Facile Production of a Fenton-Like Photocatalyst by Two-Step Calcination with a Broad pH Adaptability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
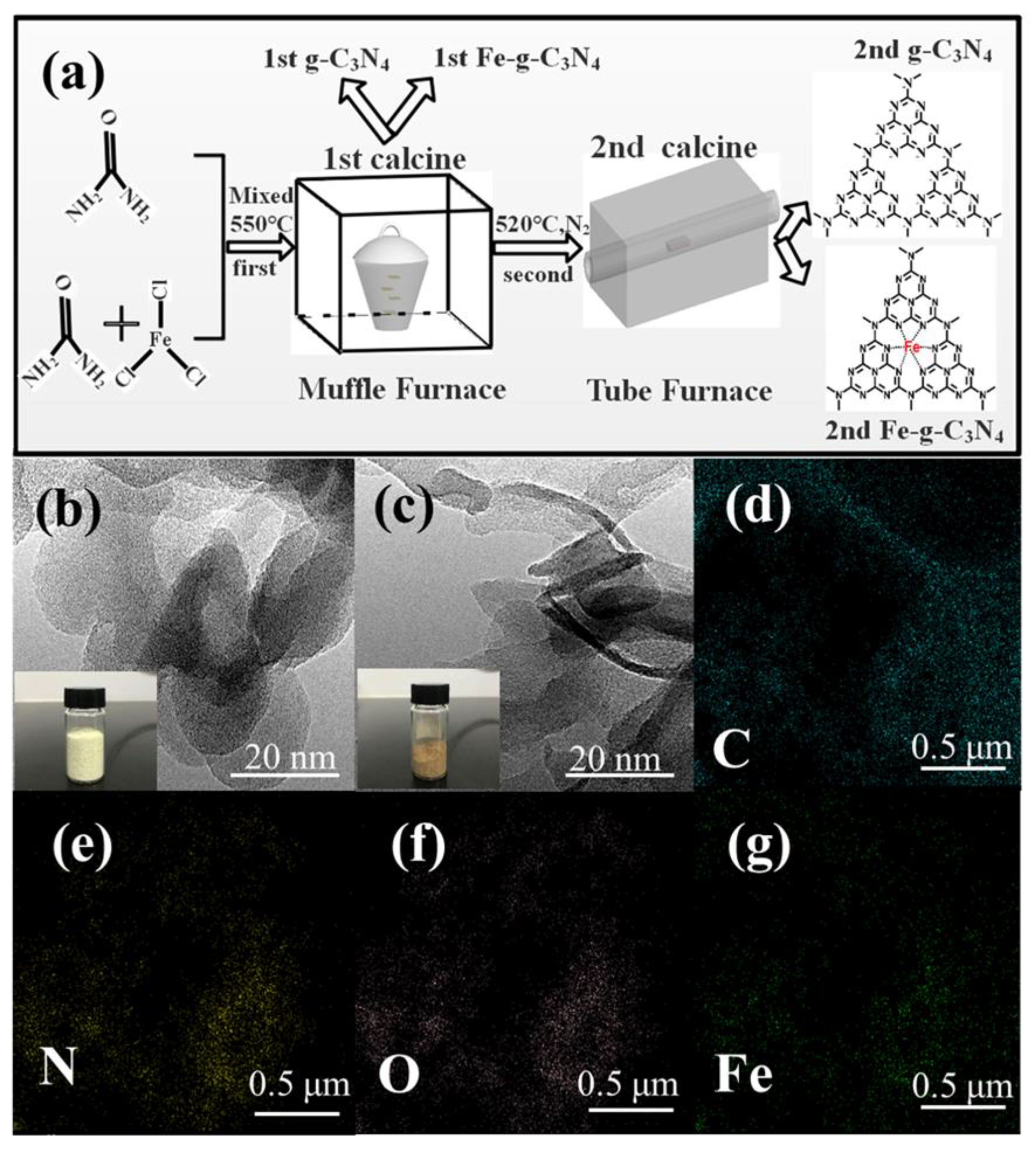
2.2. Preparation of the Catalysts
2.3. Characterization
2.4. Degradation Performance of the Catalysts
3. Results
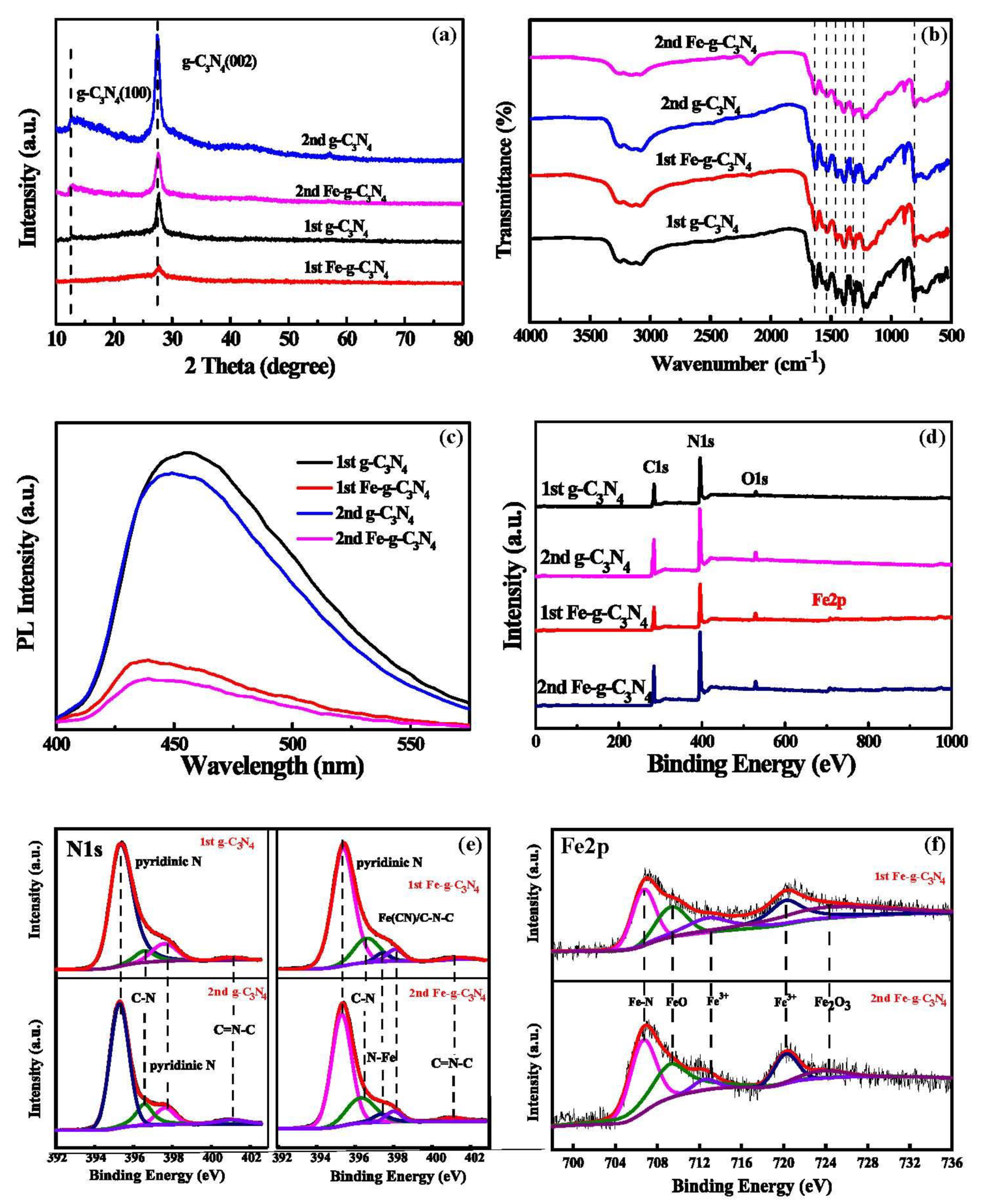
3.1. Characterization
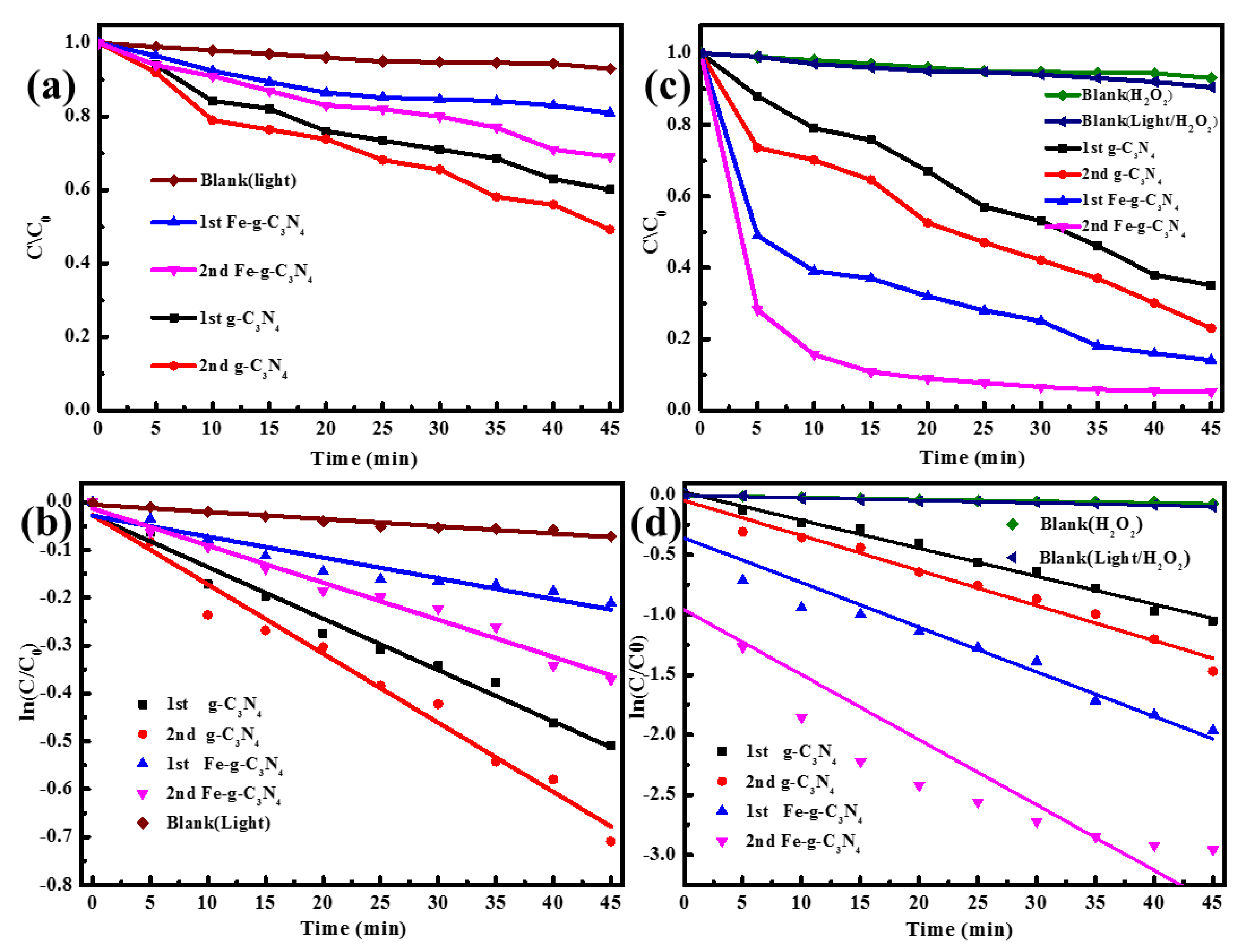
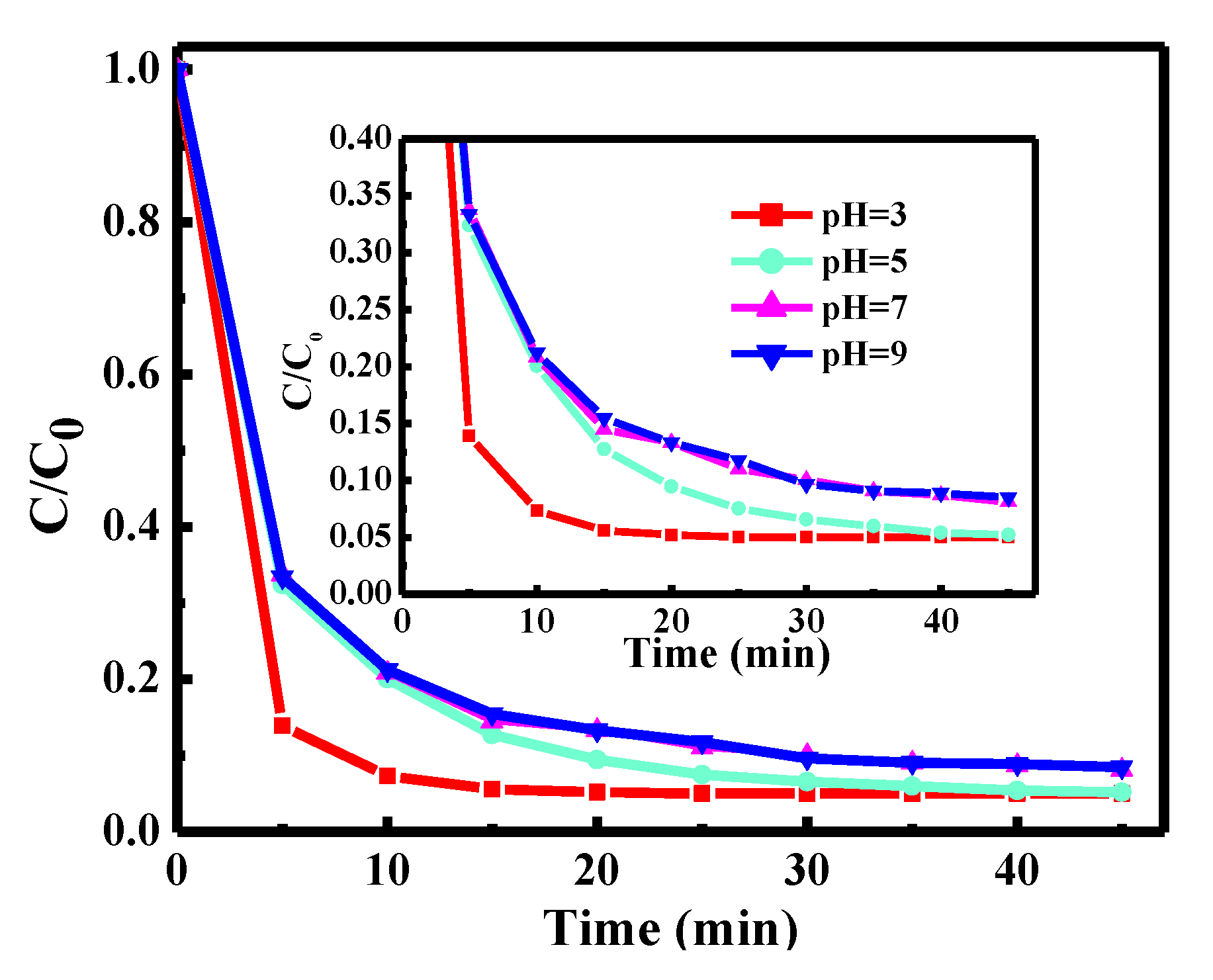
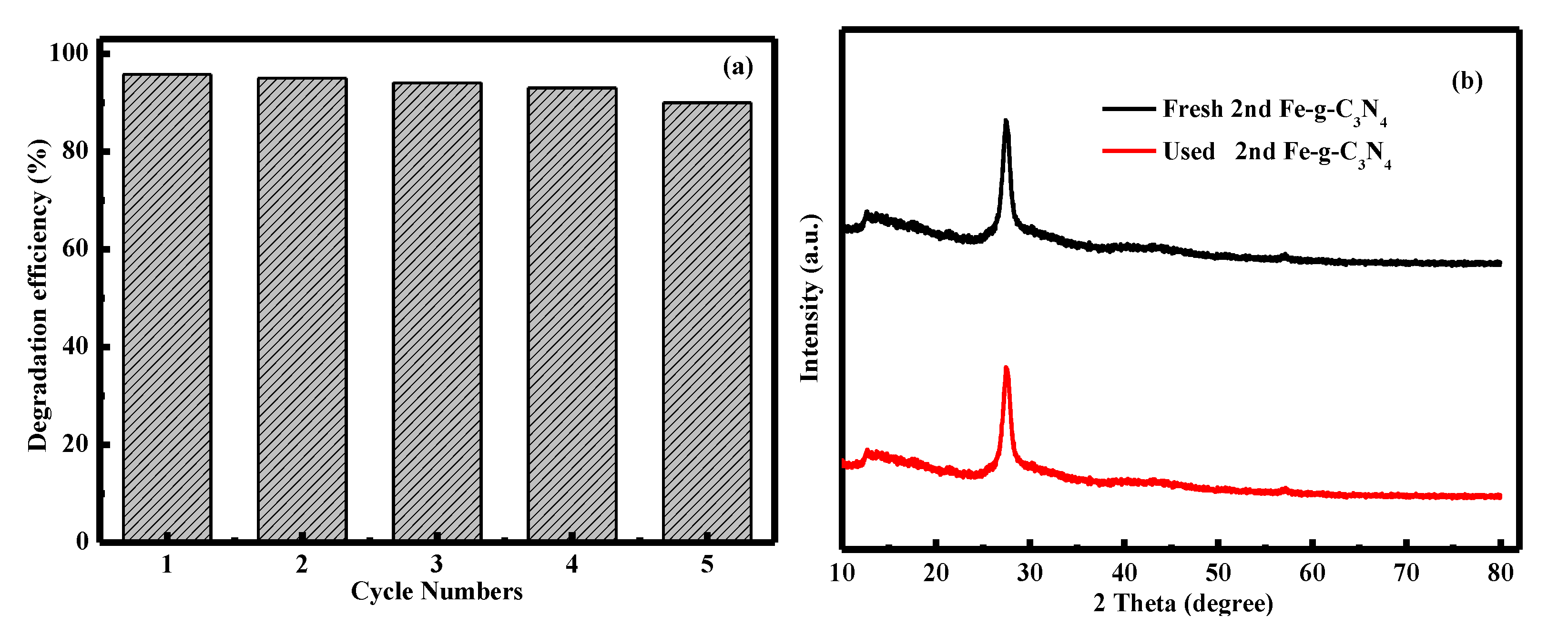
3.2. Degradation Performance of the Catalysts
3.3. Mechanism of Fenton-Like Photocatalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, L.; Wang, L.; Zhang, J.; Lei, J.; Liu, Y. Well-Dispersed Fe2O3Nanoparticles on g-C3N4 for Efficient and Stable Photo-Fenton Photocatalysis under Visible-Light Irradiation. Eur. J. Inorg. Chem. 2016, 2016, 5387–5392. [Google Scholar] [CrossRef]
- Klamerth, N.; Malato, S.; Agüera, A.; Fernández-Alba, A.; Mailhot, G. Treatment of Municipal Wastewater Treatment Plant Effluents with Modified Photo-Fenton As a Tertiary Treatment for the Degradation of Micro Pollutants and Disinfection. Environ. Sci. Technol. 2012, 46, 2885–2892. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, C.; Huang, Y.; Wang, C.; Chen, C. Degradation of azo dyes using low iron concentration of Fenton and Fenton-like system. Chemosphere 2005, 58, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Li, W.; Wang, G.; Chen, K.; Lu, L.; Hu, Q. Adsorption and heterogeneous degradation of rhodamine B on the surface of magnetic bentonite material. Appl. Surf. Sci. 2015, 349, 988–996. [Google Scholar] [CrossRef]
- Kuźniarska-Biernacka, I.; Raposo, M.M.M.; Baptista, R.M.F.; Parpot, P.; Biernacki, K.; De Magalhães, A.L.; Fonseca, A.; Neves, I. Highly efficient heterogeneous catalysts for phenol oxidation: Binuclear pyrrolyl-azine metal complexes encapsulated in NaY zeolite. Microporous Mesoporous Mater. 2016, 227, 272–280. [Google Scholar] [CrossRef]
- Georgi, A.; Schierz, A.; Trommler, U.; Horwitz, C.; Collins, T.; Kopinke, F.-D. Humic acid modified Fenton reagent for enhancement of the working pH range. Appl. Catal. B Environ. 2007, 72, 26–36. [Google Scholar] [CrossRef]
- Gupta, S.S.; Stadler, M.; Noser, C.A.; Ghosh, A.; Steinhoff, B.; Lenoir, D.; Horwitz, C.; Schramm, K.-W.; Collins, T.J. Rapid Total Destruction of Chlorophenols by Activated Hydrogen Peroxide. Science 2002, 296, 326–328. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Gu, Y.; Wu, F.; Lu, W.; Xu, T.; Chen, W. Catalytic degradation of recalcitrant pollutants by Fenton-like process using polyacrylonitrile-supported iron (II) phthalocyanine nanofibers: Intermediates and pathway. Water Res. 2016, 93, 296–305. [Google Scholar] [CrossRef]
- Ensing, B.; Buda, F.; Baerends, E.J. Fenton-like chemistry in water: Oxidation catalysis by Fe(III) and H2O2. J. Phys. Chem. A 2003, 107, 5722–5731. [Google Scholar] [CrossRef]
- Li, H.; Deng, P.; Hou, Y. Cobalt disulfide/graphitic carbon nitride as an efficient photocatalyst for hydrogen evolution reaction under visible light irradiation. Mater. Lett. 2018, 229, 217–220. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, J.; Liang, J.; Jaroniec, M.; Qiao, S.-Z. Graphitic carbon nitride materials: Controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ. Sci. 2012, 5, 6717–6731. [Google Scholar] [CrossRef]
- Wang, M.; Yang, W.-H.; Wang, H.-H.; Chen, C.; Zhou, Z.-Y.; Sun, S.-G. Pyrolyzed Fe–N–C Composite as an Efficient Non-precious Metal Catalyst for Oxygen Reduction Reaction in Acidic Medium. ACS Catal. 2014, 4, 3928–3936. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Haghighi, M.; Rahmani, F.; Dehghani, R.; Tehrani, A.M.; Miranzadeh, M.B. Photocatalytic reduction of Cr (VI) in aqueous solution over ZnO/ HZSM-5 nanocomposite: Optimization of ZnO loading and process conditions. Desalin. Water Treat. 2017, 58, 168–180. [Google Scholar] [CrossRef]
- Guo, T.; Wang, K.; Zhang, G.; Wu, X. A novel α-Fe2O3@g-C3N4 catalyst: Synthesis derived from Fe-based MOF and its superior photo-Fenton performance. Appl. Surf. Sci. 2019, 469, 331–339. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, Y.; Wang, L.; Ai, S.; Ding, H. Cu-modified alkalinized g-C3N4 as photocatalytically assisted heterogeneous Fenton-like catalyst. Appl. Surf. Sci. 2017, 426, 1133–1140. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, G.; Yu, J.C. Enhanced photo-Fenton degradation of rhodamine B using graphene oxide–amorphous FePO4 as effective and stable heterogeneous catalyst. J. Colloid Interface Sci. 2015, 448, 460–466. [Google Scholar] [CrossRef]
- Doumic, L.I.; Soares, P.; Ayude, M.A.; Cassanello, M.; Boaventura, R.; Vilar, V.J. Enhancement of a solar photo-Fenton reaction by using ferrioxalate complexes for the treatment of a synthetic cotton-textile dyeing wastewater. Chem. Eng. J. 2015, 277, 86–96. [Google Scholar] [CrossRef]
- Ortega-Liebana, M.C.; Sánchez-López, E.M.; Hidalgo-Carrillo, J.; Marinas, A.; Marinas, J.; Urbano, F.J. A comparative study of photocatalytic degradation of 3-chloropyridine under UV and solar light by homogeneous (photo-Fenton) and heterogeneous (TiO2) photocatalysis. Appl. Catal. B Environ. 2012, 127, 316–322. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Wang, Z.; Muthu, M.; Zhang, Y. Construction of an in-situ Fenton-like system based on a g-C3N4 composite photocatalyst. J. Hazard. Mater. 2019, 373, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, J. Graphene Supported Co-g-C3N4 as a Novel Metal–Macrocyclic Electrocatalyst for the Oxygen Reduction Reaction in Fuel Cells. Langmuir 2013, 29, 3821–3828. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Yin, M.; Xie, D.; Chen, J.; Yin, J.; Fu, Y.; Zhao, P.; Zhong, H.; Zhao, Y.; et al. Ultrathin g-C3N4 films supported on Attapulgite nanofibers with enhanced photocatalytic performance. Appl. Surf. Sci. 2018, 440, 170–176. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Peng, S.; Lu, G.; Li, S. Eosin Y-sensitized graphitic carbon nitride fabricated by heating urea for visible light photocatalytic hydrogen evolution: The effect of the pyrolysis temperature of urea. Phys. Chem. Chem. Phys. 2013, 15, 7657. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Shen, L.; Ma, Y.; Lei, W.; Cui, Q.; Zou, G. Preparation and characterization of graphitic carbon nitride through pyrolysis of melamine. Appl. Phys. A 2008, 94, 387–392. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B Environ. 2015, 176, 44–52. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, P.; An, W.; Liu, L.; Liang, Y.; Cui, W. In-situ Fe-doped g-C3N4 heterogeneous catalyst via photocatalysis-Fenton reaction with enriched photocatalytic performance for removal of complex wastewater. Appl. Catal. B Environ. 2019, 245, 130–142. [Google Scholar] [CrossRef]
- Leong, K.H.; Lim, P.F.; Sim, L.C.; Punia, V.; Pichiah, S. Improved solar light stimulated charge separation of g-C3N4 through self-altering acidic treatment. Appl. Surf. Sci. 2018, 430, 355–361. [Google Scholar] [CrossRef]
- Yatsimirskii, K.; Nemoshkalenko, V.; Nazarenko, Y.; Aleshin, V.; Zhilinskaya, V.; Tomashevsky, N. Use of X-ray photoelectron and Mössbauer spectroscopies in the study of iron pentacyanide complexes. J. Electron Spectrosc. Relat. Phenom. 1977, 10, 239–245. [Google Scholar] [CrossRef]
- Ma, J.Q.; Yang, Q.F.; Wen, Y.Z.; Liu, W.P. Fe-g-C3N4/graphitized mesoporous carbon composite as an effective Fenton-like catalyst in a wide pH range. Appl. Catal. B Environ. 2017, 201, 232–240. [Google Scholar] [CrossRef]
- Maya, L.; Richards, H.L. Polymeric Cyanoborane, (CNBH2)n: Single Source for Chemical Vapor Deposition of Boron Nitride Films. J. Am. Ceram. Soc. 1991, 74, 406–409. [Google Scholar] [CrossRef]
- Li, Z.; Kong, C.; Lu, G. Visible Photocatalytic Water Splitting and Photocatalytic Two-Electron Oxygen Formation over Cu- and Fe-Doped g-C3N4. J. Phys. Chem. C 2015, 120, 56–63. [Google Scholar] [CrossRef]
- Daneshvar, N.; Behnajady, M.A.; Mohammadi, M.K.A.; Dorraji, M.S.S. UV/H2O2 treatment of Rhodamine B in aqueous solution: Influence of operational parameters and kinetic modeling. Desalination 2008, 230, 16–26. [Google Scholar] [CrossRef]
- Chen, J.Y.; Xiao, X.Y.; Wang, Y.; Ye, Z.H. Fabrication of hierarchical sheet-on-sheet WO3/g-C3N4 composites with enhanced photocatalytic activity. J. Alloys Compd. 2019, 777, 325–334. [Google Scholar] [CrossRef]
- Liu, W.; Ma, J.; Shen, C.; Wen, Y.; Liu, W. A pH-responsive and magnetically separable dynamic system for efficient removal of highly dilute antibiotics in water. Water Res. 2016, 90, 24–33. [Google Scholar] [CrossRef]
- Alam, U.; Khan, A.; Raza, W.; Khan, A.; Bahnemann, D.W.; Muneer, M. Highly efficient Y and V co-doped ZnO photocatalyst with enhanced dye sensitized visible light photocatalytic activity. Catal. Today 2017, 284, 169–178. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Y.R.; Chen, Z.H.; Zhang, Z.Z.; Fang, X.M. Constructing a novel ternary Fe(III)/graphene/g-C3N4 composite photocatalyst with enhanced visible-light driven photocatalytic activity via interfacial charge transfer effect. Appl. Catal. B Environ. 2016, 183, 231–241. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, S.; Yang, Y.; Li, X.; Liu, H.; Zhou, Z. Facile Production of a Fenton-Like Photocatalyst by Two-Step Calcination with a Broad pH Adaptability. Nanomaterials 2020, 10, 676. https://doi.org/10.3390/nano10040676
Ji S, Yang Y, Li X, Liu H, Zhou Z. Facile Production of a Fenton-Like Photocatalyst by Two-Step Calcination with a Broad pH Adaptability. Nanomaterials. 2020; 10(4):676. https://doi.org/10.3390/nano10040676
Chicago/Turabian StyleJi, Siyang, Yanling Yang, Xing Li, Hang Liu, and Zhiwei Zhou. 2020. "Facile Production of a Fenton-Like Photocatalyst by Two-Step Calcination with a Broad pH Adaptability" Nanomaterials 10, no. 4: 676. https://doi.org/10.3390/nano10040676
APA StyleJi, S., Yang, Y., Li, X., Liu, H., & Zhou, Z. (2020). Facile Production of a Fenton-Like Photocatalyst by Two-Step Calcination with a Broad pH Adaptability. Nanomaterials, 10(4), 676. https://doi.org/10.3390/nano10040676




