Pillararenes Trimer for Self-Assembly
Abstract
1. Introduction
2. Fabrication Strategy for Pillararenes Trimer
2.1. Synthesis by Organic Reactions
2.2. Preparation by Noncovalent Method
2.2.1. Supramolecular Interactions
2.2.2. Mechanically Interlocked Molecules
3. Pillararenes Trimer as Building Block for Fabricating External-Stimuli Responsive Self-Assembled Materials
3.1. Interactions and Driving Forces
3.2. Multi-Dimensional Self-Assembly and Its External-Stimuli Responsiveness
4. Applications
5. Overview and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 0 D | Zero dimensional |
| 1 D | One dimensional |
| 2 D | Two dimensional |
| 3 D | Three dimensional |
| CAC | Critical assembly concentrations |
| CGC | Critical gelation concentration |
| CPC | Critical polymerization concentration |
| CV | Cyclic voltammogram |
| Cys | Cysteine |
| DLS | Dynamic light scattering |
| DOSY | Diffusion-ordered NMR spectroscopy |
| GPC | Gel permeation chromatography |
| His | Histidine |
| ICP | Inductively coupled plasma |
| Ka | Association constant |
| LOD | The detection limit |
| PXRD | X-ray powder diffusion |
| SEM | Scanning electronic microscopy |
| Ser | Serine |
| SOFs | Supramolecular organic frameworks |
| TEM | Transmission electron microscopy |
| Tgel | Gel-sol transition temperature |
References
- Yu, G.; Jie, K.; Huang, F. Supramolecular Amphiphiles Based on Host-Guest Molecular Recognition Motifs. Chem. Rev. 2015, 115, 7240–7303. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, C.; Stang, P.J. Soft Materials with Diverse Suprastructures via the Self-Assembly of Metal-Organic Complexes. Acc. Chem. Res. 2019, 52, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Nierengarten, I.; Deschenaux, R.; Nierengarten, J.F. From Pillar[n]arene Scaffolds for the Preparation of Nanomaterials to Pillar[5]arene-containing Rotaxanes. Chimia 2016, 70, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lee, J.; Lammer, A.D.; Chi, X.; Brewster, J.T.; Lynch, V.M.; Li, H.; Zhang, Z.; Sessler, J.L. Self-Assembled Pyridine-Dipyrrolate Cages. J. Am. Chem. Soc. 2016, 138, 4573–4579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lee, J.; Brewster, J.T.; Chi, X.; Lynch, V.M.; Sessler, J.L. Cation-based Structural Tuning of Pyridine Dipyrrolate Cages and Morphological Control over Their Self-assembly. J. Am. Chem. Soc. 2019, 141, 4749–4755. [Google Scholar] [CrossRef]
- Murray, J.; Kim, K.; Ogoshi, T.; Yao, W.; Gibb, B.C. The aqueous supramolecular chemistry of cucurbit[n]urils, pillar[n]arenes and deep-cavity cavitands. Chem. Soc. Rev. 2017, 46, 2479–2496. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Xu, Q.-Y.; Liu, Y. Molecular recognition and biological application of modified β-cyclodextrins. Sci. Chin. Chem. 2019, 62, 549–560. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, R.; Zhao, Y. Macrocycle-based metal-organic frameworks. Coordin. Chem. Rev. 2015, 292, 74–90. [Google Scholar] [CrossRef]
- Wang, Y.; Ping, G.; Li, C. Efficient complexation between pillar[5]arenes and neutral guests: From host-guest chemistry to functional materials. Chem. Commun. 2016, 52, 9858–9872. [Google Scholar] [CrossRef]
- Guo, F.; Sun, Y.; Xi, B.; Diao, G. Recent progress in the research on the host-guest chemistry of pillar[n]arenes. Supramol. Chem. 2018, 30, 81–92. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamagishi, T.-a.; Nakamoto, Y. Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem. Rev. 2016, 116, 7937–8002. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Meier, H. Synthesis of Pillar[6]arenes and Their Host–Guest Complexes. Synthesis 2015, 47, 1041–1056. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, L. Recent advances of functional gels controlled by pillar[n]arene-based host–guest interactions. Tetrahedron Lett. 2018, 59, 1172–1182. [Google Scholar] [CrossRef]
- Ogoshi, T.; Kakuta, T.; Yamagishi, T.-a. Applications of Pillar[n]arene-Based Supramolecular Assemblies. Angew. Chem. Int. Ed. 2019, 58, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nguyen, K.T.; Ma, X.; Yan, H.; Guo, J.; Zhu, L.; Zhao, Y. Host-guest complexation driven dynamic supramolecular self-assembly. Org. Biomol. Chem. 2013, 11, 2070–2074. [Google Scholar] [CrossRef]
- Kakuta, T.; Yamagishi, T.A.; Ogoshi, T. Stimuli-Responsive Supramolecular Assemblies Constructed from Pillar[n]arenes. Acc. Chem. Res. 2018, 51, 1656–1666. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Zhao, Y. Pillararene-based self-assembled amphiphiles. Chem. Soc. Rev. 2018, 47, 5491–5528. [Google Scholar] [CrossRef]
- Xiao, T.; Zhong, W.; Xu, L.; Sun, X.Q.; Hu, X.Y.; Wang, L. Supramolecular vesicles based on pillar[n]arenes: Design, construction, and applications. Org. Biomol. Chem. 2019, 17, 1336–1350. [Google Scholar] [CrossRef]
- Liu, L.-Z.; Qin, X.; Duan, W.-G.; Huang, H.-F.; Zhang, W.-X.; Zhou, Q.-Q.; Huang, Y. Aggregation-induced near-infrared absorption of a pillar[5]arene trimer by charge transfer interaction. Dyes Pigm. 2018, 158, 390–395. [Google Scholar] [CrossRef]
- Li, H.; Fan, X.; Shang, X.; Qi, M.; Zhang, H.; Tian, W. A triple-monomer methodology to construct controllable supramolecular hyperbranched alternating polymers. Polym. Chem. 2016, 7, 4322–4325. [Google Scholar] [CrossRef]
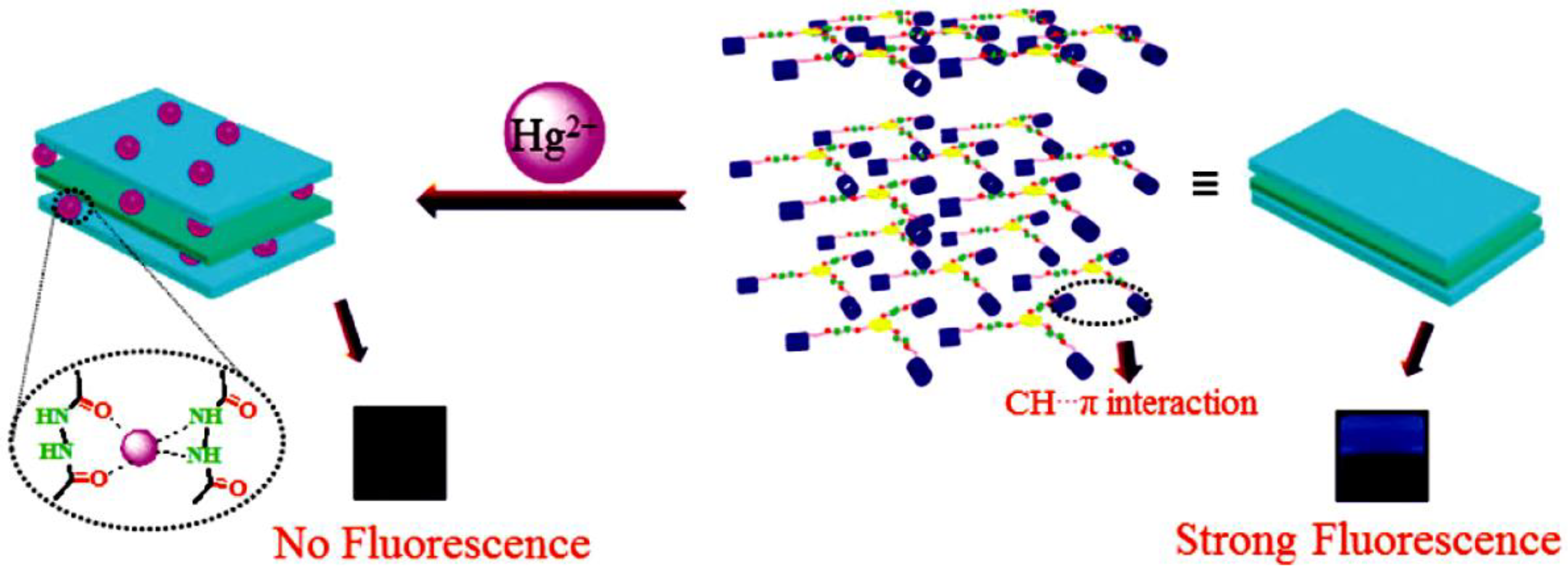
- Jiang, X.-M.; Huang, X.-J.; Song, S.-S.; Ma, X.-Q.; Zhang, Y.-M.; Yao, H.; Wei, T.-B.; Lin, Q. Tri-pillar[5]arene-based multi-stimuli-responsive supramolecular polymers for fluorescence detection and separation of Hg2+. Polym. Chem. 2018, 9, 4625–4630. [Google Scholar] [CrossRef]
- Jiang, Y.-Q.; Wu, K.; Zhang, Q.; Li, K.-Q.; Li, Y.-Y.; Xin, P.-Y.; Zhang, W.-W.; Guo, H.-M. A dual-responsive hyperbranched supramolecular polymer constructed by cooperative host–guest recognition and hydrogen-bond interactions. Chem. Commun. 2018, 54, 13821–13824. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.-B.; Ma, X.-Q.; Fan, Y.-Q.; Jiang, X.-M.; Dong, H.-Q.; Yang, Q.-Y.; Zhang, Y.-F.; Yao, H.; Lin, Q.; Zhang, Y.-M. Aggregation-induced emission supramolecular organic framework (AIE SOF) gels constructed from tri-pillar[5]arene-based foldamer for ultrasensitive detection and separation of multi-analytes. Soft Matter 2019, 15, 6753–6758. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.-Q.; Song, S.-S.; Gong, G.-F.; Wang, J.; Guan, X.-W.; Yao, H.; Zhang, Y.-M.; Wei, T.-B.; Lin, Q. Aggregation-Induced Emission Supramolecular Organic Framework (AIE SOF) Gels Constructed from Supramolecular Polymer Networks Based on Tripodal Pillar[5]arene for Fluorescence Detection and Efficient Removal of Various Analytes. ACS Sustain. Chem. Eng. 2019, 7, 11999–12007. [Google Scholar] [CrossRef]
- Khadieva, A.; Gorbachuk, V.; Shurpik, D.; Stoikov, I. Synthesis of Tris-pillar[5]arene and Its Association with Phenothiazine Dye: Colorimetric Recognition of Anions. Molecules 2019, 24, 1807. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.J.; Wang, X.Q.; Sun, B.; Li, X.; Zhang, Y.; Shi, J.; Yu, Y.; Zhang, L.; Liu, M.; et al. Organometallic rotaxane dendrimers with fourth-generation mechanically interlocked branches. Proc. Natl. Acad. Sci. USA 2015, 112, 5597–5601. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Wang, W.; Li, W.J.; Chen, L.J.; Yao, R.; Yin, G.Q.; Wang, Y.X.; Zhang, Y.; Huang, J.; Tan, H.; et al. Dual stimuli-responsive rotaxane-branched dendrimers with reversible dimension modulation. Nat. Commun. 2018, 9, 3190. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.-W.; Lin, Q.; Zhang, Y.-M.; Wei, T.-B.; Wang, J.; Fan, Y.-Q.; Yao, H. Pillar[5]arene-based spongy supramolecular polymer gel and its properties in multi-responsiveness, dye sorption, ultrasensitive detection and separation of Fe3+. Soft Matter 2019, 15, 3241–3247. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, T.; Yamagishi, T.; Ogoshi, T. Supramolecular chemistry of pillar[n]arenes functionalised by a copper(I)-catalysed alkyne-azide cycloaddition “click” reaction. Chem. Commun. 2017, 53, 5250–5266. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. High-generation organometallic rotaxane dendrimer. Sci. Chin. 2015, 58, 1089. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.-X.; Zhou, Q.-F.; Chen, L.-J.; Xu, L.; Wang, C.-H.; Li, X.; Yang, H.-B. Facile construction of organometallic rotaxane-terminated dendrimers using neutral platinum-acetylides as the main scaffold. Chem. Commun. 2018, 54, 2224–2227. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Chen, D.X.; Qiu, Y.C.; Yang, X.Y.; Xu, B.; Tian, W.; Yang, Y.W. Stimuli-responsive blue fluorescent supramolecular polymers based on a pillar[5]arene tetramer. Chem. Commun. 2014, 50, 8231–8234. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, S.; Feng, X.; Shi, J.; Hu, X.Y.; Wang, L. Controllable aggregation-induced emission based on a tetraphenylethylene-functionalized pillar[5]arene via host-guest recognition. Chem. Commun. 2014, 50, 9122–9125. [Google Scholar] [CrossRef]
- Jin, X.Y.; Song, N.; Wang, X.; Wang, C.Y.; Wang, Y.; Yang, Y.W. Monosulfonicpillar[5]arene: Synthesis, Characterization, and Complexation with Tetraphenylethene for Aggregation-Induced Emission. Sci. Rep. 2018, 8, 4035. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, B.; Wang, H.; Shangguan, L.; Li, Z.; Zhang, M.; Huang, F. Construction of Metallacage-Cored Supramolecular Gel by Hierarchical Self-Assembly of Metal Coordination and Pillar[5]arene-Based Host-Guest Recognition. Macromol. Rapid Commun. 2018, 39, e1800655. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Lou, X.Y.; Hou, W.; Wang, C.Y.; Wang, Y.; Yang, Y.W. Pillararene-Based Fluorescent Supramolecular Systems: The Key Role of Chain Length in Gelation. Macromol. Rapid Commun. 2018, 39, e1800593. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Ning, G.; Hassan, M.; Gong, W. Construction of Pillar[5]arene Tetramer-Based Cross-Linked Supramolecular Polymers through Hierarchical Charge-Transfer and Host-Guest Interactions. Asian J. Org. Chem. 2019, 8, 74–78. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhang, Y.; Zhang, C.W.; Chen, L.J.; Wang, C.; Tan, H.; Yu, Y.; Li, X.; Yang, H.B. Cross-linked supramolecular polymer gels constructed from discrete multi-pillar[5]arene metallacycles and their multiple stimuli-responsive behavior. J. Am. Chem. Soc. 2014, 136, 8577–8589. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Ou, B.; Jiang, S.-T.; Yin, G.-Q.; Chen, L.-J.; Xu, L.; Li, X.; Yang, H.-B. Cross-linked AIE supramolecular polymer gels with multiple stimuli-responsive behaviours constructed by hierarchical self-assembly. Polym. Chem. 2018, 9, 2021–2030. [Google Scholar] [CrossRef]
- Li, C.; Han, K.; Li, J.; Zhang, Y.; Chen, W.; Yu, Y.; Jia, X. Supramolecular Polymers Based on Efficient Pillar[5]arene-Neutral Guest Motifs. Eur. J. Chem. 2013, 19, 11892–11897. [Google Scholar] [CrossRef]
- Li, Q.; Han, K.; Li, J.; Jia, X.; Li, C. Synthesis of dendrimer-functionalized pillar[5]arenes and their self-assembly to dimeric and trimeric complexes. Tetrahedron Lett. 2015, 56, 3826–3829. [Google Scholar] [CrossRef]
- Wu, G.Y.; Wang, X.Q.; Chen, L.J.; Hu, Y.X.; Yin, G.Q.; Xu, L.; Jiang, B.; Yang, H.B. Supramolecular Polymer Cross-Linked by Discrete Tris-[2]pseudorotaxane Metallacycles and Its Redox-Responsive Behavior. Inorg. Chem. 2018, 57, 15414–15420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-W.; Jiang, S.-T.; Yin, G.-Q.; Li, X.; Zhao, X.-L.; Yang, H.-B. Dual Stimuli-Responsive Cross-Linked AIE Supramolecular Polymer Constructed through Hierarchical Self-Assembly. Isr. J. Chem. 2018, 58, 1265–1272. [Google Scholar] [CrossRef]
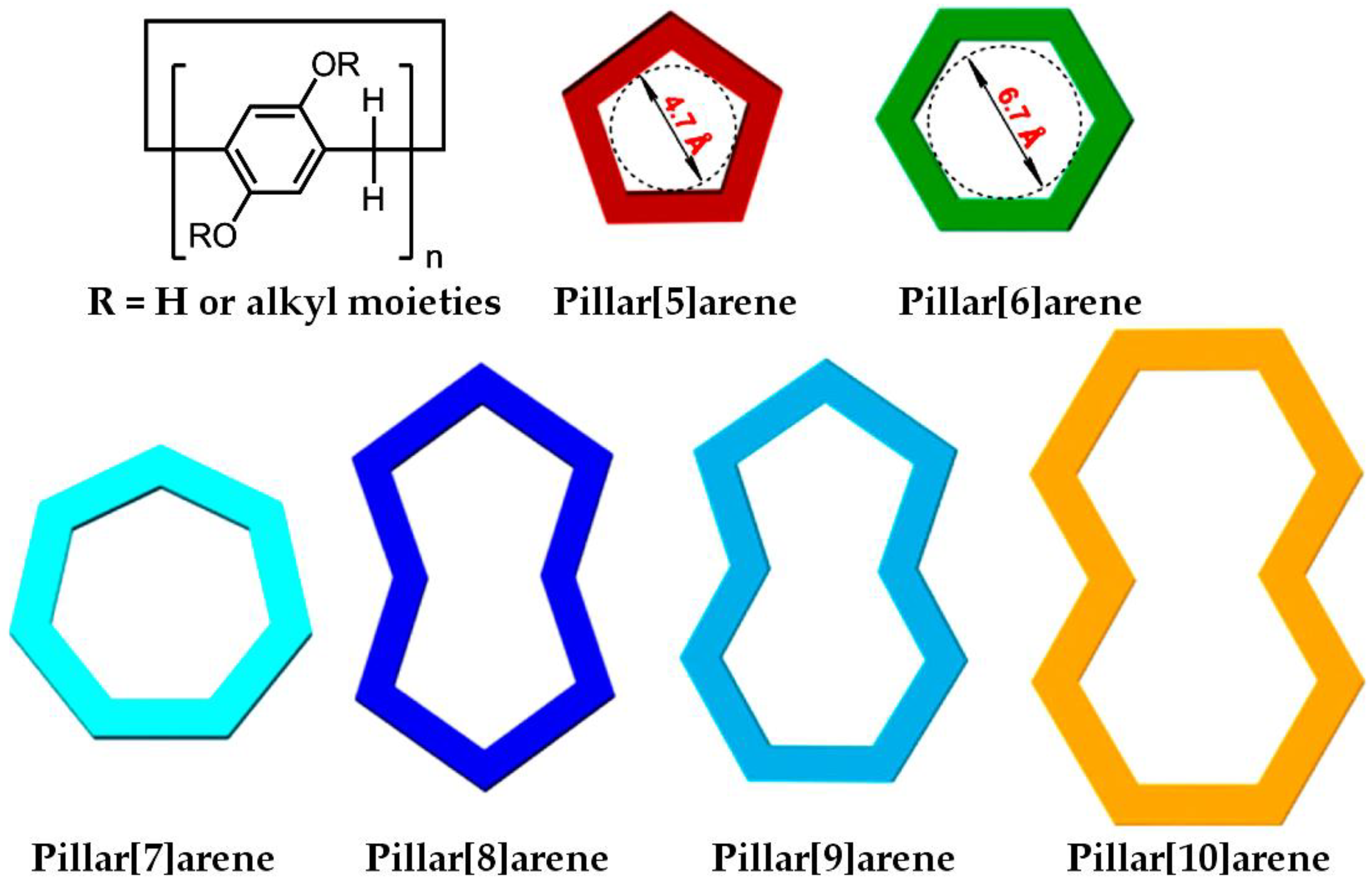
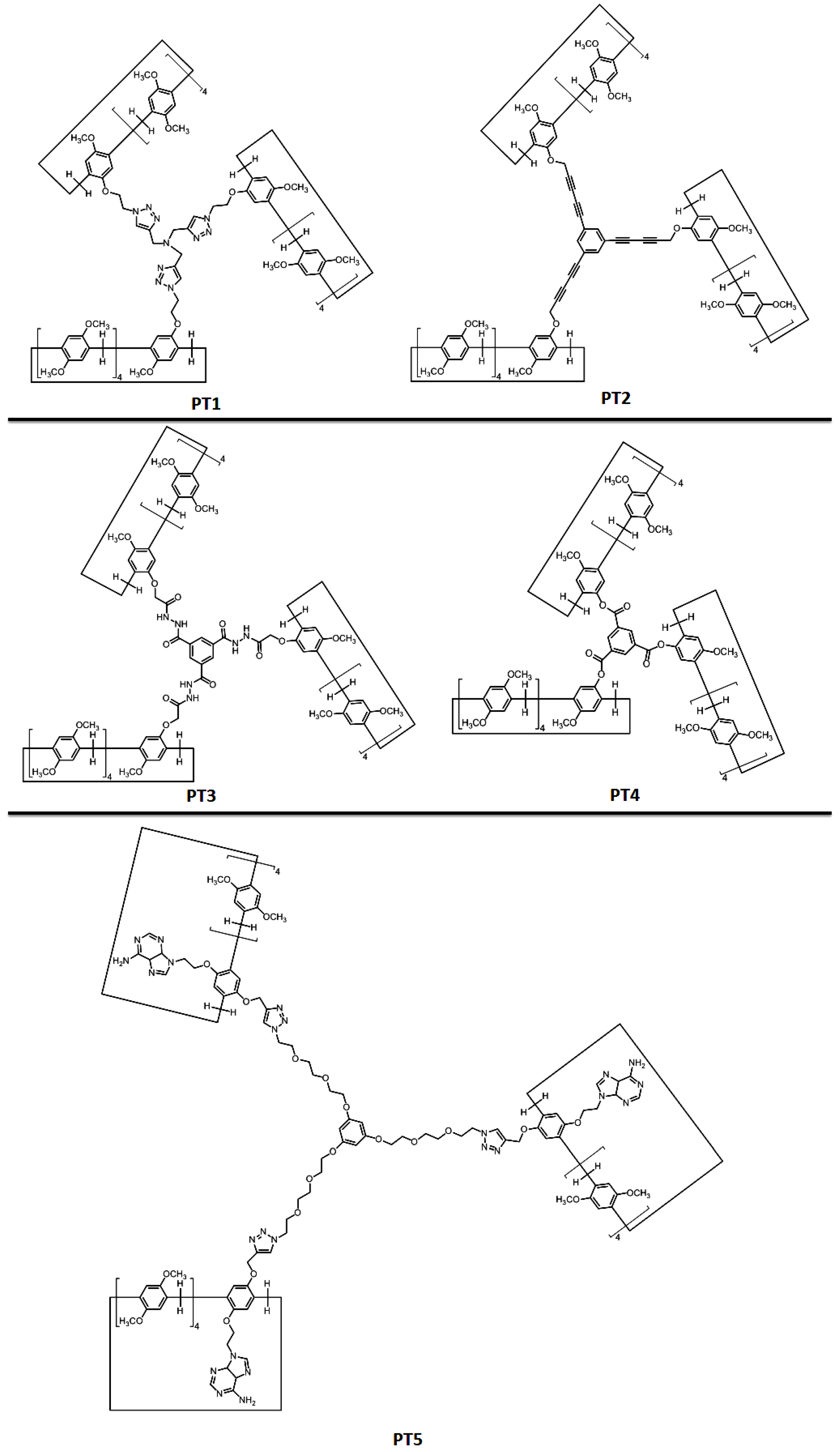

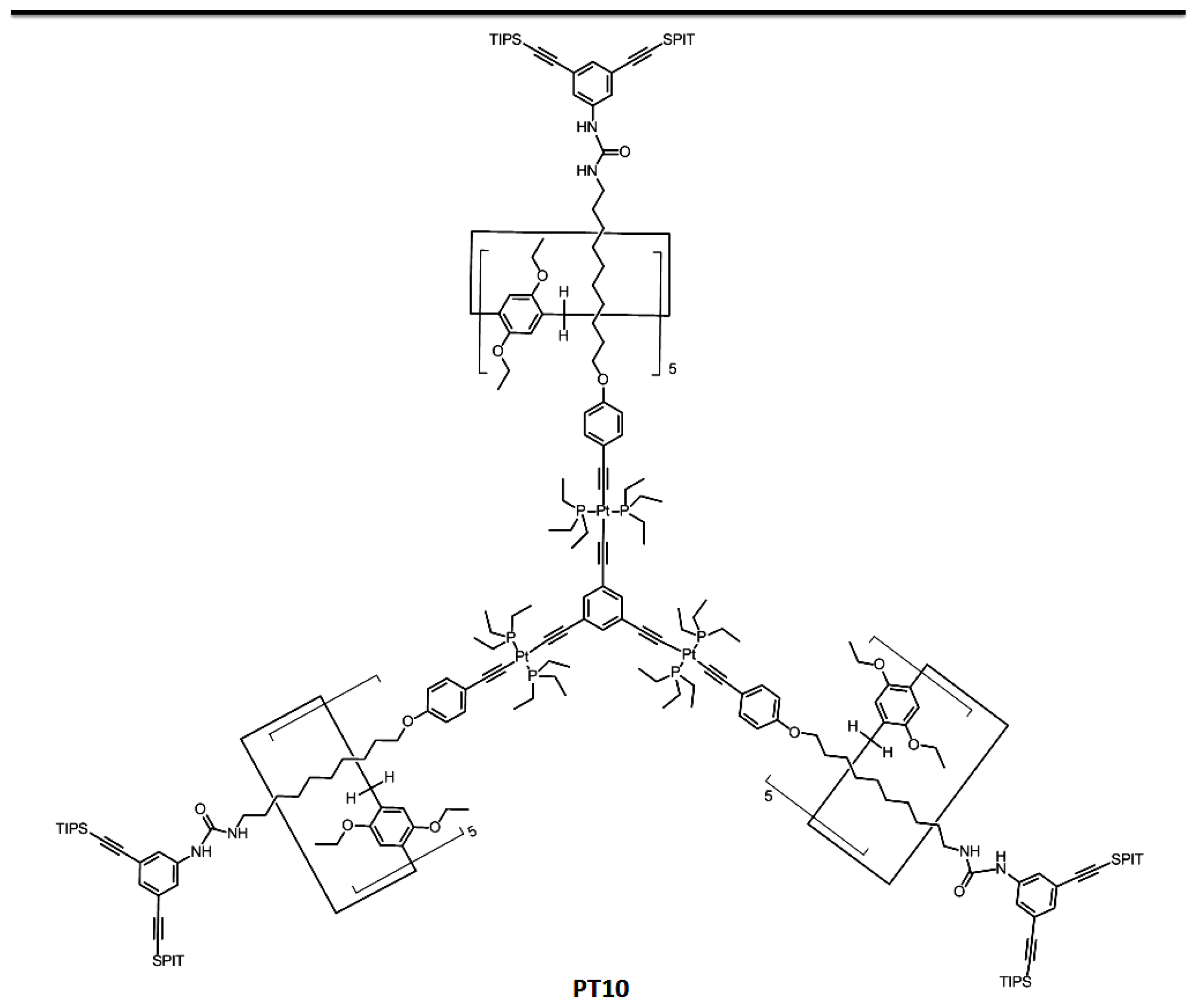
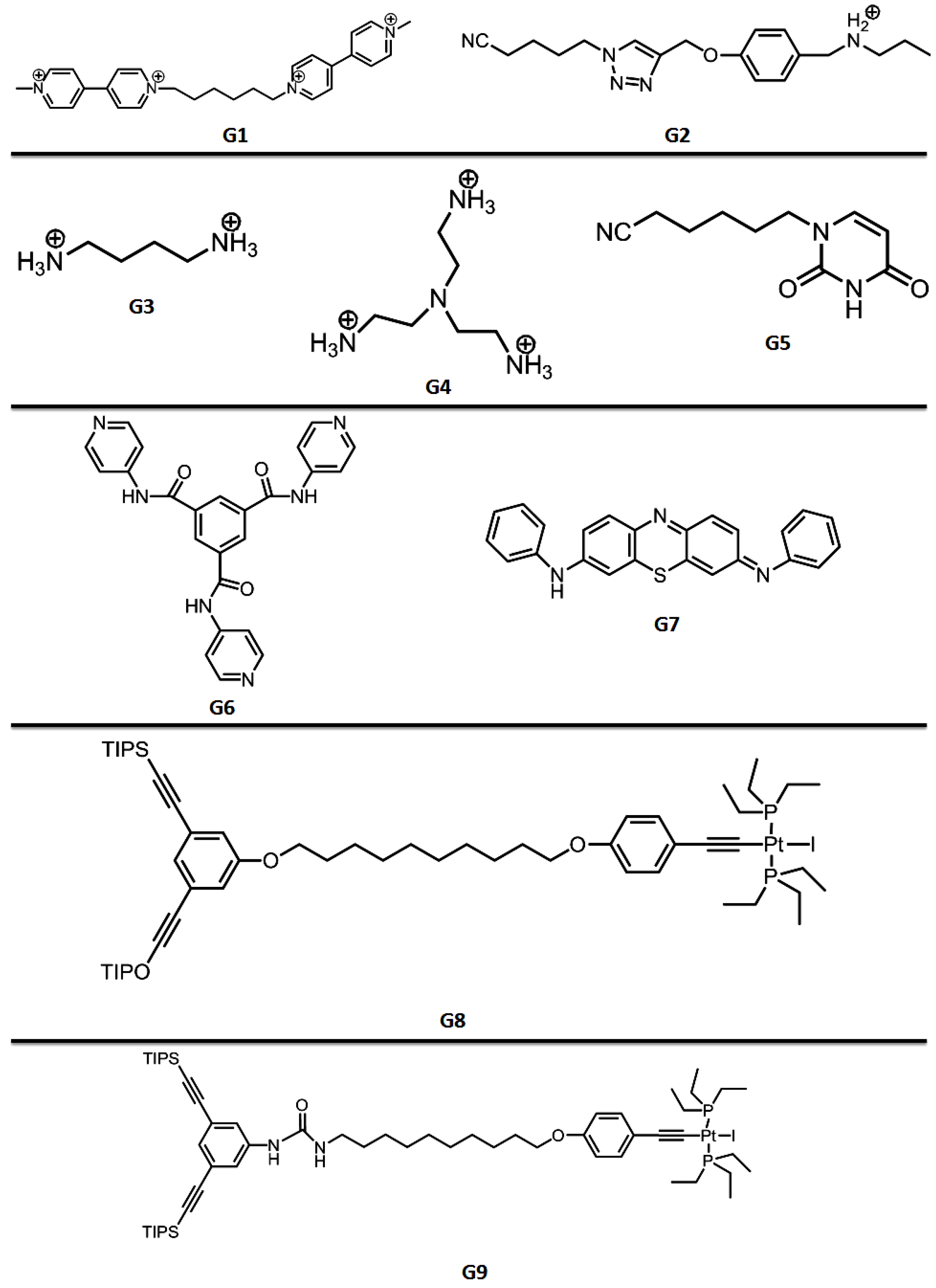
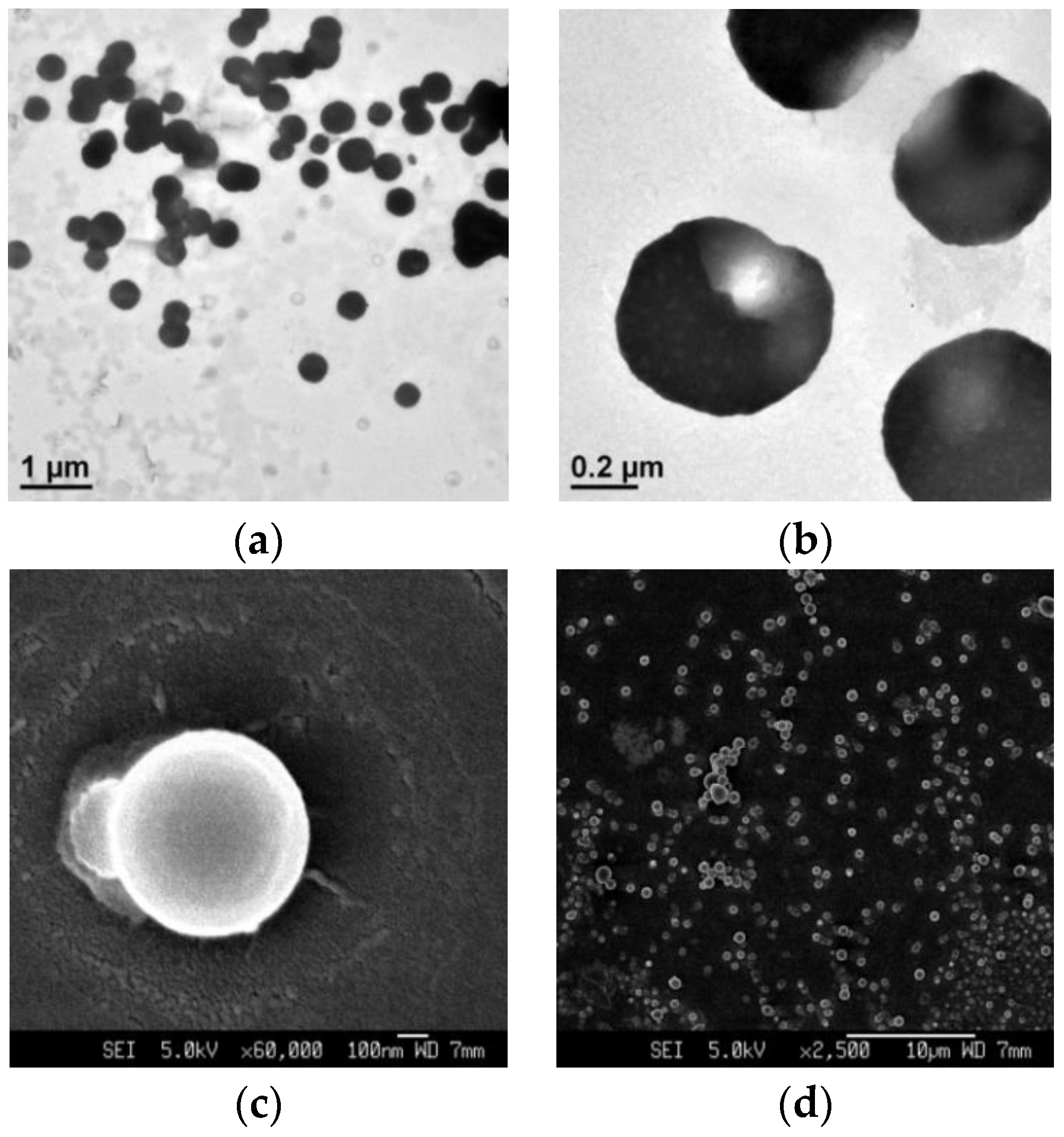
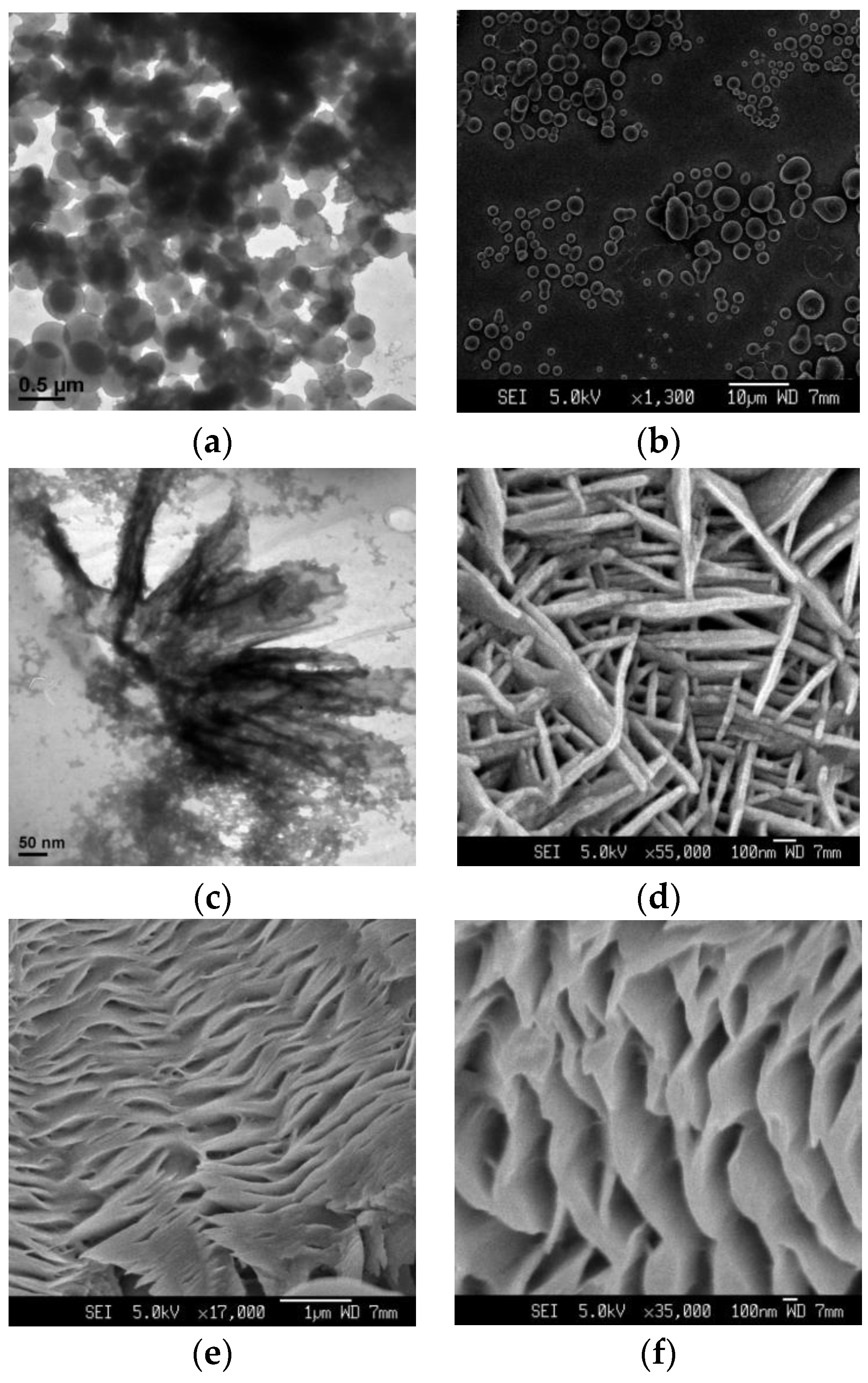
| Pillararenes Trimer | Guest | Precursor | Interactions | Assembly | External Stimuli | Applications | Ref |
|---|---|---|---|---|---|---|---|
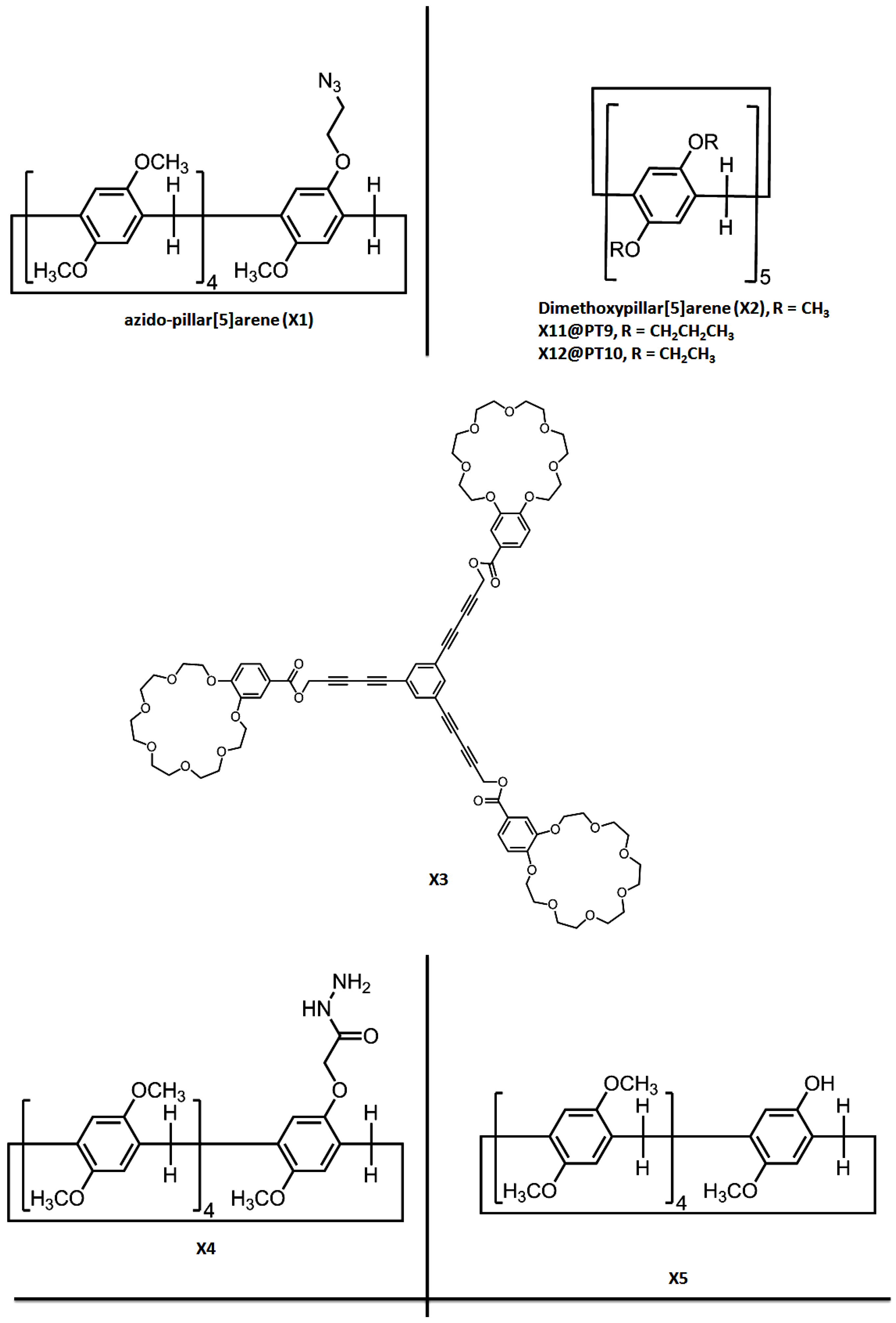
| PT1 | G1 | X1 | Host-guest interactions | Hollow spherical, tubular and layered assemblies | Concentration-dependent | Morphological control in comparison with X2 | [15] |
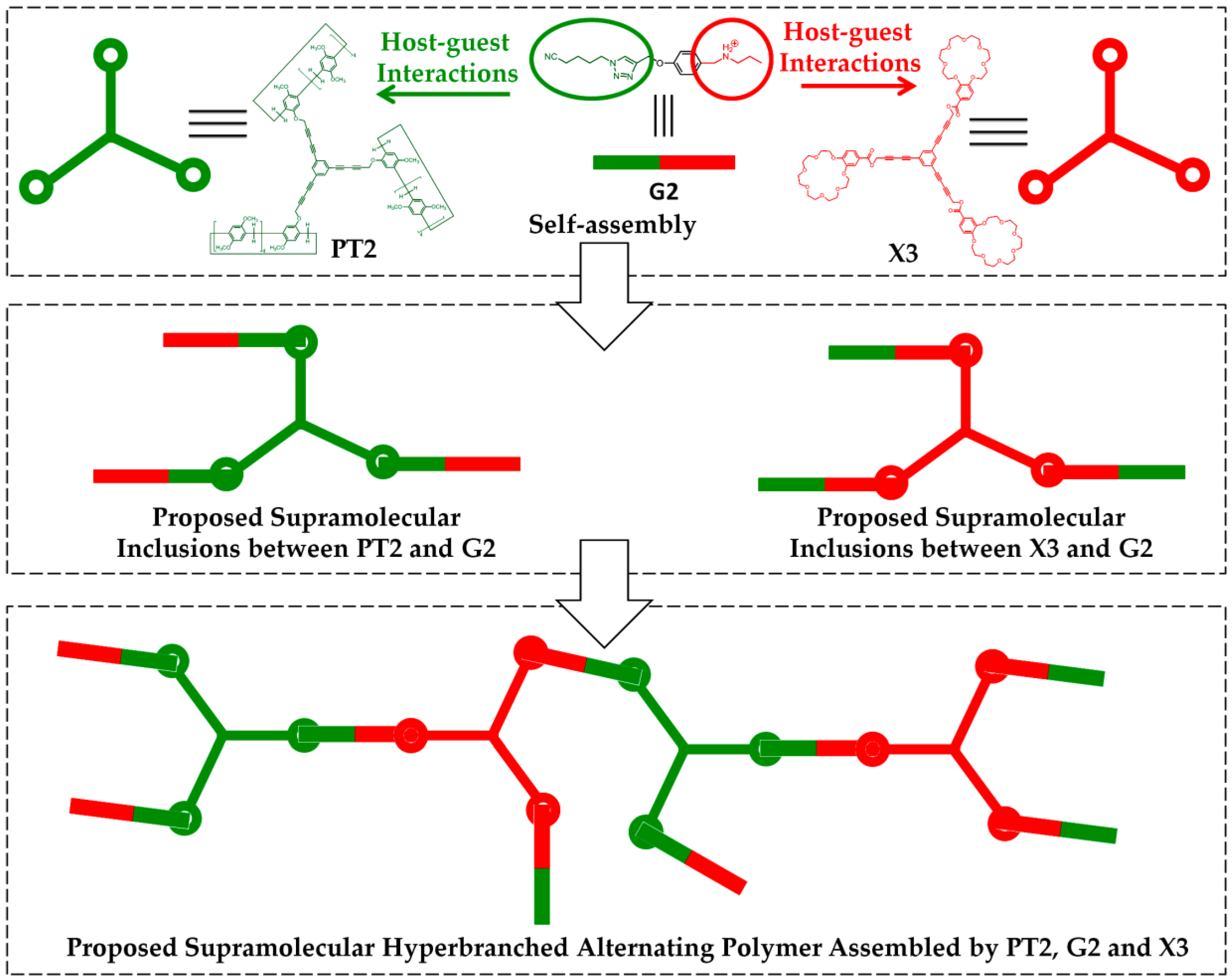
| PT2 | G2 | - | Host-guest interactions | Supramolecular hyperbranched alternating polymers | K+ (crown ether X3) | - | [20] |
| PT3 | - | X4 | Hydrogen bonding, van der Waals forces, C–H…π and π–π stacking interactions | Supramolecular polymer | Cations | Fluorescence detection and separation of Hg2+ | [21] |
| PT4 | G3/G4 | X5 | Host-guest interactions | Supramolecular polymer | - | - | [19] |
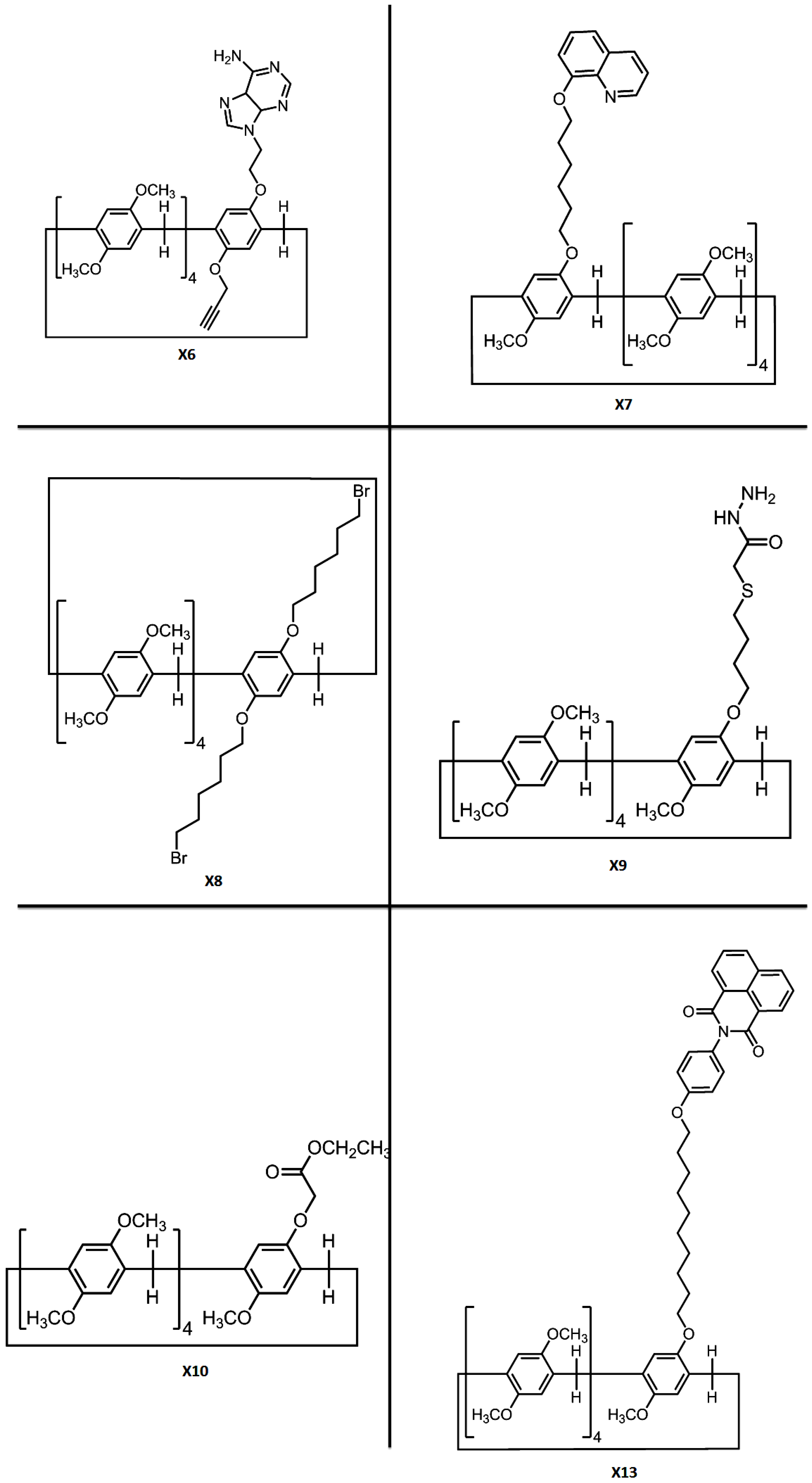
| PT5 | G5 | X6 | Hydrogen bonding, π–π stacking and host-guest interactions | Hyperbranched supramolecular polymer | Heat and acid/base | - | [22] |
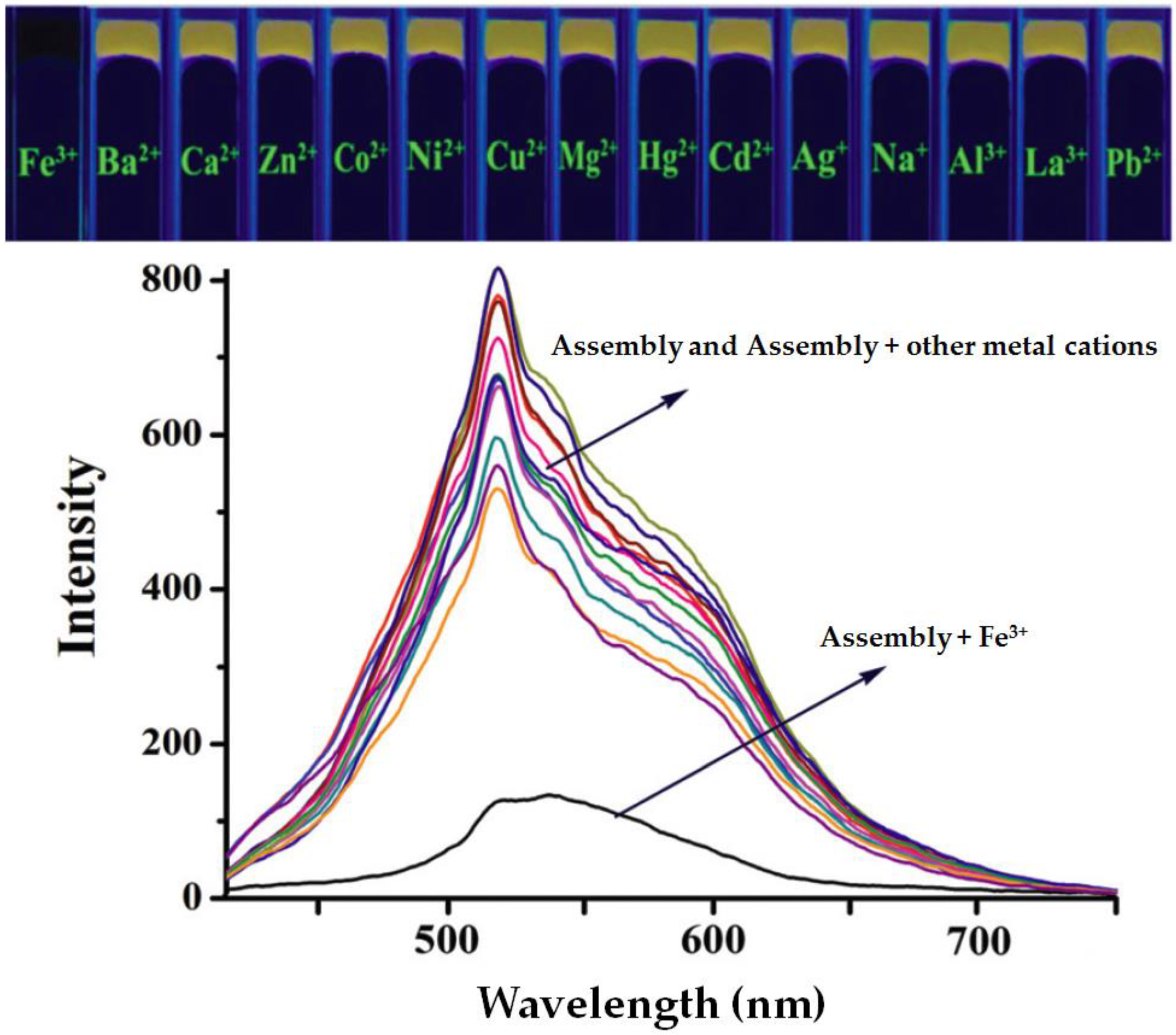
| PT6 | - | X7/X8 | π–π stacking interactions | (Metal ions coordinated) supramolecular organic frameworks | Fe3+/Hg2+/Cr3+ and CN−/H2PO4− | Fluorescence ultrasensitive detection | [23] |
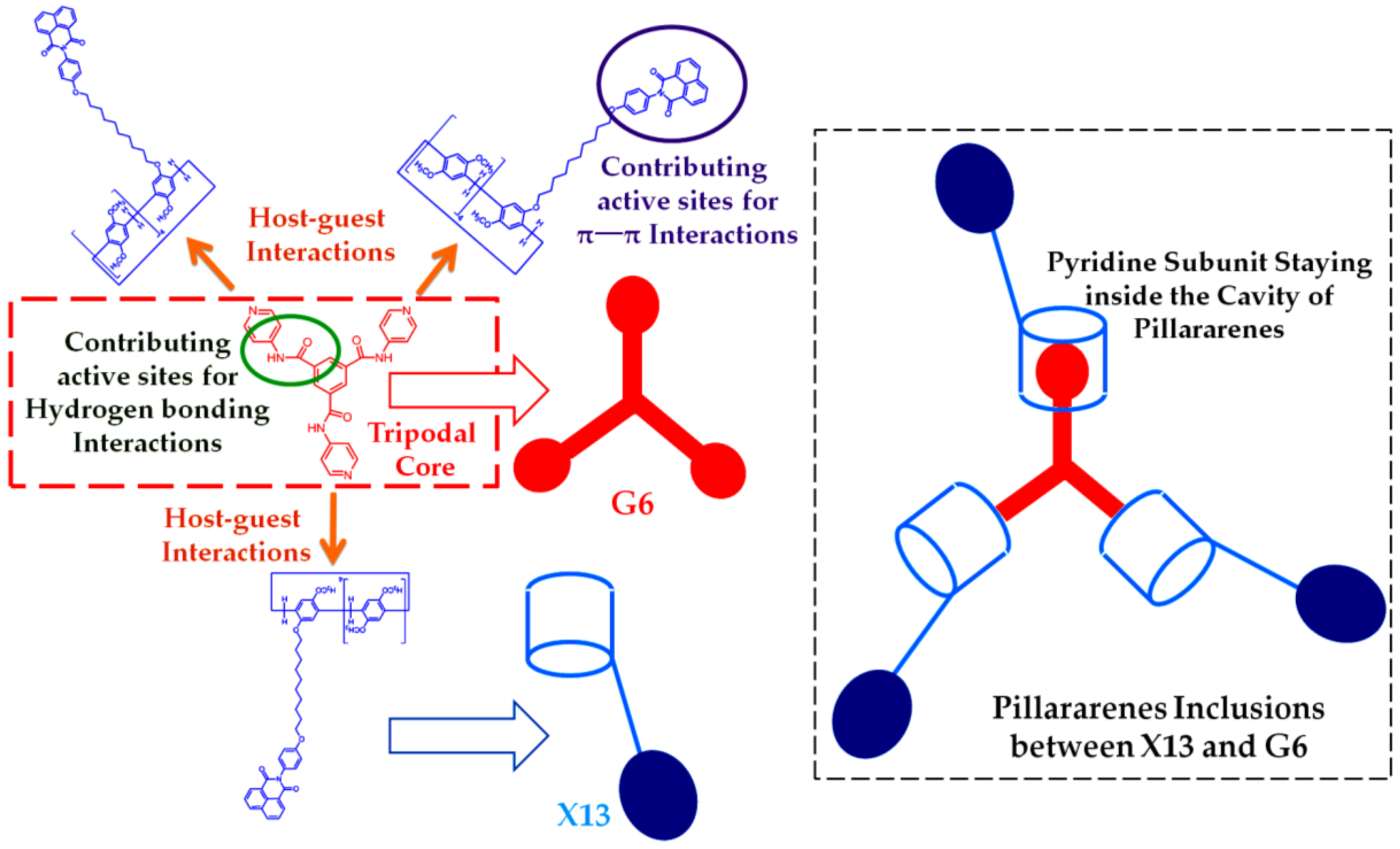
| PT7 | G6 | X9 | Hydrogen bonding, C–H…π and π–π stacking interactions | Supramolecular polymer network/supramolecular polymer framework | Metal cations/anions/amino acid | Fluorescence detection/adsorption capacity for cations | [24] |
| PT8 | G7 | X10 | - | - | Competitive complexation with Anions | Fluorescence detection of F−/AcO−/H2PO4− | [25] |
| PT9 | G8 | X11 | Mechanical interlocked molecule | Dendrimer | - | - | [26] |
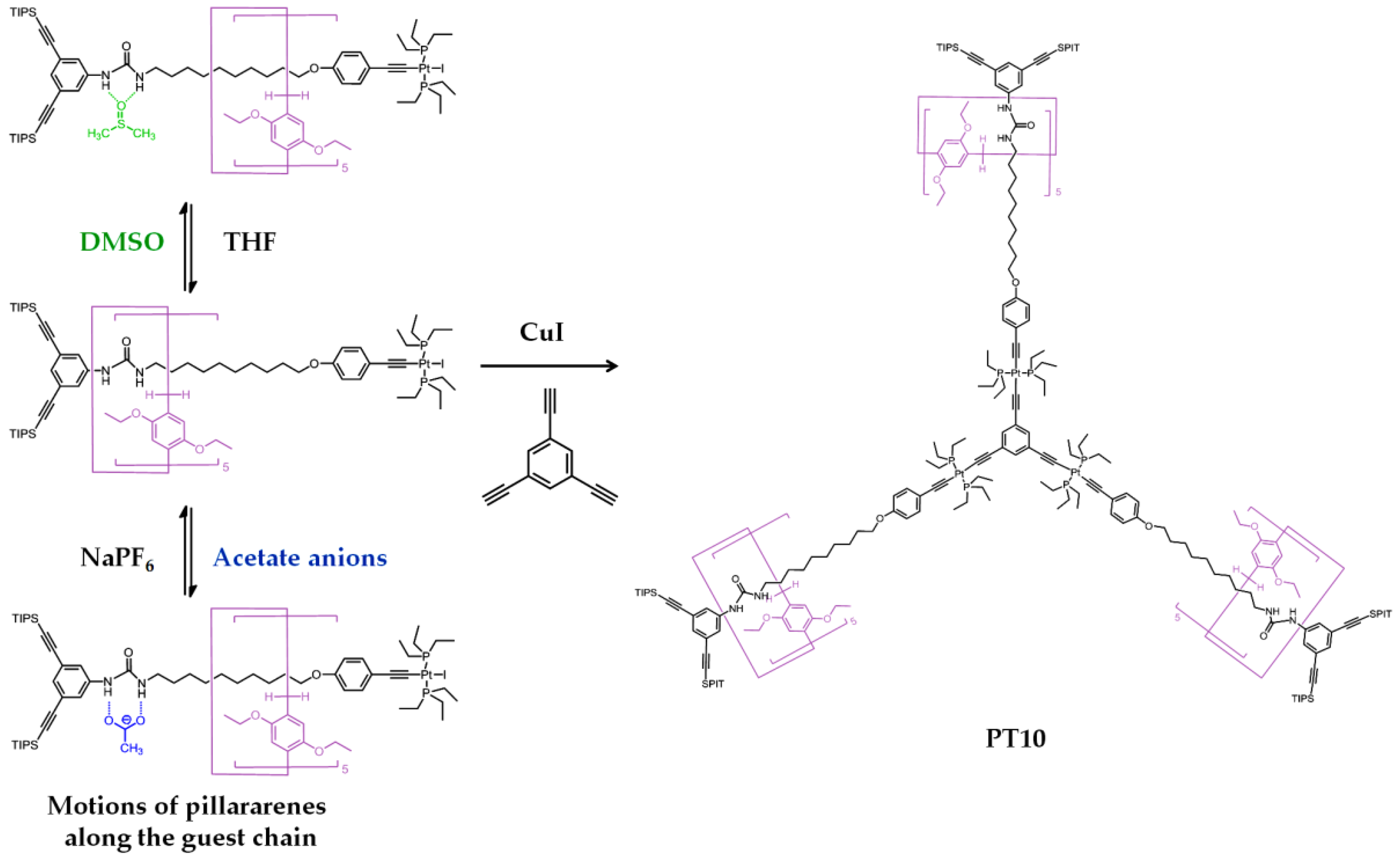
| PT10 | G9 | X12 | Mechanical interlocked molecule | Dendrimer | Dimethylsulfoxide and acetate anion | - | [27] |
| X13⸧G6 | G6 | X13 | Hydrogen bonding, π–π stacking and host-guest interactions | Supramolecular polymer networks/gel | Heat/cooling, pH, competitive guests and mechanical | Dye sorption, ultrasensitive detection and separation of Fe3+ | [28] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, Z.; Fu, H. Pillararenes Trimer for Self-Assembly. Nanomaterials 2020, 10, 651. https://doi.org/10.3390/nano10040651
Zhang H, Liu Z, Fu H. Pillararenes Trimer for Self-Assembly. Nanomaterials. 2020; 10(4):651. https://doi.org/10.3390/nano10040651
Chicago/Turabian StyleZhang, Huacheng, Zhaona Liu, and Hui Fu. 2020. "Pillararenes Trimer for Self-Assembly" Nanomaterials 10, no. 4: 651. https://doi.org/10.3390/nano10040651
APA StyleZhang, H., Liu, Z., & Fu, H. (2020). Pillararenes Trimer for Self-Assembly. Nanomaterials, 10(4), 651. https://doi.org/10.3390/nano10040651






