Designing of Chitosan Derivatives Nanoparticles with Antiangiogenic Effect for Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
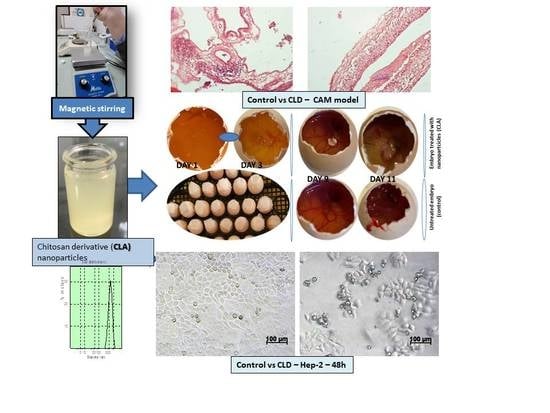
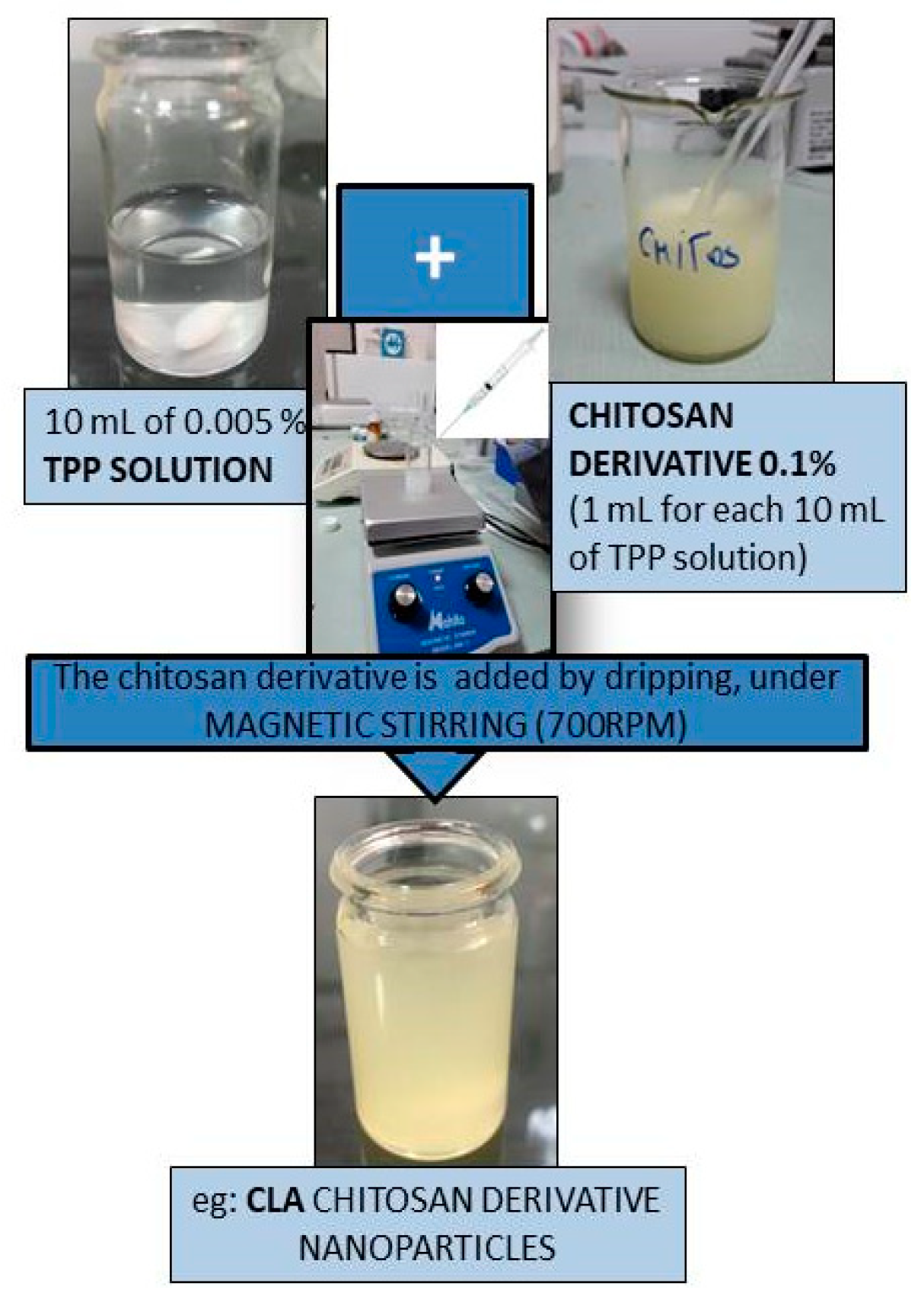
2.1. Design of Chitosan-Derived Nanoparticles
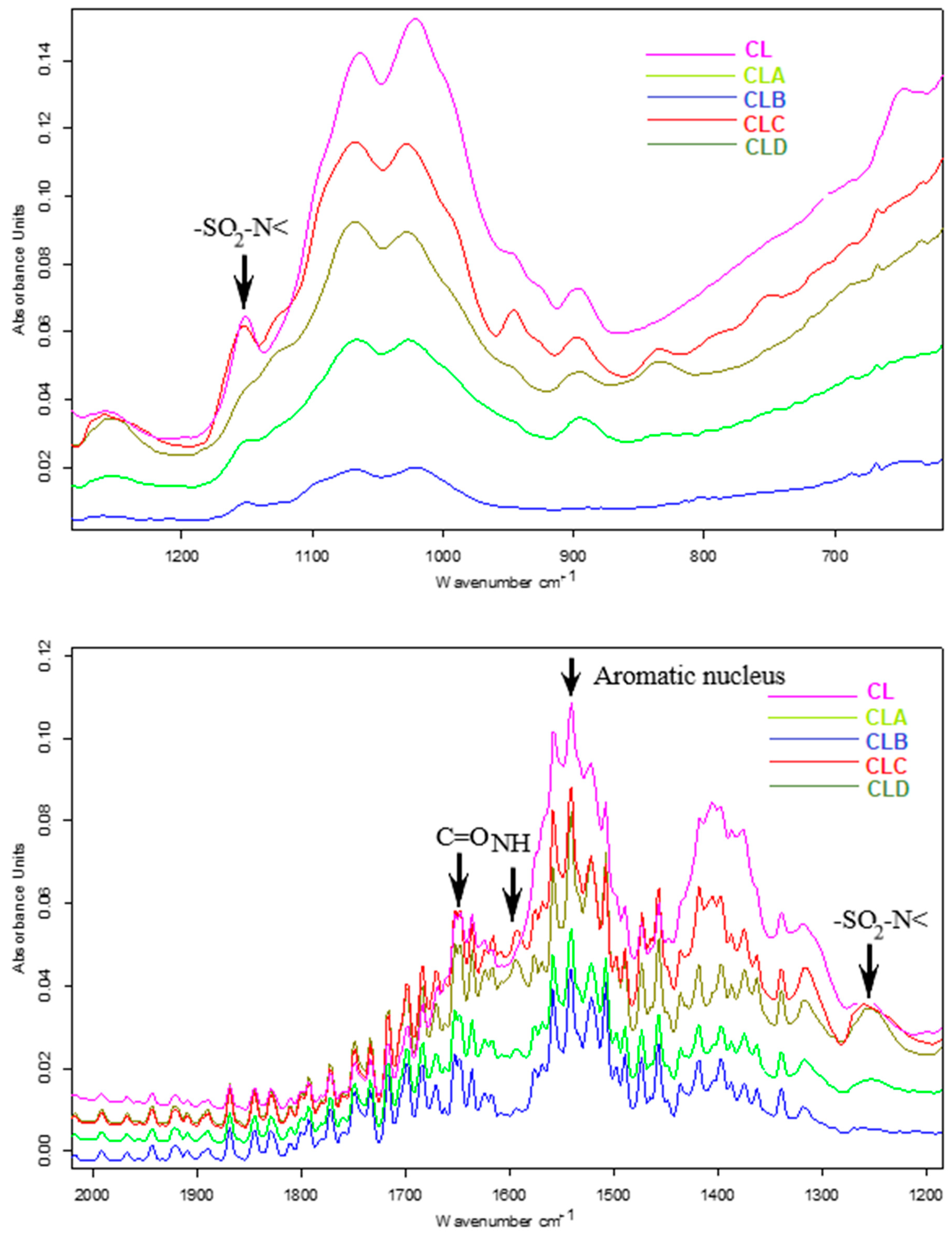
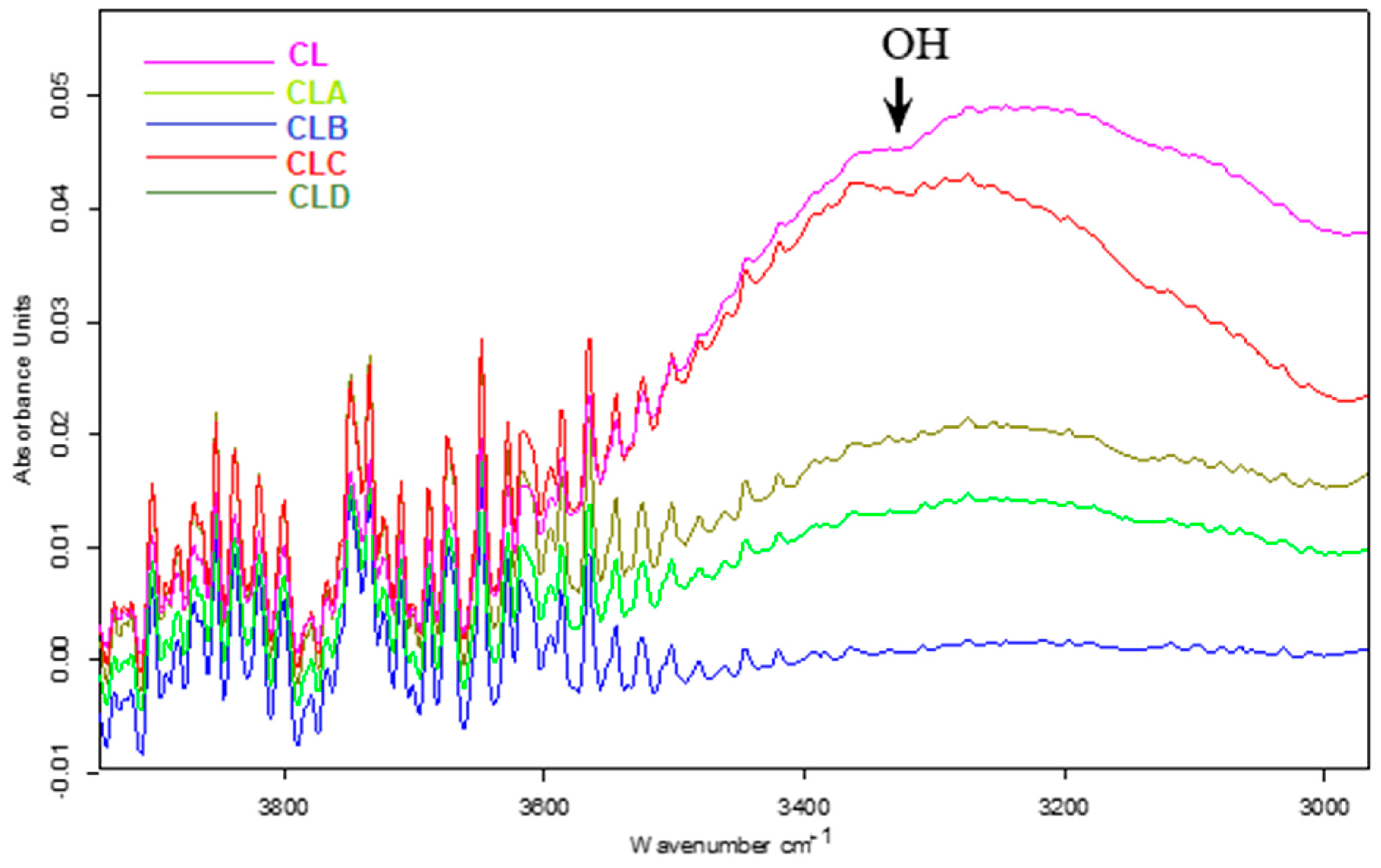
2.2. Spectral Characterization of Nanoparticles: FT-IR Analysis
2.3. Research Regarding Antioxidant Assays
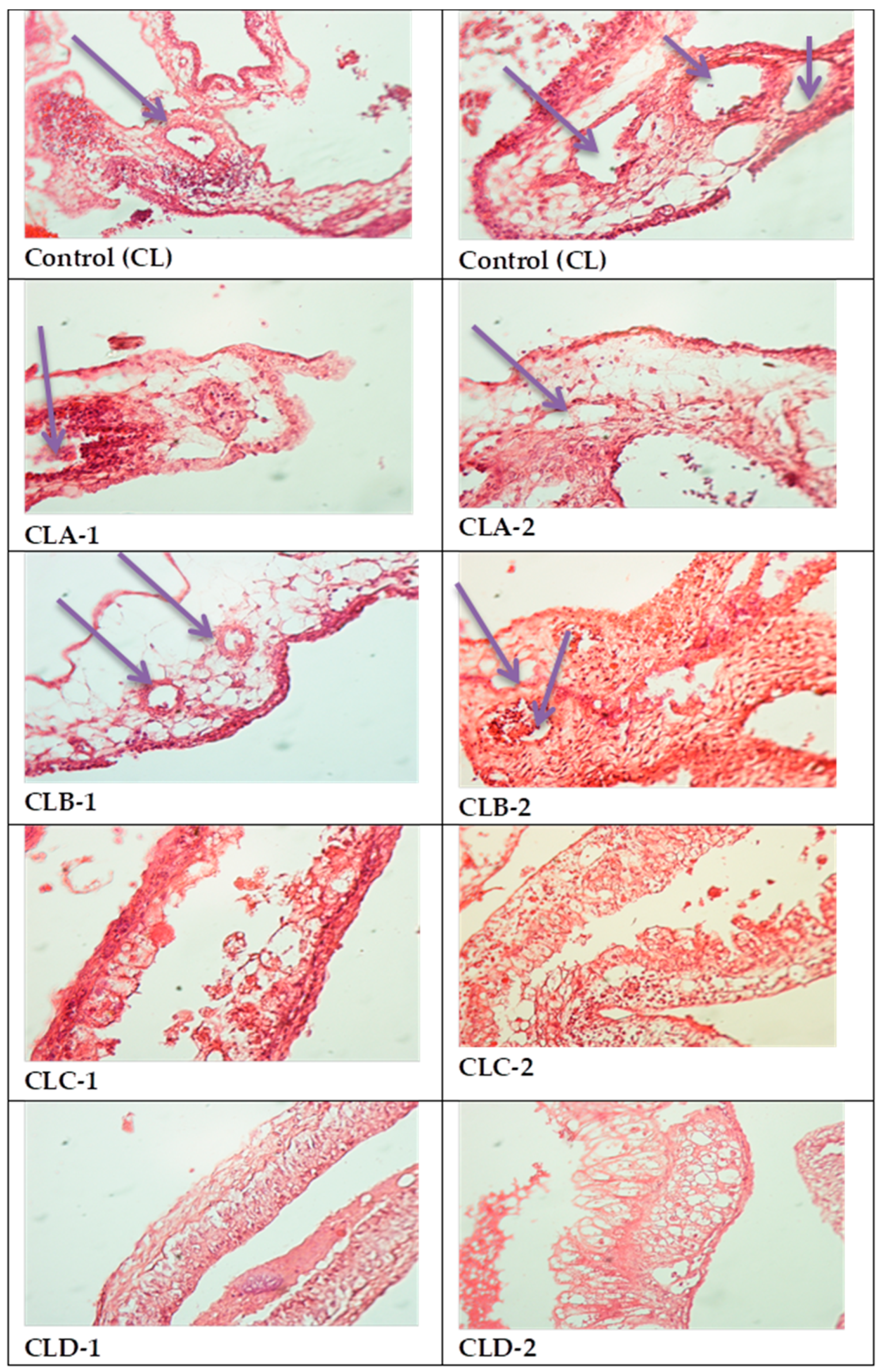
2.4. In Vivo Evaluation of Angiogenic Activity Using a CAM Model
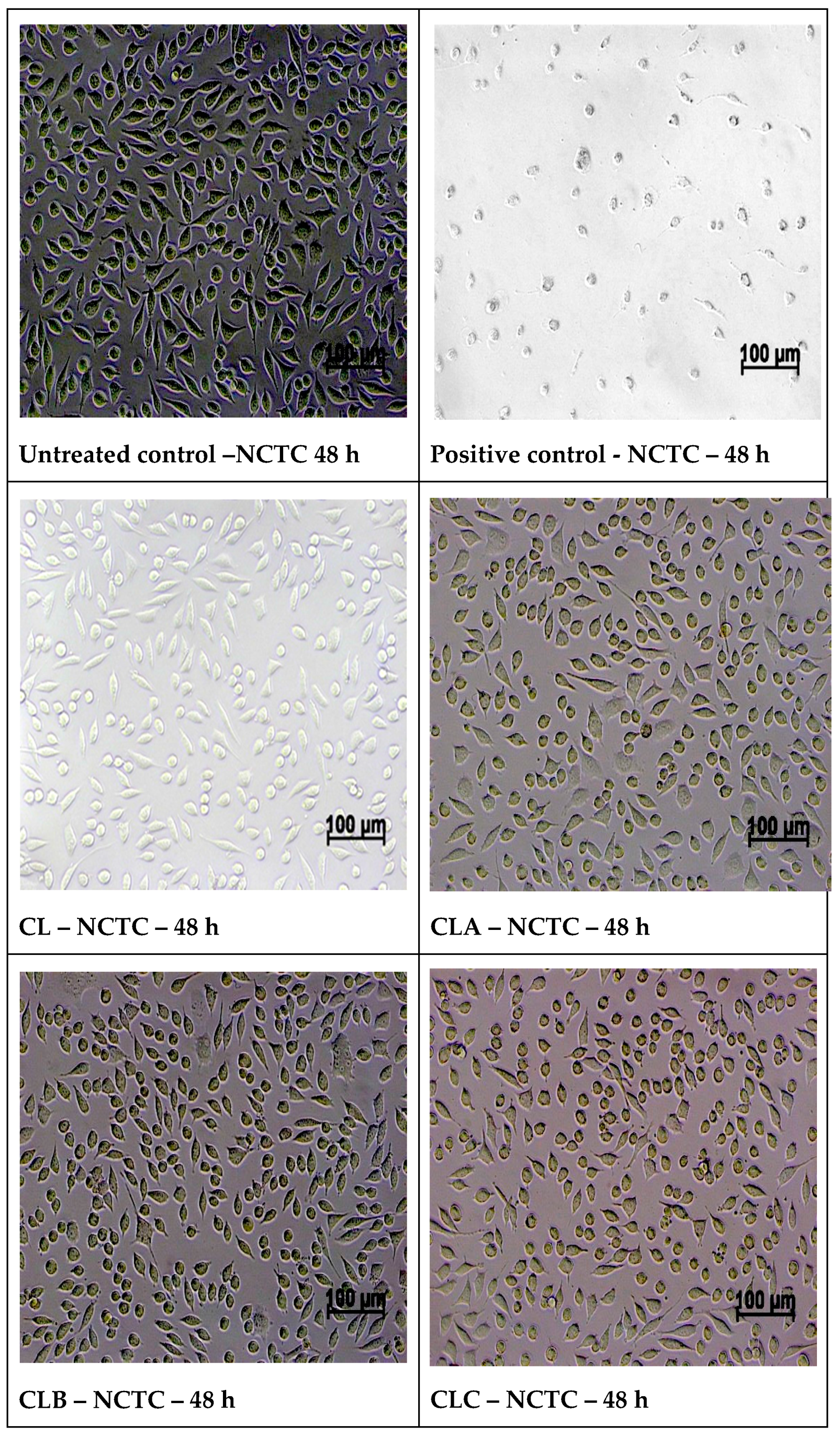
2.5. In Vitro Cytotoxicity Evaluation Using an MTT Assay
2.5.1. Cell Cultures
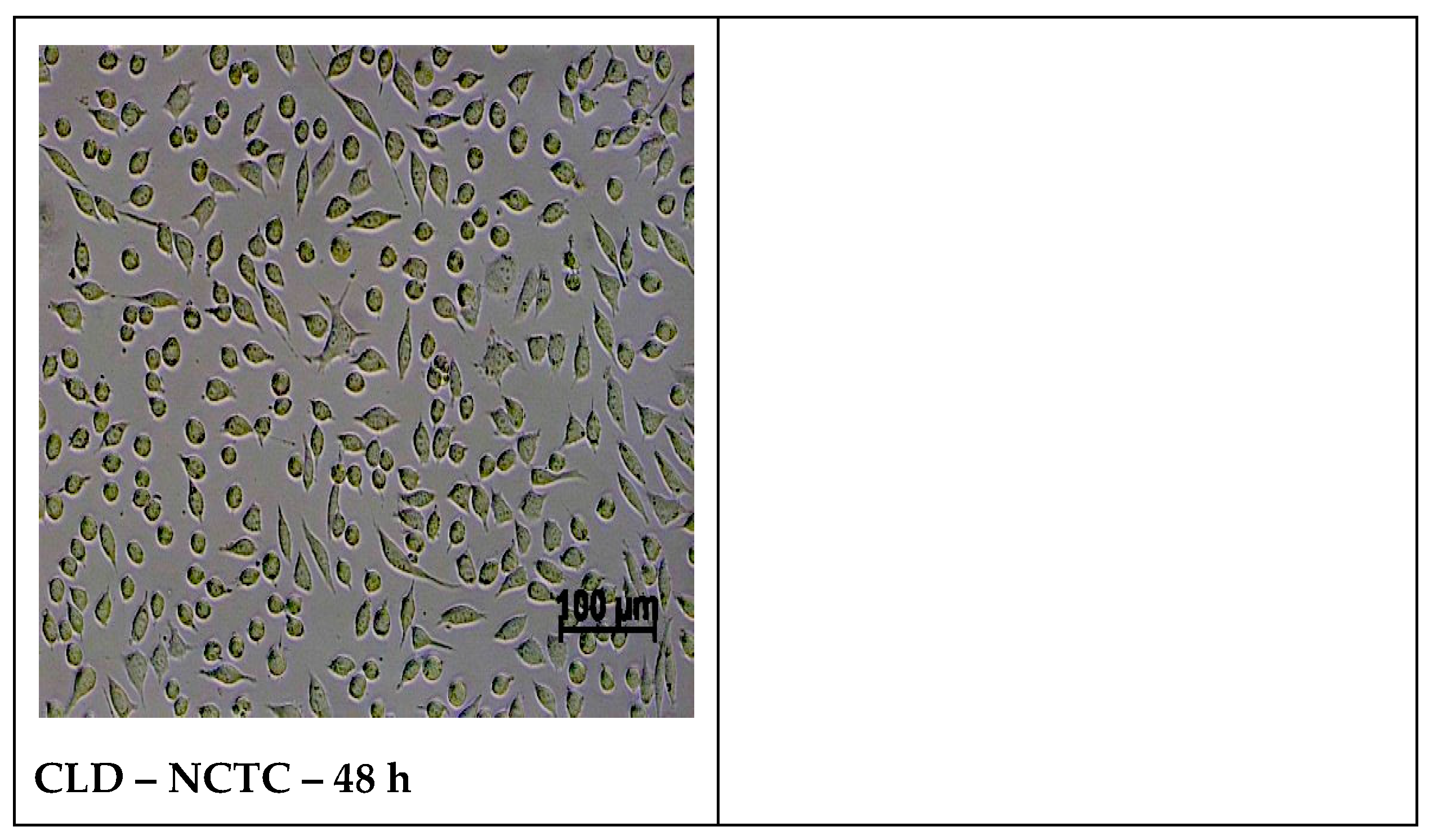
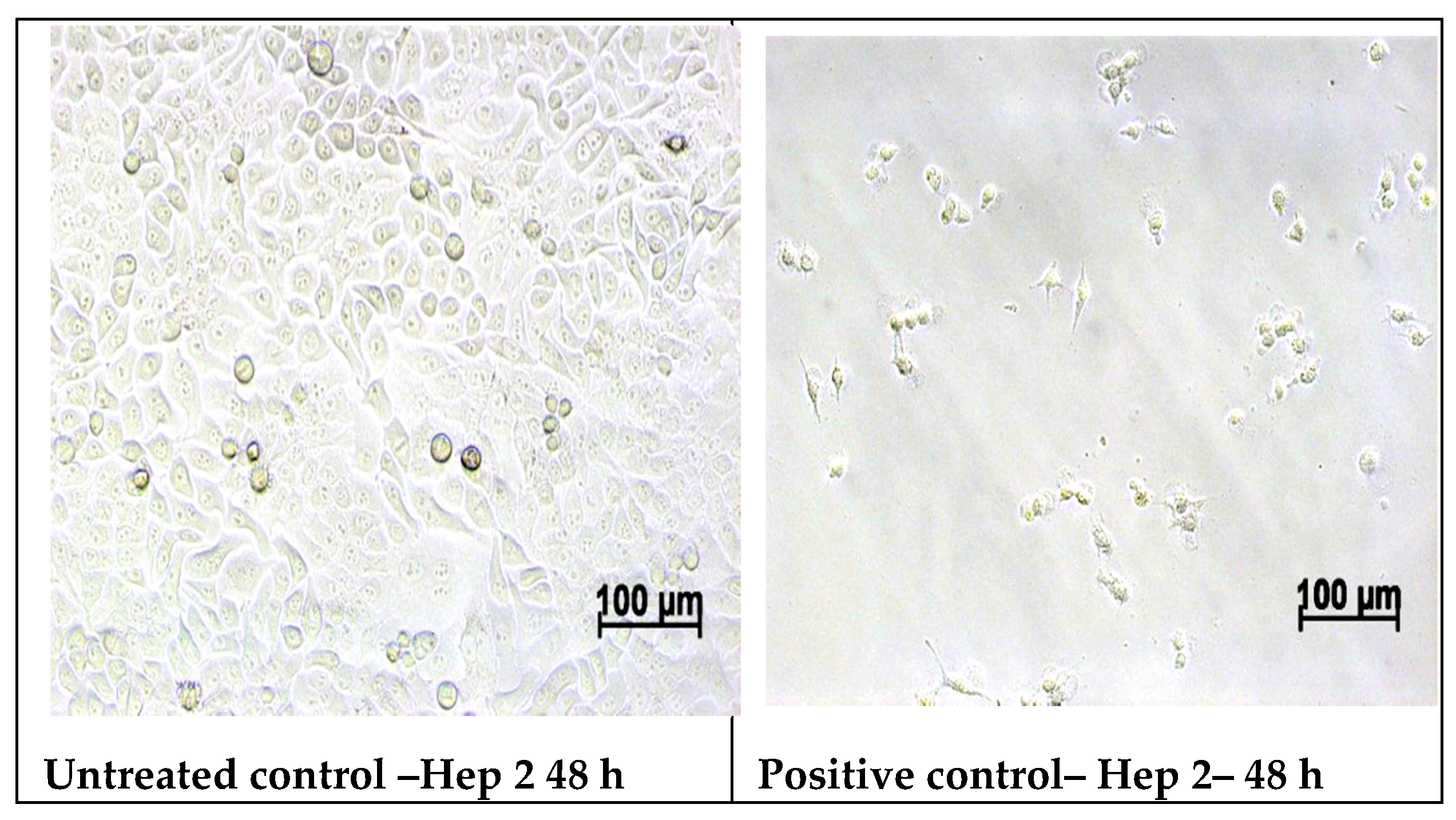
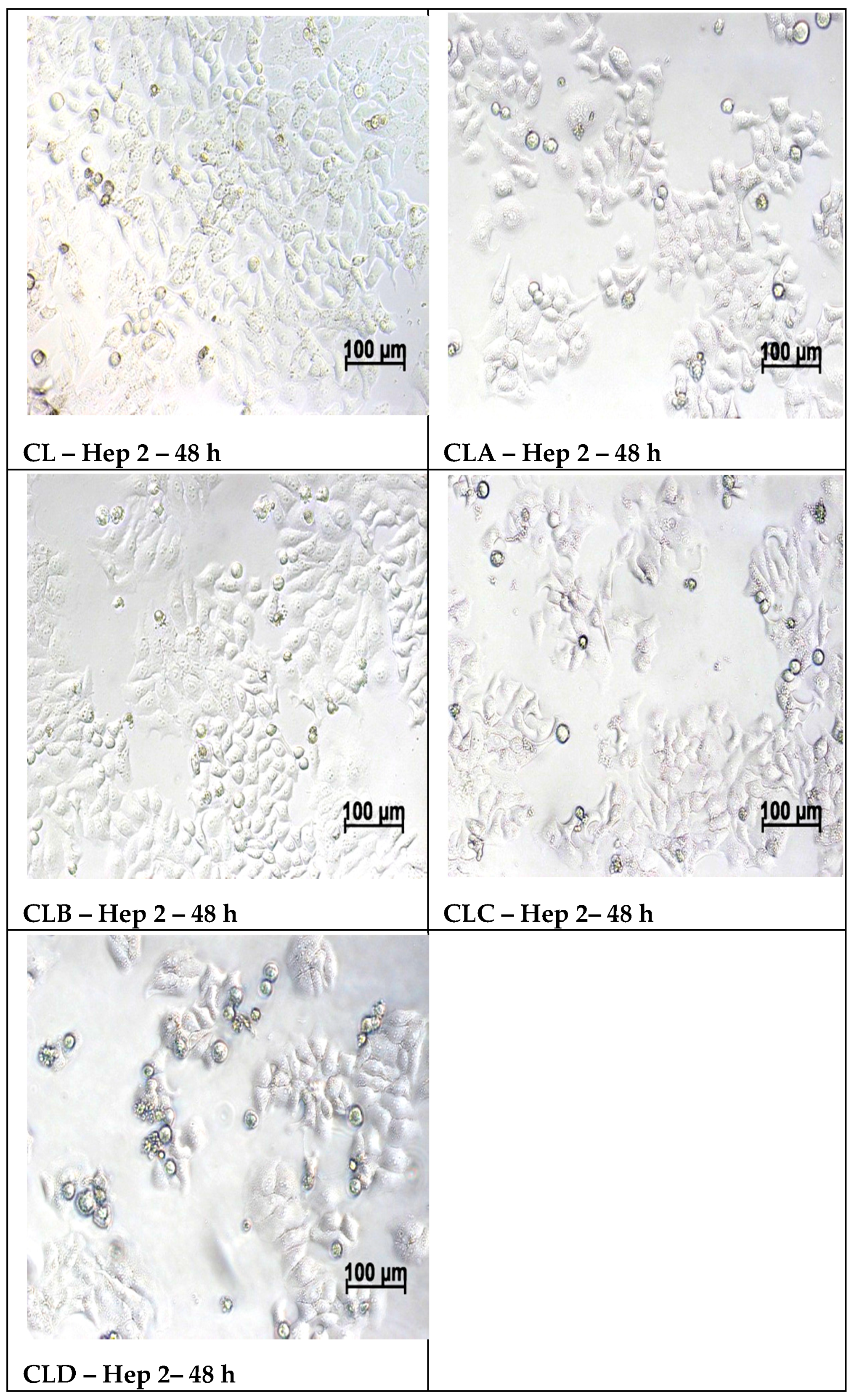
2.5.2. Cell Morphology Examination at 48 h of Treatment with Samples
2.6. Statistical Methods
- -
- one way ANOVA was conducted, for the variables X = blood vessels and Y = study batch, using an alpha of 0.05;
- -
- for multiple comparisons of normal distributed series of values, a post-hoc Bonferroni test was applied after one-way ANOVA in case of significance. One-Way ANOVA was significant (alpha of 0.05) and, in these conditions, Bonferroni correction (post-hoc Bonferroni) was performed to reduce the error rate when testing multiple hypotheses.
- -
- Skewness (−2 < p < 2) tests are tests of normality in frequentist statistics, available when using the distribution platform to examine a continuous variable.
3. Results
3.1. Chitosan-Derivatives Nanoparticles: DLS Measurements
3.2. FT-IR Characterization of Nanoparticles
3.3. Researches Regarding Antioxidant Assays
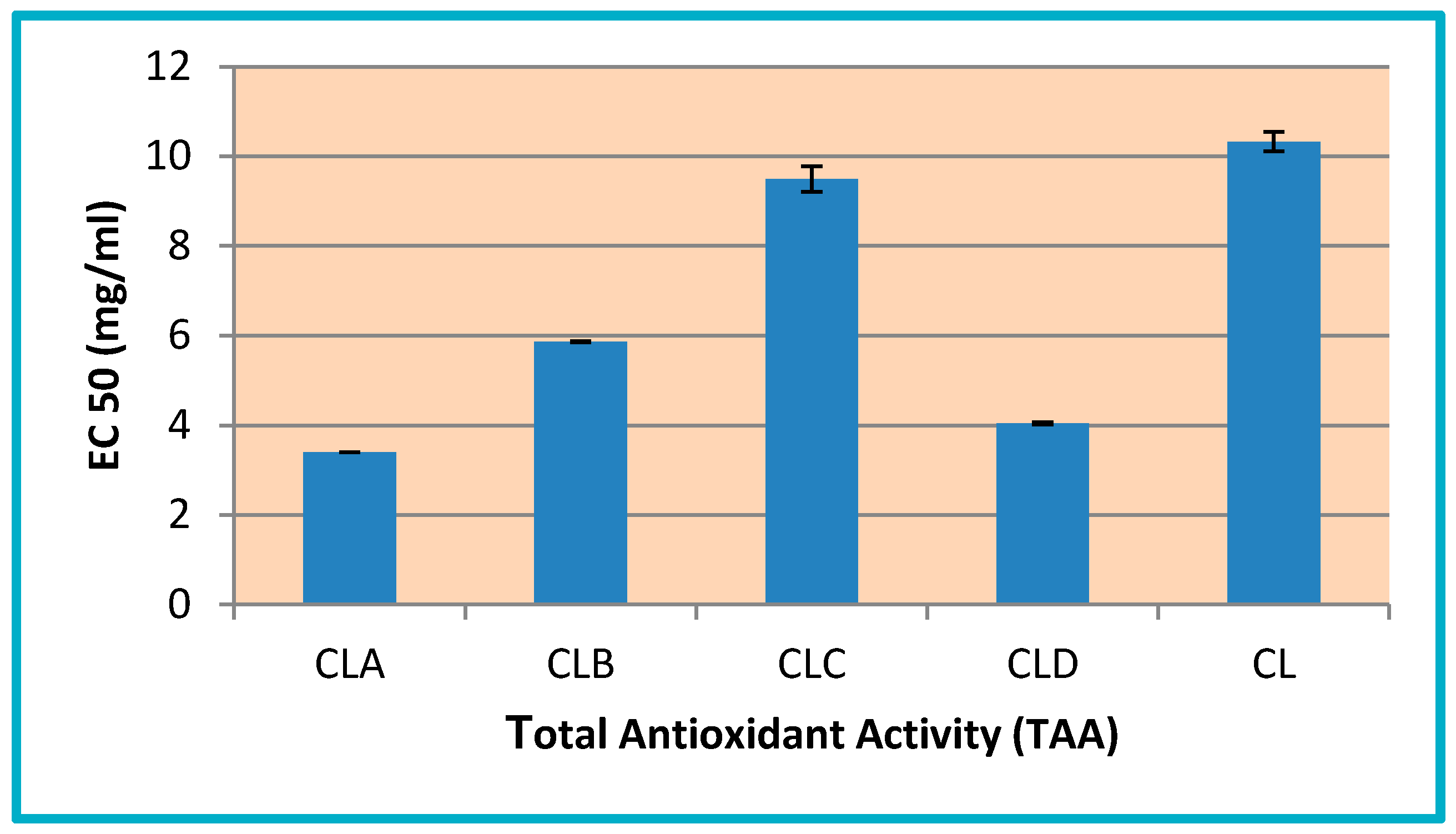
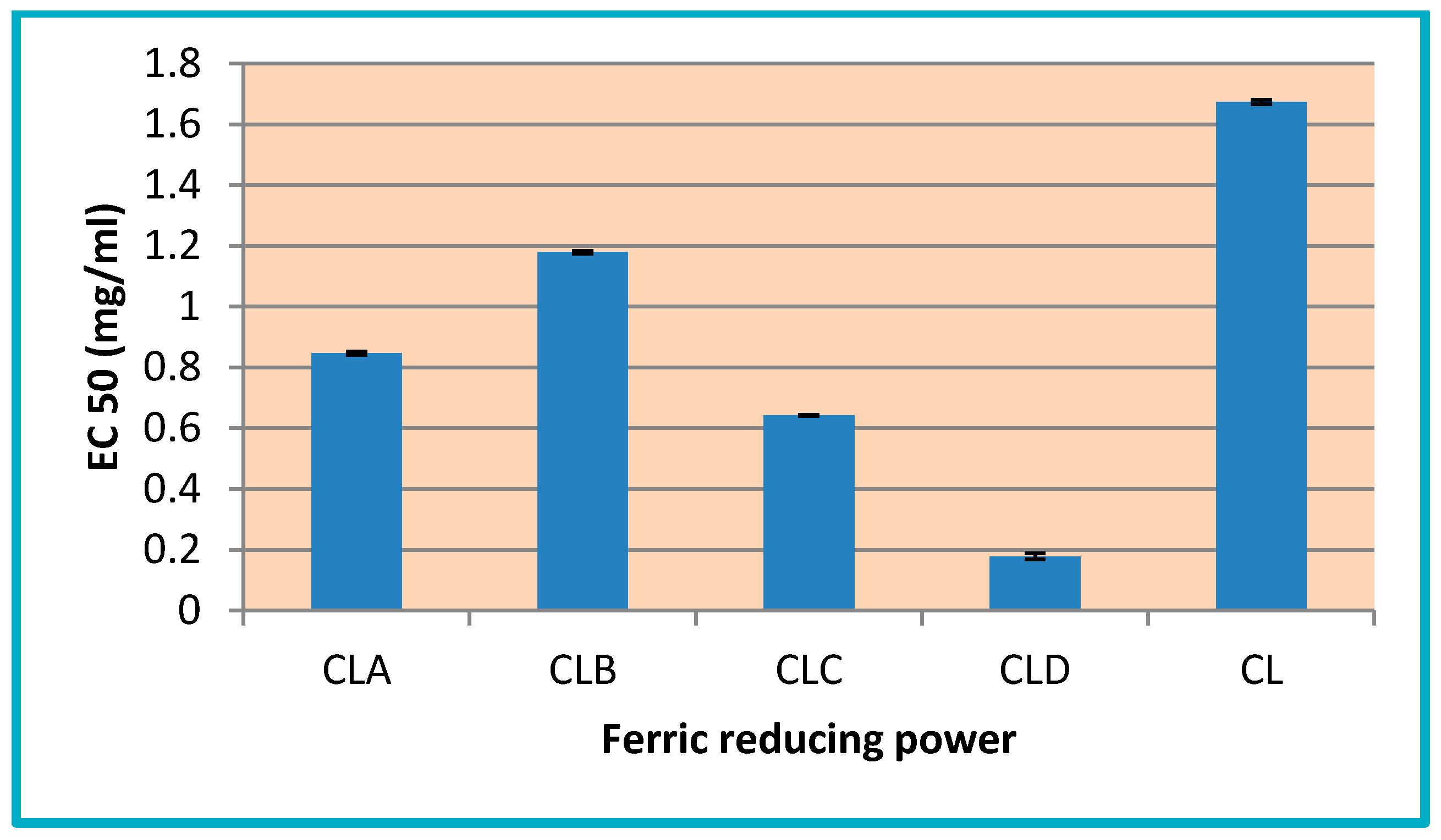
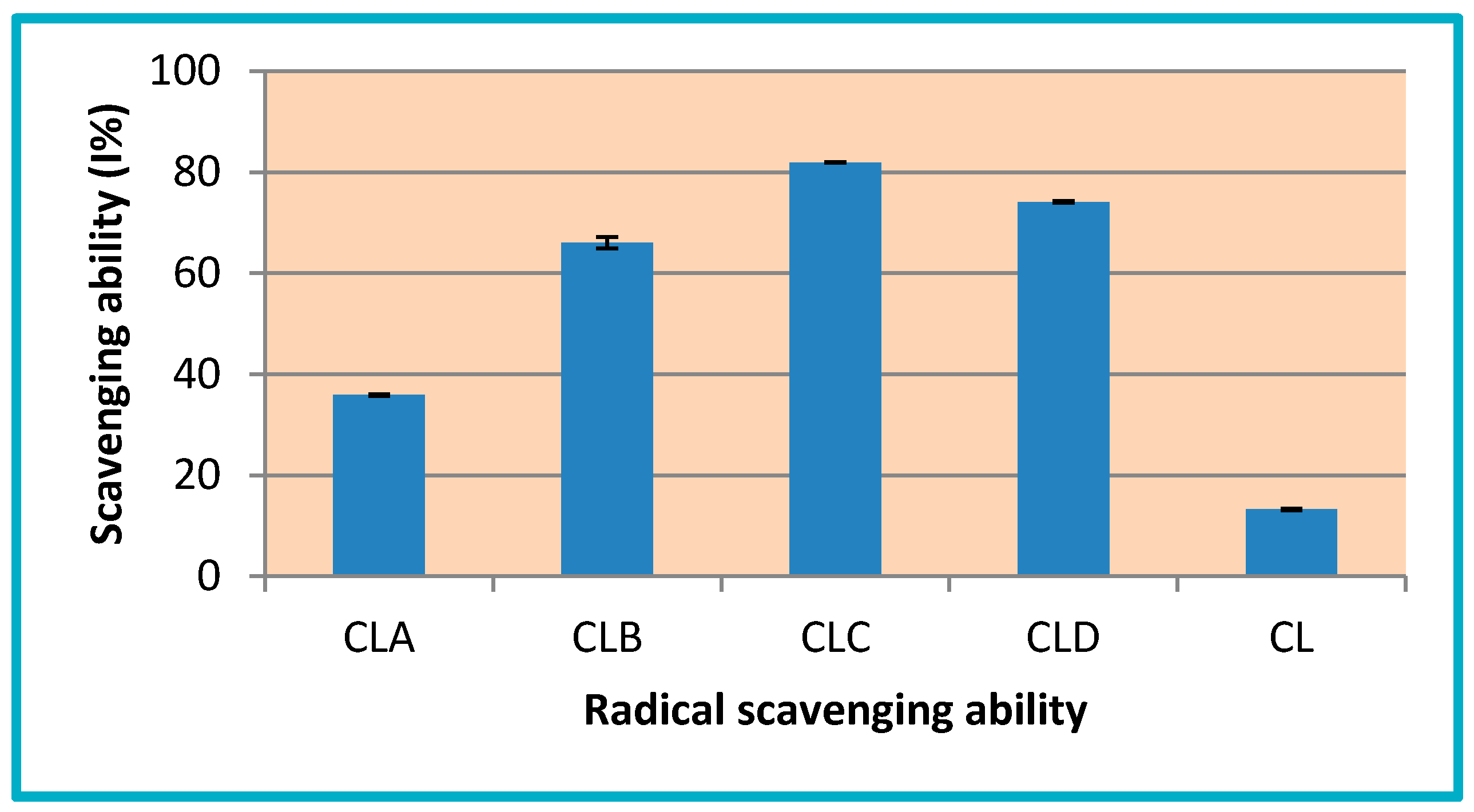
3.3.1. Determination of Total Antioxidant Activity (TAA)
3.3.2. Ferric Reducing Power
3.3.3. Radical Scavenging Ability
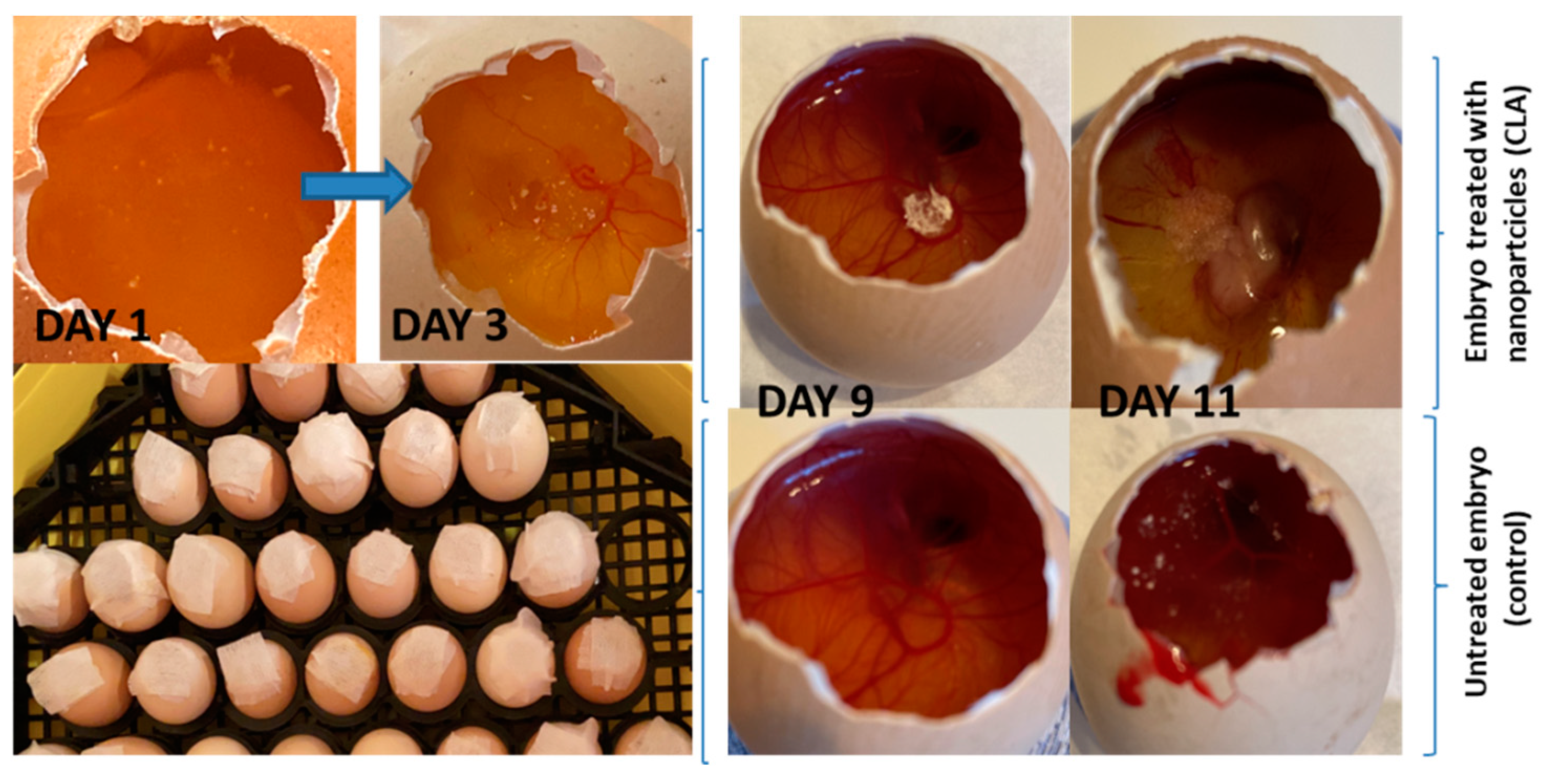
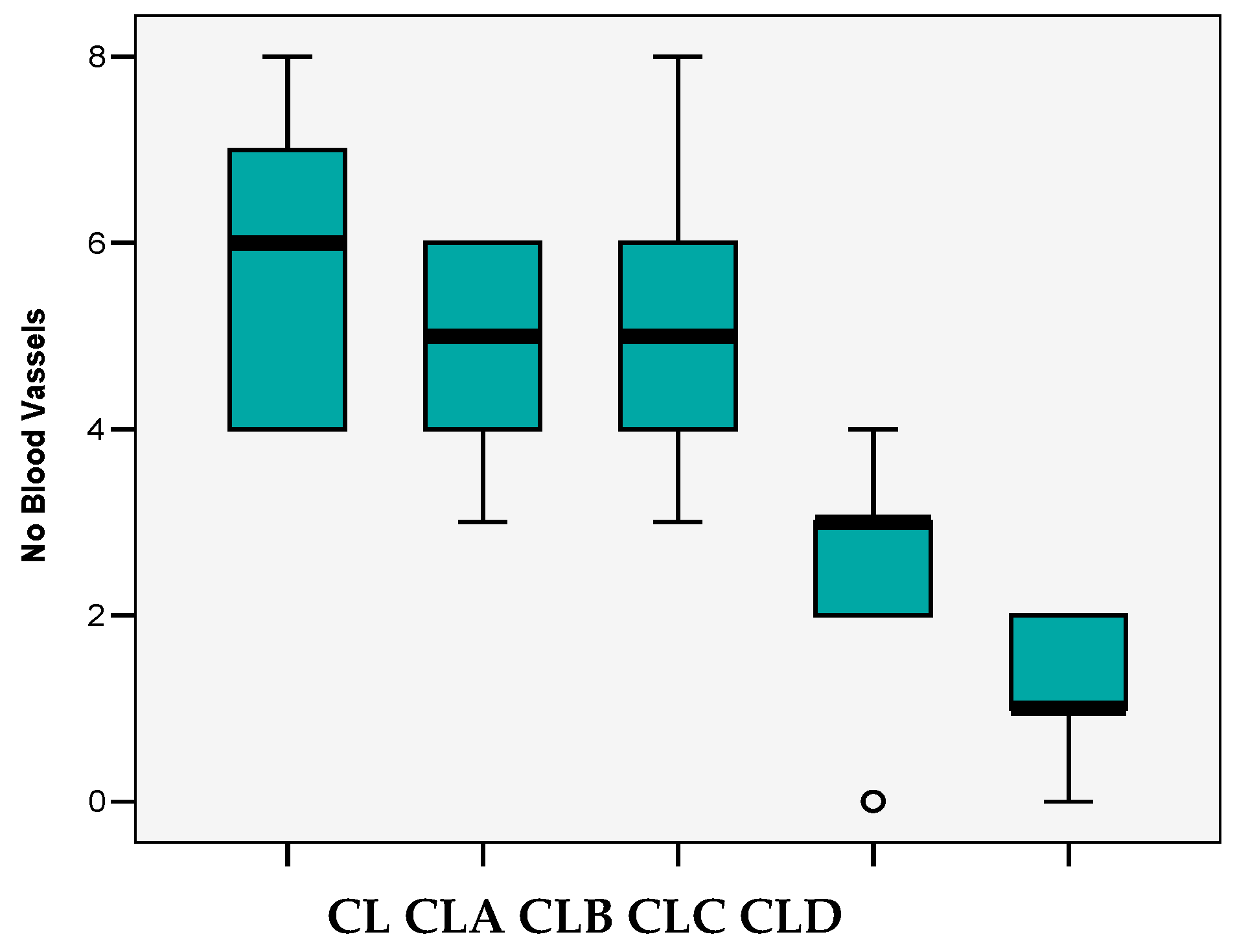
3.4. In Vivo Evaluation of Angiogenic Activity Using a CAM Model
- variations from 0 to 8 vessels/field;
- mean 3.88 ± 2.28 vessels/field;
- the median value (4 vessels/field) close to the average value and the Skewness test result <2 suggests that the series of values of the number of vessels was homogeneous, so statistical significance tests can be applied.
3.5. In Vitro Biocompatibility Evaluation Using MTT Cell Viability Assay
3.6. In Vitro MTT Assay on Human Epithelial Cervical Tumor Hep-2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Prauchner, C.A. Angiogenesis inhibition by antioxidants. J. Biomed. Sci. Eng. 2014, 2, 7–19. [Google Scholar] [CrossRef]
- Hercberg, S.; Galan, P.; Preziosi, P.; Bertrais, S.; Mennen, L.; Malvy, D.; Roussel, A.-M.; Alain Favier, A.; Briançon, S. The SU.VI.MAX Study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch. Intern. Med. 2004, 164, 2335–2342. [Google Scholar] [CrossRef]
- Lee, I.M.; Cook, N.R.; Gaziano, J.M.; Gordon, D.; Ridker, P.M.; Manson, J.A.; Hennekens, C.H.; Buring, J.E. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women’s Health Study: A randomized controlled trial. JAMA 2005, 294, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.M. High Surface Area Materials. Chapter 4. Nanostructure Science and Technology, 1st ed.; Kluwer Academic Publishers Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 49–66. [Google Scholar]
- Samal, S.K.; Dash, M.; Chiellini, F.; Wang, X.; Chiellini, E.; Declercq, H.A.; Kaplan, D.L. Silk/chitosan biohybrid hydrogels and scaffolds via green technology. RSC Advances 2014, 96, 53547–53556. [Google Scholar] [CrossRef]
- Dash, M.; Samal, S.K.; Declercq, H.A.; Douglas, T.E.L.; Schaubroeck, D.; Vander Voort, P.; Leeuwenburgh, S.C.; Dubruel, P. Enzymatically Biomineralized Chitosan Scaffolds for Tissue-Engineering Applications. J. Tissue Eng. Regen. M 2015, 11, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Samal, S.K.; Morelli, A.; Bartoli, C.; Declercq, H.A.; Douglas, T.E.L.; Dubruel, P.; Chiellini, F. Ulvan-Chitosan Polyelectrolyte Complexes as Matrices for Enzyme Induced Biomimetic Mineralization. Carbohydr. Polym. 2018, 182, 254–264. [Google Scholar] [CrossRef]
- Yadav, V.; Sharma, A.; Singh, S.K. Microencapsulation techniques applicable to food flavours research and development: A comprehensive review. Int. J. Food Nutr. Sci. 2015, 4, 119–124. [Google Scholar]
- Dragostin, I.; Dragostin, O.M.; Dragan, M.; Stan, C.D.; Zamfir, C.L. Drug hypersensitivity reduction using encapsulation method with chitosan-cetirizine derivatives. Rev. De Chimie 2018, 69, 3731–3735. [Google Scholar] [CrossRef]
- Jyothi, S.S.; Seethadevi, A.; Prabha, K.S.; Muthuprasanna, P.; Pavitra, P. Microencapsulation: A review. Int. J. Pharm. Bio. Sci. 2012, 3, 509–531. [Google Scholar]
- Xu, Y.; Wen, Z.; Xu, Z. Chitosan nanoparticles inhibit the growth of human hepatocellular carcinoma Xenografts through an Antiangiogenic mechanism. Anticancer Res. 2010, 30, 5103–5110. [Google Scholar]
- Kim, J.H.; Kim, Y.S.; Park, K.; Kang, E.; Lee, S.; Nam, H.Y.; Kim, K.; Park, J.H.; Chi, D.Y.; Park, R.W.; et al. Self-assembled glycol chitosan nanoparticles for the sustained and prolonged delivery of antiangiogenic small peptide drugs in cancer therapy. Biomaterials 2008, 29, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Katas, H.; Ghafoor Raja, M.A.; Lam, K.L. Development of chitosan nanoparticles as a stable drug delivery system for protein/siRNA. Int. J. Biomater. 2013, 9. [Google Scholar] [CrossRef]
- Dragostin, O.M.; Samal, S.K.; Lupascu, F.; Pânzariu, A.; Dubruel, P.; Lupascu, D.; Tuchilus, C.; Vasile, C.; Profire, L. Development and characterization of novel films based on sulfonamide-chitosan derivatives for potential wound dressing. Int. J. Molec. Sci. 2015, 16, 29843–29855. [Google Scholar] [CrossRef]
- Dragostin, O.M.; Samal, S.K.; Dash, M.; Lupascu, F.; Pânzariu, A.; Tuchilus, C.; Ghetu, N.; Danciu, M.; Dubruel, P.; Pieptu, D.; et al. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydr. Polym. 2016, 141, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Norrby, K. In vivo models of angiogenesis. J. Cell. Mol. Med. 2006, 10, 588–612. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Zeisser-Labouèbe, M.; Lange, N.; Gurny, R.; Delie, F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef]
- Stephens, F.O.; Aigner, K.R. Basics of Oncology; Springer: Berlin, Germany, 2009. [Google Scholar]
- Okolotowicz, K.J.; Dwyer, M.; Ryan, D.; Cheng, J.; Cashman, E.A.; Moore, S.; Mercola, M.; Cashman, J.R. Novel tertiary sulfonamides as potent anti-cancer agents. Bioorg. Med. Chem. 2018, 2, 4441–4451. [Google Scholar] [CrossRef]
- Lupascu, F.; Dash, M.; Samal, S.K.; Dubruel, P.; Lupusoru, C.E.; Lupusoru, R.V.; Dragostin, O.; Profire, L. Development, optimization and biological evaluation of chitosan scaffold formulations of new xanthine derivatives for treatment of type-2 diabetes mellitus. Eur. J. Pharm. Sci. 2015, 77, 122–134. [Google Scholar] [CrossRef]
- Samal, S.K.; Dash, M.; Chiellini, F.; Kaplan, D.L.; Chiellini, E. Silk Microgels formed by Proteolytic Enzyme Activity. Acta Biomater 2013, 9, 8192–8199. [Google Scholar] [CrossRef]
- Samal, S.K.; Dash, M.; Declercq, H.A.; Gheysens, T.; Dendooven, J.; Vander Voort, P.; Cornelissen, R.; Dubruel, P.; Kaplan, D.L. Enzymatic Mineralization of Silk Scaffolds. Macromol. Biosci. 2014, 14, 991–1003. [Google Scholar] [CrossRef]
- Samal, S.K.; Goranov, V.; Dash, M.; Russo, A.; Shelyakova, T.; Graziosi, P.; Lungaro, L.; Riminucci, A.; Uhalarz, M.; Banobre-Lopez, M.; et al. Multilayered Magnetic Gelatin Membrane Scaffolds. ACS App. Mater. Interfaces 2015, 7, 23098–23109. [Google Scholar] [CrossRef]
- Dragan, M.; Dragostin, O.; Iacob, A.; Profire, L.; Stan, C.D.; Tuchiluş, C. Antioxidant and antimicrobial potential of new azetidin-2-one of ferulic acid. Farmacia 2019, 67, 789–793. [Google Scholar] [CrossRef]
- Panzariu, A.; Vasincu, I.M.; Dragostin, O.M.; Dragan, M.; Buron, F.; Routier, S.; Profire, L. New arginine derivatives-synthesis and biological evaluation. Farmacia 2015, 63, 581–585. [Google Scholar]
- Kue, C.S.; Tan, K.Y.; LaM, M.L.; Lee, H.B. Chick embryo chorioallantoic membrane (CAM): An alternative predictive model in acute toxicological studies for anti-cancer drugs. Exp. Anim. 2015, 64, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.T.; Fong, L.Y.; Tan, J.J.; Rajab, N.F.; Abas, F.; Shaari, K.; Chan, K.M.; Juliana, F.; Yong, Y.K. Water extract of Clinacanthus nutans leaves exhibits in vitro, ex vivo and in vivo anti- angiogenic activities in endothelial cell via suppression of cell proliferation. BMC Complement Altern. Med. 2018, 18, 210. [Google Scholar] [CrossRef] [PubMed]
- Lokman, N.A.; Elder, A.S.; Ricciardelli, C.; Oehler, M.K. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, A.P.; Diaconu, A.; Nita, L.E.; Tudorachi, N.; Mititelu-Tartau, L.; Creteanu, A.; Dragostin, O.; Rusu, D.; Popa, G. The influence of excipients on physical and pharmaceutical properties of oral lyophilisates containing a pregabalin-acetaminophen combination. Expert Opin. Drug Del. 2017, 14, 589–599. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Silva, S.M.L.; Braga, C.R.; Fook, M.V.; Raposo, C.M.; Carvalho, L.H.; Canedo, E.L. Application of Infrared Spectroscopy to Analysis of Chitosan/Clay Nanocomposites, Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophile, P., Ed.; InTech: Rijeka, Croatia; ISBN 978-953-51-0537-4.
- Kumar, V. Neoplasia—Metastasis. In Robbins and Cotran’s Pathologic Basis of Disease. Philadelphia, 7th ed.; Kumar, V., Abbas, A.K., Fausto, N., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2005; pp. 279–281. [Google Scholar]
- Ruddon, R.W. Cancer Biology, 4th ed.; Oxford University Press: Oxford, UK, 2007; pp. 207–256. [Google Scholar]
- di Leo, N.; Battaglini, M.; Berger, L.; Giannaccini, M.; Dente, L.; Hampel, S.; Vittorio, O.; Cirillo, G.; Raffa, V. A catechin nanoformulation inhibits WM266 melanoma cell proliferation, migration and associated neo-angiogenesis. Eur. J. Pharm. Biopharm. 2017, 114, 1–10. [Google Scholar] [CrossRef]
- Ho, Y.; Wu, C.Y.; Chin, Y.T.; Li, Z.L.; Pan, Y.S.; Huang, T.Y.; Su, P.Y.; Lee, S.Y.; Crawford, D.R.; Su, K.W.; et al. NDAT suppresses pro-inflammatory gene expression to enhance resveratrol induced anti-proliferation in oral cancer cells. Food Chem. Toxicol. 2020, 136, 111092. [Google Scholar] [CrossRef]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.-S.; Jiang, F.-S.; Zhang, K.; Zhu, X.-X.; Jin, B.; Lu, J.-J.; Ding, Z.-S. Flavonoids from the leaves of Carya cathayensis Sarg. Inhibit vascular endothelial growth factor-induced angiogenesis. Fitoterapia 2014, 92, 34–40. [Google Scholar] [CrossRef]
- Mohan, C.D.; Hari, S.; Preetham, H.D.; Rangappa, S.; Barash, U.; Ilan, N.; Nayak, S.C.; Gupta, V.K.; Vlodavsky, I.; Rangappa, K.S. Targeting heparanase in cancer: Inhibition by synthetic, chemically modified, and natural compounds. iScience 2019, 15, 360–390. [Google Scholar] [CrossRef] [PubMed]
- Badyal, D.K.; Desai, C. Animal use in pharmacology education and research: The changing scenario. Indian J. Pharmacol. 2014, 46, 257–265. [Google Scholar] [CrossRef] [PubMed]
- IACUC. Policy on Protocol Approval for Use of Chicken Embryos and Eggs. An Association of New England Medical Center and Tufts. 2001. Available online: https://www.bu.edu/researchsupport/forms-policies/iacuc-use-of-live-embryonated-eggs-of-egg-laying-vertebrate-species (accessed on 5 March 2020).
- Sahu, D.; Sharma, S.; Singla, R.K.; Panda, A.K. Antioxidant activity and protective effect of suramin against oxidative stress in collagen induced arthritis. Eur. J. Pharm. Sci. 2017, 101, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Çalışkan, B.; Sinoplu, E.; İbiş, K.; Akhan Güzelcan, E.; Çetin Atalay, R.; Banoglu, E. Synthesis and cellular bioactivities of novel isoxazole derivatives incorporating an arylpiperazine moiety as anticancer agents. J. Enzyme Inhib. Med. Chem. 2018, 33, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Dai, F.; Chen, Z.; Wang, S.; Cheng, X.; Sheng, Q.; Lin, J.; Chen, W. Dimethoxy curcumin induces apoptosis by suppressing Survivin and inhibits invasion by enhancing E-Cadherin in colon cancer cells. Med. Sci. Monit. 2016, 22, 3215–3222. [Google Scholar] [CrossRef]
- Zessel, K.; Mohring, S.; Hamscher, G.; Kietzmann, M.; Stahl, J. Biocompatibility and antibacterial activity of photolytic products of sulfonamides. Chemosphere 2014, 100, 167–174. [Google Scholar] [CrossRef]
| Compound | CL (chitosan) | CLA | CLB | CLC | CLD |
|---|---|---|---|---|---|
| Dimension (nm) | 390.2 | 622.2 | 571 | 351 | 435 |
| All Groups | CL | CLA | CLB | CLC | CLD | ||
|---|---|---|---|---|---|---|---|
| N | 25 | 5 | 5 | 5 | 5 | 5 | |
| Mean | 3.88 | 5.80 | 4.80 | 5.20 | 2.40 | 1.20 | |
| p post-hoc Bonferroni | ns) | ns) ns) | b) ns) ns) | a) b) b) ns) | |||
| Median | 4.00 | 6.00 | 5.00 | 5.00 | 3.00 | 1.00 | |
| Std.Deviation | 2.28 | 1.79 | 1.30 | 1.92 | 1.52 | 0.84 | |
| Variance | 5.19 | 3.20 | 1.70 | 3.70 | 2.30 | 0.70 | |
| Skewness Test | 0.092 | 0.052 | −0.541 | 0.590 | −1.118 | −0.512 | |
| Std.Err.Skewness | 0.464 | 0.913 | 0.913 | 0.913 | 0.913 | 0.913 | |
| Cell Culture | Time | Untreated Control | Positive Control | CL | CLA | CLB | CLC | CLD |
|---|---|---|---|---|---|---|---|---|
| NCTC | 24 h | 100% | 11.45% | 95.72% | 105.79% | 100.00% | 98.07% | 104.83% |
| 48 h | 100% | 7.31% | 89.29% | 99.28% | 101.96% | 107.52% | 107.52% | |
| Hep-2 | 24 h | 100% | 13.86% | 113.69% | 85.00% | 111.96% | 99.35% | 98.15% |
| 48 h | 100% | 6.95% | 87.85% | 66.16% | 79.70% | 69.91% | 66.89% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragostin, O.-M.; Tatia, R.; Samal, S.K.; Oancea, A.; Zamfir, A.S.; Dragostin, I.; Lisă, E.-L.; Apetrei, C.; Zamfir, C.L. Designing of Chitosan Derivatives Nanoparticles with Antiangiogenic Effect for Cancer Therapy. Nanomaterials 2020, 10, 698. https://doi.org/10.3390/nano10040698
Dragostin O-M, Tatia R, Samal SK, Oancea A, Zamfir AS, Dragostin I, Lisă E-L, Apetrei C, Zamfir CL. Designing of Chitosan Derivatives Nanoparticles with Antiangiogenic Effect for Cancer Therapy. Nanomaterials. 2020; 10(4):698. https://doi.org/10.3390/nano10040698
Chicago/Turabian StyleDragostin, Oana-Maria, Rodica Tatia, Sangram Keshari Samal, Anca Oancea, Alexandra Simona Zamfir, Ionuț Dragostin, Elena-Lăcrămioara Lisă, Constantin Apetrei, and Carmen Lăcrămioara Zamfir. 2020. "Designing of Chitosan Derivatives Nanoparticles with Antiangiogenic Effect for Cancer Therapy" Nanomaterials 10, no. 4: 698. https://doi.org/10.3390/nano10040698
APA StyleDragostin, O.-M., Tatia, R., Samal, S. K., Oancea, A., Zamfir, A. S., Dragostin, I., Lisă, E.-L., Apetrei, C., & Zamfir, C. L. (2020). Designing of Chitosan Derivatives Nanoparticles with Antiangiogenic Effect for Cancer Therapy. Nanomaterials, 10(4), 698. https://doi.org/10.3390/nano10040698