Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of TiO2 Samples
2.3. Characterization
2.4. Photocatalytic Experiments
3. Results
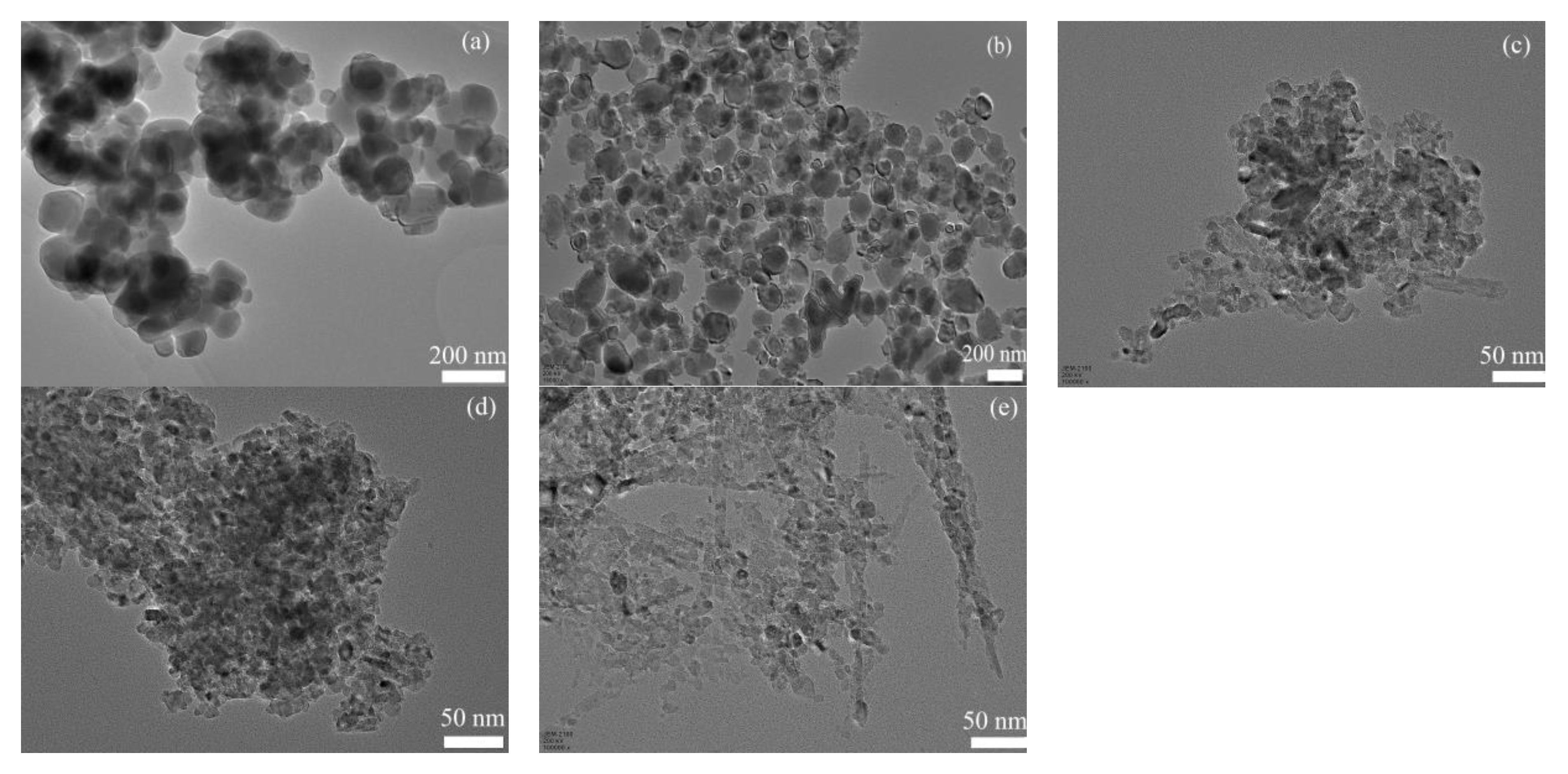
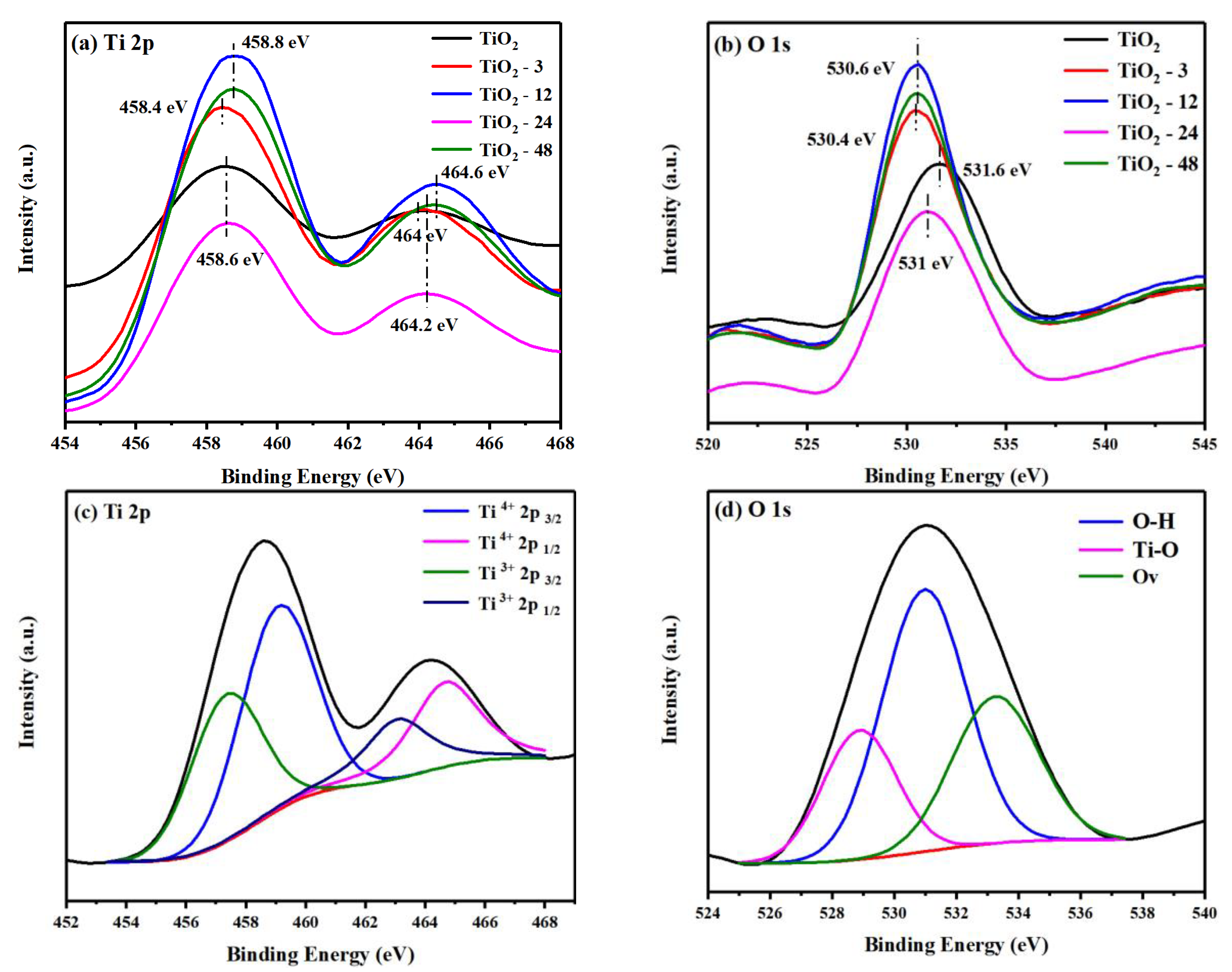
3.1. Characterization of Prepared TiO2-X
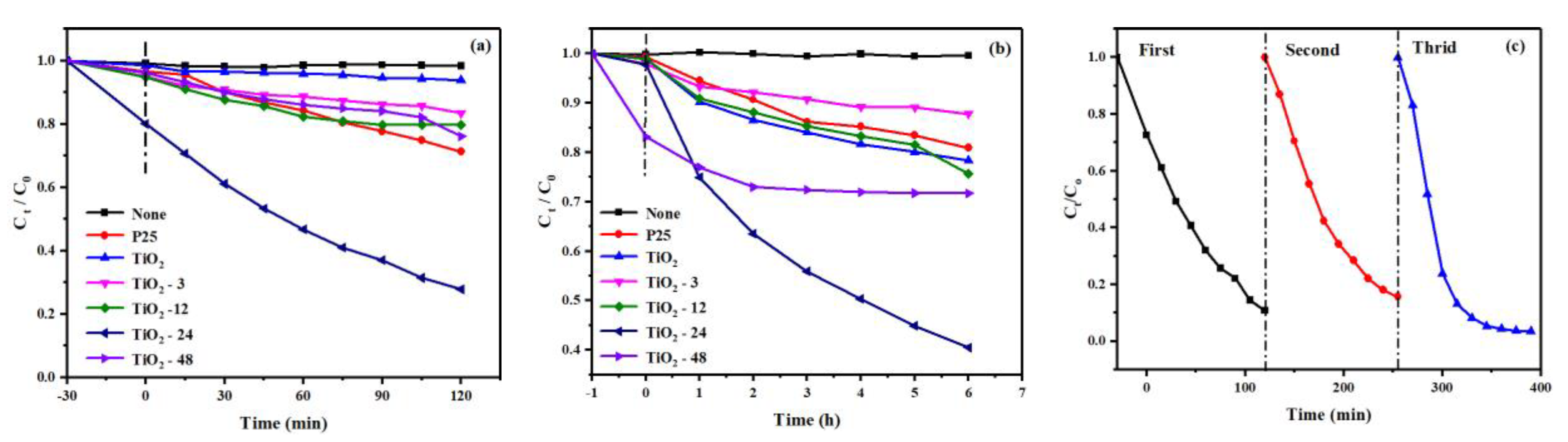
3.2. Evaluation of the Photocatalytic Efficiency
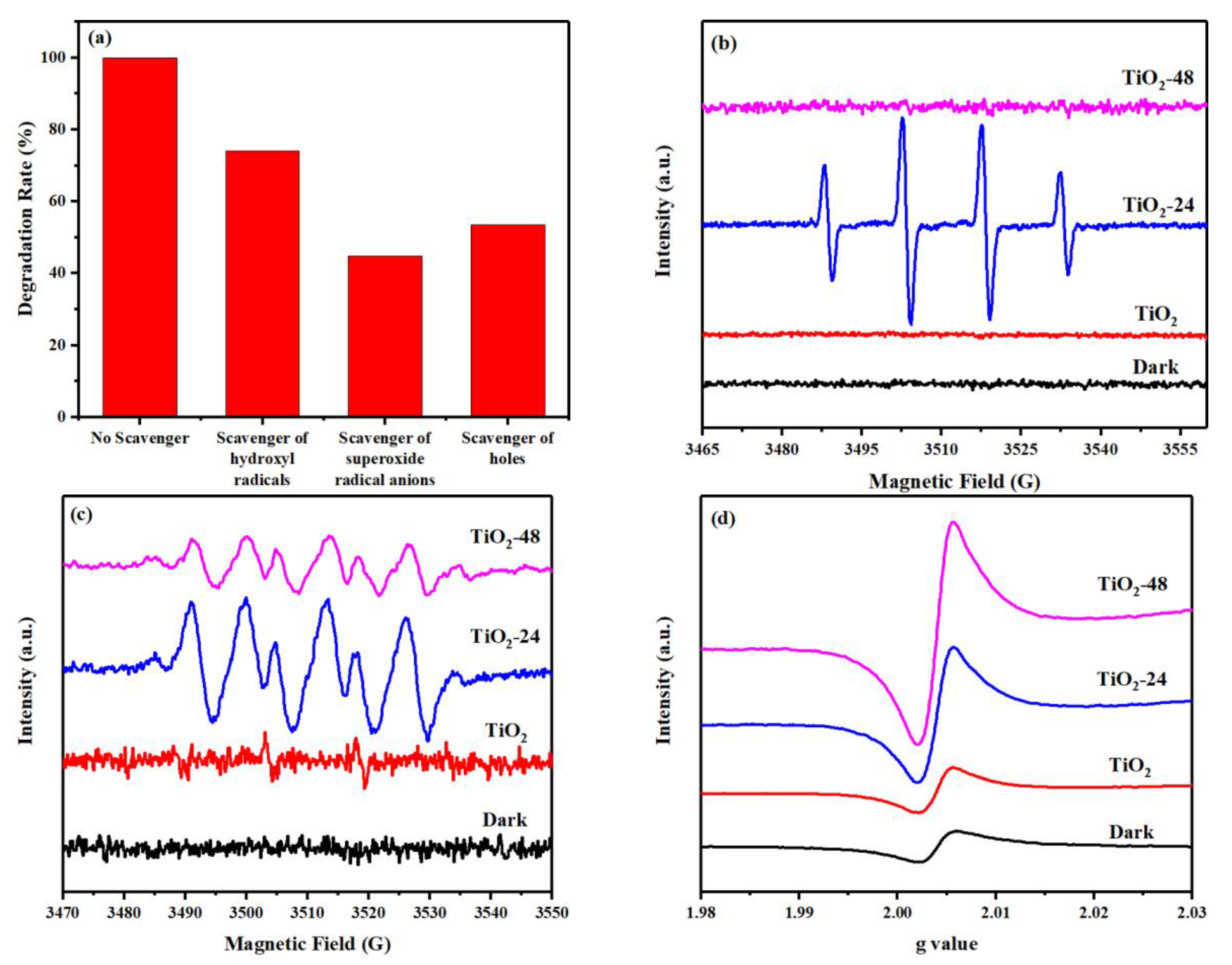
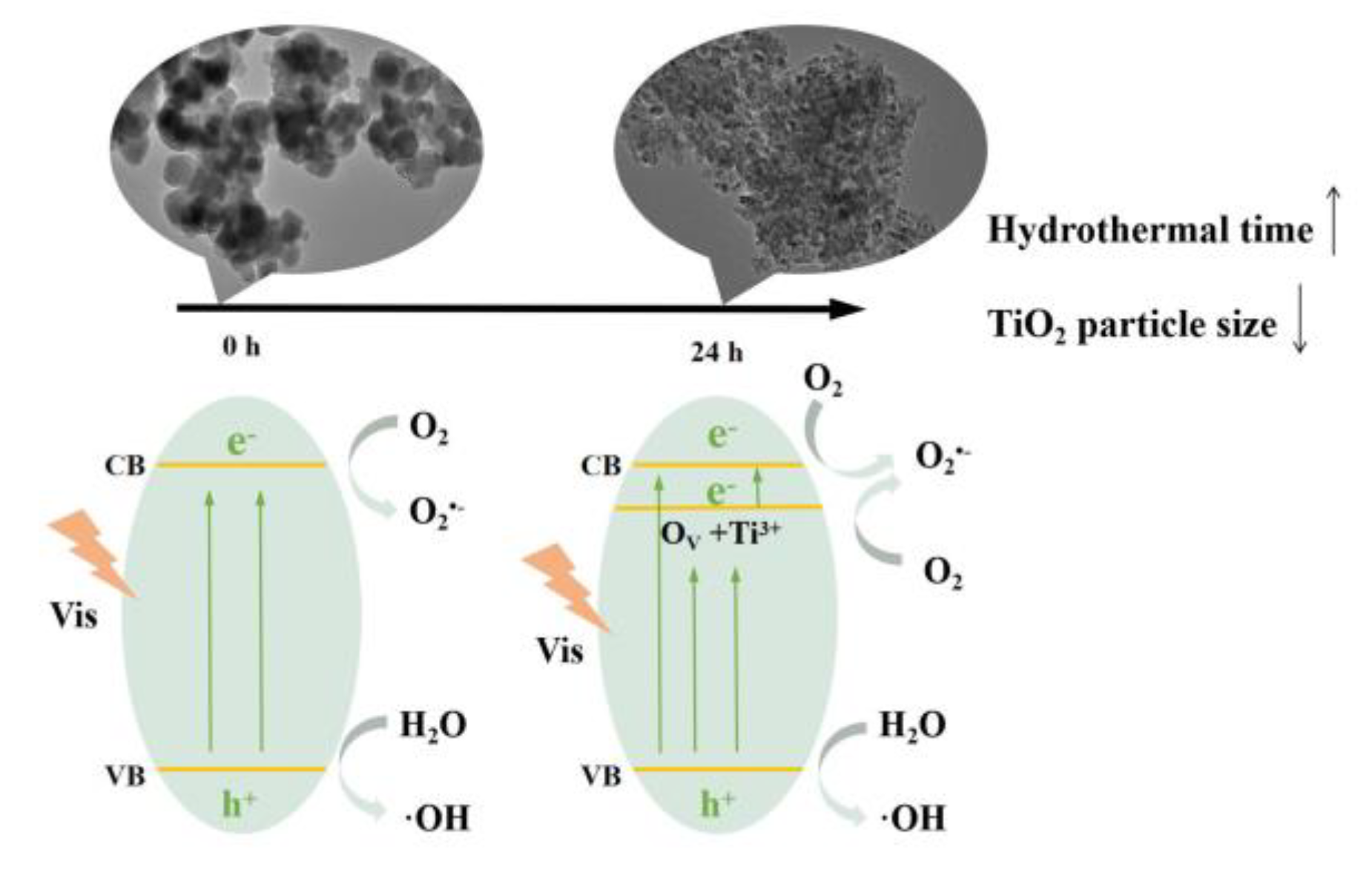
3.3. The mechanism of Photocatalytic Performance Improvement of the TiO2-X.
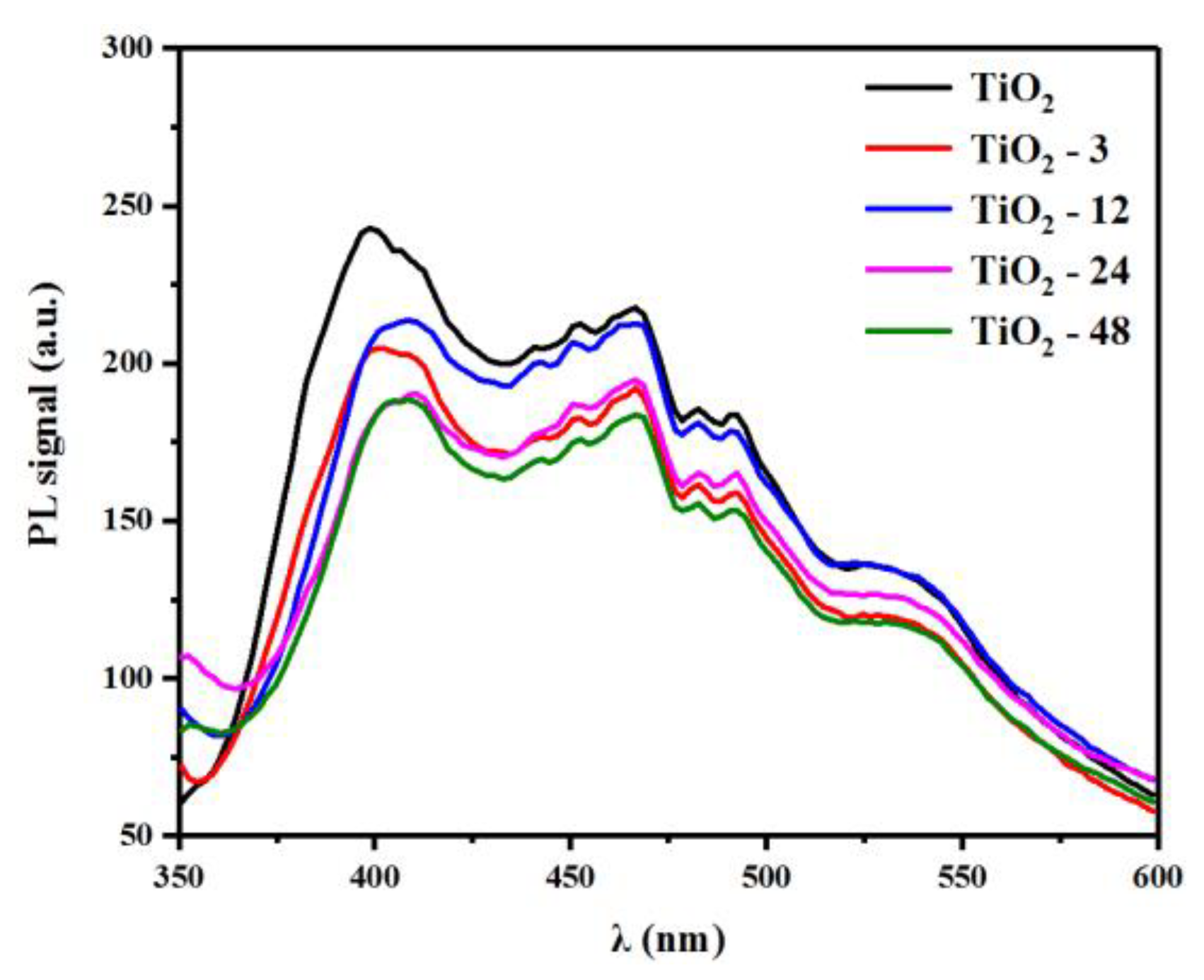
3.4. Photoinduced Electron Transfer Properties in the TiO2-X.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Highly reactive {001} facets of TiO2-based composites: Synthesis, formation mechanism and characterization. Nanoscale 2014, 6, 1946–2008. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Ren, Z.; Fan, H.; Yang, X. Elementary photocatalytic chemistry on TiO2 surfaces. Chem. Soc. Rev. 2016, 45, 3701–3730. [Google Scholar] [CrossRef]
- Li, W.; Elzatahry, A.; Aldhayan, D.; Zhao, D. Core–shell structured titanium dioxide nanomaterials for solar energy utilization. Chem. Soc. Rev. 2018, 47, 8203–8237. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Cao, S.; Tao, F.F.; Tang, Y.; Li, Y.; Yu, J. Size-and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem. Soc. Rev. 2016, 45, 4747–4765. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Yang, S.; Lee, H. Surface analysis of N-doped TiO2 nanorods and their enhanced photocatalytic oxidation activity. Appl. Catal. B Environ. 2017, 204, 209–215. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Z.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef]
- Bianchi, C.; Gatto, S.; Pirola, C.; Naldoni, A.; Di Michele, A.; Cerrato, G.; Crocellà, V.; Capucci, V. Photocatalytic degradation of acetone, acetaldehyde and toluene in gas-phase: Comparison between nano and micro-sized TiO2. Appl. Catal. B Environ. 2014, 146, 123–130. [Google Scholar] [CrossRef]
- Lin, H.; Huang, C.; Li, W.; Ni, C.; Shah, S.I.; Tseng, Y.-H. Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Appl. Catal. B Environ. 2006, 68, 1–11. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, W.; Huang, B.; Wang, Z.; Zhan, J.; Qin, X.; Zhang, X.; Dai, Y. Tailoring AgI nanoparticles for the assembly of AgI/BiOI hierarchical hybrids with size-dependent photocatalytic activities. J. Mater. Chem. A 2013, 1, 7131–7136. [Google Scholar] [CrossRef]
- Kočí, K.; Obalová, L.; Matějová, L.; Plachá, D.; Lacný, Z.; Jirkovský, J.; Šolcová, O. Effect of TiO2 particle size on the photocatalytic reduction of CO2. Appl. Catal. B Environ. 2009, 89, 494–502. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Hydrothermal/solvothermal synthesis and treatment of TiO2 for photocatalytic degradation of air pollutants: Preparation, characterization, properties, and performance. Chemosphere 2019, 219, 804–825. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Guo, R.-T.; Pan, W.-G.; Tang, J.-Y.; Zhou, W.-G.; Liu, X.-Y.; Qin, H.; Jia, P.-Y. One-dimension TiO2 nanostructures with enhanced activity for CO2 photocatalytic reduction. Appl. Surf. Sci. 2019, 464, 534–543. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Arruda, L.B.; Santos, C.M.; Orlandi, M.O.; Schreiner, W.H.; Lisboa-Filho, P.N. Formation and evolution of TiO2 nanotubes in alkaline synthesis. Ceram. Int. 2015, 41, 2884–2891. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Navarrete, J.; Gomez, R. Synthesis, characterization and photocatalytic activity of TiO2 nanostructures: Nanotubes, nanofibers, nanowires and nanoparticles. Catal. Today 2016, 266, 90–101. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, H.; Guo, L.-H.; Zhao, L. Two-dimensional interface engineering of a titania–graphene nanosheet composite for improved photocatalytic activity. ACS Appl. Mater. Interfaces 2013, 5, 13035–13041. [Google Scholar] [CrossRef]
- Lee, J.; Li, Z.; Zhu, L.; Xie, S.; Cui, X. Ti3+ self-doped TiO2 via facile catalytic reduction over Al (acac)3 with enhanced photoelectrochemical and photocatalytic activities. Appl. Catal. B Environ. 2018, 224, 715–724. [Google Scholar] [CrossRef]
- Li, X.; Wan, T.; Qiu, J.; Wei, H.; Qin, F.; Wang, Y.; Liao, Y.; Huang, Z.; Tan, X. In-situ photocalorimetry-fluorescence spectroscopy studies of RhB photocatalysis over Z-scheme g-C3N4@ Ag@ Ag3PO4 nanocomposites: A pseudo-zero-order rather than a first-order process. Appl. Catal. B Environ. 2017, 217, 591–602. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L.; Cheng, B.; Su, Y. Hydrothermal preparation and photocatalytic activity of hierarchically sponge-like macro-/mesoporous titania. J. Phys. Chem. C 2007, 111, 10582–10589. [Google Scholar] [CrossRef]
- Zheng, X.; Kuang, Q.; Yan, K.; Qiu, Y.; Qiu, J.; Yang, S. Mesoporous TiO2 single crystals: Facile shape-, size-, and phase-controlled growth and efficient photocatalytic performance. ACS Appl. Mater. Interfaces 2013, 5, 11249–11257. [Google Scholar] [CrossRef]
- Li, X.; Zheng, W.; He, G.; Zhao, R.; Liu, D. Morphology control of TiO2 nanoparticle in microemulsion and its photocatalytic property. ACS Sustain. Chem. Eng. 2014, 2, 288–295. [Google Scholar] [CrossRef]
- Dong, W.; Yao, Y.; Li, L.; Sun, Y.; Hua, W.; Zhuang, G.; Zhao, D.; Yan, S.; Song, W. Three-dimensional interconnected mesoporous anatase TiO2 exhibiting unique photocatalytic performances. Appl. Catal. B Environ. 2017, 217, 293–302. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, X.; Zhang, H.; Liao, Y.; Xiang, Q. Enhanced photocatalytic hydrogen evolution activity of carbon and nitrogen self-doped TiO2 hollow sphere with the creation of oxygen vacancy and Ti3+. Mater. Today Energy 2018, 10, 132–140. [Google Scholar] [CrossRef]
- Liu, D.; Cai, W.; Wang, Y.; Zhu, Y. Constructing a novel Bi2SiO5/BiPO4 heterostructure with extended light response range and enhanced photocatalytic performance. Appl. Catal. B Environ. 2018, 236, 205–211. [Google Scholar] [CrossRef]
- Likodimos, V.; Chrysi, A.; Calamiotou, M.; Fernández-Rodríguez, C.; Doña-Rodríguez, J.M.; Dionysiou, D.; Falaras, P. Microstructure and charge trapping assessment in highly reactive mixed phase TiO2 photocatalysts. Appl. Catal. B Environ. 2016, 192, 242–252. [Google Scholar] [CrossRef]
- Kong, M.; Li, Y.; Chen, X.; Tian, T.; Fang, P.; Zheng, F.; Zhao, X. Tuning the relative concentration ratio of bulk defects to surface defects in TiO2 nanocrystals leads to high photocatalytic efficiency. J. Am. Chem. Soc. 2011, 133, 16414–16417. [Google Scholar] [CrossRef]
- Yang, G.; Yan, Z.; Xiao, T.; Yang, B. Low-temperature synthesis of alkalis doped TiO2 photocatalysts and their photocatalytic performance for degradation of methyl orange. J. Alloys Compd. 2013, 580, 15–22. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, X.; Li, Y.; Chen, H.; Ye, X.; Wang, Y.; Ye, J. Anatase TiO2 Mesocrystals: Green Synthesis, In Situ Conversion to Porous Single Crystals, and Self-Doping Ti3+ for Enhanced Visible Light Driven Photocatalytic Removal of NO. Chem. Eur. J. 2017, 23, 5478–5487. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, W.; Fang, J.; Xu, X.; Wu, S.; Zhu, X.; Chen, Z. Decoration of BiOI quantum size nanoparticles with reduced graphene oxide in enhanced visible-light-driven photocatalytic studies. Appl. Surf. Sci. 2012, 259, 441–447. [Google Scholar] [CrossRef]
- Jiang, Y.; Ning, H.; Tian, C.; Jiang, B.; Li, Q.; Yan, H.; Zhang, X.; Wang, J.; Jing, L.; Fu, H. Single-crystal TiO2 nanorods assembly for efficient and stable cocatalyst-free photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 229, 1–7. [Google Scholar] [CrossRef]
- Chen, D.; Hao, Q.; Wang, Z.; Ding, H.; Zhu, Y. Influence of phase structure and morphology on the photocatalytic activity of bismuth molybdates. Cryst. Eng. Comm. 2016, 18, 1976–1986. [Google Scholar] [CrossRef]
- Ziarati, A.; Badiei, A.; Luque, R. Black hollow TiO2 nanocubes: Advanced nanoarchitectures for efficient visible light photocatalytic applications. Appl. Catal. B Environ. 2018, 238, 177–183. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, J.; Wang, Y.; Wu, M.; Meng, M.; Tian, Y.; Li, X.; Zhang, J.; Zheng, L.; Jiang, Z. Insights into the effects of surface/bulk defects on photocatalytic hydrogen evolution over TiO2 with exposed {001} facets. Appl. Catal. B Environ. 2018, 220, 126–136. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P.; Huang, B. Self-doped TiO2- x nanowires with enhanced photocatalytic activity: Facile synthesis and effects of the Ti3+. Appl. Surf. Sci. 2015, 356, 391–398. [Google Scholar] [CrossRef]
- Moradi, V.; Jun, M.B.; Blackburn, A.; Herring, R.A. Significant improvement in visible light photocatalytic activity of Fe doped TiO2 using an acid treatment process. Appl. Surf. Sci. 2018, 427, 791–799. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, M.; Guan, Z.; Li, Q.; Yang, J. Effect of annealing ambience on the formation of surface/bulk oxygen vacancies in TiO2 for photocatalytic hydrogen evolution. Appl. Surf. Sci. 2018, 428, 640–647. [Google Scholar] [CrossRef]
- Wang, K.; Yao, W.; Teng, F.; Zhu, Y. Photocatalytic activity enhancement of LaPO4 via surface oxygen vacancies. RSC Adv. 2015, 5, 56711–56716. [Google Scholar] [CrossRef]
- Pan, J.; Dong, Z.; Wang, B.; Jiang, Z.; Zhao, C.; Wang, J.; Song, C.; Zheng, Y.; Li, C. The enhancement of photocatalytic hydrogen production via Ti3+ self-doping black TiO2/g-C3N4 hollow core-shell nano-heterojunction. Appl. Catal. B Environ. 2019, 242, 92–99. [Google Scholar] [CrossRef]
- Li, K.; Huang, Z.; Zeng, X.; Huang, B.; Gao, S.; Lu, J. Synergetic effect of Ti3+ and oxygen doping on enhancing photoelectrochemical and photocatalytic properties of TiO2/g-C3N4 heterojunctions. ACS Appl. Mater. Interfaces 2017, 9, 11577–11586. [Google Scholar] [CrossRef]
- Lv, Y.; Yao, W.; Ma, X.; Pan, C.; Zong, R.; Zhu, Y. The surface oxygen vacancy induced visible activity and enhanced UV activity of a ZnO1−x photocatalyst. Catal. Sci. Technol. 2013, 3, 3136–3146. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, G.; Wan, Z. Visible-light-driven g-C3N4/Ti3+-TiO2 photocatalyst co-exposed {0 0 1} and {1 0 1} facets and its enhanced photocatalytic activities for organic pollutant degradation and Cr (VI) reduction. Appl. Surf. Sci. 2015, 358, 223–230. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Zhang, K.; Wang, J.; Sun, B.; Li, H.; Qiao, P.; Wang, L.; Zhou, W. Ti3+ self-doped black TiO2 nanotubes with mesoporous nanosheet architecture as efficient solar-driven hydrogen evolution photocatalysts. ACS Sustain. Chem. Eng. 2017, 5, 6894–6901. [Google Scholar] [CrossRef]
- Kumar, R.; Govindarajan, S.; Siri Kiran Janardhana, R.K.; Rao, T.N.; Joshi, S.V.; Anandan, S. Facile one-step route for the development of in situ cocatalyst-modified Ti3+ self-doped TiO2 for improved visible-light photocatalytic activity. ACS Appl. Mater. Interfaces 2016, 8, 27642–27653. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.; Tian, Y.; Lan, K.; Liu, Q.; Wang, C.; Liu, Y.; Elzatahry, A.; Che, R.; Li, W. Synthesis of uniform ordered mesoporous TiO2 microspheres with controllable phase junctions for efficient solar water splitting. Chem. Sci. 2019, 10, 1664–1670. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Yao, W.; Zong, R.; Zhu, Y. New insights into the relationship between photocatalytic activity and TiO2-GR composites. RSC Adv. 2015, 5, 29201–29208. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; An, W.; Liu, L.; Liang, Y.; Zhu, Y. Combination of photoelectrocatalysis and adsorption for removal of bisphenol A over TiO2-graphene hydrogel with 3D network structure. Appl. Catal. B Environ. 2018, 221, 36–46. [Google Scholar] [CrossRef]
- Zhang, A.-Y.; Lin, T.; He, Y.-Y.; Mou, Y.-X. Heterogeneous activation of H2O2 by defect-engineered TiO2-x single crystals for refractory pollutants degradation: A Fenton-like mechanism. J. Hazard. Mater. 2016, 311, 81–90. [Google Scholar] [CrossRef]
- Cushing, S.K.; Meng, F.; Zhang, J.; Ding, B.; Chen, C.K.; Chen, C.-J.; Liu, R.-S.; Bristow, A.D.; Bright, J.; Zheng, P. Effects of defects on photocatalytic activity of hydrogen-treated titanium oxide nanobelts. ACS Catal. 2017, 7, 1742–1748. [Google Scholar] [CrossRef]
- Wan, J.; Chen, W.; Jia, C.; Zheng, L.; Dong, J.; Zheng, X.; Wang, Y.; Yan, W.; Chen, C.; Peng, Q. Defect effects on TiO2 nanosheets: Stabilizing single atomic site Au and promoting catalytic properties. Adv. Mater. 2018, 30, 1705369. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, F.; Lin, H.; Xie, Y.; Tong, N.; Lin, J.; Zhang, X.; Zhang, Z.; Wang, X. Surface oxygen vacancy and defect engineering of WO3 for improved visible light photocatalytic performance. Catal. Sci. Technol. 2018, 8, 4399–4406. [Google Scholar] [CrossRef]
- Liu, D.; Lv, Y.; Zhang, M.; Liu, Y.; Zhu, Y.; Zong, R.; Zhu, Y. Defect-related photoluminescence and photocatalytic properties of porous ZnO nanosheets. J. Mater. Chem. A 2014, 2, 15377–15388. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cell. 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J., Jr. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Li, X.; Gao, C.; Wang, J.; Lu, B.; Chen, W.; Song, J.; Zhang, S.; Zhang, Z.; Pan, X.; Xie, E. TiO2 films with rich bulk oxygen vacancies prepared by electrospinning for dye-sensitized solar cells. J. Power Sources. 2012, 214, 244–250. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Jiang, J.; Rong, Y.; Wang, Y.; Wu, Y.; Pan, C. Characterization of oxygen vacancy associates within hydrogenated TiO2: A positron annihilation study. J. Phys. Chem. C 2012, 116, 22619–22624. [Google Scholar] [CrossRef]
- Hamandi, M.; Berhault, G.; Guillard, C.; Kochkar, H. Reduced graphene oxide/TiO2 nanotube composites for formic acid photodegradation. Appl. Catal. B Environ. 2017, 209, 203–213. [Google Scholar] [CrossRef]
- Gao, H.; Hu, G.; Sui, J.; Mu, C.; Shangguan, W.; Kong, M.; Shentu, W. Scalable preparation of defect-rich free-standing TiO2 sheets with visible-light photocatalytic activity. Appl. Catal. B Environ. 2018, 226, 337–345. [Google Scholar] [CrossRef]
- Liqiang, J.; Xiaojun, S.; Baifu, X.; Baiqi, W.; Weimin, C.; Honggang, F. The preparation and characterization of La doped TiO2 nanoparticles and their photocatalytic activity. J. Solid State Chem. 2004, 177, 3375–3382. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, J.; Peng, C.; Xiang, Y.; Chen, H. Enhanced photocatalytic hydrogen production over conjugated polymer/black TiO2 hybrid: The impact of constructing active defect states. Appl. Surf. Sci. 2019, 465, 288–296. [Google Scholar] [CrossRef]
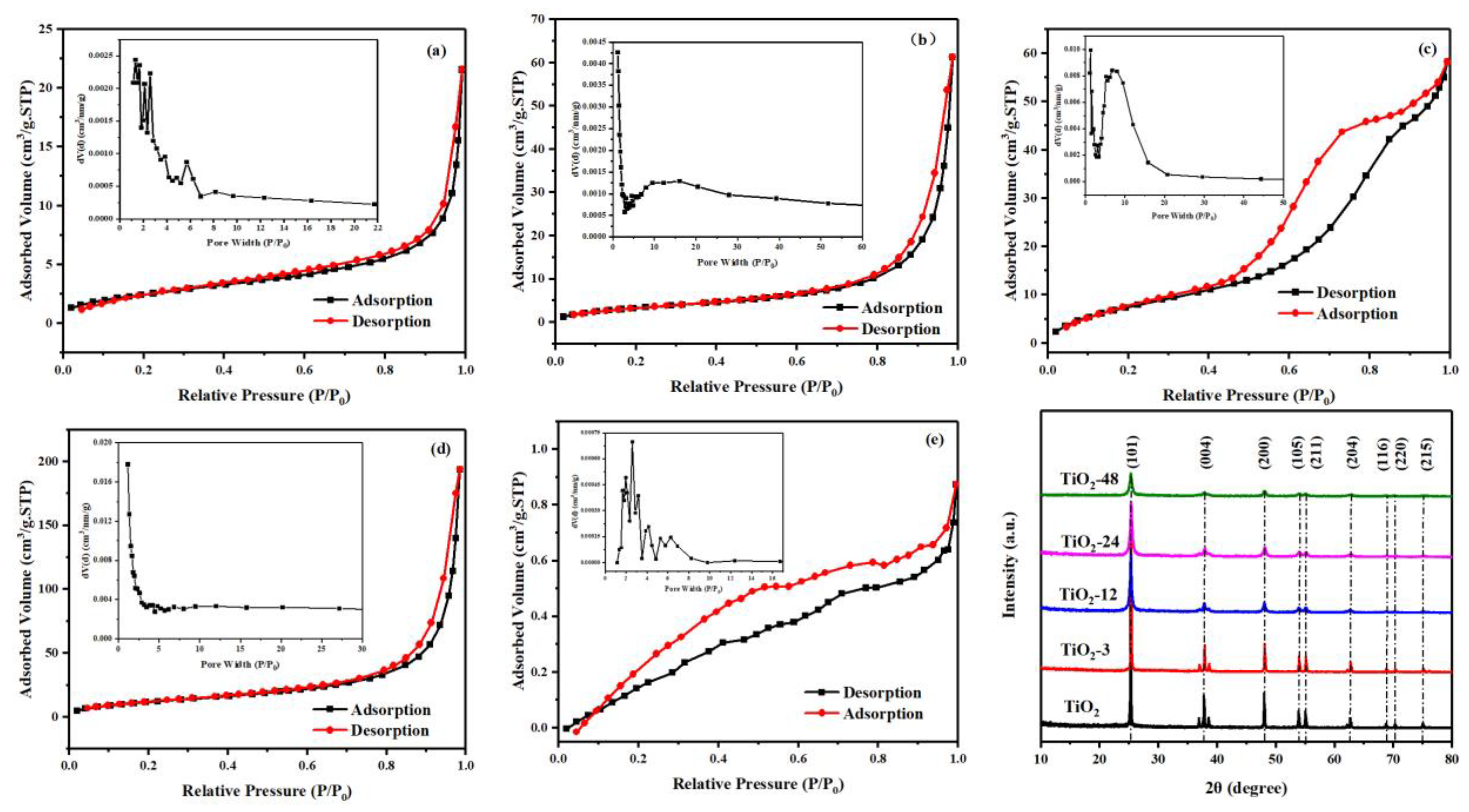

| Sample | SBET 1 (m2/g) | Pore Volume 2 (cm3/g) | Pore Diameter 2 (nm) | Eg 3 (eV) | dXRD 4 (nm) |
|---|---|---|---|---|---|
| TiO2 | 13.060 | 0.035 | 1.335 | 3.27 | 12.96 |
| TiO2-3 | 23.164 | 0.099 | 1.149 | 3.26 | 7.92 |
| TiO2-12 | 59.742 | 0.102 | 1.336 | 3.16 | 3.94 |
| TiO2-24 | 78.888 | 0.312 | 1.145 | 3.42 | 3.44 |
| TiO2-48 | 1.592 | 0.002 | 2.592 | 3.09 | 3.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials 2020, 10, 546. https://doi.org/10.3390/nano10030546
Li D, Song H, Meng X, Shen T, Sun J, Han W, Wang X. Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials. 2020; 10(3):546. https://doi.org/10.3390/nano10030546
Chicago/Turabian StyleLi, Danqi, Hongchen Song, Xia Meng, Tingting Shen, Jing Sun, Wenjia Han, and Xikui Wang. 2020. "Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2" Nanomaterials 10, no. 3: 546. https://doi.org/10.3390/nano10030546
APA StyleLi, D., Song, H., Meng, X., Shen, T., Sun, J., Han, W., & Wang, X. (2020). Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials, 10(3), 546. https://doi.org/10.3390/nano10030546






