Nanoarchitectonics of Nanoporous Carbon Materials in Supercapacitors Applications
Abstract
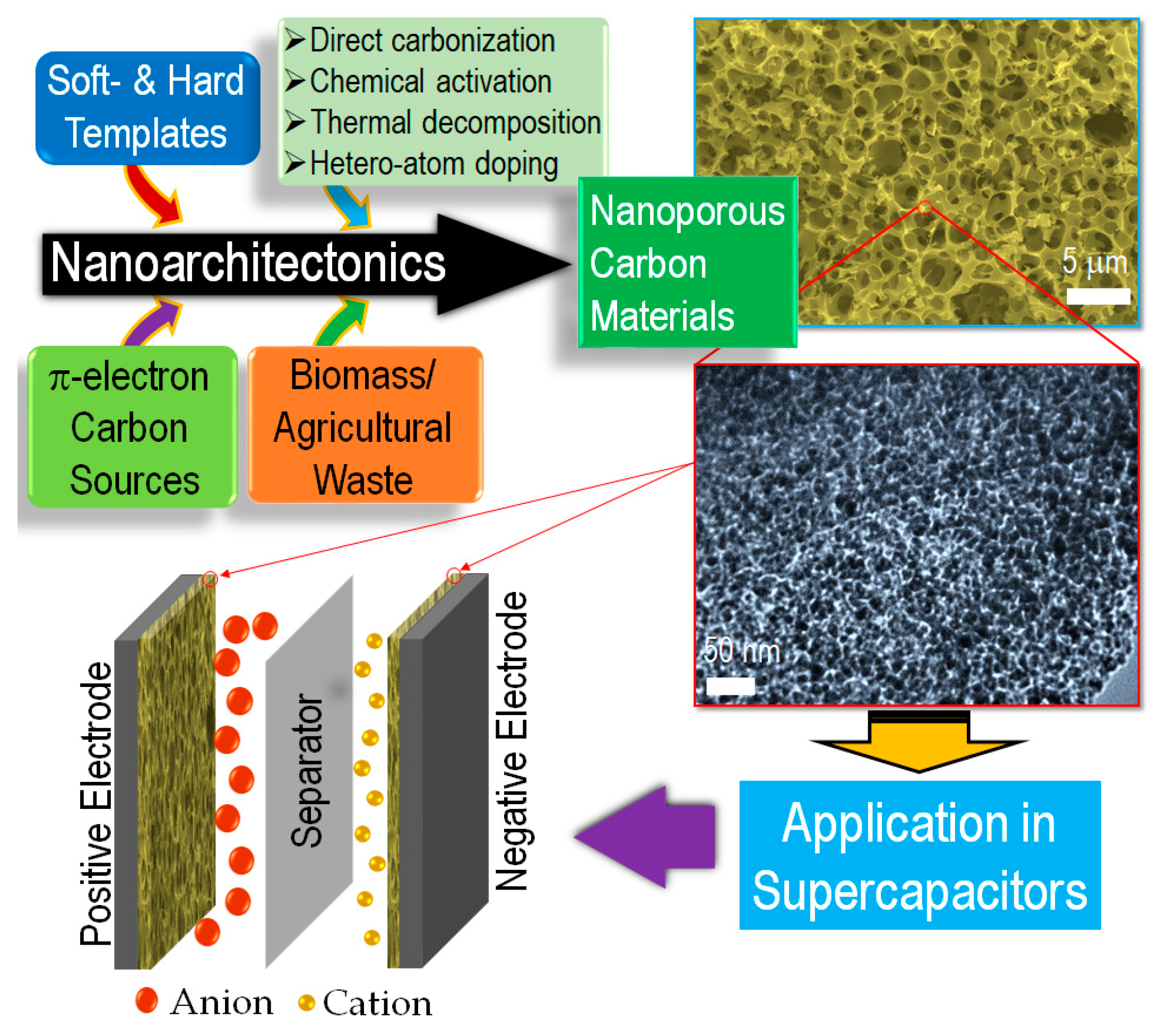
:1. Introduction
2. Nanoporous Carbons Materials
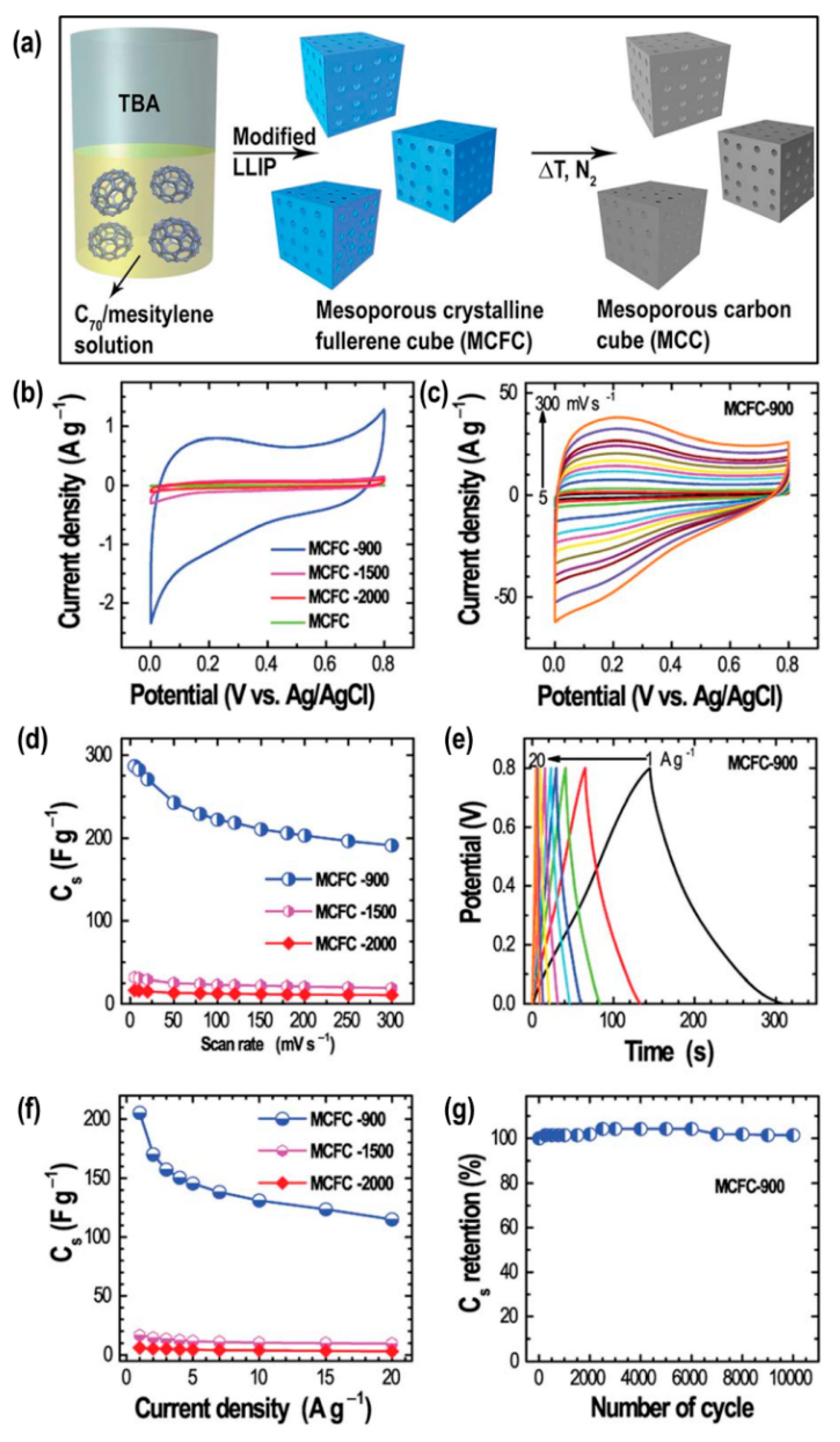
2.1. Nanoporous Carbons from Self-Assembled Fullerene Crystals
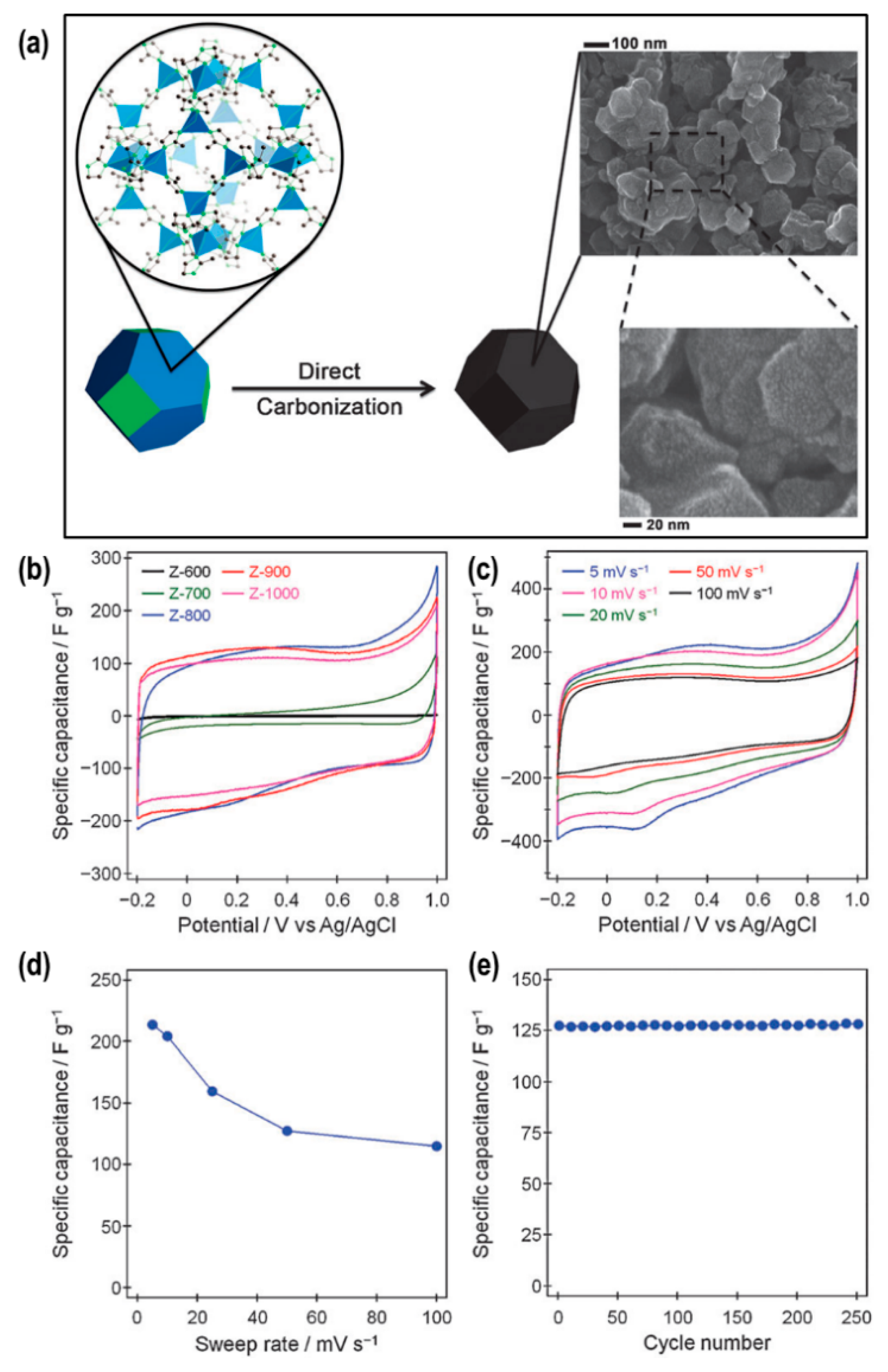
2.2. Nanoporous Carbons from Metal−Organic Frameworks
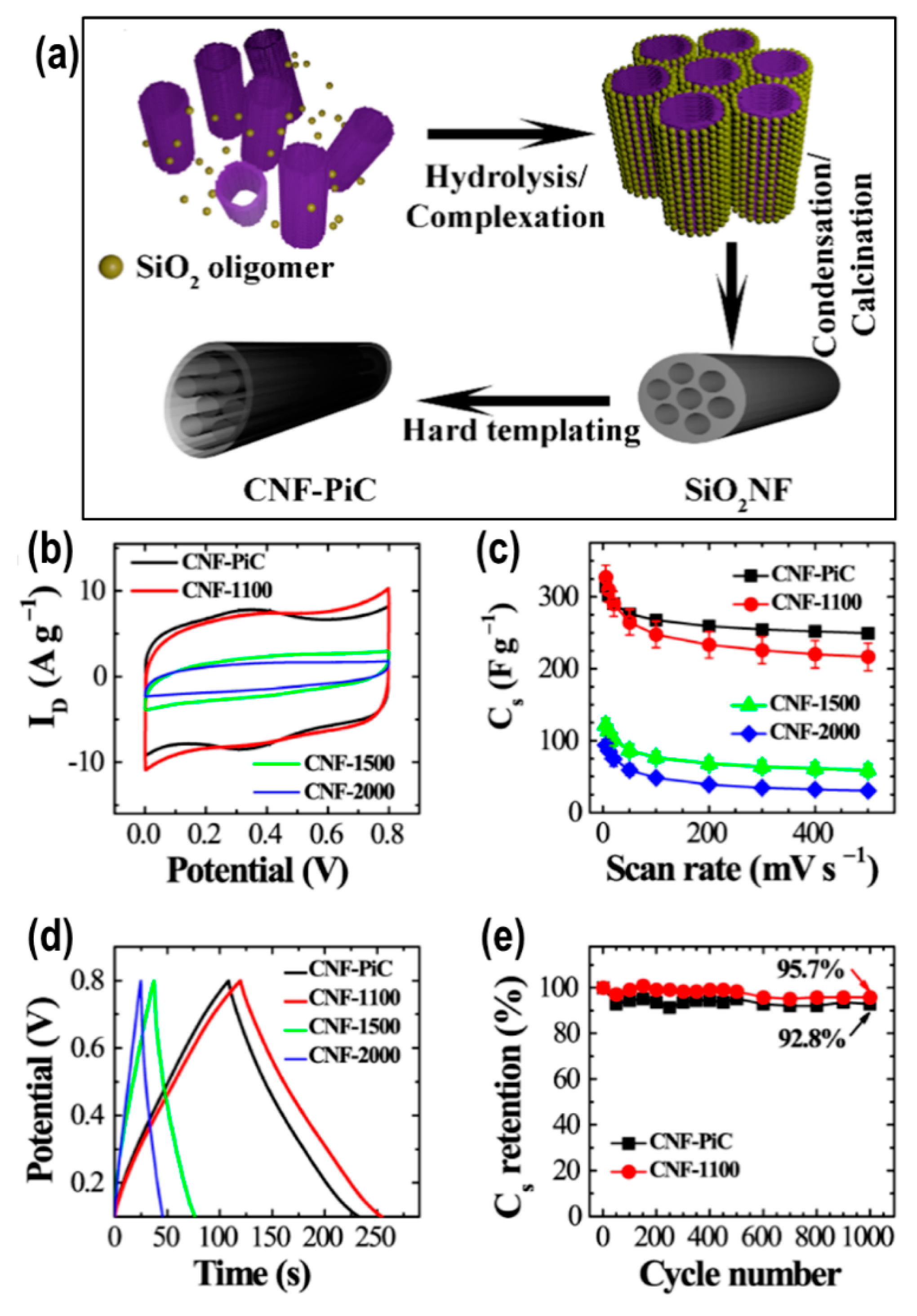
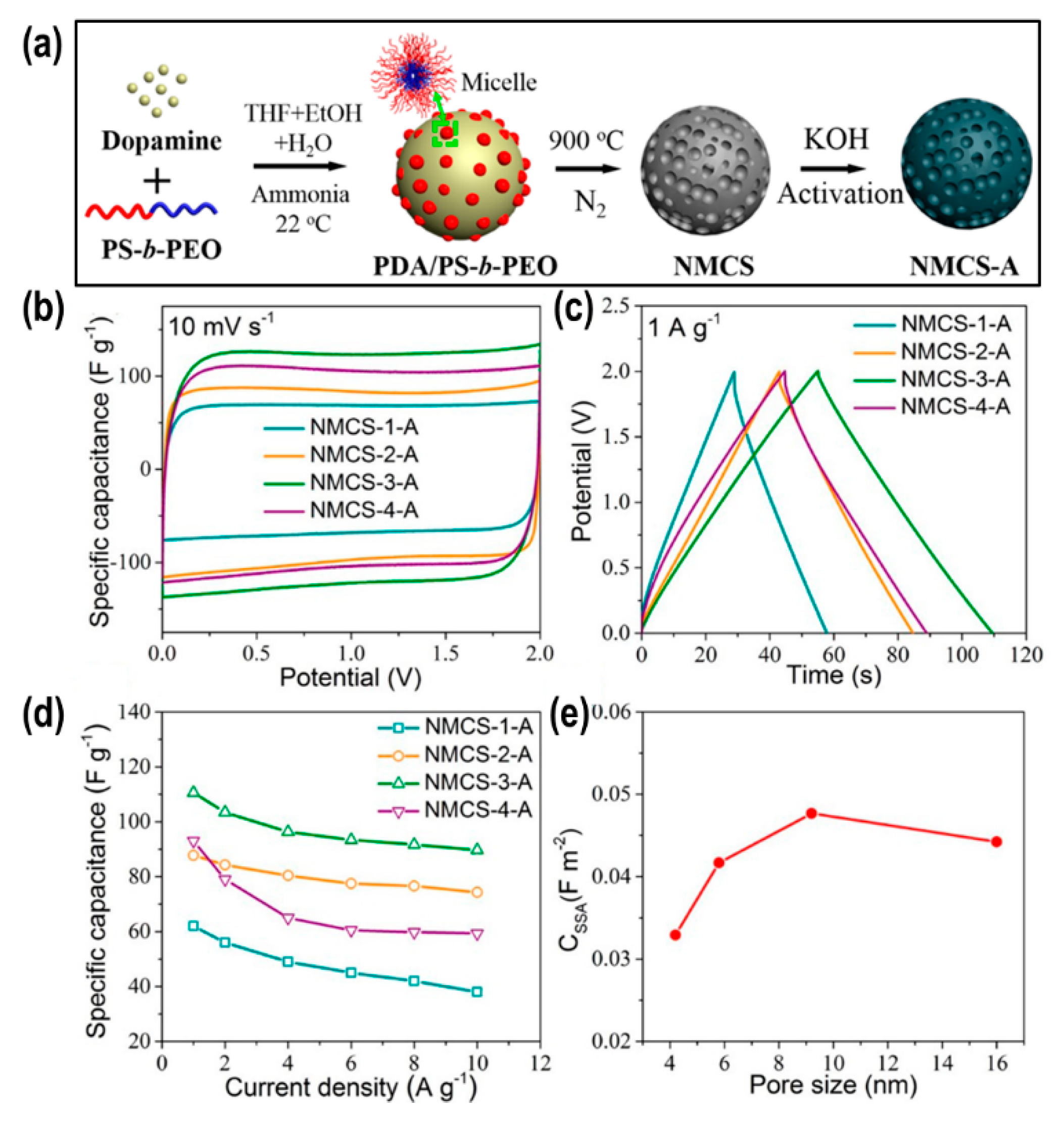
2.3. Nanoporous Carbons from Hard- and Soft-Templates
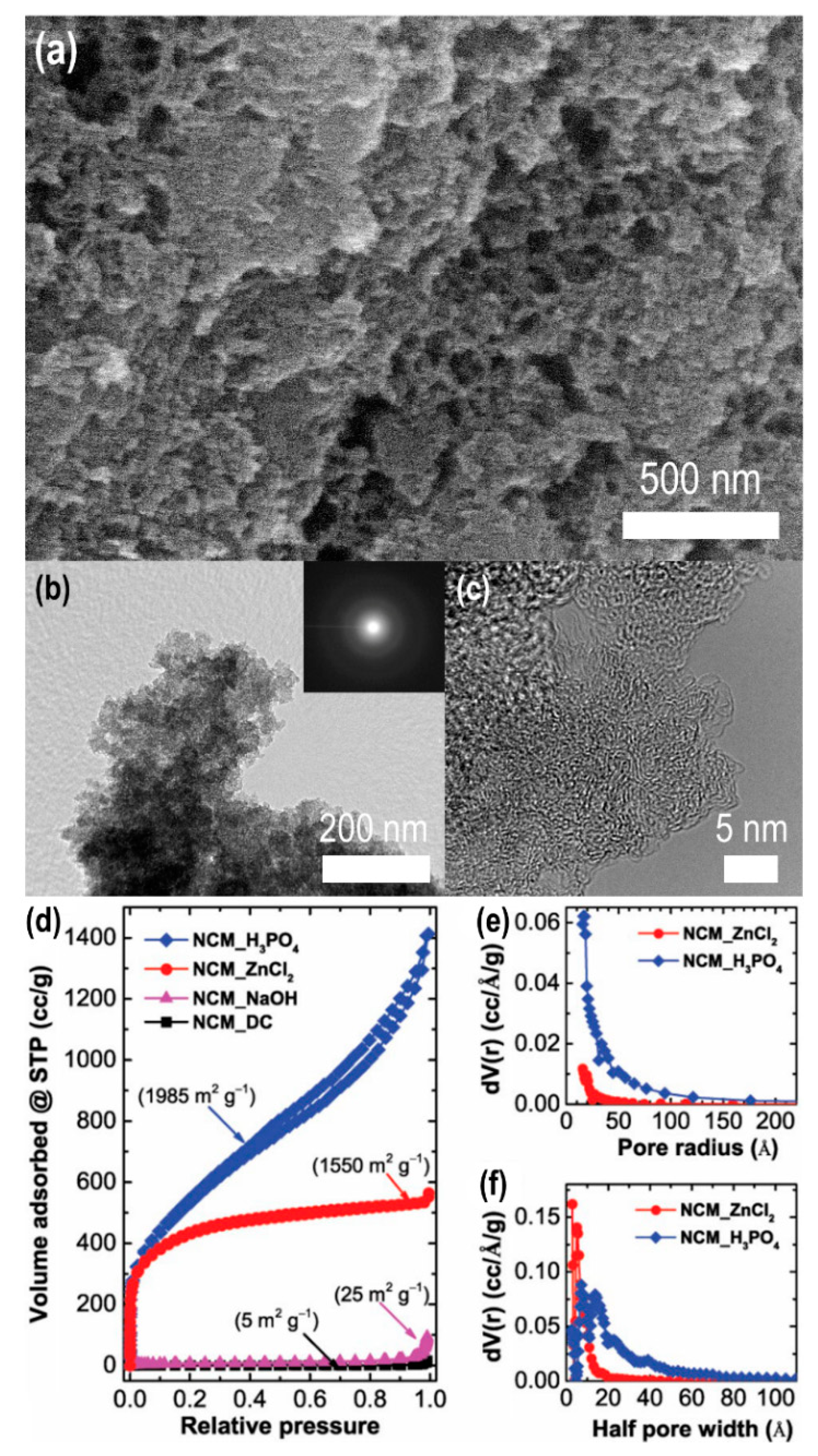
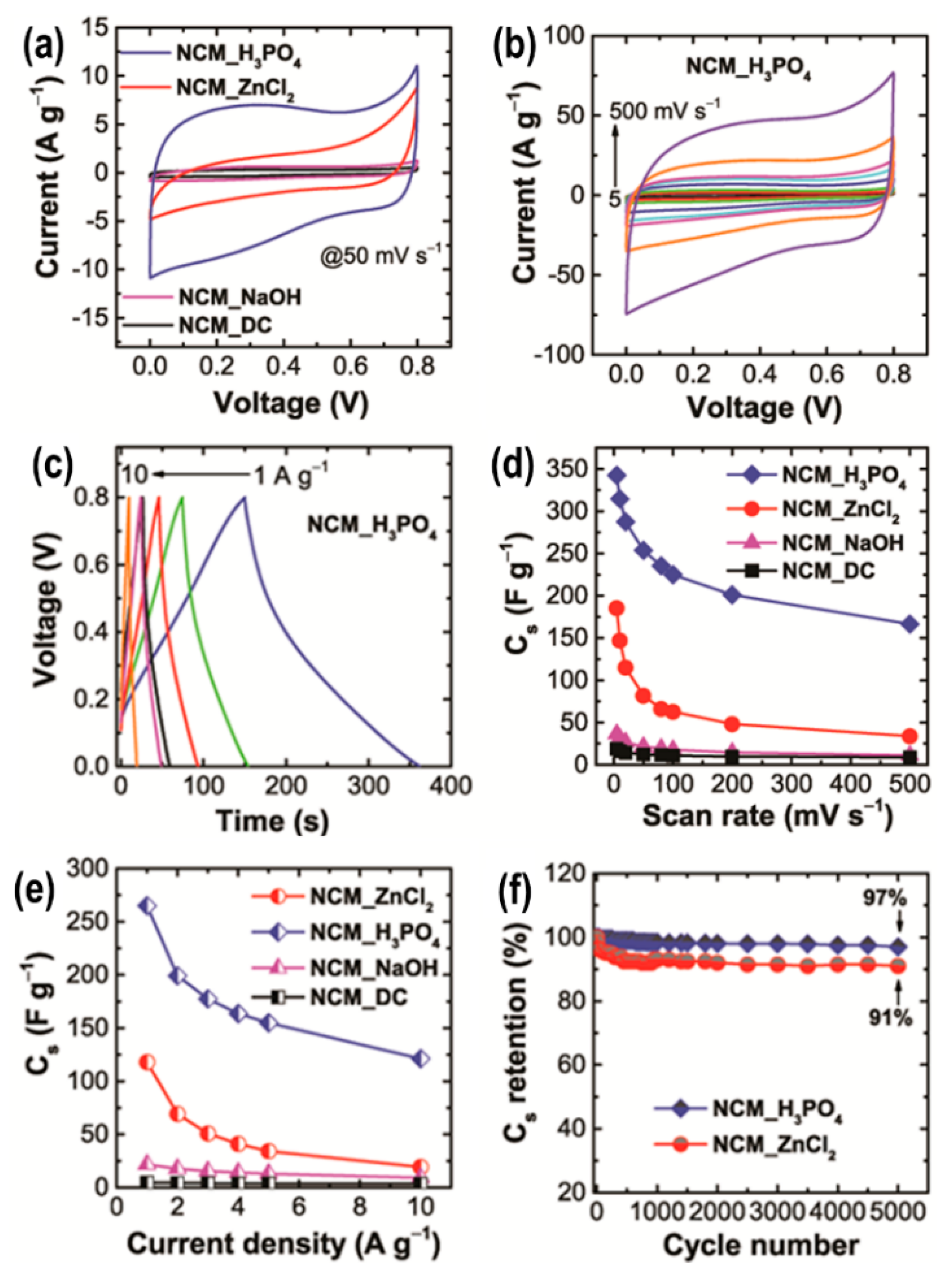
2.4. Nanoporous Carbons from Natural Biomass
3. Conclusions and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Ghanbari, F.; Moradi, M. Application of Peroxymonosulfate and Its Activation Methods for Degradation of Environmental Organic Pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, A.; Ciacchi, L.C.; Wei, G. Recent Advances in Nanoporous Membranes for Water Purification. Nanomaterials 2018, 8, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Fukuoka, A. Development of Solid Catalyst-Solid Substrate Reactions for Efficient Utilization of Biomass. Bull. Chem. Soc. Jpn. 2018, 91, 29–43. [Google Scholar] [CrossRef]
- Haque, E.; Ward, A.C. Zebrafish as a Model to Evaluate Nanoparticle Toxicity. Nanomaterials 2018, 8, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Lou, Y.; Chen, J. New UiO-66/CuxS Heterostructures: Surface Functionalization Synthesis and Their Application in Photocatalytic Degradation of RhB. Bull. Chem. Soc. Jpn. 2018, 91, 515–522. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active Sites of Nitrogen-doped Carbon Materials for Oxygen Reduction Reaction Clarified Using Model Catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Theerthagiri, J.; Karuppasamy, K.; Durai, G.; Rana, A.S.; Arunachalam, P.; Sangeetha, K.; Kuppusami, P.; Kim, H.-S. Recent Advances in Metal Chalcogenides (MX; X = S, Se) Nanostructures for Electrochemical Supercapacitor Applications: A Brief Review. Nanomaterials 2018, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Mallouk, T.E. Two-Dimensional Metal Oxide Nanosheets as Building Blocks for Artificial Photosynthetic Assemblies. Bull. Chem. Soc. Jpn. 2019, 92, 38–54. [Google Scholar] [CrossRef]
- Ohno, H.; Yoshizawa-Fujita, M.; Kohno, Y. Functional Design of Ionic Liquids: Unprecedented Liquids that Contribute to Energy Technology, Bioscience and Materials Sciences. Bull. Chem. Soc. Jpn. 2019, 92, 852–868. [Google Scholar] [CrossRef]
- Malka, D.; Katz, G. An Eight-Channel C-Band Demux Based on Multicore Photonic Crystal Fiber. Nanomaterials 2018, 8, 845. [Google Scholar] [CrossRef] [Green Version]
- Pearton, S.J.; Yang, J.; Cary, P.H.; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A Review of Ga2O3 Materials, Processing, and Devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef] [Green Version]
- Kitamori, T. Thermal Lens Microscope and Microchip Chemistry. Bull. Chem. Soc. Jpn. 2019, 92, 469–473. [Google Scholar] [CrossRef]
- Gong, C.; Zhang, X. Two-Dimensional Magnetic Crystals and Emergent Heterostructure Devices. Science 2019, 363, eaav4450. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Sasabe, H.; Kido, J. Review of Molecular Engineering for Horizontal Molecular Orientation in Organic Light-Emitting Devices. Bull. Chem. Soc. Jpn. 2019, 92, 716–728. [Google Scholar] [CrossRef] [Green Version]
- Burdusel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoanta, L.; Ficai, A.; Andronescu, E. Ecaterina Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Al-Enizi, A.M.; Zagho, M.M.; Elzatahry, A.A. Polymer-Based Electrospun Nanofibers for Biomedical Applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, J.; Okano, T. Design of Temperature-Responsive Polymer-Grafted Surfaces for Cell Sheet Preparation and Manipulation. Bull. Chem. Soc. Jpn. 2019, 92, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Wei, Z.; Song, C.H.; Tang, C.C.; Han, W.; Dong, X.C. Optical Nano-Agents in the Second Near-Infrared Window for Biomedical Applications. Chem. Soc. Rev. 2019, 48, 22–37. [Google Scholar] [CrossRef]
- Tanaka, M.; Kobayashi, S.; Murakami, D.; Aratsu, F.; Kashiwazaki, A.; Hoshiba, T.; Fukushima, K. Design of Polymeric Biomaterials: The "Intermediate Water Concept". Bull. Chem. Soc. Jpn. 2019, 92, 2043–2057. [Google Scholar] [CrossRef]
- Povie, G.; Segawa, Y.; Nishihara, T.; Miyauchi, Y.; Itami, K. Synthesis of a Carbon Nanobelt. Science 2017, 356, 172–175. [Google Scholar] [CrossRef]
- Takimiya, K.; Nakano, M. Thiophene-Fused Naphthalene Diimides: New Building Blocks for Electron Deficient π-Functional Materials. Bull. Chem. Soc. Jpn. 2018, 91, 121–140. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Matsuno, T.; Isobe, H. Stereoisomerism and Structures of Rigid Cylindrical Cycloarylenes. Bull. Chem. Soc. Jpn. 2018, 91, 907–921. [Google Scholar] [CrossRef]
- Sun, Z.; Ikemoto, K.; Fukunaga, T.M.; Koretsune, T.; Arita, R.; Sato, S.; Isobe, H. Finite Phenine Nanotubes with Periodic Vacancy Defects. Science 2019, 363, 151–154. [Google Scholar] [CrossRef]
- Kawamura, S.; Sodeoka, M. Fluoroalkylation Methods for Synthesizing Versatile Building Blocks. Bull. Chem. Soc. Jpn. 2019, 92, 1245–1262. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, J.; Kanbara, T. Facile Synthesis of pi-Conjugated Polymers via Direct Arylation Polycondensation. Bull. Chem. Soc. Jpn. 2019, 92, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Jia, C.; Wang, D.; Deng, J.; Xu, G.; Wu, C.; Dong, M.; Guo, Z. Synthesis and Characterization of Porous Tree Gum Grafted Copolymer Derived from Prunus Cerasifera Gum Polysaccharide. Int. J. Biol. Macromol. 2019, 133, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Akagi, K. Interdisciplinary Chemistry Based on Integration of Liquid Crystals and Conjugated Polymers: Development and Progress. Bull. Chem. Soc. Jpn. 2019, 92, 1509–1655. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Li, S.; Liu, W.; Ran, M.; Lu, H.; Yang, Y. Recent Advances of Rare-Earth Ion Doped Luminescent Nanomaterials in Perovskite Solar Cells. Nanomaterials 2018, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Miyasaka, T. Lead Halide Perovskites in Thin Film Photovoltaics: Background and Perspectives. Bull. Chem. Soc. Jpn. 2018, 91, 1058–1068. [Google Scholar] [CrossRef]
- Wadsworth, A.; Moser, M.; Marks, A.; Little, M.S.; Gasparini, N.; Brabec, C.J.; Baran, D.; McCulloch, I. Critical Review of the Molecular Design Progress in Non-Fullerene Electron Acceptors towards Commercially Viable Organic Solar Cells. Chem. Soc. Rev. 2019, 48, 1596–1625. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Pramoda, K. Borocarbonitrides, BxCyNz, 2D Nanocomposites with Novel Properties. Bull. Chem. Soc. Jpn. 2019, 92, 441–468. [Google Scholar] [CrossRef] [Green Version]
- Yan, A.-L.; Wang, X.-C.; Cheng, J.-P. Research Progress of NiMn Layered Double Hydroxides for Supercapacitors: A Review. Nanomaterials 2018, 8, 747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stauss, S.; Honma, I. Biocompatible Batteries-Materials and Chemistry, Fabrication, Applications, and Future Prospects. Bull. Chem. Soc. Jpn. 2018, 91, 492–505. [Google Scholar] [CrossRef]
- Kawai, T.; Nakao, S.; Nishide, H.; Oyaizu, K. Poly(Diphenanthrenequinone-Substituted Norbornene) for Long Life and Efficient Lithium Battery Cathodes. Bull. Chem. Soc. Jpn. 2018, 91, 721–727. [Google Scholar] [CrossRef]
- Ji, Q.; Honma, I.; Paek, S.-M.; Akada, M.; Hill, J.P.; Vinu, A.; Ariga, K. Layer-by-Layer Films of Graphene and Ionic Liquid for Highly Selective Gas Sensing. Angew. Chem. Int. Ed. 2010, 49, 9737–9739. [Google Scholar] [CrossRef]
- Ariga, K.; Ito, H.; Hill, J.P.; Tsukube, H. Molecular Recognition: From Solution Science to Nano/Materials Technology. Chem. Soc. Rev. 2012, 41, 5800–5835. [Google Scholar] [CrossRef]
- Bhattarai, J.K.; Neupane, D.; Nepal, B.; Mikhaylov, V.; Demchenko, A.V.; Stine, K.J. Preparation, Modification, Characterization, and Biosensing Application of Nanoporous Gold Using Electrochemical Techniques. Nanomaterials 2018, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Sai-Anand, G.; Sivanesan, A.; Benzigar, M.R.; Singh, G.; Gopalan, A.-I.; Baskar, A.V.; Ilbeygi, H.; Ramadass, K.; Kambala, V.; Vinu, A. Recent Progress on the Sensing of Pathogenic Bacteria Using Advanced Nanostructures. Bull. Chem. Soc. Jpn. 2019, 92, 216–244. [Google Scholar] [CrossRef] [Green Version]
- Jackman, J.A.; Ferhan, A.R.; Cho, N.-J. Surface-Based Nanoplasmonic Sensors for Biointerfacial Science Applications. Bull. Chem. Soc. Jpn. 2019, 92, 1404–1412. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Chen, H.; Peng, H.; Wang, Z.; Huang, W. Graphene Modified TiO2 Composite Photocatalysts: Mechanism, Progress and Perspective. Nanomaterials 2018, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Roy, N.; Suzuki, N.; Terashima, C.; Fujishima, A. Recent Improvements in the Production of Solar Fuels: From CO2 Reduction to Water Splitting and Artificial Photosynthesis. Bull. Chem. Soc. Jpn. 2019, 92, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 Step-scheme H2-Production Photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Leow, W.R.; Chen, X. Surface Complexation for Photocatalytic Organic Transformations. Bull. Chem. Soc. Jpn. 2019, 92, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Datta, K.K.R.; Reddy, B.V.S.; Ariga, K.; Vinu, A. Gold Nanoparticles Embedded in a Mesoporous Carbon Nitride Stabilizer for Highly Efficient Three-Component Coupling Reaction. Angew. Chem. Int. Ed. 2010, 49, 5961–5965. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K. Development and Application of New Iridium Catalysts for Efficient Dehydrogenative Reactions of Organic Molecules. Bull. Chem. Soc. Jpn. 2019, 92, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Ohkuma, T.; Kurono, N.; Arai, N. Development of Asymmetric Reactions Catalyzed by Ruthenium Complexes with Two Kinds of Ligands. Bull. Chem. Soc. Jpn. 2019, 92, 475–504. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Ariga, K.; Hill, J.P.; Lee, M.V.; Vinu, A.; Charvet, R.; Acharya, S. Challenges and Breakthroughs in Recent Research on Self-Assembly. Sci. Technol. Adv. Mater. 2008, 9, 014109. [Google Scholar] [CrossRef] [Green Version]
- Cherumukkil, S.; Vedhanarayanan, B.; Das, G.; Praveen, V.K.; Ajayaghosh, A. Self-Assembly of Bodipy-Derived Extended π-Systems. Bull. Chem. Soc. Jpn. 2018, 91, 100–120. [Google Scholar] [CrossRef]
- Shimizu, T. Self-Assembly of Discrete Organic Nanotubes. Bull. Chem. Soc. Jpn. 2018, 91, 623–668. [Google Scholar] [CrossRef]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-Assembly as a Key Player for Materials Nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariga, K.; Ji, Q.; Mori, T.; Naito, M.; Yamauchi, Y.; Abe, H.; Hill, J.P. Enzyme Nanoarchitectonics: Organization and Device Application. Chem. Soc. Rev. 2013, 42, 6322–6345. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xu, B. Instructed-Assembly (iA): A Molecular Process for Controlling Cell Fate. Bull. Chem. Soc. Jpn. 2018, 91, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Tsutsumi, H.; Mihara, H. Self-Assembling Peptides as Building Blocks of Functional Materials for Biomedical Applications. Bull. Chem. Soc. Jpn. 2019, 92, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.; Liz-Marzan, L.M. Recent Advances in Chiral Plasmonics-Towards Biomedical Applications. Bull. Chem. Soc. Jpn. 2019, 92, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Yamazoe, S.; Tsukuda, T. X-Ray Absorption Spectroscopy on Atomically Precise Metal Clusters. Bull. Chem. Soc. Jpn. 2019, 92, 193–204. [Google Scholar] [CrossRef]
- Ozaki, Y. Recent Advances in Molecular Spectroscopy of Electronic and Vibrational Transitions in Condensed Phase and Its Application to Chemistry. Bull. Chem. Soc. Jpn. 2019, 92, 629–654. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Gui, Y.; Kang, J.; Wang, W.; Tang, C. A DFT Study on the Adsorption of H2S and SO2 on Ni Doped MoS2 Monolayer. Nanomaterials 2018, 8, 646. [Google Scholar] [CrossRef] [Green Version]
- Hosoya, H. Mathematical Features of the Genealogy of Acyclic Conjugated Polyenes. Bull. Chem. Soc. Jpn. 2019, 92, 205–215. [Google Scholar] [CrossRef]
- Chen, X.; Hou, T.; Persson, K.A.; Zhang, Q. Combining Theory and Experiment in Lithium-Sulfur Batteries: Current Progress and Future Perspectives. Mater. Today 2019, 22, 142–158. [Google Scholar] [CrossRef]
- Matubayasi, N. Energy-Representation Theory of Solutions: Its Formulation and Application to Soft, Molecular Aggregates. Bull. Chem. Soc. Jpn. 2019, 92, 1910–1927. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Hill, J.P. Mechanical Control of Nanomaterials and Nanosystems. Adv. Mater. 2012, 24, 158–176. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Xu, Q. Metal Nanoparticle-Catalyzed Hydrogen Generation from Liquid Chemical Hydrides. Bull. Chem. Soc. Jpn. 2018, 91, 1606–1617. [Google Scholar] [CrossRef] [Green Version]
- Imaoka, T.; Yamamoto, K. Wet-Chemical Strategy for Atom-Precise Metal Cluster Catalysts. Bull. Chem. Soc. Jpn. 2019, 92, 941–948. [Google Scholar] [CrossRef] [Green Version]
- Kageyama, H.; Yajima, T.; Tsujimoto, Y.; Yamamoto, T.; Tassel, C.; Kobayashi, Y. Exploring Structures and Properties through Anion Chemistry. Bull. Chem. Soc. Jpn. 2019, 92, 1349–1357. [Google Scholar] [CrossRef] [Green Version]
- Ariga, K.; Ji, Q.; Nakanishi, W.; Hill, J.P.; Aono, M. Nanoarchitectonics: A New Materials Horizon for Nanotechnology. Mater. Horizons 2015, 2, 406–413. [Google Scholar] [CrossRef]
- Ariga, K.; Minami, K.; Ebara, M.; Nakanishi, J. What Are Emerging Concepts and Challenges in NANO?: Nanoarchitectonics, Hand-Operating Nanotechnology, and Mechanobiology. Polym. J. 2016, 48, 371–389. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Hill, J.P.; Bando, Y.; Aono, M. Forming Nanomaterials as Layered Functional Structures towards Materials Nanoarchitectonics. NPG Asia Mater. 2012, 4, e17. [Google Scholar] [CrossRef] [Green Version]
- Aono, M.; Ariga, K. The Way to Nanoarchitectonics & the Way of Nanoarchitectonics. Adv. Mater. 2016, 28, 989–992. [Google Scholar]
- Ariga, K.; Li, J.; Fei, J.; Ji, Q.; Hill, J.P. Nanoarchitectonics for Dynamic Functional Materials from Atomic/Molecular-Level Manipulation to Macroscopic Action. Adv. Mater. 2016, 28, 1251–1286. [Google Scholar] [CrossRef]
- Ariga, K.; Yusuke, Y. Nanoarchitectonics from Atom to Life. Chem. Asian. J. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, M.; Shrestha, L.K.; Mori, T.; Ji, Q.; Hill, J.P.; Ariga, K. Amphiphile Nanoarchitectonics: From Basic Physical Chemistry to Advanced Applications. Phys. Chem. Chem. Phys. 2013, 15, 10580–10611. [Google Scholar] [CrossRef] [PubMed]
- Azhar, A.; Li, Y.; Cai, Z.; Zakaria, M.B.; Masud, M.K.; Hossain, M.S.A.; Kim, J.; Zhang, W.; Na, J.; Yamauchi, Y.; et al. Nanoarchitectonics: A New Materials Horizon for Prussian Blue and Its Analogues. Bull. Chem. Soc. Jpn. 2019, 92, 875–904. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Kitao, T.; Uemura, T. Supramolecular Chiral Nanoarchitectonics. Adv. Mater. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Rydzek, G.; Ji, Q.; Li, M.; Schaaf, P.; Hill, J.P.; Boulmedais, F.; Ariga, K. Electrochemical Nanoarchitectonics and Layer-by-Layer Assembly: From Basics to Future. Nano Today 2015, 10, 138–167. [Google Scholar] [CrossRef] [Green Version]
- Ariga, K.; Mori, T.; Li, J. Langmuir Nanoarchitectonics from Basic to Frontier. Langmuir 2019, 35, 3585–3599. [Google Scholar] [CrossRef]
- Abe, H.; Liu, J.; Ariga, K. Catalytic Nanoarchitectonics for Environmentally-Compatible Energy Generation. Mater. Today 2016, 19, 12–18. [Google Scholar] [CrossRef]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.P.; Ariga, K. Porphyrin-Based Sensor Nanoarchitectonics in Diverse Physical Detection Modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Yang, W.; Ariga, K. Soft Nanoarchitectonics for Enantioselective Biosensing. Acc. Chem. Res. 2020, in press. [Google Scholar] [CrossRef]
- Ariga, K.; Watanabe, S.; Mori, T.; Takeya, J. Soft 2D Nanoarchitectonics. NPG Asia Mater. 2018, 10, 90–106. [Google Scholar] [CrossRef]
- Ariga, K.; Ito, M.; Mori, T.; Watanabe, S.; Takeya, J. Atom/Molecular Nanoarchitectonics for Devices and Related Applications. Nano Today 2019, 28, 100762. [Google Scholar] [CrossRef]
- Rajendran, R.; Shrestha, L.K.; Minami, K.; Subramanian, M.; Jayavel, R.; Ariga, K. Dimensionally Integrated Nanoarchitectonics for Novel Composite from 0D, 1D, and 2D Nanomaterials: RGO/CNT/CeO2 Ternary Nanocomposite with Electrochemical Performance. J. Mater. Chem. A 2014, 2, 18480–18487. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Ariga, K. Redox Active Polymers for Energy Storage Nanoarchitectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef] [Green Version]
- Ariga, K.; Ishihara, S.; Abe, H.; Li, M.; Hill, J.P. Materials Nanoarchitectonics for Environmental Remediation and Sensing. J. Mater. Chem. 2012, 22, 2369–2377. [Google Scholar] [CrossRef]
- Nakanishi, W.; Minami, K.; Shrestha, L.K.; Ji, Q.; Hill, J.P.; Ariga, K. Bioactive Nanocarbon Assemblies: Nanoarchitectonics and Applications. Nano Today 2014, 9, 378–394. [Google Scholar] [CrossRef] [Green Version]
- Ariga, K.; Leong, D.T.; Mori, T. Nanoarchitectonics for Hybrid and Related Materials for Bio-Oriented Applications. Adv. Funct. Mater. 2018, 28, 1702905. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for Electrochemical Capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xia, Y. Recent Progress in Supercapacitors: From Materials Design to System Construction. Adv. Mater. 2013, 25, 5336–5342. [Google Scholar] [CrossRef]
- Wang, J.; Dong, S.; Ding, B.; Wang, Y.; Hao, X.; Dou, H.; Xia, Y.; Zhang, X. Pseudocapacitive Materials for Electrochemical Capacitors: From Rational Synthesis to Capacitance Optimization. Nat. Sci. Rev. 2017, 4, 71–90. [Google Scholar] [CrossRef]
- Chen, D.; Wang, W.; Wang, R.; Shen, G. Ternary Oxide Nanostructured Materials for Supercapacitors: A Review. J. Mater. Chem. A 2015, 3, 10158–10173. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Yaïci, W.; Entchev, E. Hybrid Battery/Supercapacitor Energy Storage System for the Electric Vehicles. J. Power Sources 2018, 374, 237–248. [Google Scholar] [CrossRef]
- Pell, W.G.; Conway, B.E. Peculiarities and Requirements of Asymmetric Capacitor Devices based on Combination of Capacitor and Battery-type Electrodes. J. Powder Sources 2004, 136, 334–345. [Google Scholar] [CrossRef]
- Burke, A. R&D Considerations for the Performance and Application of Electrochemical Capacitors. Electrochim. Acta 2007, 53, 1083–1091. [Google Scholar]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fua, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest Advances in Supercapacitors: From New Electrode Materials to Novel Device Designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Vinu, A.; Miyahara, M.; Hill, J.P.; Mori, T. One-Pot Separation of Tea Components through Selective Adsorption on Pore-Engineered Nanocarbon, Carbon Nanocage. J. Am. Chem. Soc. 2007, 129, 11022–11023. [Google Scholar] [CrossRef]
- Maji, S.; Shrestha, L.K.; Ariga, K. Nanoarchitectonics for Nanocarbon Assembly and Composite. J. Inorg. Organomet. Polym. Mater. 2020, 30, 42–55. [Google Scholar] [CrossRef]
- Akiyama, T. Development of Fullerene Thin-Film Assemblies and Fullerene-Diamine Adducts towards Practical Nanocarbon-based Electronic Materials. Bull. Chem. Soc. Jpn. 2019, 92, 1181–1199. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Torad, N.L.; Li, C.; Imura, M.; Suzuki, N.; Ishihara, S.; Ariga, K.; Yamauchi, Y. Synthesis of Nanoporous Carbon-Cobalt-Oxide Hybrid Electrocatalysts by Thermal Conversion of Metal-Organic Frameworks. Chem. Eur. J. 2014, 20, 4217–4221. [Google Scholar] [CrossRef]
- Sengottaiyan, C.; Jayavel, R.; Shrestha, R.G.; Subramani, T.; Maji, S.; Kim, J.H.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Indium Oxide Carbon Nanotube/Reduced Graphene Oxide Ternary Nanocomposite with Enhanced Electrochemical Supercapacitance. Bull. Chem. Soc. Jpn. 2019, 92, 521–528. [Google Scholar] [CrossRef]
- Han, S.A.; Lee, J.; Shim, K.; Lin, J.J.; Shahabuddin, M.; Lee, J.W.; Kim, S.W.; Park, M.S.; Kim, J.H. Strategically Designed Zeolitic Imidazolate Frameworks for Controlling the Degree of Graphitization. Bull. Chem. Soc. Jpn. 2018, 91, 1474–1480. [Google Scholar] [CrossRef] [Green Version]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.G.; Jones, R.T.; Fichtner, M. Calcined Chicken Eggshell Electrode for Battery and Supercapacitor Applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef] [Green Version]
- Minakshi, M.; Visbal, H.; Mitchell, D.R.G.; Fichtner, M. Bio-Waste Chicken Eggshell to Store Energy. Dalton Trans. 2018, 47, 16828–16834. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.K.; Ji, Q.; Mori, T.; Miyazawa, K.; Yamauchi, Y.; Hill, J.P.; Ariga, K. Fullerene Nanoarchitectonics: From Zero to Higher Dimensions. Chem. Asian. J. 2013, 8, 1662–1679. [Google Scholar] [CrossRef]
- Miyazawa, K. Synthesis of Fullerene Nanowhiskers Using the Liquid-Liquid Interfacial Precipitation Method and Their Mechanical, Electrical and Superconducting Properties. Sci. Technol. Adv. Mater. 2015, 16, 013502. [Google Scholar] [CrossRef]
- Shrestha, R.G.; Shrestha, L.K.; Khan, A.H.; Kumar, G.S.; Acharya, S.; Ariga, K. Demonstration of ultrarapid interfacial formation of 1D fullerene nanorods with photovoltaic properties. ACS Appl. Mater. Interfaces 2014, 6, 15597–15603. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.K.; Shrestha, R.G.; Yamauchi, Y.; Hill, J.P.; Nishimura, T.; Miyazawa, K.; Kawai, T.; Okada, S.; Wakabayashi, K.; Ariga, K. Nanoporous Carbon Tubes from Fullerene Crystals as the π-Electron Carbon Source. Angew. Chem. Int. Ed. 2015, 54, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Bairi, P.; Shrestha, R.G.; Hill, J.P.; Nishimura, T.; Ariga, K.; Shrestha, L.K. Mesoporous Graphitic Carbon Microtubes Derived from Fullerene C70 Tubes as a High Performance Electrode Material for Advanced Supercapacitors. J. Mater. Chem. A 2016, 4, 13899–13906. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, L.K.; Shrestha, R.G.; Hill, J.P.; Tsuruoka, T.; Ji, Q.; Nishimura, T.; Ariga, K. Surfactant-Triggered Nanoarchitectonics of Fullerene C60 Crystals at a Liquid-Liquid Interface. Langmuir 2016, 32, 12511–12519. [Google Scholar] [CrossRef]
- Tang, Q.; Bairi, P.; Shrestha, R.G.; Hill, J.P.; Ariga, K.; Zeng, H.; Ji, Q.; Shrestha, L.K. Quasi 2D Mesoporous Carbon Microbelts Derived from Fullerene Crystals as an Electrode Material for Electrochemical Supercapacitors. ACS Appl. Mater. Interface 2017, 9, 44458–44465. [Google Scholar] [CrossRef]
- Bairi, P.; Maji, S.; Hill, J.P.; Kim, J.H.; Ariga, K.; Shrestha, L.K. Mesoporous Carbon Cubes Derived from Fullerene Crystals as a High Rate Performance Electrode Material for Supercapacitors. J. Mater. Chem. A 2019, 7, 12654–12660. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Kim, J.; Kim, J.H.; Yamauchi, Y. Nanoarchitectures for Metal-Organic Framework-Derived Nanoporous Carbons toward Supercapacitor Applications. Acc. Chem. Res. 2016, 49, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-Organic Framework as a Template for Porous Carbon Synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef] [PubMed]
- Chaikittisilp, W.; Hu, M.; Wang, H.; Huang, H.-S.; Fujita, T.; Wu, C.-W.K.; Chen, L.C.; Yamauchi, Y.; Ariga, K. Nanoporous Carbons through Direct Carbonization of a Zeolitic Imidazolate Framework for Supercapacitor Electrodes. Chem. Commun. 2012, 48, 7259–7261. [Google Scholar] [CrossRef] [PubMed]
- Kitao, T.; Bracco, S.; Comotti, A.; Sozzani, P.; Naito, M.; Seki, S.; Uemura, T.; Kitagawa, S. Confinement of Single Polysilane Chains in Coordination Nanospaces. J. Am. Chem. Soc. 2015, 137, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Marpaung, F.; Park, T.; Kim, M.; Yi, J.W.; Lin, J.; Wang, J.; Ding, B.; Lim, H.; Konstantinov, K.; Yamauchi, Y.; et al. Gram-Scale Synthesis of Bimetallic ZIFs and Their Thermal Conversion to Nanoporous Carbon Materials. Nanomaterials 2019, 9, 1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.; Reboul, J.; Furukawa, S.; Torad, N.L.; Ji, Q.; Srinivasu, P.; Ariga, K.; Kitagawa, S.; Yamauchi, Y. Direct Carbonization of Al-based Porous Coordination Polymer for Synthesis of Nanoporous Carbon. J. Am. Chem. Soc. 2012, 134, 2864–2867. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Hill, J.P.; Ji, Q.; Shrestha, L.K.; Ariga, K. Supercapacitive Hybrid Materials from the Thermolysis of Porous Coordination Nanorods Based on a Catechol Porphyrin. J. Mater. Chem. A 2016, 4, 5737–5744. [Google Scholar] [CrossRef]
- Knox, J.H.; Kaur, B.; Millward, G.R. Structure and Performance of Porous Graphitic Carbon in Liquid Chromatography. J. Chromatogr. 1986, 352, 3. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of Highly Ordered Carbon Molecular Sieves via Template-Mediated Structural Transformation. J. Phys. Chem. B 1999, 103, 77434–77746. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M. Ordered Mesoporous Carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar]
- Jun, S.; Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec, M.; Liu, Z.; Ohsuna, T.; Terasaki, O. Synthesis of New, Nanoporous Carbon with Hexagonally Ordered Mesostructure. J. Am. Chem. Soc. 2000, 122, 10712–10713. [Google Scholar] [CrossRef]
- Kim, T.-W.; Kleitz, F.; Paul, B.; Ryoo, R. MCM-48-like Large Mesoporous Silicas with Tailored Pore Structure: Facile Synthesis Domain in a Ternary Triblock Copolymer-Butanol-Water System. J. Am. Chem. Soc. 2005, 127, 7601–7610. [Google Scholar] [CrossRef] [PubMed]
- Che, S.; Garcia-Bennett, A.E.; Liu, X.; Hodgkins, R.P.; Wright, P.A.; Zhao, D.; Terasaki, O.; Tatsumi, T. Synthesis of Large-Pore Ia3 −d Mesoporous Silica and Its Tubelike Carbon Replica. Angew. Chem., Int. Ed. 2003, 42, 3930–3934. [Google Scholar] [CrossRef] [PubMed]
- Vinu, A.; Miyahara, M.; Sivamurugan, V.; Mori, T.; Ariga, K. Large Pore Cage Type Mesoporous Carbon, Carbon Nanocage: A Superior Adsorbent for Biomaterials. J. Mater. Chem. 2005, 15, 5122–5127. [Google Scholar] [CrossRef]
- Vinu, A.; Miyahara, M.; Mori, T.; Ariga, K. Carbon Nanocage: A Large-Pore Cage-Type Mesoporous Carbon Material as an Adsorbent for Biomolecules. J Porous Mater 2006, 13, 379–383. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Wang, Y.; Hosono, E.; Zhou, H. Mesoporous Carbon Nanofibers for Supercapacitor Application. J. Phys. Chem. C 2009, 113, 1093–1097. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Mokaya, R. Templated Nanoscale Porous Carbons. Nanoscale 2010, 2, 639–659. [Google Scholar] [CrossRef]
- He, X.; Li, R.; Qiu, J.; Xie, K.; Ling, P.; Yu, M.; Zhang, X.; Zheng, M. Synthesis of Mesoporous Carbons for Supercapacitors from Coal Tar Pitch by Coupling Microwave-Assisted KOH activation with a MgO Template. Carbon 2012, 50, 4911–4921. [Google Scholar] [CrossRef]
- He, X.; Li, R.; Han, J.; Yu, M.; Wu, M. Facile Preparation of Mesoporous Carbons for Supercapacitors by One-Step Microwave-Assisted ZnCl2 Activation. Mater. Lett. 2013, 94, 158–160. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, L.-b.; Li, X.; Ran, F.; Luo, Y.-C.; Kang, L. Mesoporous Carbons for Supercapacitors Obtained by the Pyrolysis of Block Copolymers. New Carbon Mater. 2015, 30, 302–309. [Google Scholar] [CrossRef]
- Tang, D.; Hu, S.; Dai, F.; Yi, R.; Gordin, M.L.; Chen, S.; Song, J.; Wang, D. Self-Templated Synthesis of Mesoporous Carbon from Carbon Tetrachloride Precursor for Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 6779–6783. [Google Scholar] [CrossRef] [PubMed]
- Magana, J.R.; Kolen’ko, Y.V.; Deepak, F.L.; Solans, C.; Shrestha, R.G.; Hill, J.P.; Ariga, K.; Shrestha, L.K.; Rodriguez-Abreu, C. From Chromonic Self-Assembly to Hollow Carbon Nanofibers: Efficient Materials in Supercapacitor and Vapor-Sensing Applications. ACS Appl. Mater. Interfaces 2016, 8, 31231–31238. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, J.; Shrestha, L.K.; Hossain, Md. S.A.; Alothman, Z.A.; Yamauchi, Y.; Ariga, K. Activated Porous Carbon Spheres with Customized Mesopores through Assembly of Diblock Copolymers for Electrochemical Capacitor. ACS Appl. Mater. Interfaces 2017, 9, 18986–18993. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhang, L.; Liu, J.; Wen, X.; Chen, X.; Ma, J.; Tang, T.; Mijowska, E. Hierarchical Porous Carbon Sheets Derived on a MgO Template for High-performance Supercapacitor Applications. Nanotechnology 2019, 30, 295703. [Google Scholar] [CrossRef] [PubMed]
- Nanaji, K.; Rao, T.N.; Varadaraju, U.V.; Anandan, S. Pore Size-Engineered Three-Dimensional Ordered Mesoporous Carbons with Improved Electrochemical Performance for Supercapacitor and Lithium-ion Battery Applications. Chem. Select 2019, 4, 10104–10112. [Google Scholar] [CrossRef]
- Tian, W.; Zhu, J.; Dong, Y.; Zhao, J.; Li, J.; Guo, N.; Lin, H.; Zhang, S.; Jia, D. Micelle-Induced Assembly of Graphene Quantum Dots into Conductive Porous Carbon for High Rate Supercapacitor Electrodes at High Mass Loadings. Carbon 2020, 161, 89–96. [Google Scholar] [CrossRef]
- Rajbhandari, R.; Shrestha, L.K.; Pokharel, B.P.; Pradhananga, R.R. Development of Nanoporous Structure in Carbons by Chemical Activation with Zinc Chloride. J. Nanosci. Nanotechnol. 2013, 13, 2613–2623. [Google Scholar] [CrossRef]
- Adhikari, M.P.; Adhikari, R.; Shrestha, R.G.; Rajendran, R.; Adhikari, L.; Bairi, P.; Pradhananga, R.R.; Shrestha, L.K.; Ariga, K. Nanoporous Activated Carbons Derived from Agro-Waste Corncob for Enhanced Electrochemical and Sensing Performance. Bull. Chem. Soc. Jpn. 2015, 88, 1108–1115. [Google Scholar] [CrossRef]
- Genovese, M.; Jiang, J.; Lian, K.; Holmb, N. High Capacitive Performance of Exfoliated Biochar Nanosheets from Biomass Waste Corn Cob. J. Mater. Chem. A 2015, 3, 2903–2913. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Adhikari, L.; Shrestha, R.G.; Adhikari, M.P.; Adhikari, R.; Hill, J.P.; Pradhananga, R.R.; Ariga, K. Nanoporous Carbon Materials with Enhanced Supercapacitance Performance and Non-Aromatic Chemical Sensing with C1/C2 Alcohol Discrimination. Sci. Technol. Adv. Mater. 2016, 17, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, L.K.; Shrestha, R.G.; Joshi, S.; Rajbhandari, R.; Shrestha, N.; Adhikari, M.P.; Pradhananga, R.R.; Ariga, K. Nanoarchitectonics of Nanoporous Carbon Materials from Natural Resource for Supercapacitor Application. J. Inorg. Organomet. Polym. 2017, 27 (Suppl 1), S48–S56. [Google Scholar] [CrossRef]
- Feng, H.; Zheng, M.; Dong, H.; Xiao, Y.; Hu, H.; Sun, Z.; Long, C.; Cai, Y.; Zhao, X.; Zhang, H.; et al. Three-dimensional Honeycomb-like Hierarchically Structured Carbon for High-performance Supercapacitors Derived from High-Ash-Content Sewage Sludge. J. Mater. Chem. A 2015, 3, 15225–15234. [Google Scholar] [CrossRef]
- Wei, X.; Li, Y.; Gao, S. Biomass-Derived Interconnected Carbon Nanoring Electrochemical Capacitors with High Performance in Both Strongly Acidic and Alkaline Electrolytes. J. Mater. Chem. A 2017, 5, 181–188. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Z.; Liu, Y.; Fan, L.-Z. Biowaste-Derived 3D Honeycomb-like Porous Carbon with Binary-Heteroatom Doping for High-performance Flexible Solid-State Supercapacitors. J. Mater. Chem. A 2018, 6, 160–166. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, K. Converting Corncob to Activated Porous Carbon for Supercapacitor Application. Nanomaterials 2018, 8, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, G.; Huang, W.; Guan, K.; Li, H.; Xie, Y.; Liang, Y.; Liu, Y.; Zheng, M. A Universal KOH-Free Strategy towards Nitrogen Doped Carbon Nanosheets for High-Rate and High Energy Storage Devices. J. Mater. Chem. A 2019, 7, 26469–26478. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, W.; Zhou, W.; Chen, S.; Zhang, Y. Hierarchical Porous Carbon Derived from Sichuan Pepper for High-performance Symmetric Supercapacitor with Decent Rate Capability and Cycling Stability. Nanomaterials 2019, 9, 553. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Zequine, C.; Bhoyate, S.; Lin, W.; Li, X.; Kahol, P.; Gupta, R. Waste Coffee Management: Deriving High-performance Supercapacitors Using Nitrogen-doped Coee-Derived Carbon. C J. Carbon Res. 2019, 5, 44. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Lei, Y.; Li, Y.; Zhang, Y.; Xiao, W. Nitrogen-doped Hierarchical Meso/Microporous Carbon from Bamboo Fungus for Symmetric Supercapacitor Applications. Molecules 2019, 24, 3677. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Chen, Z.; Yu, C.; Zhong, W. Facile Synthesis of High Nitrogen-doped Content, Mesopore-Dominated Biomass-Derived Hierarchical Porous Graphitic Carbon for High Performance Supercapacitors. Electrochim. Acta 2020, 334, 135615. [Google Scholar] [CrossRef]

| Biomass | Electrolyte | Current Density/Scan Rate | Specific Capacitance (F g−1) | Reference |
|---|---|---|---|---|
| Batata leaves and stalks | 1 M H2SO4 | 1 A g−1 | 532.5 | [143] |
| Batata leaves and stalks | 6 M KOH | 1 A g−1 | 350 | [143] |
| Peach gum | 6 M KOH | 0.5 A g−1 | 426 | [150] |
| Peanut dregs | 6 M KOH | 0.5 A g−1 | 340 | [146] |
| Corncob | 6 M KOH | 0.1 A g−1 | 404 | [144] |
| Areca catechu nut | 1 M H2SO4 | 5 mV s−1 | 342 | [141] |
| Bamboo | 1 M H2SO4 | 5 mV s−1 | 256 | [140] |
| Corncob | 1 M H2SO4 | 5 mV s−1 | 340.8 | [138] |
| Sewage sludge | 1 M Na2SO4 | 0.5 A g−1 | 379 | [142] |
| Corn cob | 0.5 M H2SO4 | 0.5 A g−1 | 210 | [139] |
| Lapsi seed | 1 M H2SO4 | 5 mV s−1 | 328 | [137] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, R.G.; Maji, S.; Shrestha, L.K.; Ariga, K. Nanoarchitectonics of Nanoporous Carbon Materials in Supercapacitors Applications. Nanomaterials 2020, 10, 639. https://doi.org/10.3390/nano10040639
Shrestha RG, Maji S, Shrestha LK, Ariga K. Nanoarchitectonics of Nanoporous Carbon Materials in Supercapacitors Applications. Nanomaterials. 2020; 10(4):639. https://doi.org/10.3390/nano10040639
Chicago/Turabian StyleShrestha, Rekha Goswami, Subrata Maji, Lok Kumar Shrestha, and Katsuhiko Ariga. 2020. "Nanoarchitectonics of Nanoporous Carbon Materials in Supercapacitors Applications" Nanomaterials 10, no. 4: 639. https://doi.org/10.3390/nano10040639
APA StyleShrestha, R. G., Maji, S., Shrestha, L. K., & Ariga, K. (2020). Nanoarchitectonics of Nanoporous Carbon Materials in Supercapacitors Applications. Nanomaterials, 10(4), 639. https://doi.org/10.3390/nano10040639