Solar Cell Applications of Solution-Processed AgInGaSe2 Thin Films and Improved Properties by Sodium Doping
Abstract
:1. Introduction
2. Experimental Approach
2.1. Fabrication of Nanoparticle Inks
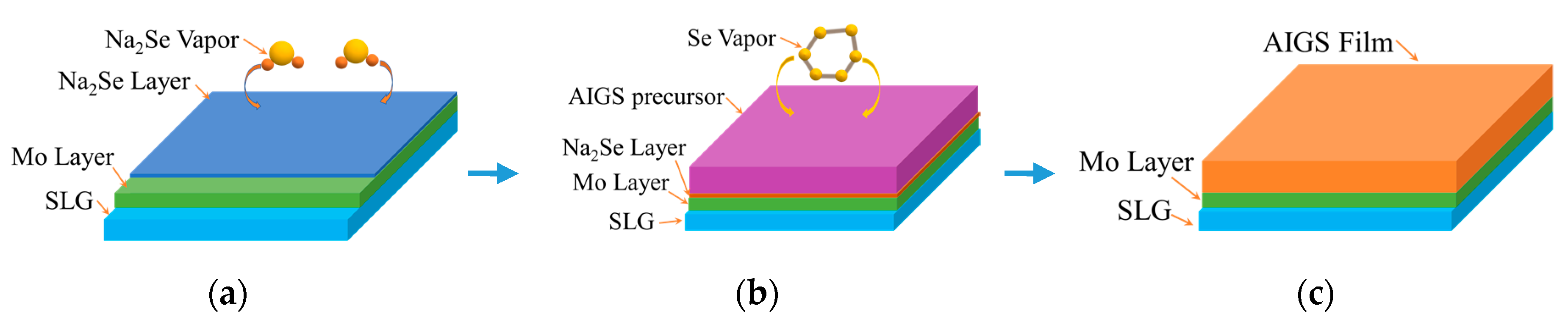
2.2. Sodium Doping Process
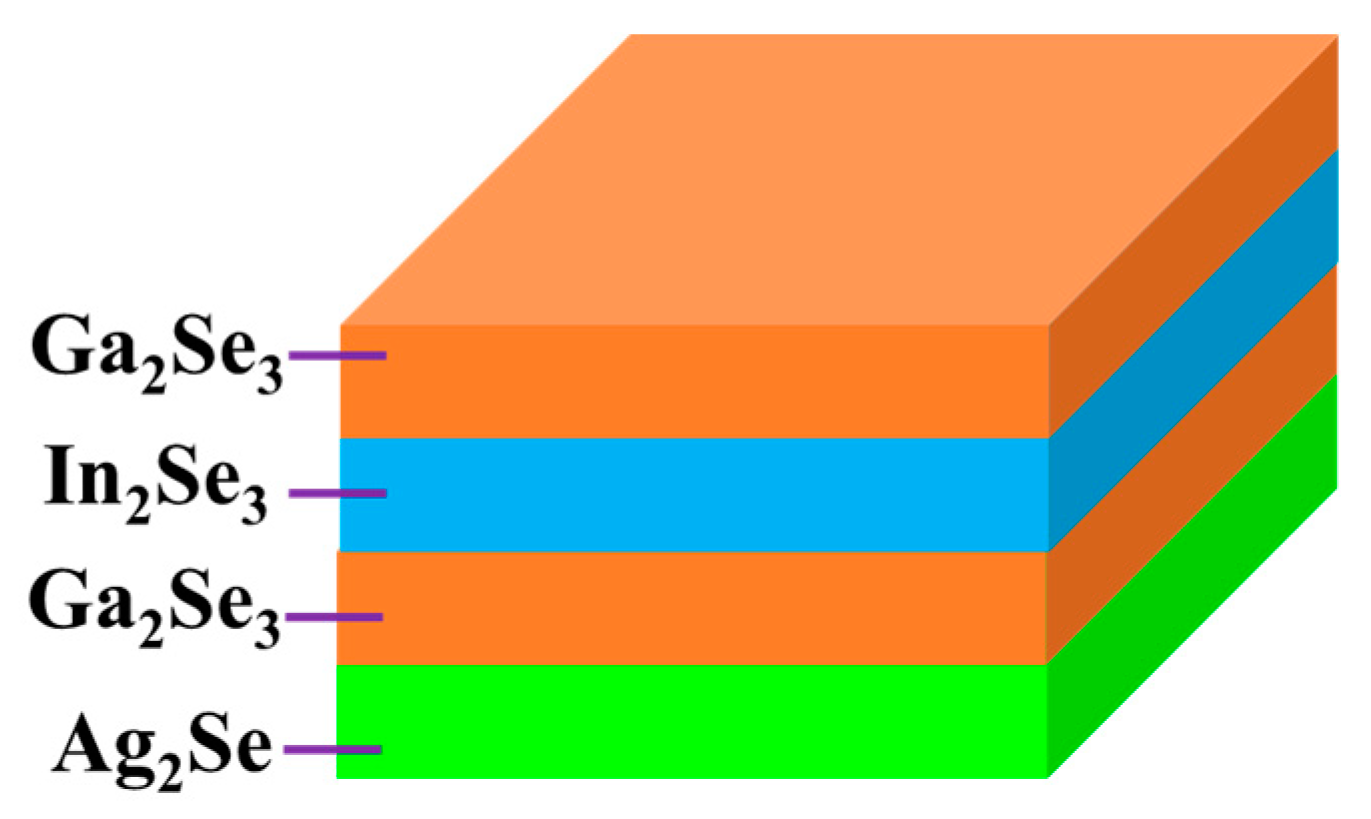
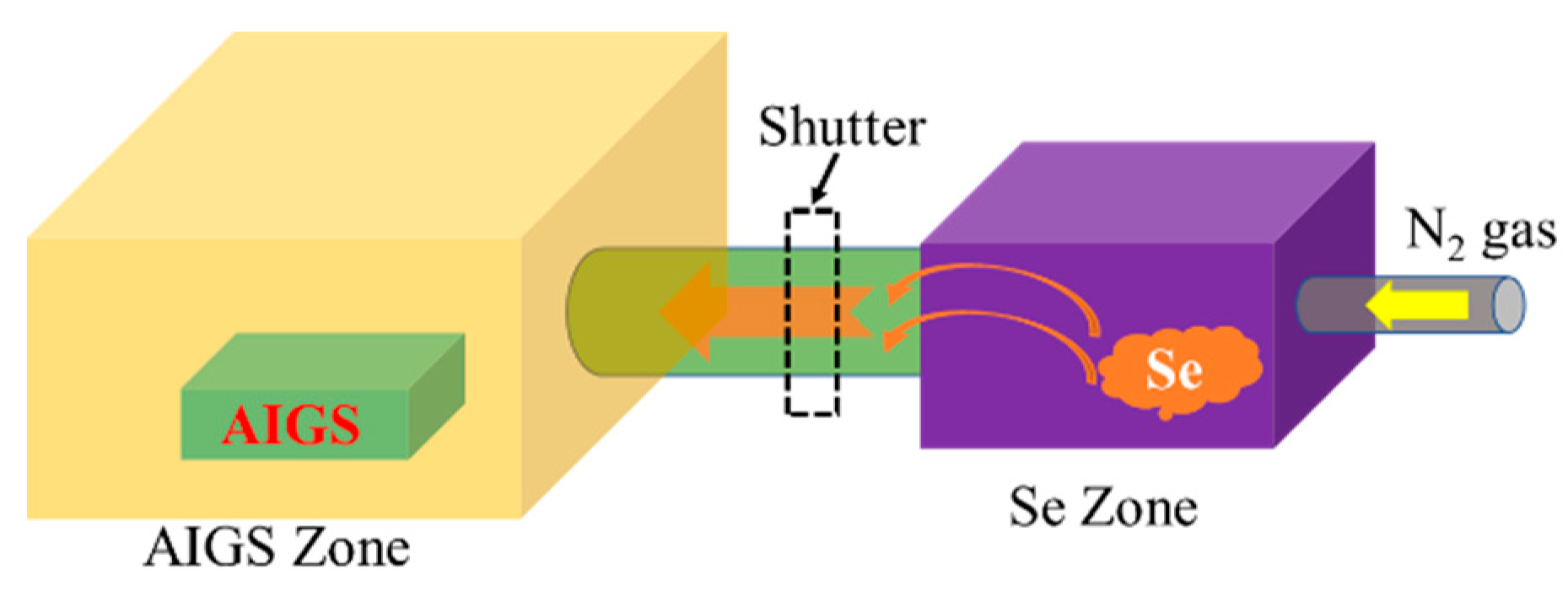
2.3. Fabrication of AIGS Absorbers and Solar Cells
2.4. Characterization
3. Results and Discussion
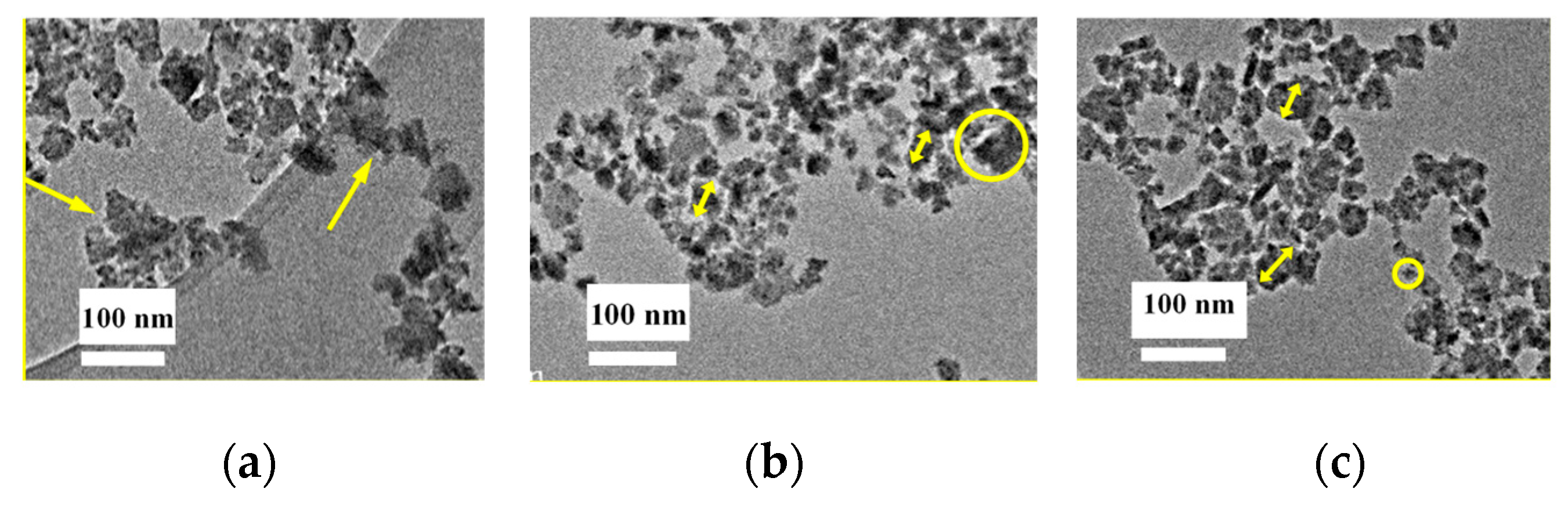
3.1. Nanoparticle Ink Characterization
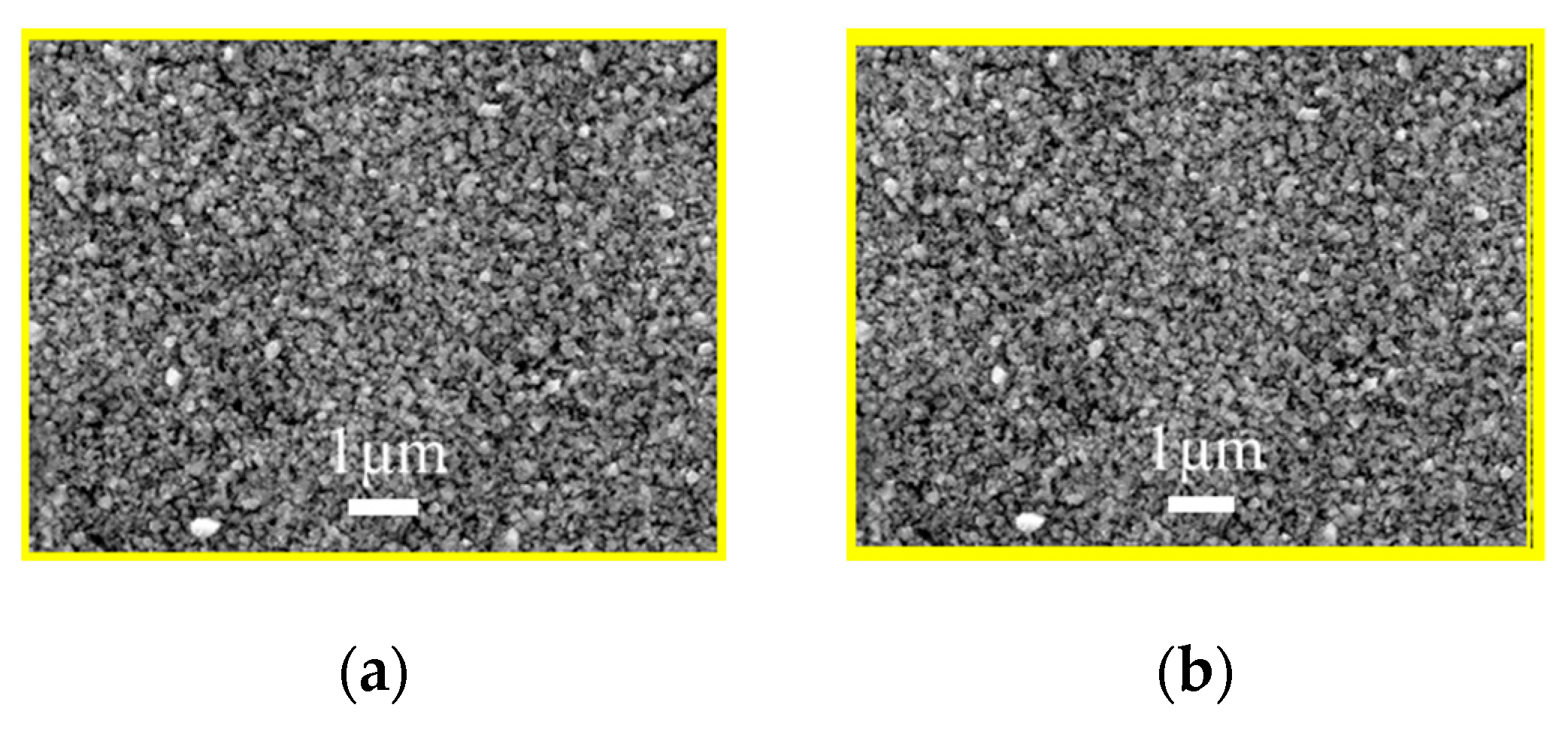
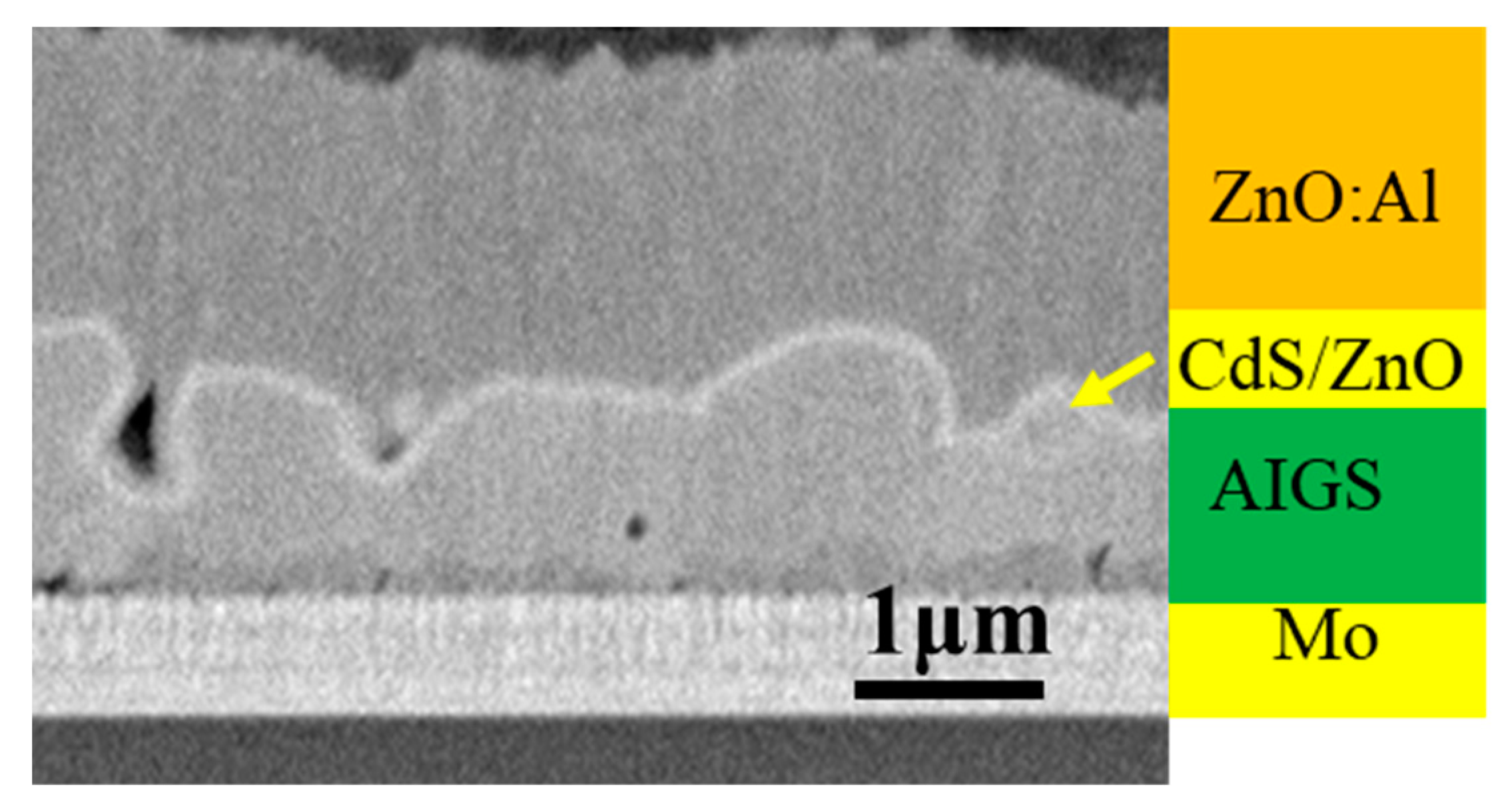
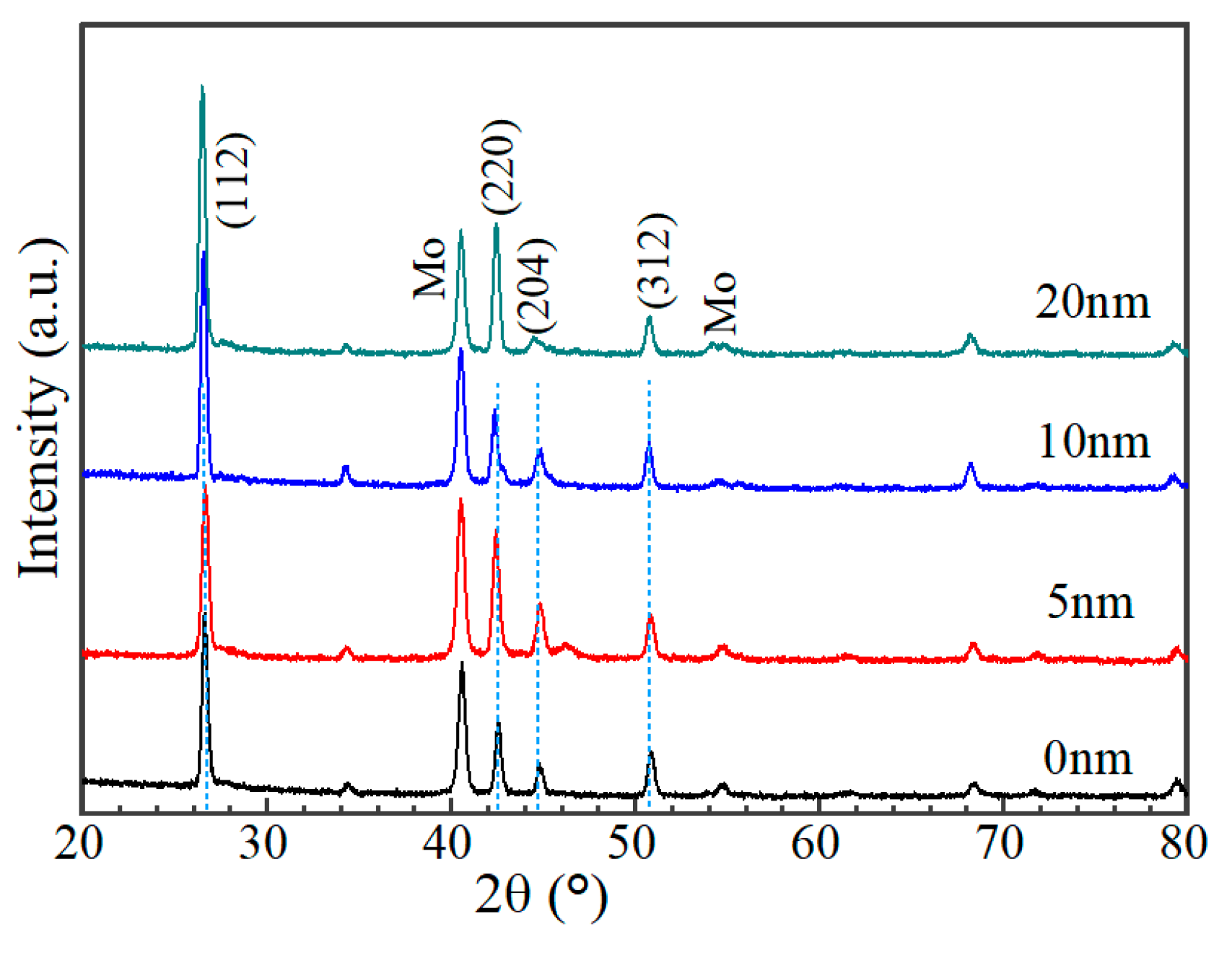
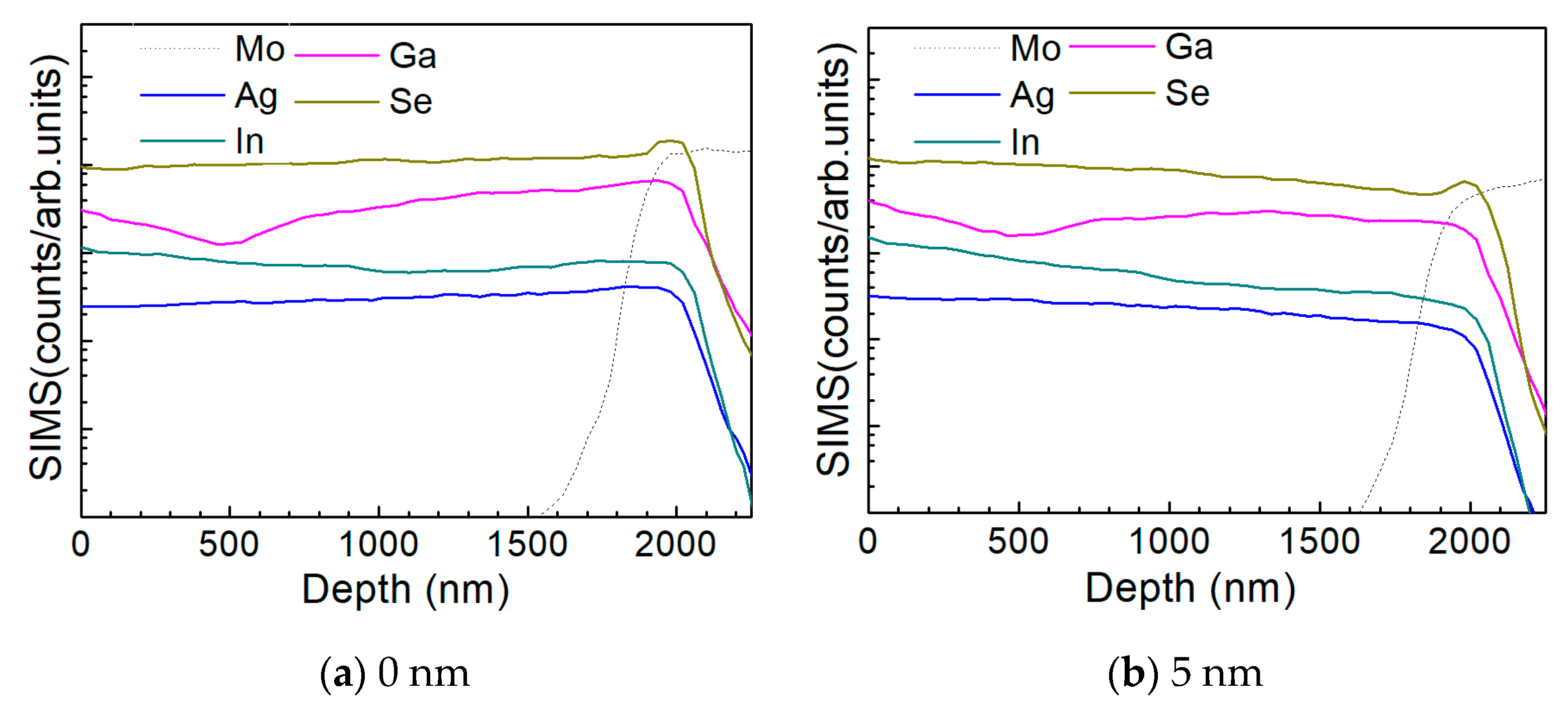
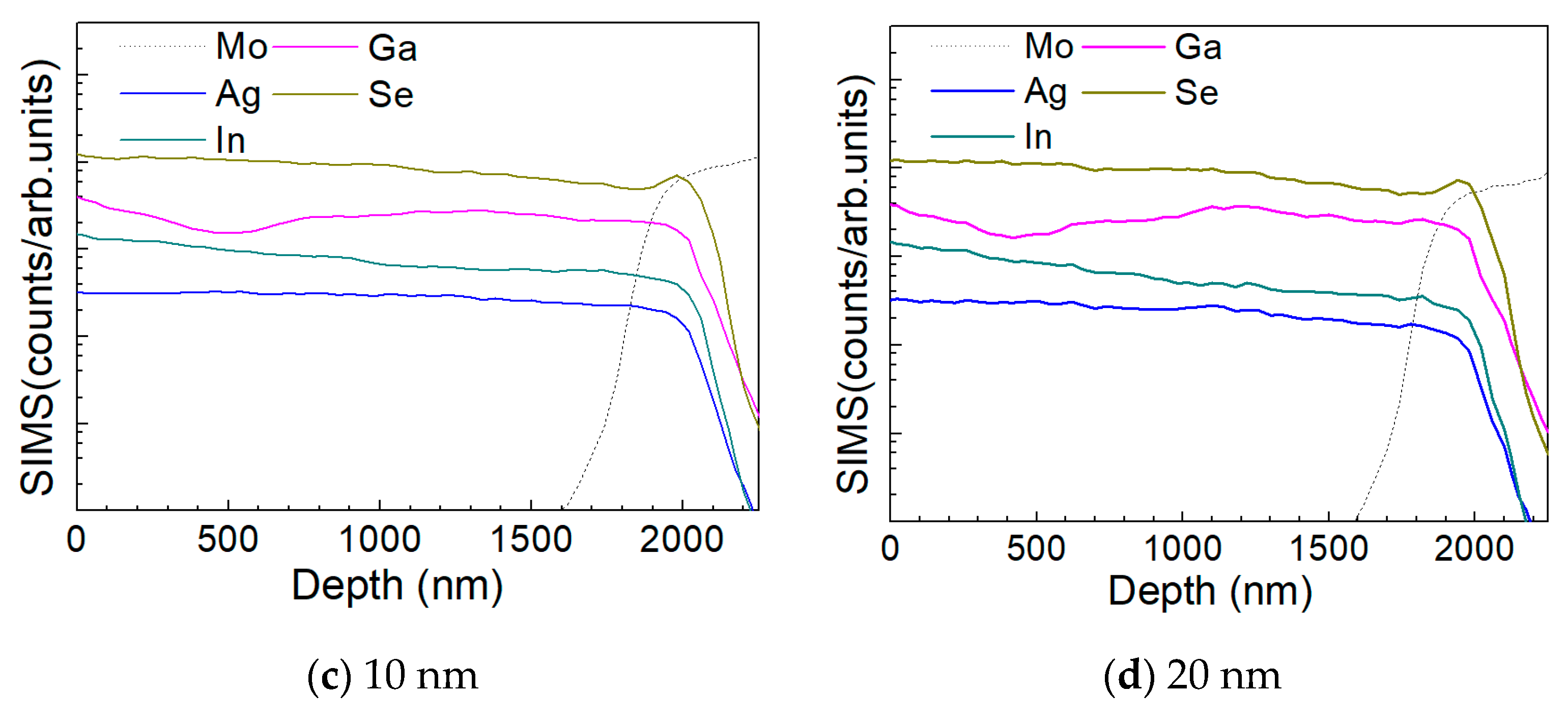
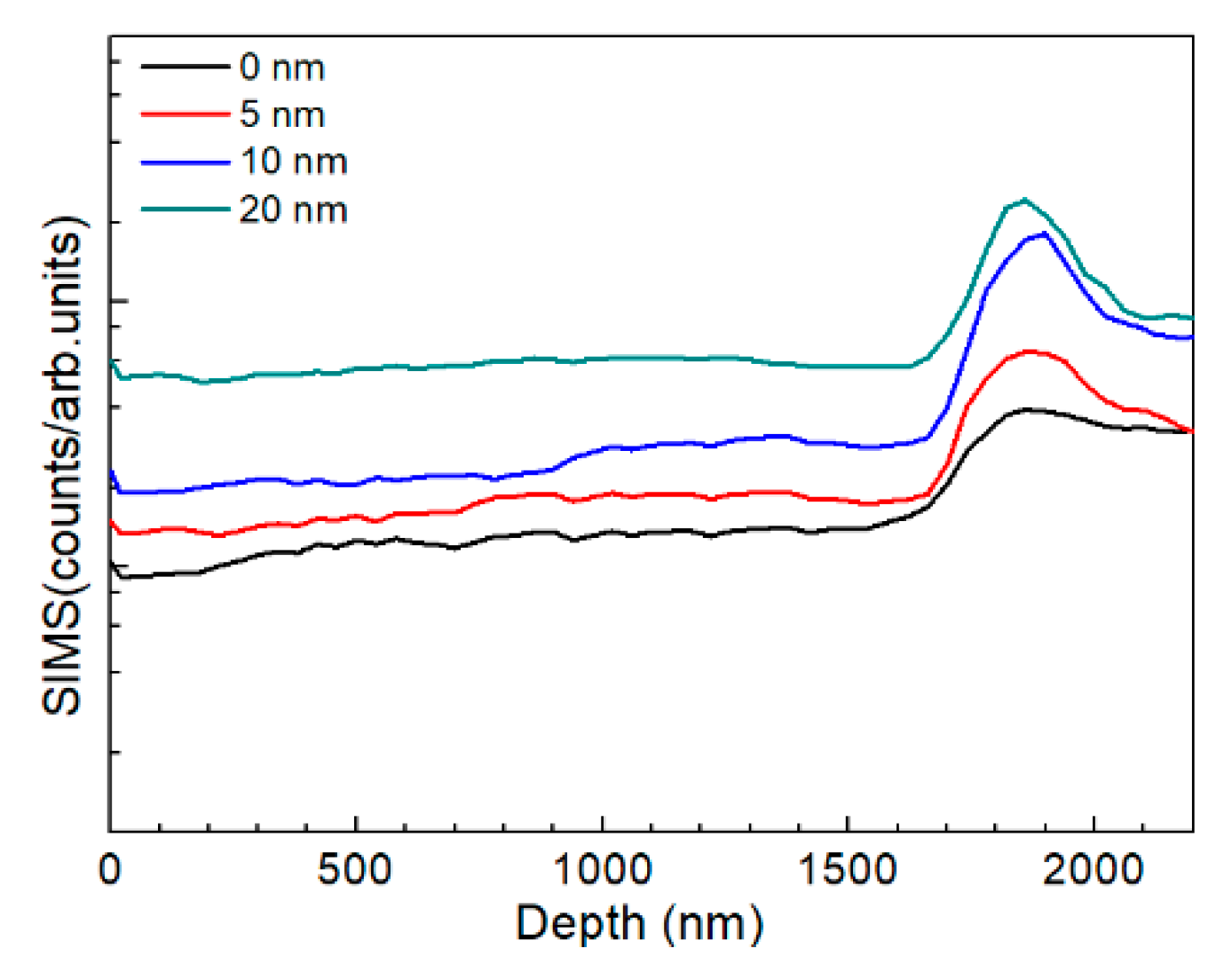
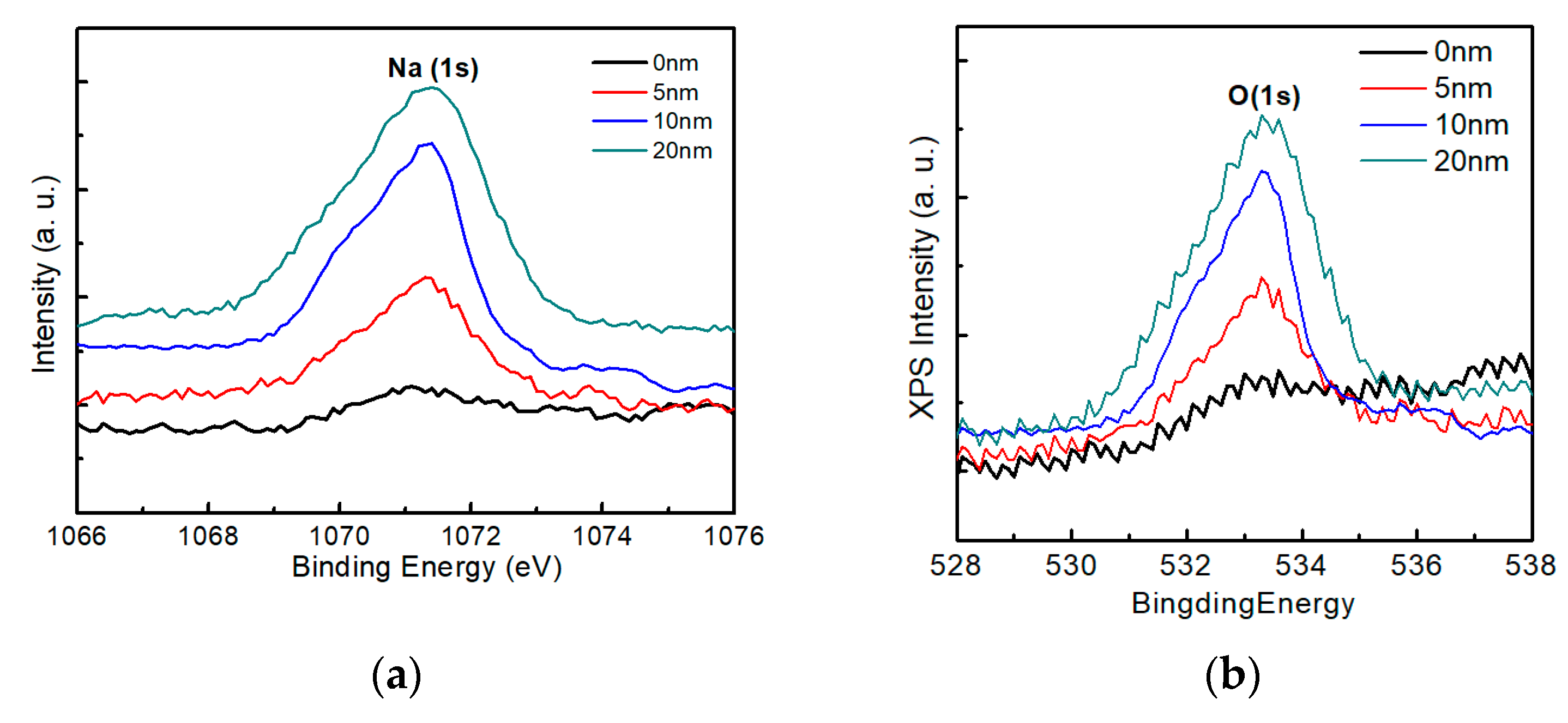
3.2. Characterization of AIGS Films
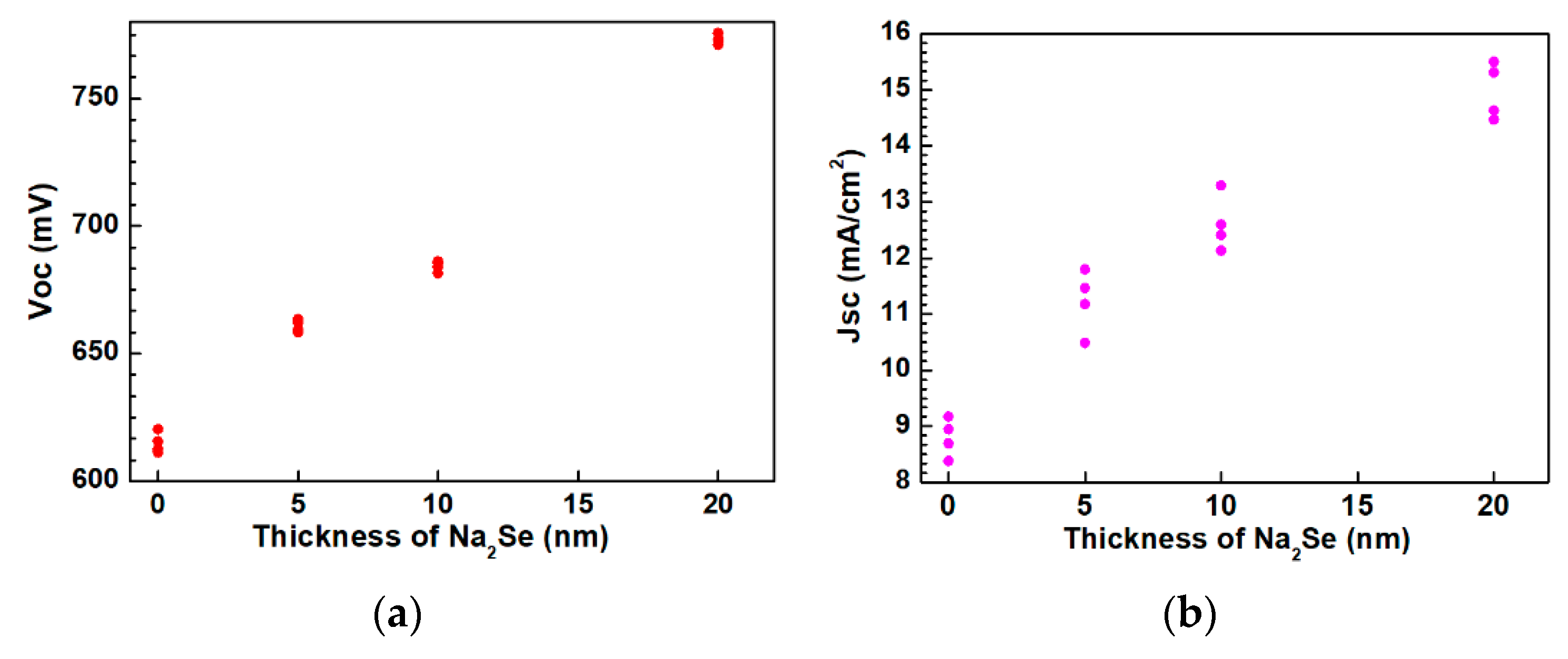
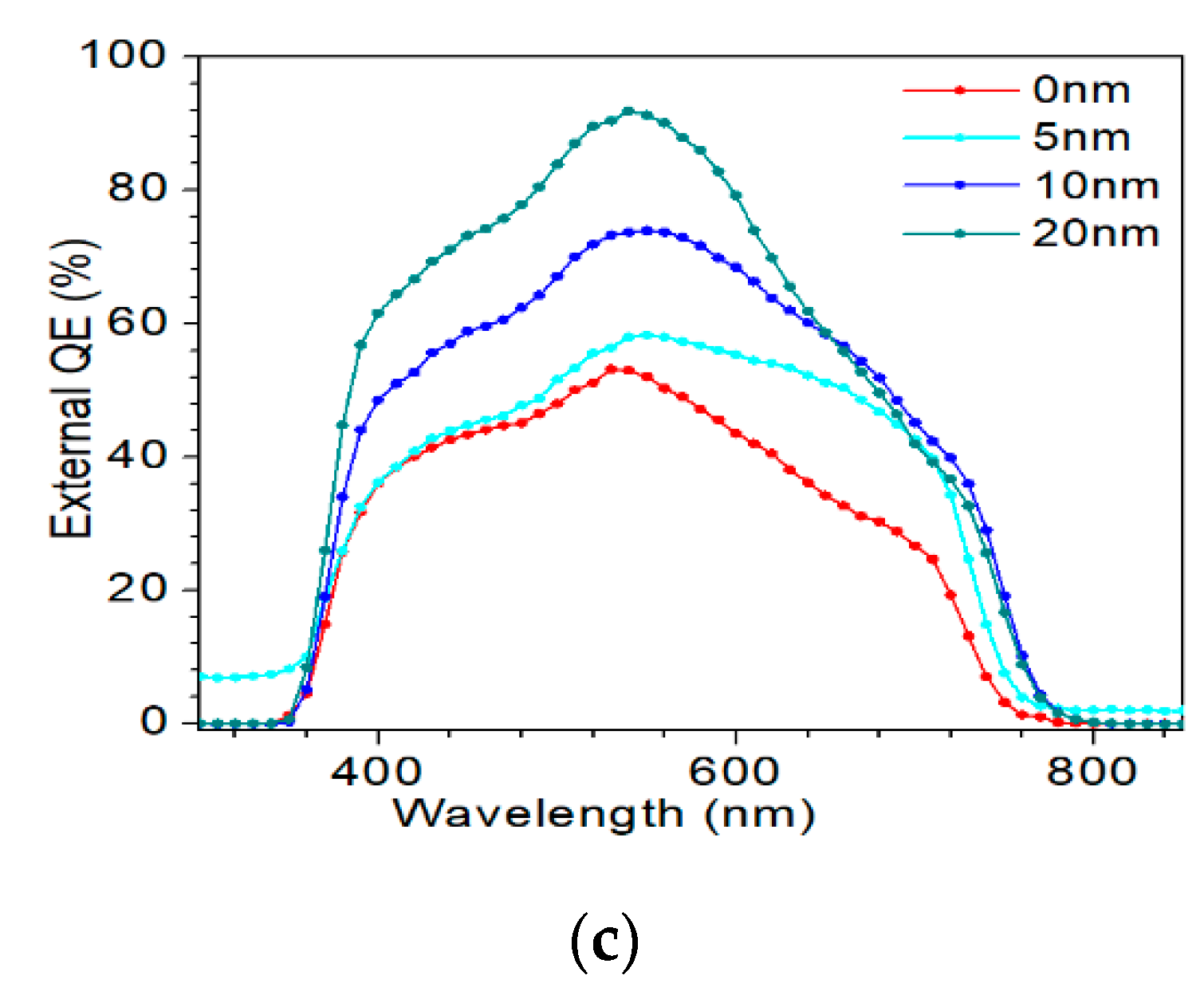
3.3. Properties of AIGS Solar Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yoshida, S. Solar Frontier Achieves World Record Thin-Film Solar Cell Efficiency of 23.35%. Available online: http://www.solar-frontier.com/eng/news/2019/0117_press.html (accessed on 17 January 2019).
- Martin, A.G.; Ewan, D.D.; Dean, H.L.; Jochen, H.E.; Masahiro, Y.; Anita, W.Y.H. Solar Cell Efficiency Tables (version 54). Prog. Photovolt. Res. Appl. 2019, 27, 565–575. [Google Scholar]
- Teodor, K.T.; Douglas, M.B.; Yun, S.L. Materials Perspectives for Next-generation Low-cost Tandem Solar Cells. Sol. Energy Mater. Sol. Cells 2018, 180, 350–357. [Google Scholar]
- Zhang, X.F.; Kobayashi, T.; Kurokawa, Y.; Miyajima, S.; Yamada, A. Deposition of Ag(In, Ga)Se2Solar Cells by a Modified Three-Stage Method Using a Low-Temperature-Deposited Ag–Se Cap Layer. Jpn. J. Appl. Phys. 2013, 52, 055801. [Google Scholar]
- Zhang, X.F.; Kobayashi, T.; Kurokawa, Y.; Yamada, A. Growth of Ag(In, Ga)Se2 Films by Modified Three-Stage Method and Influence of Annealing on Performance of Solar Cells. Jpn. J. Appl. Phys. 2012, 51, 1–5. [Google Scholar]
- Yamada, K.; Hoshino, N.; Nakada, T. Crystallographic and electrical properties of wide gap Ag(In1−x, Gax)Se2 thin films and solar cells. Sci. Technol. Adv. Mater. 2006, 7, 42–45. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.P.; Jiang, J.J.; Yu, S.T.; Gong, Y.C.; Yan, W.B.; Xin, H.; Huang, W. Over 12% Efficient Low-Bandgap CuIn(S, Se)2 Solar Cells with the Absorber Processed from Aqueous Metal Complexes Solution in Air. Nano Energy 2019, 62, 818–822. [Google Scholar] [CrossRef]
- Taylor, B.H.; Isao, M.; Stolle, C.J.; Bogart, T.D.; Ostrowski, D.P.; Glaz, M.S.; Du, J.; Pernik, D.R.; Akhavan, V.A.; Kesrouani, H.; et al. Copper Indium Gallium Selenide (CIGS) Photovoltaic Devices Made Using Multistep Selenization of Nanocrystal Films. ACS Appl. Mater. Interfaces 2013, 5, 9134–9140. [Google Scholar]
- Barkhouse, D.A.R.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Mitzi, D.B. Device characteristics of a 10.1% hydrazine-processed Cu2ZnSn(Se, S)4 solar cell. Prog. Photovolt. Res. Appl. 2011, 20, 6–11. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, E.; Zheng, M.; Wang, Y. Fabrication of Cu2ZnSnS4 Thin Films from Ball-Milled Nanoparticle inks under Various Annealing Temperatures. Nanomaterials 2019, 9, 1615. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.; Stone, P.; Woodruff, J.; Cardozo, B.; Jackrel, D. Device characteristics of a 17.1% efficient solar cell deposited by a non-vacuum printing method on flexible foil. In Proceedings of the 38th IEEE Photovoltaic Specialists Conference, Austin, TX, USA, 3–8 June 2012; pp. 3230–3233. [Google Scholar]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device Characteristics of CZTSSe Thin-Film Solar Cells with 12.6% Efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Raguse, J.M.; Muzzillo, C.P.; Sites, J.R.; Mansfield, L. Effects of Sodium and Potassium on the Photovoltaic Performance of CIGS Solar Cells. IEEE J. Photovolt. 2017, 7, 303–306. [Google Scholar] [CrossRef]
- Zhang, X.; Kobayashi, M. Effect of Sodium on the Properties of Ag(In, Ga)Se2 Thin Films and Solar Cells. IEEE J. Photovolt. 2017, 7, 1426–1432. [Google Scholar] [CrossRef]
- Fraga, D.; Lyubenova, T.S.; Martí, R.; Calvet, I.; Barrachina, E.; Carda, J. Effect of alkali doping on CIGS photovoltaic ceramic tiles. Sol. Energy 2017, 147, 1–7. [Google Scholar] [CrossRef]
- Rockett, A.; Britt, J.; Gillespie, T.; Marshall, C.; Al Jassim, M.; Hasoon, F.; Matson, R.; Basol, B. Na in selenized Cu(In, Ga)Se2 on Na-containing and Na-free glasses: Distribution, grain structure, and device performances. Thin Solid Films 2000, 372, 212–217. [Google Scholar] [CrossRef]
- Talia, G.; Byungha, S.; Nestor, B.; Marinus, H.; David, B.M. The Role of Sodium as a Surfactant and Suppressor of Non-Radiative Recombination at Internal Surfaces in Cu2ZnSnS4. Adv. Energy Mater. 2015, 5, 1400849. [Google Scholar]
- Singh, O.P.; Gour, K.S.; Parmar, R.; Singh, V.N. Sodium induced grain growth, defect passivation and enhancement in the photovoltaic properties of Cu2ZnSnS4 thin film solar cell. Mater. Chem. Phys. 2016, 177, 293–298. [Google Scholar] [CrossRef]
- Bi, C.; Zheng, X.; Chen, B.; Wei, H.; Huang, J. Spontaneous Passivation of Hybrid Perovskite by Sodium Ions from Glass Substrates: Mysterious Enhancement of Device Efficiency Revealed. ACS Energy Lett. 2017, 2, 1400–1406. [Google Scholar] [CrossRef]
- Mungan, E.S.; Wang, X.; Alam, M.A. Modeling the effects of Na incorporation on CIGS solar cells. IEEE J. Photovolt. 2013, 3, 451–456. [Google Scholar] [CrossRef]
- Tuttle, J.R.; Contreras, M.A.; Gabor, A.M.; Ramanathan, K.R.; Tennant, A.L.; Albin, D.S.; Keane, J.; Noufi, R. Perspective on High-efficiency Cu(In, Ga)Se2-based Thin-film Solar Cells Fabricated by Simple, Scalable Processes. Prog. Photovolt. Res. Appl. 1995, 3, 383–391. [Google Scholar] [CrossRef]
- Zhang, X.F.; Fu, E.G.; Wang, Y.H.; Zhang, C. Fabrication of Cu2ZnSnS4 (CZTS) Nanoparticle Inks for Growth of CZTS Films for Solar Cells. Nanomaterials 2019, 9, 336. [Google Scholar] [CrossRef] [Green Version]
- Reinhard, P.; Bissig, B.; Pianezzi, F.; Avancini, E.; Hagendorfer, H.; Keller, D.; Fuchs, P.; Döbeli, M.; Vigo, C.; Crivelli, P.; et al. Features of KF and NaF Postdeposition Treatments of Cu(In, Ga)Se2 Absorbers for High Efficiency Thin Film Solar Cells. Chem. Mater. 2015, 27, 5755–5764. [Google Scholar] [CrossRef]
- Sutter-Fella, C.M.; Stückelberger, J.A.; Hagendorfer, H.; La Mattina, F.; Kranz, L.; Nishiwaki, S.; Uhl, A.R.; Romanyuk, Y.E.; Tiwari, A.N. Sodium Assisted Sintering of Chalcogenides and Its Application to Solution Processed Cu2ZnSn(S, Se)4 Thin Film Solar Cells. Chem. Mater. 2014, 26, 1420–1425. [Google Scholar] [CrossRef]
- Nishiwaki, S.; Kohara, N.; Negami, T.; Wada, T. MoSe2 layer formation at Cu(In, Ga)Se2/Mo Interfaces in High Efficiency Cu(In1−x, Gax)Se2 Solar Cells. Jpn. J. Appl. Phys. 1998, 37, L71–L73. [Google Scholar] [CrossRef]
- Nakada, T.; Yamada, K.; Arai, R.; Ishizaki, H.; Yamada, N. Novel Wide-Band-Gap Ag(In1-x, Gax)Se2 Thin Film Solar Cells. MRS Online Proc. Libr. Arch. 2005, 865. [Google Scholar] [CrossRef]
- Ando, Y.; Ishizuka, S.; Wang, S.; Chen, J.; Islam, M.M.; Shibata, H.; Akimoto, K.; Sakurai, T. Relationship between bandgap grading and carrier recombination for Cu(In, Ga)Se2-based solar cells. Jpn. J. Appl. Phys. 2018, 57. [Google Scholar] [CrossRef]
- Noikaew, B.; Sukaiem, S.; Namnuan, B.; Chatraphorn, S. CIGS Thin Film Solar Cells with Graded-Bandgap Fabricated by CIS/CGS bilayer and CGS/CIS/CGS Trilayer Systems. J. Phys. Conf. Ser. 2018, 1144, 012069. [Google Scholar] [CrossRef]
- Neelkanth, G.D.; Ashwani, K.; Helio, M. Effect of Location of Sodium Precursor on the Morphological and Device Properties of CIGS Solar Cells. MRS Online Proc. Libr. Arch. 2013, 1538, 51–60. [Google Scholar]
- Salomé, P.; Fjällström, V.; Hultqvist, A.; Edoff, M. Na Doping of CIGS Solar Cells Using Low Sodium-Doped Mo Layer. IEEE J. Photovolt. 2013, 3, 509–513. [Google Scholar] [CrossRef]
- Haight, R.; Shao, X.; Wang, W.; Mitzi, D.B. Electronic and elemental properties of the Cu2ZnSn(S, Se)4 surface and grain boundaries. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef]
- Ramanathan, K.; Contreras, M.A.; Perkins, C.L.; Asher, S.; Hasoon, F.S.; Keane, J.; Young, D.; Romero, M.; Metzger, W.; Noufi, R.; et al. Properties of 19.2% efficiency ZnO/CdS/CuInGaSe2 thin-film solar cells. Prog. Photovolt. Res. Appl. 2003, 11, 225–230. [Google Scholar] [CrossRef]
- Nelson, J. The Physics of Solar Cells, 1st ed.; World Scientific: Singapore, 2003; p. 7. [Google Scholar]
- Christians, J.A.; Manser, J.S.; Kamat, P.V. Best Practices in Perovskite Solar Cell Efficiency Measurements. Avoiding the Error of Making Bad Cells Look Good. J. Phys. Chem. Lett. 2015, 6, 852–857. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Sun, Q.; Zheng, M.; Duan, Z.; Wang, Y. Solar Cell Applications of Solution-Processed AgInGaSe2 Thin Films and Improved Properties by Sodium Doping. Nanomaterials 2020, 10, 547. https://doi.org/10.3390/nano10030547
Zhang X, Sun Q, Zheng M, Duan Z, Wang Y. Solar Cell Applications of Solution-Processed AgInGaSe2 Thin Films and Improved Properties by Sodium Doping. Nanomaterials. 2020; 10(3):547. https://doi.org/10.3390/nano10030547
Chicago/Turabian StyleZhang, Xianfeng, Qingxuan Sun, Maoxi Zheng, Zhuohua Duan, and Yuehui Wang. 2020. "Solar Cell Applications of Solution-Processed AgInGaSe2 Thin Films and Improved Properties by Sodium Doping" Nanomaterials 10, no. 3: 547. https://doi.org/10.3390/nano10030547
APA StyleZhang, X., Sun, Q., Zheng, M., Duan, Z., & Wang, Y. (2020). Solar Cell Applications of Solution-Processed AgInGaSe2 Thin Films and Improved Properties by Sodium Doping. Nanomaterials, 10(3), 547. https://doi.org/10.3390/nano10030547





