Controlled Growth and Bandstructure Properties of One Dimensional Cadmium Sulfide Nanorods for Visible Photocatalytic Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CdS Nanorods (CdS NRs)
2.3. Characterization Methods
2.4. Photocatalytic H2 Evolution Measurements
3. Results and Discussion
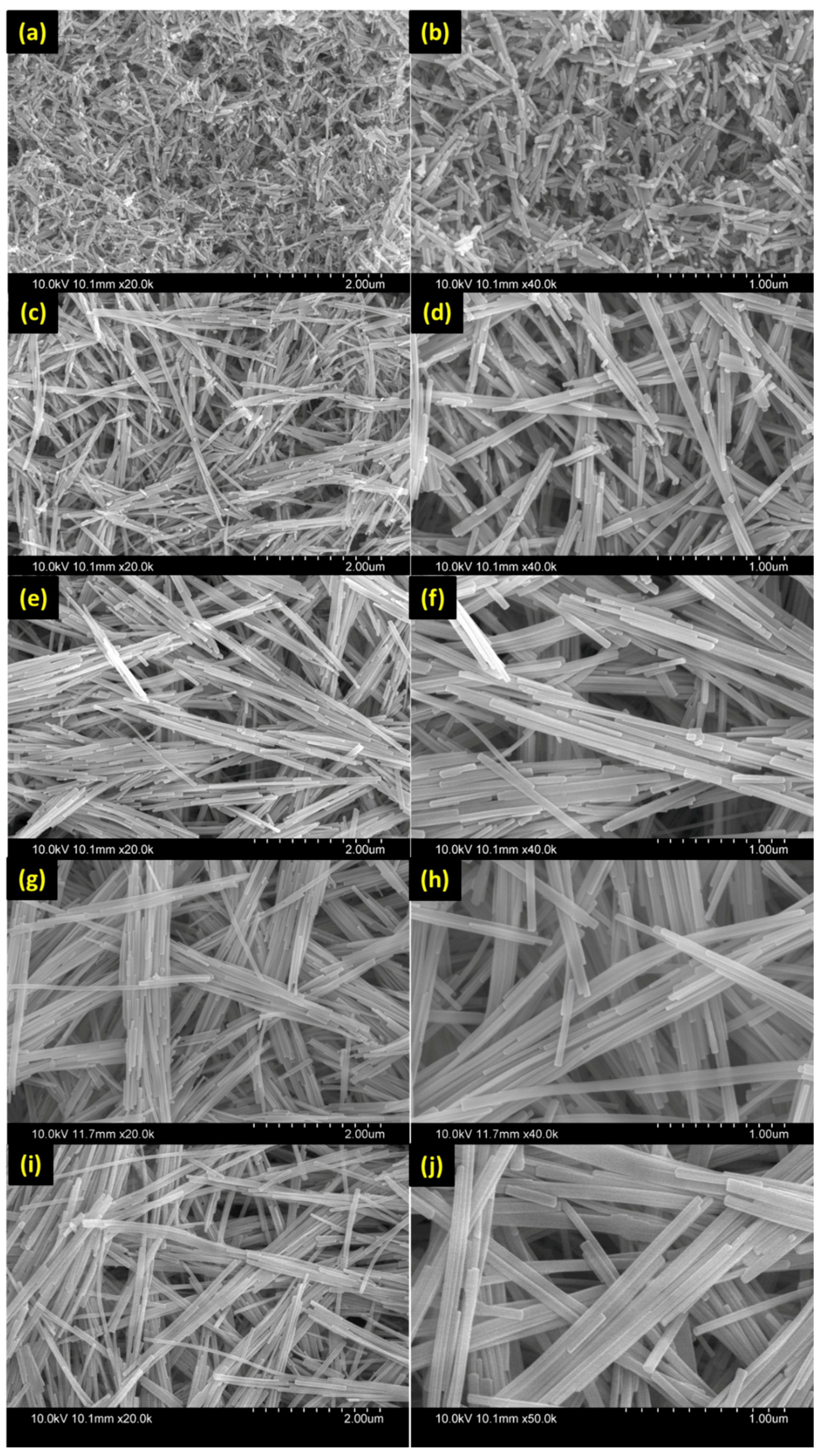
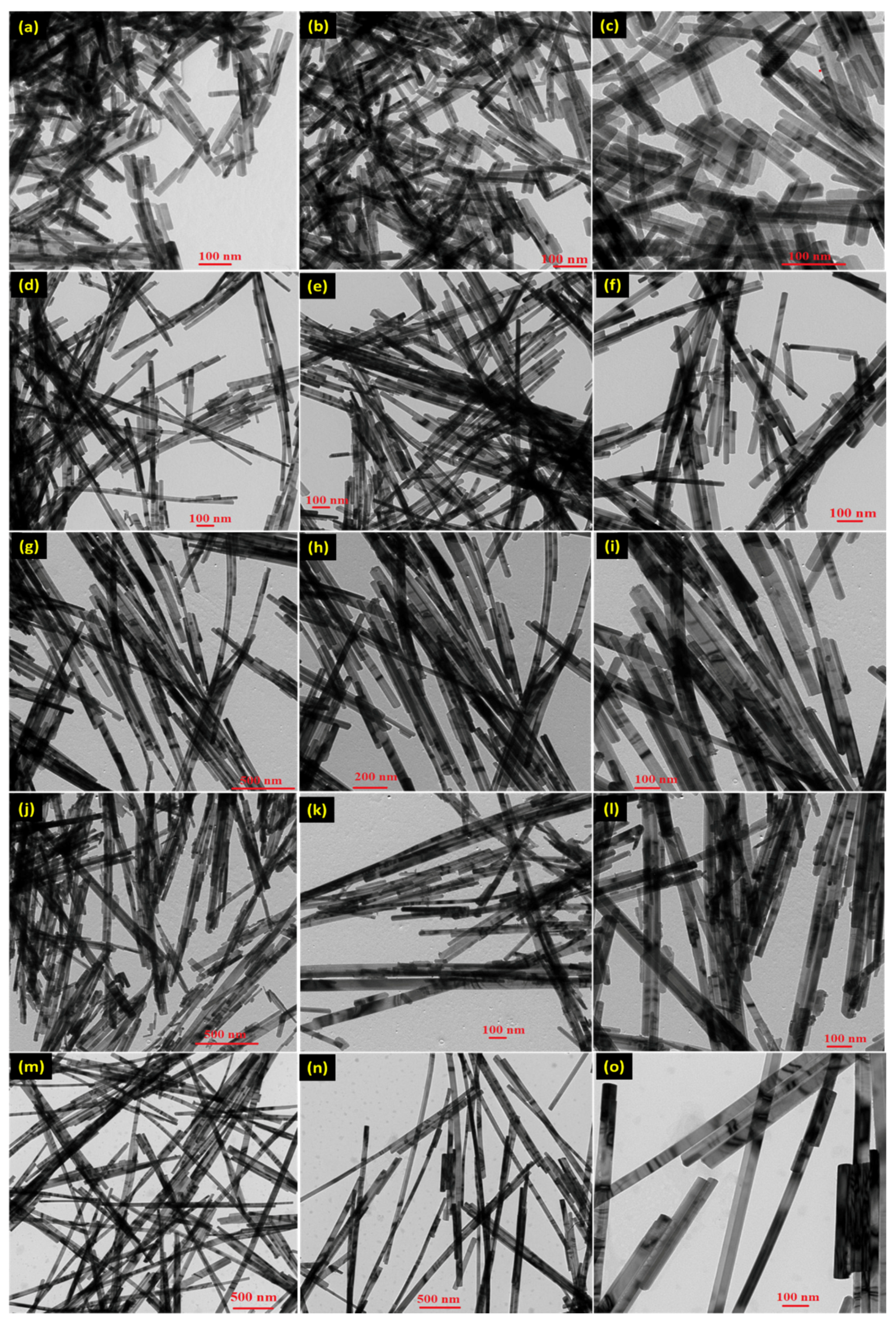
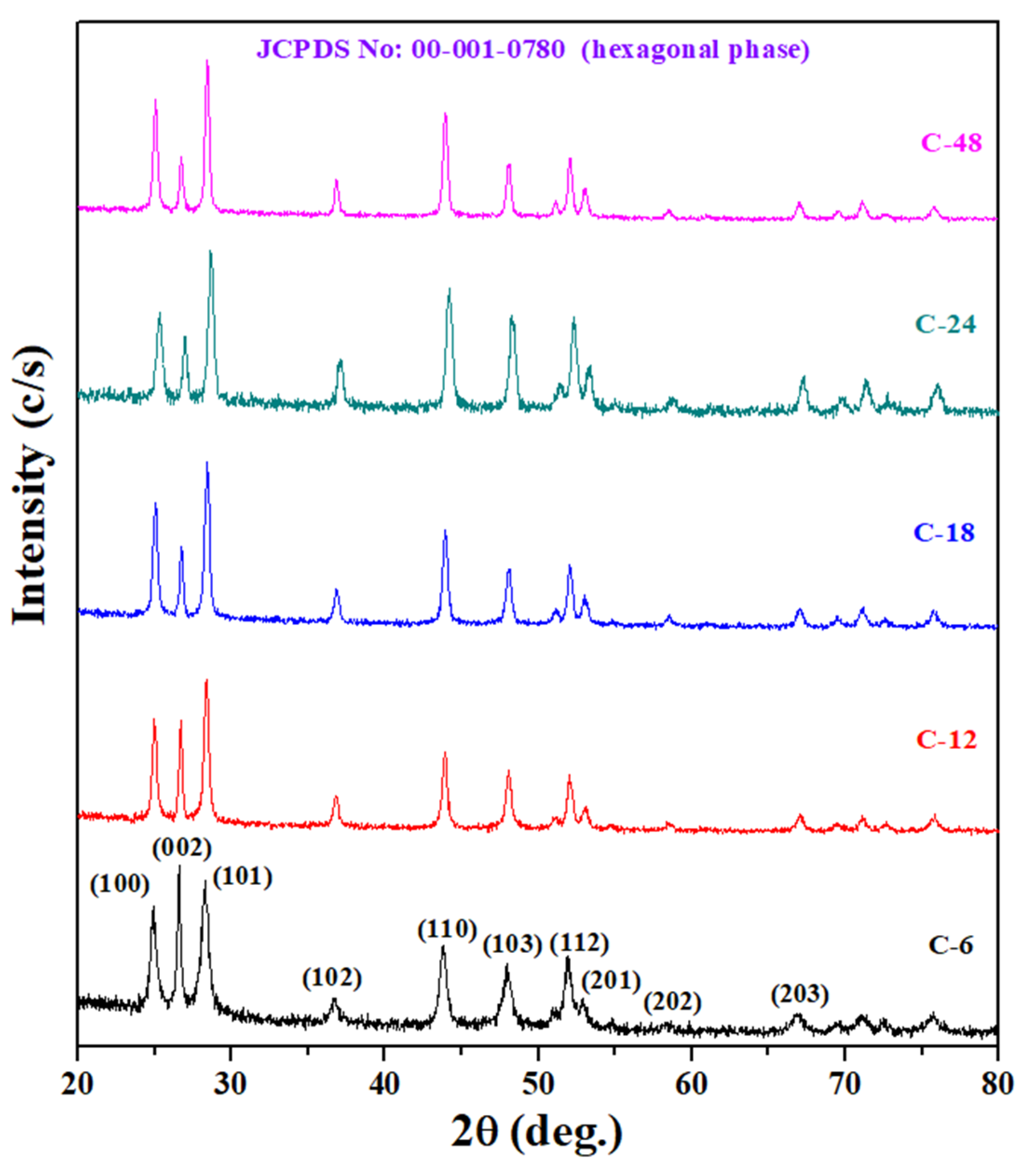
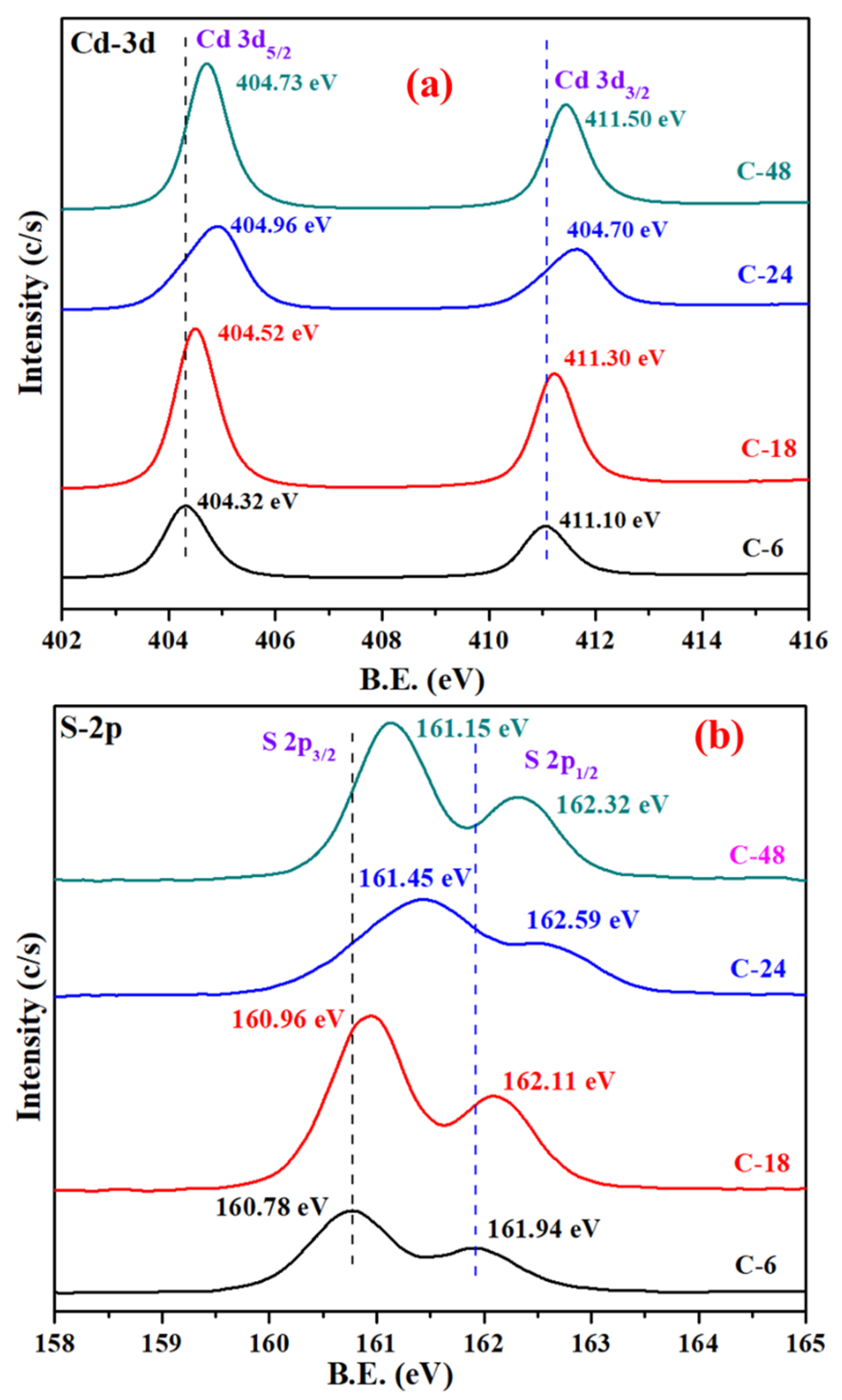
3.1. Morphological and Structural Studies
3.2. UV-vis Absorption, Bandgap and Bandstructure Studies
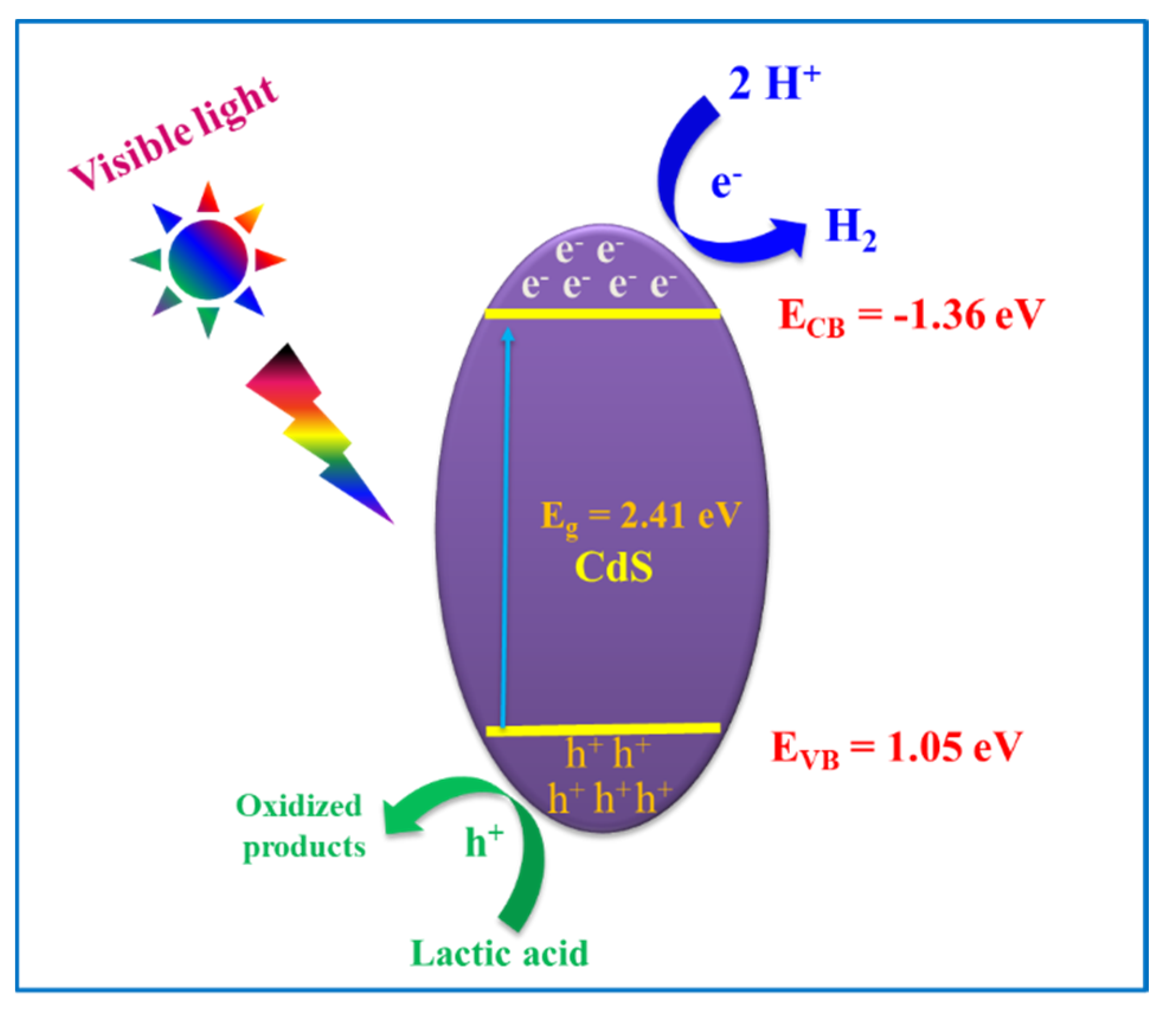
3.3. Visible Photocatalytic H2 Evolution Activity and Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luo, J.M.; Zhang, S.Q.; Sun, M.; Yang, L.X.; Luo, S.L.; Crittenden, J.C. A Critical Review on Energy Conversion and Environmental Remediation of Photocatalysts with Remodeling Crystal Lattice, Surface, and Interface. ACS Nano 2019, 13, 9811–9840. [Google Scholar] [CrossRef]
- Tilley, S.D. Recent Advances and Emerging Trends in PhotoElectrochemical Solar Energy Conversion. Adv. Energy Mater. 2018, 8, 1802877. [Google Scholar]
- Tian, X.Y.; Zhao, P.C.; Sheng, W.C. Hydrogen Evolution and Oxidation: Mechanistic Studies and Material Advances. Adv. Mater. 2019, 31, 1808066. [Google Scholar] [CrossRef] [PubMed]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.-D.; Liu, S.H.; Teng, C.P.; Han, M.-Y. Recent Progress in Energy-Driven Water Splitting. Adv. Sci. 2017, 4, 1600337. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Zhou, Y.; Tu, W.G.; Ye, J.H.; Zou, Z.G. State-of-the-Art Progress in Diverse Heterostructured Photocatalysts toward Promoting Photocatalytic Performance. Adv. Funct. Mater. 2015, 25, 998–1013. [Google Scholar] [CrossRef]
- Xu, C.P.; Anusuyadevi, P.R.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Hu, W.Y.; Zhou, W.; Zhang, K.F.; Zhang, X.C.; Wang, L.; Jiang, B.J.; Tian, G.H.; Zhao, D.Y.; Fu, H.G. Facile strategy for controllable synthesis of stable mesoporous black TiO2 hollow spheres with efficient solar-driven photocatalytic hydrogen evolution. J. Mater. Chem. 2016, 4, 7495–7502. [Google Scholar] [CrossRef]
- Xiong, H.L.; Wu, L.L.; Yu, L.; Gao, T.; Li, K.Q.; Long, Y.; Zhang, R.; Zhang, L.; Qiao, Z.-A.; Huo, Q.S.; et al. Controllable Synthesis of Mesoporous TiO2 Polymorphs with Tunable Crystal Structure for Enhanced Photocatalytic H2 Production. Adv. Energy Mater. 2019, 9, 1901634. [Google Scholar] [CrossRef]
- Zhang, G.G.; Li, G.S.; Lan, Z.-A.; Lin, L.H.; Savateev, A.; Heil, T.; Zafeiratos, S.; Wang, X.C.; Antoniett, M. Optimizing Optical Absorption, Exciton Dissociation, and Charge Transfer of a Polymeric Carbon Nitride with Ultrahigh Solar Hydrogen Production Activity. Angew. Chem. Int. Ed. 2017, 129, 13630–13634. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhang, G.G.; Chen, X.F.; Lin, S.; Möhlmann, L.; Dołęga, G.; Lipner, G.; Antonietti, M.; Blechert, S.; Wang, X.C. Co-Monomer Control of Carbon Nitride Semiconductors to Optimize Hydrogen Evolution with Visible Light. Angew. Chem. Int. Ed. 2012, 124, 3237–3241. [Google Scholar] [CrossRef]
- Xu, Q.C.; Yu, L.; Jiang, H.; Hu, Y.J.; Liu, H.L.; Li, C.Z. Unsaturated Sulfur Edge Engineering of Strongly Coupled MoS2 Nanosheet–Carbon Macroporous Hybrid Catalyst for Enhanced Hydrogen Generation. Adv. Energy Mater. 2018, 8, 1802553. [Google Scholar] [CrossRef]
- Yang, L.; Guo, S.H.; Li, X.H. Au nanoparticles@MoS2 core-shell structures with moderate MoS2 coverage for efficient photocatalytic water splitting. J. Alloy. Compd. 2017, 706, 82–88. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Wang, X.; Lou, X.W.D. Formation of Hierarchical Co9S8@ZnIn2S4 Heterostructured Cages as an Efficient Photocatalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2018, 140, 15145–15148. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Q.; Lin, Z.Y.; Yan, B.; Xi, C.X.; Yang, G.W. Half-unit-cell ZnIn2S4 monolayer with sulfur vacancies for photocatalytic hydrogen evolution. Appl. Catal. B 2019, 248, 193–201. [Google Scholar] [CrossRef]
- Li, Y.; Tang, L.; Peng, S.; Li, Z.; Lu, G. Phosphate-assisted hydrothermal synthesis of hexagonal CdS for efficient photocatalytic hydrogen evolution. CrystEngComm 2012, 14, 6974–6982. [Google Scholar] [CrossRef]
- Qi, L.; Yu, J.; Jaroniec, M. Preparation and enhanced visible-light photocatalytic H2-production activity of CdS-sensitized Pt/TiO2 nanosheets with exposed (001) facets. Phys. Chem. 2011, 13, 8915–8923. [Google Scholar] [CrossRef]
- Yu, J.G.; Yu, Y.F.; Zhou, P.; Xiao, W.; Cheng, B. Morphology-dependent photocatalytic H2-production activity of CdS. Appl. Catal. B 2014, 156, 184–191. [Google Scholar] [CrossRef]
- Li, Y.L.; Jin, T.; Ma, G.; Li, Y.C.; Fan, L.Z.; Li, X.H. Metal–organic framework assisted and in situ synthesis of hollow CdS nanostructures with highly efficient photocatalytic hydrogen evolution. Dalton Trans. 2019, 48, 5649–5655. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, B.; Yu, J. Hierarchical porous CdS nanosheet-assembled flowers with enhanced visible-light photocatalytic H2-production performance. Appl. Catal. B 2013, 138, 299–303. [Google Scholar] [CrossRef]
- Sun, Q.M.; Wang, N.; Yu, J.H.; Yu, J.C. A Hollow Porous CdS Photocatalyst. Adv. Mater. 2018, 30, 1804368. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, W.; Xu, R.; Shi, Y.; Zhang, B. Synthesis of ultrathin CdS nanosheets as efficient visible-light-driven water splitting photocatalysts for hydrogen evolution. Chem. Commun. 2013, 49, 9803–9805. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Qian, X.F.; Zhou, P.; Yu, X.B.; Du, W.M.; Xu, S.H. In Situ Sacrificial Template Approach to the Synthesis of Octahedral CdS Microcages. J. Phys. Chem. C 2007, 111, 1935–1940. [Google Scholar] [CrossRef]
- Ashokkumar, M. An overview on semiconductor particulate systems for photoproduction of hydrogen. Int. J. Hydrog. Energy 1998, 23, 427–438. [Google Scholar] [CrossRef]
- Xie, J.L.; Guo, C.X.; Li, C.M. Construction of one-dimensional nanostructures on graphene for efficient energy conversion and storage. Energy Environ. Sci. 2014, 7, 2559–2579. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Z.-R.; Sun, Y.; Colmenares, J.C.; Xu, Y.-J. One-dimension-based spatially ordered architectures for solar energy conversion. Chem. Soc. Rev. 2015, 44, 5053–5075. [Google Scholar] [CrossRef]
- Morales, A.M.; Lieber, C.M. A Laser Ablation Method for the Synthesis of Crystalline Semiconductor Nanowires. Science 1998, 279, 208–211. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Zhang, Y.; Xu, Y.-J. Tuning the Optical Property and Photocatalytic Performance of Titanate Nanotube toward Selective Oxidation of Alcohols under Ambient Conditions. ACS Appl. Mater. Interfaces 2012, 4, 1512–1520. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Yin, X.; Zhang, Y.; Xu, Y.-J. Synthesis of Titanate Nanotube–CdS Nanocomposites with Enhanced Visible Light Photocatalytic Activity. Inorg. Chem. 2013, 52, 11758–11766. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Li, F.; Zhang, Y.; Fu, X.; Xu, Y.-J. Composites of Titanate Nanotube and Carbon Nanotube as Photocatalyst with High Mineralization Ratio for Gas-Phase Degradation of Volatile Aromatic Pollutant. J. Phys. Chem. C 2011, 115, 7880–7886. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Yin, X.; Zhang, Y.; Xu, Y.-J. One-pot, high-yield synthesis of one-dimensional ZnO nanorods with well-defined morphology as a highly selective photocatalys. RSC Adv. 2013, 3, 5956–5965. [Google Scholar] [CrossRef]
- Pan, Z.W.; Dai, Z.R.; Wang, Z.L. Nanobelts of Semiconducting Oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.D.; Song, S.Y.; Zhang, H.J. Hydrothermal synthetic strategies of inorganic semiconducting nanostructures. Chem. Soc. Rev. 2013, 42, 5714–5743. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.S.; Joshi, U.A.; Lee, J.S. Solvothermal Synthesis of CdS Nanowires for Photocatalytic Hydrogen and Electricity Production. J. Phys. Chem. C 2007, 111, 13280–13287. [Google Scholar] [CrossRef]
- Li, Y.X.; Hu, Y.F.; Peng, S.Q.; Lu, G.X.; Li, S.B. Synthesis of CdS Nanorods by an Ethylenediamine Assisted Hydrothermal Method for Photocatalytic Hydrogen Evolution. J. Phys. Chem. C 2009, 113, 9352–9358. [Google Scholar] [CrossRef]
- Loudon, R. The Raman effect in crystals. Adv. Phys. 1964, 13, 423–482. [Google Scholar] [CrossRef]
- Fan, H.M.; Fan, X.F.; Ni, Z.H.; Shen, Z.X.; Feng, Y.P.; Zou, B.S. Orientation-Dependent Raman Spectroscopy of Single Wurtzite CdS Nanowires. J. Phys. Chem. C 2008, 112, 1865–1870. [Google Scholar] [CrossRef]
- Zeiri, L.; Patla, I.; Acharya, S.; Golan, Y.; Efrima, S. Raman Spectroscopy of Ultranarrow CdS Nanostructures. J. Phys. Chem. C 2007, 111, 11843–11848. [Google Scholar] [CrossRef]
- Shi, R.; Ye, H.F.; Liang, F.; Wang, Z.; Li, K.; Weng, Y.X.; Lin, Z.S.; Fu, W.-F.; Che, C.-M.; Chen, Y. Interstitial P-Doped CdS with Long-Lived Photogenerated Electrons for Photocatalytic Water Splitting without Sacrificial Agents. Adv. Mater. 2018, 30, 1705941. [Google Scholar] [CrossRef]
- Hu, C.; Zeng, X.H.; Cui, J.Y.; Chen, H.T.; Lu, J.F. Size Effects of Raman and Photoluminescence Spectra of CdS Nanobelts. J. Phys. Chem. C 2013, 117, 20998–21005. [Google Scholar] [CrossRef]
- Li, S.S.; Wang, L.; Li, Y.D.; Zhang, L.H.; Wang, A.; Xiao, N.; Gao, Y.Q.; Li, N.; Song, W.Y.; Ge, L.; et al. Novel photocatalyst incorporating Ni-Co layered double hydroxides with P-doped CdS for enhancing photocatalytic activity towards hydrogen evolution. Appl. Catal. B 2019, 254, 145–155. [Google Scholar] [CrossRef]
- Lv, J.L.; Zhang, J.F.; Liu, J.; Li, Z.; Dai, K.; Liang, C.H. Bi SPR-Promoted Z-Scheme Bi2MoO6/CdS-Diethylenetriamine Composite with Effectively Enhanced Visible Light Photocatalytic Hydrogen Evolution Activity and Stability. ACS Sustain. Chem. Eng. 2018, 6, 696–706. [Google Scholar] [CrossRef]
- Lee, H.; Reddy, D.A.; Kim, Y.J.; Chun, S.Y.; Ma, R.; Kumar, D.P.; Song, J.K.; Kim, T.K. Drastic Improvement of 1D-CdS Solar-Driven Photocatalytic Hydrogen Evolution Rate by Integrating with NiFe Layered Double Hydroxide Nanosheets Synthesized by Liquid-Phase Pulsed-Laser Ablation. ACS Sustain. Chem. Eng. 2018, 6, 16734–16743. [Google Scholar] [CrossRef]
- Gopannagari, M.; Praveen Kumar, D.; Park, H.; Kim, E.H.; Bhavani, P.; Reddy, D.A.; Kim, T.K. Influence of surface-functionalized multi-walled carbon nanotubes on CdS nanohybrids for effective photocatalytic hydrogen production. Appl. Catal. B 2018, 236, 294–303. [Google Scholar] [CrossRef]
- Chava, R.K.; Do, J.Y.; Kang, M. Enhanced photoexcited carrier separation in CdS–SnS2 heteronanostructures: A new 1D–0D visible light photocatalytic system for the hydrogen evolution reaction. J. Mater. Chem. 2019, 7, 13614–13628. [Google Scholar] [CrossRef]
- Sing, K.; Everett, D.; Haul, R.; Moscou, L.; Pierotti, R.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Yuan, J.L.; Wen, J.Q.; Gao, Q.Z.; Chen, S.C.; Li, J.M.; Li, X.; Fang, Y.P. Amorphous Co3O4 modified CdS nanorods with enhanced visible-light photocatalytic H2-production activity. Dalton Trans. 2015, 44, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Poh, C.K.; Zhu, Y.W.; Xing, G.C.; Chin, K.C.; Feng, Y.P.; Lin, J.; Sow, C.H.; Ji, W.; Wee, A.T.S. Novel CdS Nanostructures: Synthesis and Field Emission. J. Phys. Chem. C 2008, 112, 11227–11230. [Google Scholar] [CrossRef]
- Moniruddin, M.D.; Oppong, E.; Stewart, D.; McCleese, C.; Roy, A.; Warzywoda, J.; Nuraje, N. Designing CdS-Based Ternary Heterostructures Consisting of Co Metal and CoOx Cocatalysts for Photocatalytic H2 Evolution under Visible Light. Inorg. Chem. 2019, 58, 12325–12333. [Google Scholar] [CrossRef]
- Wu, T.F.; Wang, P.F.; Qian, J.; Ao, Y.H.; Wang, C.; Hou, J. Noble-metal-free nickel phosphide modified CdS/C3N4 nanorods for dramatically enhanced photocatalytic hydrogen evolution under visible light irradiation. Dalton Trans. 2017, 46, 13793–13801. [Google Scholar] [CrossRef]
- Deng, J.; Su, Y.; Liu, D.; Yang, P.D.; Liu, B.; Liu, C. Nanowire Photoelectrochemistry. Chem. Rev. 2019, 119, 9221–9259. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Han, B.; Han, C.; Xu, Y.-J. One dimensional CdS based materials for artificial photoredox reactions. J. Mater. Chem. 2017, 5, 2387–2410. [Google Scholar] [CrossRef]
- Zhang, G.P.; Chen, D.G.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. Fabrication of Bi2MoO6/ZnO hierarchical heterostructures with enhanced visible-light photocatalytic activity. Appl. Catal. B 2019, 250, 313–324. [Google Scholar] [CrossRef]
- Chava, R.K.; Do, J.Y.; Kang, M. Smart Hybridization of Au Coupled CdS Nanorods with Few Layered MoS2 Nanosheets for High Performance Photocatalytic Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 6, 6445–6457. [Google Scholar] [CrossRef]
- Yin, X.-L.; He, G.-Y.; Sun, B.; Jiang, W.-J.; Xue, D.-J.; Xia, A.-D.; Wan, L.-J.; Hu, J.-S. Rational design and electron transfer kinetics of MoS2/CdS nanodots-on-nanorods for efficient visible-light-driven hydrogen generation. Nano Energy 2016, 28, 319–329. [Google Scholar] [CrossRef]
- Zhang, K.; Fujitsuka, M.; Du, Y.; Majima, T. 2D/2D Heterostructured CdS/WS2 with Efficient Charge Separation Improving H2 Evolution under Visible Light Irradiation. ACS Appl. Mater. Interfaces 2018, 10, 20458–20466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Q.; Luo, Y. Impact of Element Doping on Photoexcited Electron Dynamics in CdS Nanocrystals. J. Phys. Chem. Lett. 2017, 8, 5680–5686. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.L.; Zhang, L.; Liu, L.H.; Li, H.; Meng, S.G.; Ye, X.G.; Chen, S.F. In situ photodeposition of MoSx on CdS nanorods as a highly efficient cocatalyst for photocatalytic hydrogen production. J. Mater. Chem. 2017, 5, 15287–15293. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Tu, W.G.; Wu, S.Y.; Liu, Y.; Tan, Y.Z.; Luo, H.J.; Yuan, X.Z.; Chew, J.W. Petal-like CdS nanostructures coated with exfoliated sulfur-doped carbon nitride via chemically activated chain termination for enhanced visible-light–driven photocatalytic water purification and H2 generation. Appl. Catal. B 2018, 229, 181–191. [Google Scholar] [CrossRef]
- Bao, N.Z.; Shen, L.M.; Takata, T.; Domen, K. Self-Templated Synthesis of Nanoporous CdS Nanostructures for Highly Efficient Photocatalytic Hydrogen Production under Visible Light. Chem. Mater. 2008, 20, 110–117. [Google Scholar] [CrossRef]


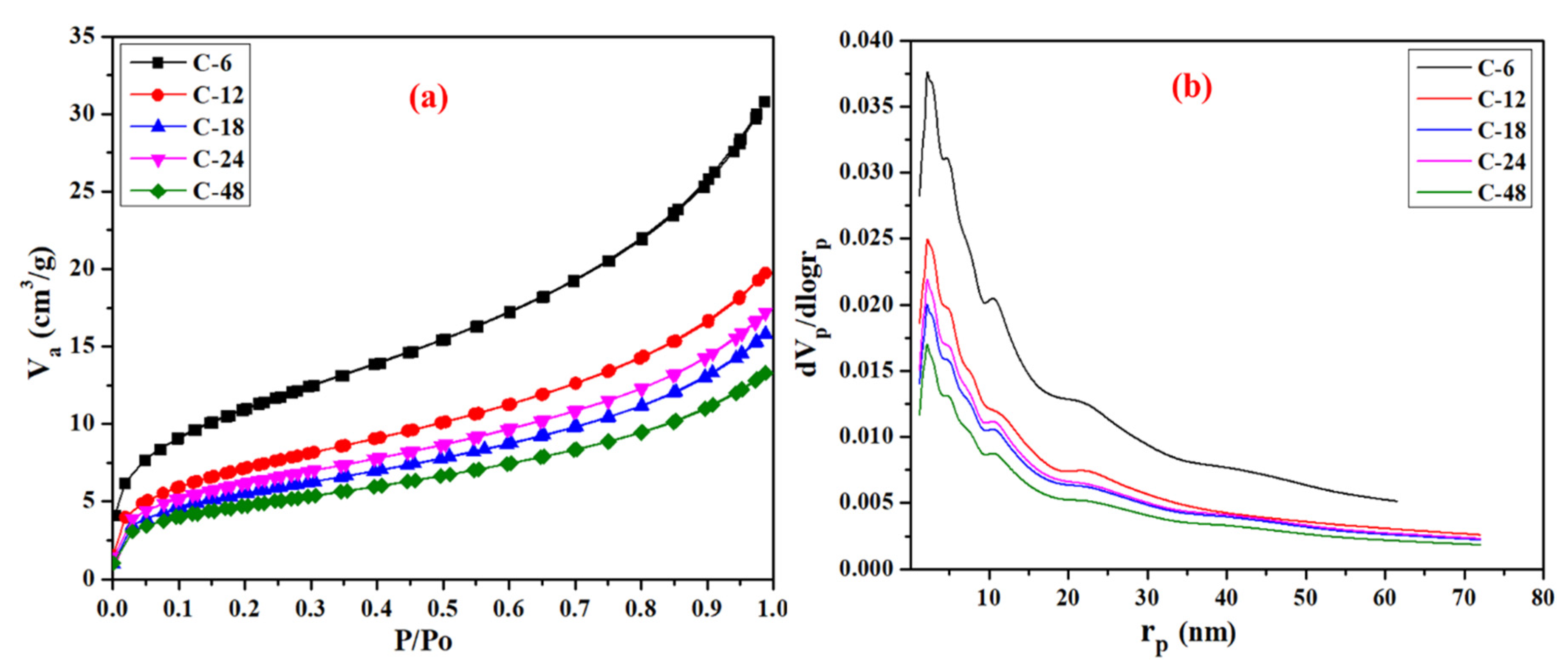

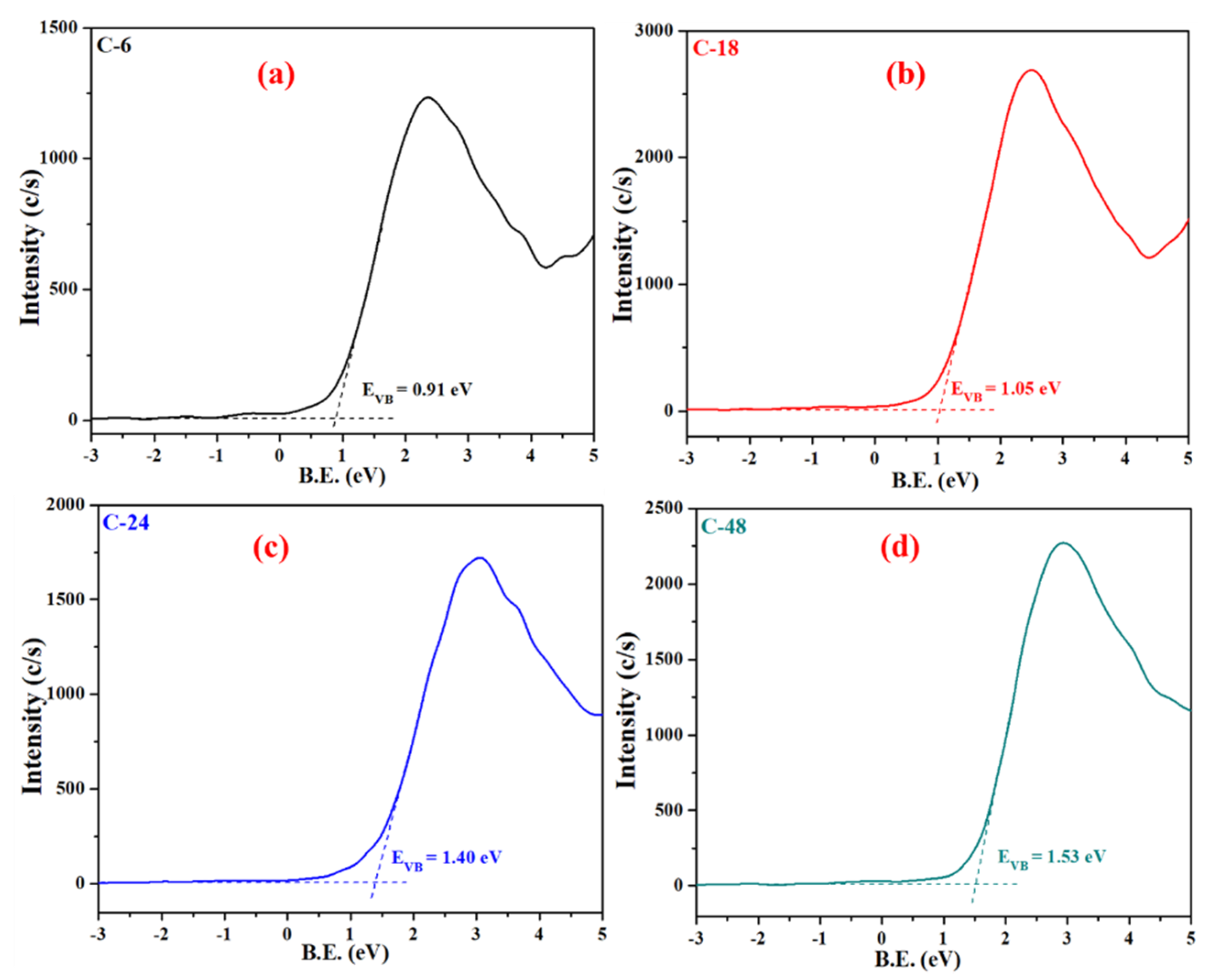

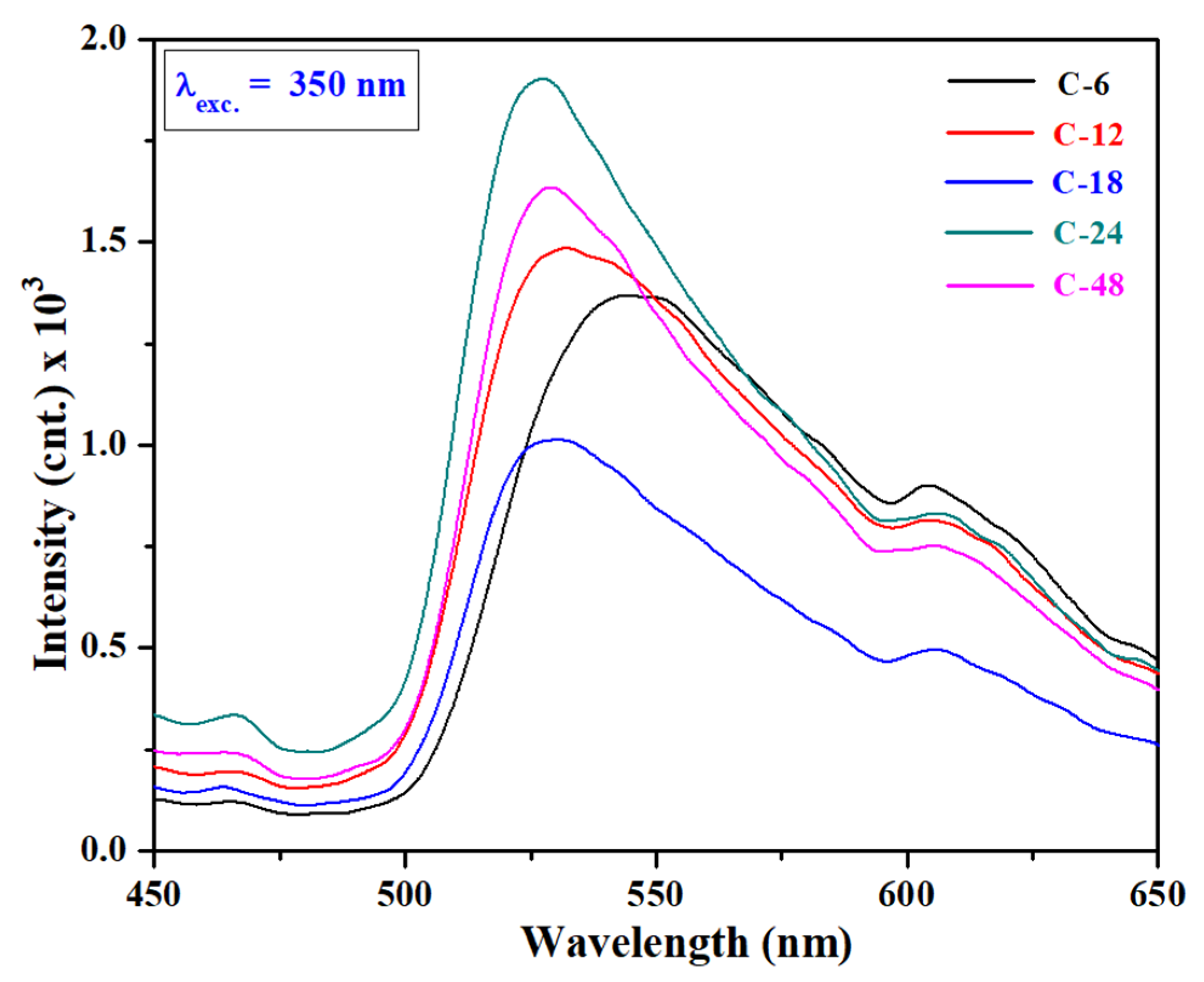


| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| C-6 | 39 | 0.047 | 4.8 |
| C-12 | 26 | 0.030 | 4.7 |
| C-18 | 19 | 0.024 | 4.9 |
| C-24 | 21 | 0.026 | 4.8 |
| C-48 | 16 | 0.020 | 4.9 |
| Sample | H2 Evolution Rate | Reference |
|---|---|---|
| CdS nanowires | 803 μmol.h-1 | [18] |
| CdS hollowstructures | 21654 μmol.g-1.h-1 | [19] |
| CdS nanoflowers | 468.7 μmol.h-1 | [20] |
| CdS hollowstructures | 3.52 mmol.g−1.h−1 | [21] |
| CdS nanosheets | 6.7 mmol.g−1.h−1 | [22] |
| CdS nanowires | 4 μmol.g−1.h−1 | [34] |
| CdS nanostructures | 223 μmol.h−1 | [35] |
| CdS petal-like nanostructures | 117 μmol.g−1.h−1 | [59] |
| CdS nanoporous structures | 4 μmol.g−1.h−1 | [60] |
| CdS nanorods | 206 μmol.g−1.h−1 | Present work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chava, R.K.; Son, N.; Kim, Y.S.; Kang, M. Controlled Growth and Bandstructure Properties of One Dimensional Cadmium Sulfide Nanorods for Visible Photocatalytic Hydrogen Evolution Reaction. Nanomaterials 2020, 10, 619. https://doi.org/10.3390/nano10040619
Chava RK, Son N, Kim YS, Kang M. Controlled Growth and Bandstructure Properties of One Dimensional Cadmium Sulfide Nanorods for Visible Photocatalytic Hydrogen Evolution Reaction. Nanomaterials. 2020; 10(4):619. https://doi.org/10.3390/nano10040619
Chicago/Turabian StyleChava, Rama Krishna, Namgyu Son, Yang Soo Kim, and Misook Kang. 2020. "Controlled Growth and Bandstructure Properties of One Dimensional Cadmium Sulfide Nanorods for Visible Photocatalytic Hydrogen Evolution Reaction" Nanomaterials 10, no. 4: 619. https://doi.org/10.3390/nano10040619
APA StyleChava, R. K., Son, N., Kim, Y. S., & Kang, M. (2020). Controlled Growth and Bandstructure Properties of One Dimensional Cadmium Sulfide Nanorods for Visible Photocatalytic Hydrogen Evolution Reaction. Nanomaterials, 10(4), 619. https://doi.org/10.3390/nano10040619








