, 2, 7680–7685. [Google Scholar] [CrossRef]
- Yao, Y.J.; Zhao, Q.; Wei, W.; Chen, Z.; Zhu, Y.; Zhang, P.; Zhang, Z.T.; Gao, Y.F. WO3 quantum-dots electrochromism. Nano Energy 2020, 68, 104350. [Google Scholar] [CrossRef]
- Zhou, Y.; Ramanathan, S. Heteroepitaxial VO2 thin films on GaN: Structure and metal-insulator transition characteristics. J. Appl. Phys. 2012, 112, 4621–4628. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, Y.; Gao, Y.F.; Chen, D.L.; Shao, G.S. Large Scale Synthesis of Nanopyramidal-Like VO2 Films by an Oxygen-Assisted Etching Growth Method with Significantly Enhanced Field Emission Properties. Nanomaterials 2019, 9, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.D.; Gao, Y.F.; Zhang, Z.T.; Luo, H.J.; Cao, C.X.; Chen, Z.; Dai, L.; Liu, X.L. VO2 thermochromic smart window for energy savings and generation. Sci. Rep. 2013, 3, 3029. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Zhu, Q.Y.; Huang, S.N.; Jiang, S.S.; Lu, S.; Chen, W.; Zakharova, G.S. Interpenetrating Network of V2O5 Nanosheets/Carbon Nanotubes Nanocomposite for Fast Lithium Storage. RSC Adv. 2014, 4, 46624–46630. [Google Scholar] [CrossRef]
- Sathiya, M.; Prakash, A.S.; Ramesha, K.; Tarascon, J.M.; Shukla, A.K. V2O5-Anchored Carbon Nanotubes for Enhanced Electrochemical Energy Storage. J. Am. Chem. Soc. 2011, 133, 16291. [Google Scholar] [CrossRef]
- Ren, Q.Y.; Qin, N.; Liu, B.; Yao, Y.; Zhao, X.; Deng, Z.; Li, Y.; Dong, Y.C.; Qian, D.; Su, B.L.; et al. An Oxygen-Deficient Vanadium Oxide@N-doped Carbon Heterostructure for Sodium-Ion Batteries: Insights into the Charge Storage Mechanism and Enhanced Reaction Kinetics. J. Mater. Chem. A 2020, 8, 3450. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, C.; Meng, Q.; Chen, Z.; Liu, H.; Guo, Z. Facile Synthesis of Hierarchical Networks Composed of Highly Interconnected V2O5 Nanosheets Assembled on Carbon Nanotubes and Their Superior Lithium Storage Properties. ACS Appl. Mater. Interfaces 2013, 5, 12394–12399. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; He, Q.; Xu, X.; Zhao, Y.; Liu, X.; Zhou, C.; Ai, D.; Xia, L.; Mai, L. A 3D Nitrogen-Doped Graphene/TiN Nanowires Composite as a Strong Polysulfide Anchor for Lithium–Sulfur Batteries with Enhanced Rate Performance and High Areal Capacity. Adv. Mater. 2018, 30, 1804089. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.; Yi, Z.; Liu, Y.; Xin, Y.; Zhang, Z.; Huang, Y. A dual coaxial nanocable sulfur composite for high-rate lithium–sulfur batteries. Nanoscale 2014, 6, 1653–1660. [Google Scholar] [CrossRef] [Green Version]
- Papandrea, B.; Xu, X.; Xu, Y.; Chen, C.Y.; Lin, Z.; Wang, G.; Luo, Y.; Liu, M.; Huang, Y.; Mai, L.; et al. Three-dimensional graphene framework with ultra-high sulfur content for a robust lithium–sulfur battery. Nano Res. 2016, 9, 240–248. [Google Scholar] [CrossRef]
- Peng, H.J.; Wang, D.W.; Huang, J.Q.; Cheng, X.B.; Yuan, Z.; Wei, F.; Zhang, Q. Janus Separator of Polypropylene-Supported Cellular Graphene Framework for Sulfur Cathodes with High Utilization in Lithium–Sulfur Batteries. Adv. Sci. 2016, 3, 1500268. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Q.; Ma, Z.; Liu, Q.; Wu, Z.; Wang, S. Oxygen plasma modified separator for lithium sulfur battery. RSC Adv. 2015, 5, 79473–79478. [Google Scholar] [CrossRef]
- Qi, B.; Zhao, X.S.; Wang, S.G.; Chen, K.; Wei, Y.J.; Chen, G.; Gao, Y.; Zhang, D.; Sun, Z.H.; Li, F. Mesoporous TiN microspheres as an efficient polysulfide barrier for lithium-sulfur batteries. J. Mater. Chem. A 2018, 6, 14359–14366. [Google Scholar] [CrossRef]
- Ghazi, Z.A.; He, X.; Khattak, A.M.; Khan, N.A.; Liang, B.; Iqbal, A.; Wang, J.X.; Sin, H.S.; Li, L.S.; Tang, Z.Y. MoS2/Celgard Separator as Efficient Polysulfide Barrier for Long-Life Lithium-Sulfur Batteries. Adv Mater. 2017, 29, 1606817. [Google Scholar] [CrossRef]
- Li, N.; Chen, Z.X.; Chen, F.; Hu, G.J.; Wang, S.G.; Sun, Z.H.; Sun, X.D.; Li, F. From interlayer to lightweight capping layer: Rational design of mesoporous TiO2 threaded with CNTs for advanced Li-S batteries. Carbon 2019, 143, 523–530. [Google Scholar] [CrossRef]
- Liu, F.; Xiao, Q.F.; Wu, H.B.; Sun, F.; Liu, X.Y.; Li, Y.; Le, Z.Y.; Shen, L.; Wang, G.; Cai, M.; et al. Regenerative Polysulfide-Scavenging Layers Enabling Lithium–Sulfur Batteries with High Energy Density and Prolonged Cycling Life. ACS Nano 2017, 11, 2697–2705. [Google Scholar] [CrossRef]
- Wu, D.S.; Shi, F.; Zhou, G.; Zu, C.; Liu, C.; Liu, K.; Liu, Y.; Wang, J.; Peng, Y.; Cui, Y. Quantitative investigation of polysulfide adsorption capability of candidate materials for Li-S batteries. Energy Storage Mater. 2018, 13, 241–246. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wu, G.; Ji, H.; Chen, D.; Xia, D.; Gao, K.; Xu, J.; Mao, B.; Yi, S.; Zhang, L.; et al. 2D/1D V2O5 Nanoplates Anchored Carbon Nanofibers as Efficient Separator Interlayer for Highly Stable Lithium–Sulfur Battery. Nanomaterials 2020, 10, 705. https://doi.org/10.3390/nano10040705
Zhang Z, Wu G, Ji H, Chen D, Xia D, Gao K, Xu J, Mao B, Yi S, Zhang L, et al. 2D/1D V2O5 Nanoplates Anchored Carbon Nanofibers as Efficient Separator Interlayer for Highly Stable Lithium–Sulfur Battery. Nanomaterials. 2020; 10(4):705. https://doi.org/10.3390/nano10040705
Chicago/Turabian StyleZhang, Zongtao, Guodong Wu, Haipeng Ji, Deliang Chen, Dengchao Xia, Keke Gao, Jianfei Xu, Bin Mao, Shasha Yi, Liying Zhang, and et al. 2020. "2D/1D V2O5 Nanoplates Anchored Carbon Nanofibers as Efficient Separator Interlayer for Highly Stable Lithium–Sulfur Battery" Nanomaterials 10, no. 4: 705. https://doi.org/10.3390/nano10040705
APA StyleZhang, Z., Wu, G., Ji, H., Chen, D., Xia, D., Gao, K., Xu, J., Mao, B., Yi, S., Zhang, L., Wang, Y., Zhou, Y., Kang, L., & Gao, Y. (2020). 2D/1D V2O5 Nanoplates Anchored Carbon Nanofibers as Efficient Separator Interlayer for Highly Stable Lithium–Sulfur Battery. Nanomaterials, 10(4), 705. https://doi.org/10.3390/nano10040705